The Association of EEG μ Rhythm Phase and Power with TMS-Assessed Cortical Excitability States
Abstract
1. Introduction
2. Methods
2.1. Subjects
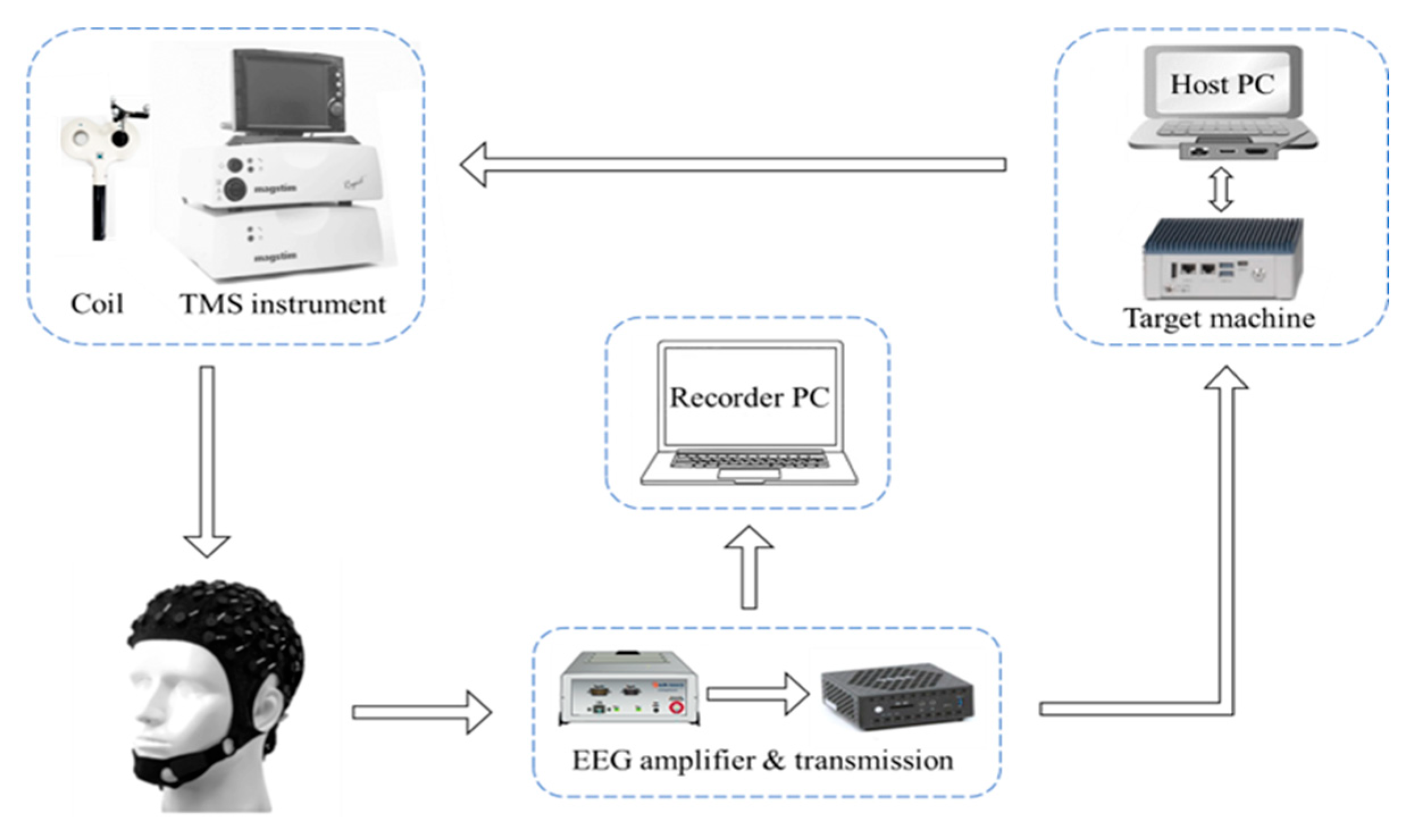
2.2. Experimental Set-Up
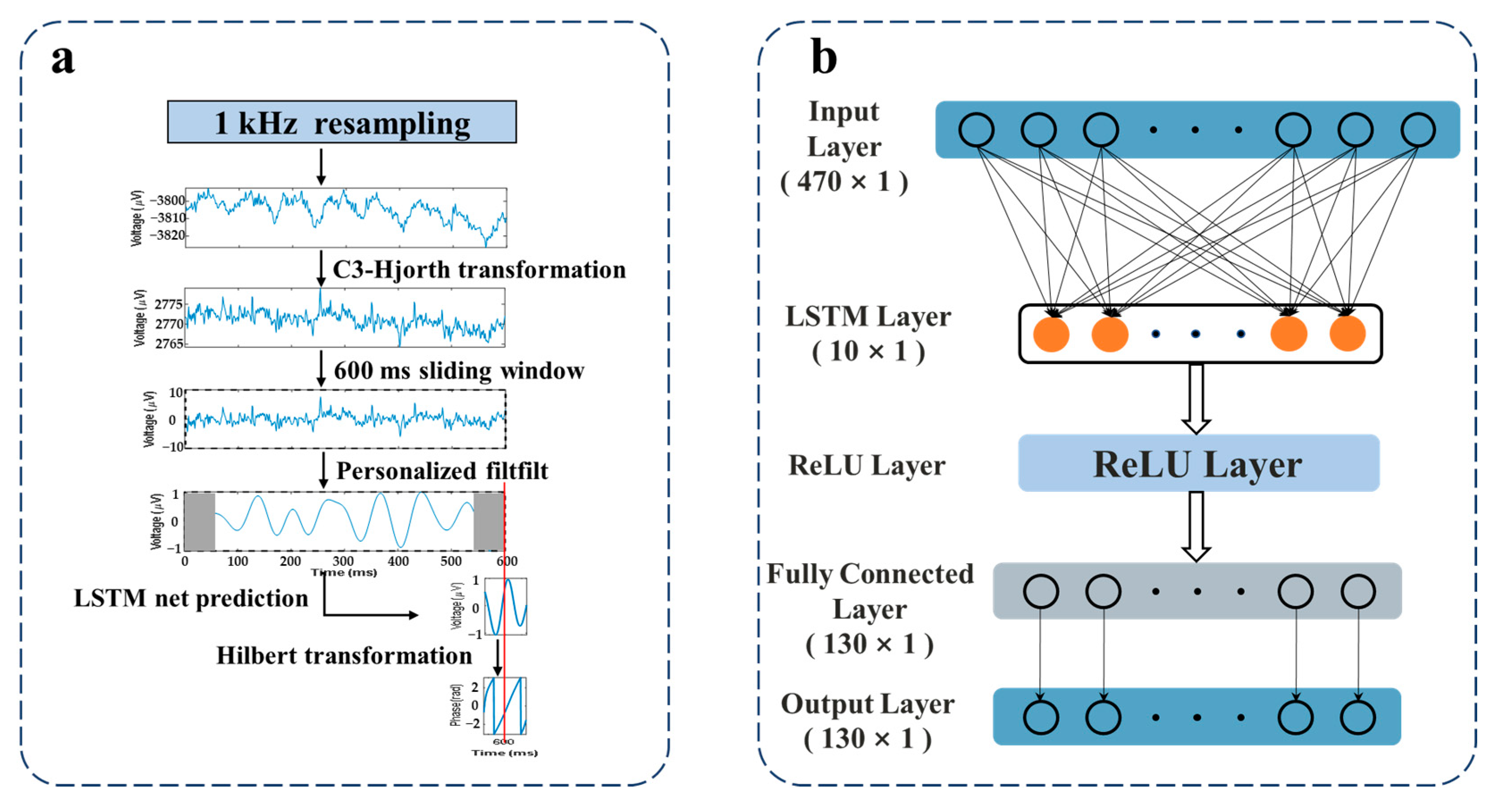
2.3. EEG Signal Processing
2.4. EEG and Electromyography (EMG) Recording
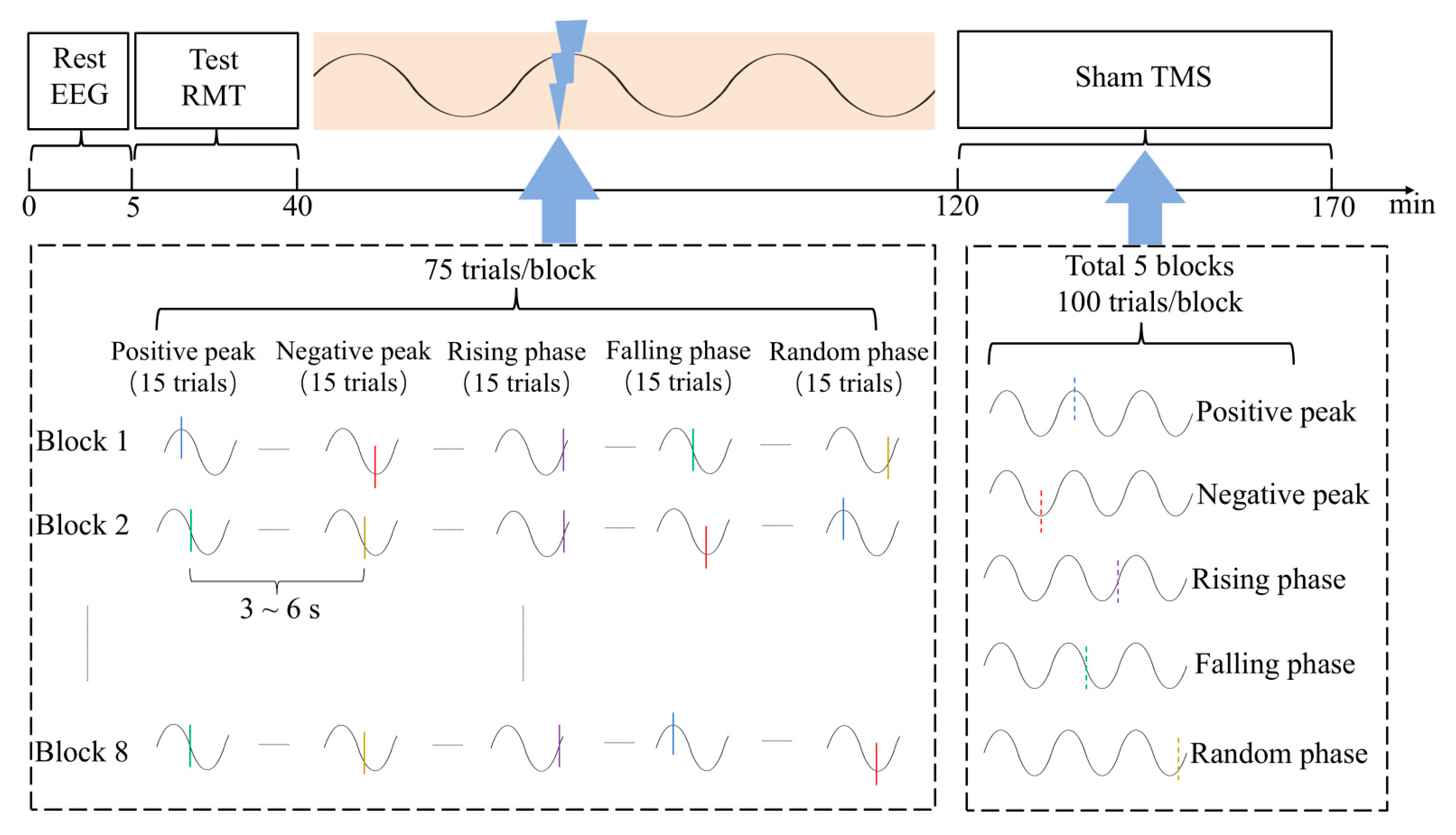
2.5. Experimental Session
2.6. Pre-Stimulus Power
2.7. MEP Analysis
2.8. TEPs of Left Sensorimotor Cortex
2.9. Statistics
3. Results
3.1. Online Phase-Triggered EEG-TMS
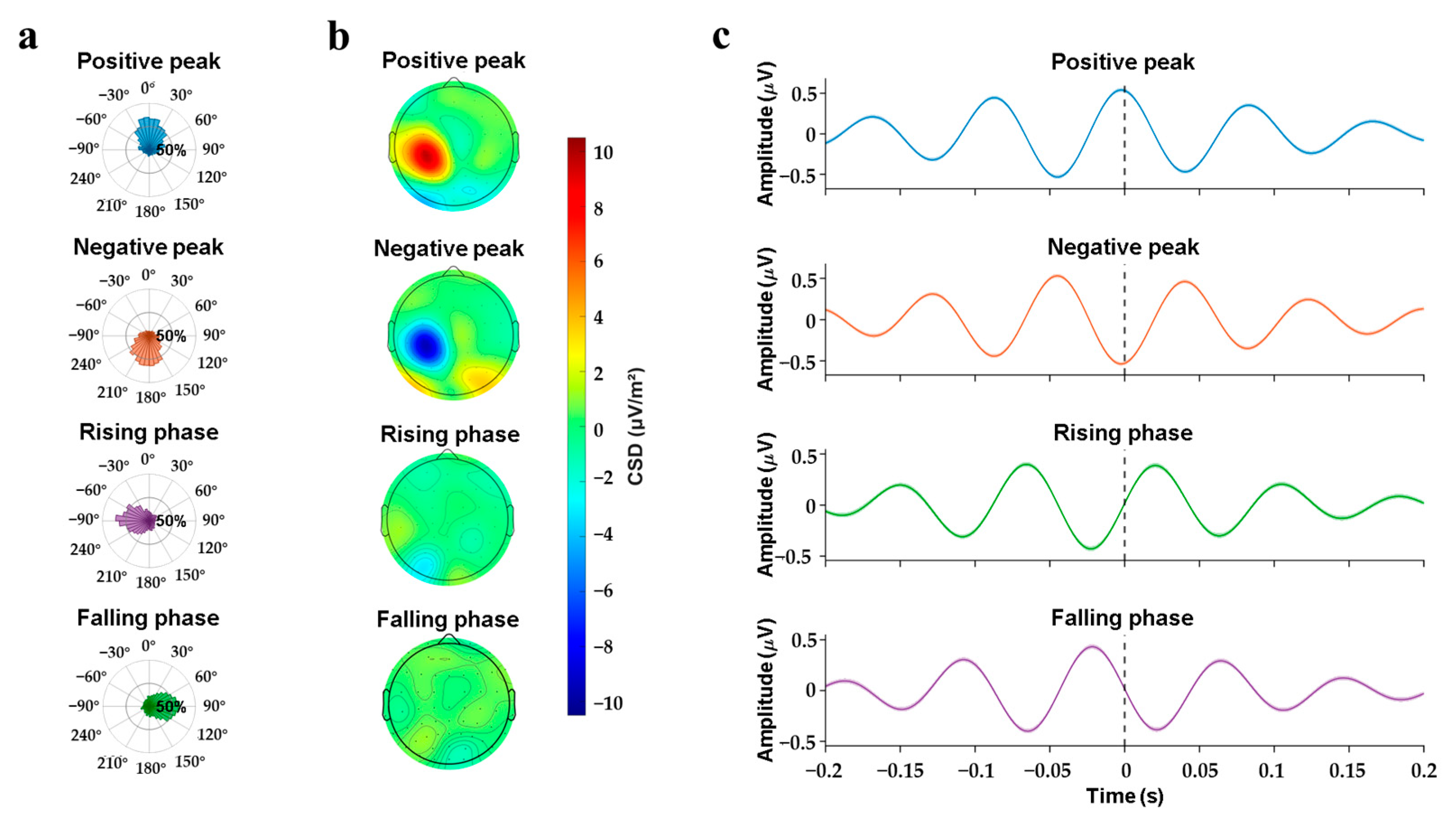
3.2. Phase-Modulation
3.3. Power-Modulation
3.4. Consistency of Coil Position During Closed-Loop TMS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrarelli, F.; Phillips, M.L. Examining and Modulating Neural Circuits in Psychiatric Disorders with Transcranial Magnetic Stimulation and Electroencephalography: Present Practices and Future Developments. Am. J. Psychiatry 2021, 178, 400–413. [Google Scholar] [CrossRef]
- Miyauchi, E.; Ide, M.; Tachikawa, H.; Nemoto, K.; Arai, T.; Kawasaki, M. A novel approach for assessing neuromodulation using phase-locked information measured with TMS-EEG. Sci. Rep. 2019, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Shirinpour, S.; Alekseichuk, I.; Mantell, K.; Opitz, A. Experimental evaluation of methods for real-time EEG phase-specific transcranial magnetic stimulation. J. Neural Eng. 2020, 17, 046002. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, C.; Desideri, D.; Belardinelli, P.; Ziemann, U. Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul. 2018, 11, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, Y.; Li, J.; Li, X. Closed-loop TMS-EEG reactivity with occipital alpha-phase synchronized. J. Neural Eng. 2022, 19, 056027. [Google Scholar] [CrossRef]
- Poorganji, M.; Zomorrodi, R.; Zrenner, C.; Bansal, A.; Hawco, C.; Hill, A.T.; Hadas, I.; Rajji, T.K.; Chen, R.; Zrenner, B.; et al. Pre-Stimulus Power but Not Phase Predicts Prefrontal Cortical Excitability in TMS-EEG. Biosensors 2023, 13, 220. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Yu, Y.; Si, X.; Hu, C.; Zhang, J. A Review of Recurrent Neural Networks: LSTM Cells and Network Architectures. Neural Comput. 2019, 31, 1235–1270. [Google Scholar] [CrossRef]
- Hernandez-Pavon, J.C.; Veniero, D.; Bergmann, T.O.; Belardinelli, P.; Bortoletto, M.; Casarotto, S.; Casula, E.P.; Farzan, F.; Fecchio, M.; Julkunen, P.; et al. TMS combined with EEG: Recommendations and open issues for data collection and analysis. Brain Stimul. 2023, 16, 567–593. [Google Scholar] [CrossRef]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10-10 system. Neuroimage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Murray, P.D.; Keller, A. Somatosensory response properties of excitatory and inhibitory neurons in rat motor cortex. J. Neurophysiol. 2011, 106, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.M.; Heyl, M.; Mejer, L.; Vinding, M.C.; Christiansen, L.; Tomasevic, L.; Siebner, H.R. Methodological Choices Matter: A Systematic Comparison of TMS-EEG Studies Targeting the Primary Motor Cortex. Hum. Brain Mapp. 2024, 45, e70048. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of TMS in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; André-Obadia, N.; Poulet, E.; Devanne, H.; Haffen, E.; Londero, A.; Cretin, B.; Leroi, A.-M.; Radtchenko, A.; Saba, G.; et al. Recommandations françaises sur l’utilisation de la stimulation magnétique transcrânienne répétitive (rTMS): Règles de sécurité et indications thérapeutiques. Clin. Neurophysiol. 2011, 41, 221–295. [Google Scholar] [CrossRef]
- Antal, A.; Luber, B.; Brem, A.K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljević, V.; Fecteau, S.; Ferreri, F.; Flöel, A.; et al. Non-invasive brain stimulation and neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef]
- Madsen, K.H.; Karabanov, A.N.; Krohne, L.G.; Safeldt, M.G.; Tomasevic, L.; Siebner, H.R. No trace of phase: Corticomotor excitability is not tuned by phase of pericentral mu-rhythm. Brain Stimul. 2019, 12, 1261–1270. [Google Scholar] [CrossRef]
- Schilberg, L.; Ten Oever, S.; Schuhmann, T.; Sack, A.T. Phase and power modulations on the amplitude of TMS-induced motor evoked potentials. PLoS ONE 2021, 16, e0255815. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Sullivan, C.; Thomson, R.H.; Rose, N.S.; Bailey, N.W.; Fitzgerald, P.B.; Farzan, F.; Hernandez-Pavon, J.C. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: A review and introduction to the open-source TESA software. Neuroimage 2017, 147, 934–951. [Google Scholar] [CrossRef]
- Esser, S.K.; Huber, R.; Massimini, M.; Peterson, M.J.; Ferrarelli, F.; Tononi, G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res. Bull. 2006, 69, 86–94. [Google Scholar] [CrossRef]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci. Biobehav. Rev. 2016, 64, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Knowles, K.S.; Beausejour, J.P.; Harmon, K.K.; Girts, R.M.; Fukuda, D.H.; Kidgell, D.J.; Stock, M.S. The Influence of Transcranial Magnetic Stimulation Interpulse Interval Duration on Knee Extensor Corticospinal Excitability. Brain Connect. 2023, 13, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Nguyen, H.; Vrana, A.; Baldwin, S.; Li, C.Q.; Giles, A.; Wang, J.; Yang, Y.; Lu, H. A high-density theta burst paradigm enhances the aftereffects of transcranial magnetic stimulation: Evidence from focal stimulation of rat motor cortex. Brain Stimul. 2022, 15, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Schaworonkow, N.; Triesch, J.; Ziemann, U.; Zrenner, C. EEG-triggered TMS reveals stronger brain state-dependent modulation of motor evoked potentials at weaker stimulation intensities. Brain Stimul. 2019, 12, 110–118. [Google Scholar] [CrossRef]
- Zrenner, C.; Belardinelli, P.; Ermolova, M.; Gordon, P.C.; Stenroos, M.; Zrenner, B.; Ziemann, U. µ-rhythm phase from somatosensory but not motor cortex correlates with corticospinal excitability in EEG-triggered TMS. J. Neurosci. Methods 2022, 379, 109662. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Desideri, D.; Belardinelli, P.; Zrenner, C.; Ziemann, U. Phase synchronicity of mu-rhythm determines efficacy of interhemispheric communication between human motor cortices. J. Neurosci. 2018, 38, 10525–10534. [Google Scholar] [CrossRef]
- Miller, K.J.; Hermes, D.; Honey, C.J.; Hebb, A.O.; Ramsey, N.F.; Knight, R.T.; Ojemann, J.G.; E Fetz, E. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput. Biol. 2012, 8, e1002655. [Google Scholar] [CrossRef]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef]
- Zrenner, C.; Ziemann, U. Closed-Loop Brain Stimulation. Biol. Psychiatry 2024, 95, 545–552. [Google Scholar] [CrossRef]
- Gogulski, J.; Ross, J.M.; Talbot, A.; Cline, C.C.; Donati, F.L.; Munot, S.; Kim, N.; Gibbs, C.; Bastin, N.; Yang, J.; et al. Personalized Repetitive Transcranial Magnetic Stimulation for Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 351–360. [Google Scholar] [CrossRef]
- Biabani, M.; Fornito, A.; Coxon, J.P.; Fulcher, B.D.; Rogasch, N.C. The correspondence between EMG and EEG measures of changes in cortical excitability following transcranial magnetic stimulation. J. Physiol. 2021, 599, 2907–2932. [Google Scholar] [CrossRef]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical utility and prospective of TMS-EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef]
- Volz, L.J.; Hamada, M.; Rothwell, J.C.; Grefkes, C. What Makes the Muscle Twitch: Motor System Connectivity and TMS-Induced Activity. Cereb. Cortex 2015, 25, 2346–2353. [Google Scholar] [CrossRef]
- Gordon, P.C.; Desideri, D.; Belardinelli, P.; Zrenner, C.; Ziemann, U. Comparison of cortical EEG responses to realistic sham versus real TMS of human motor cortex. Brain Stimul. 2018, 11, 1322–1330. [Google Scholar] [CrossRef]
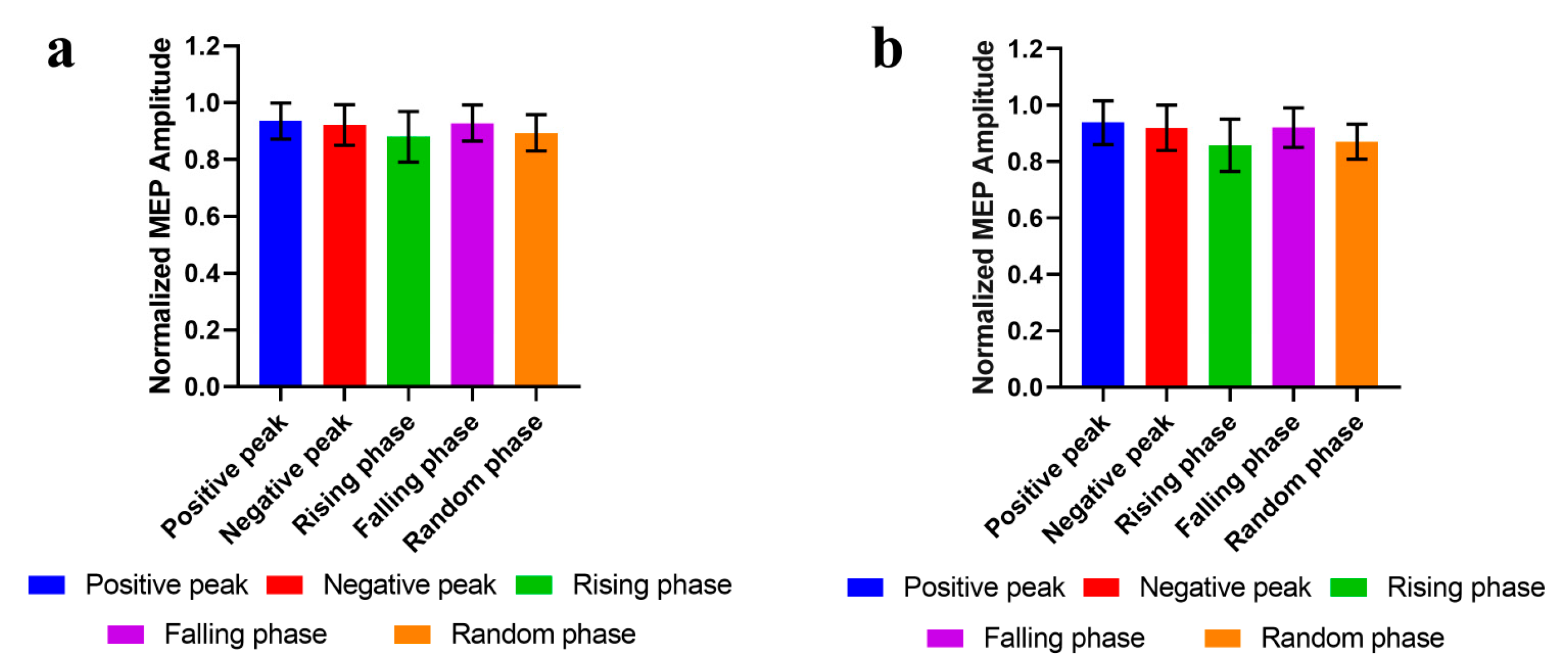
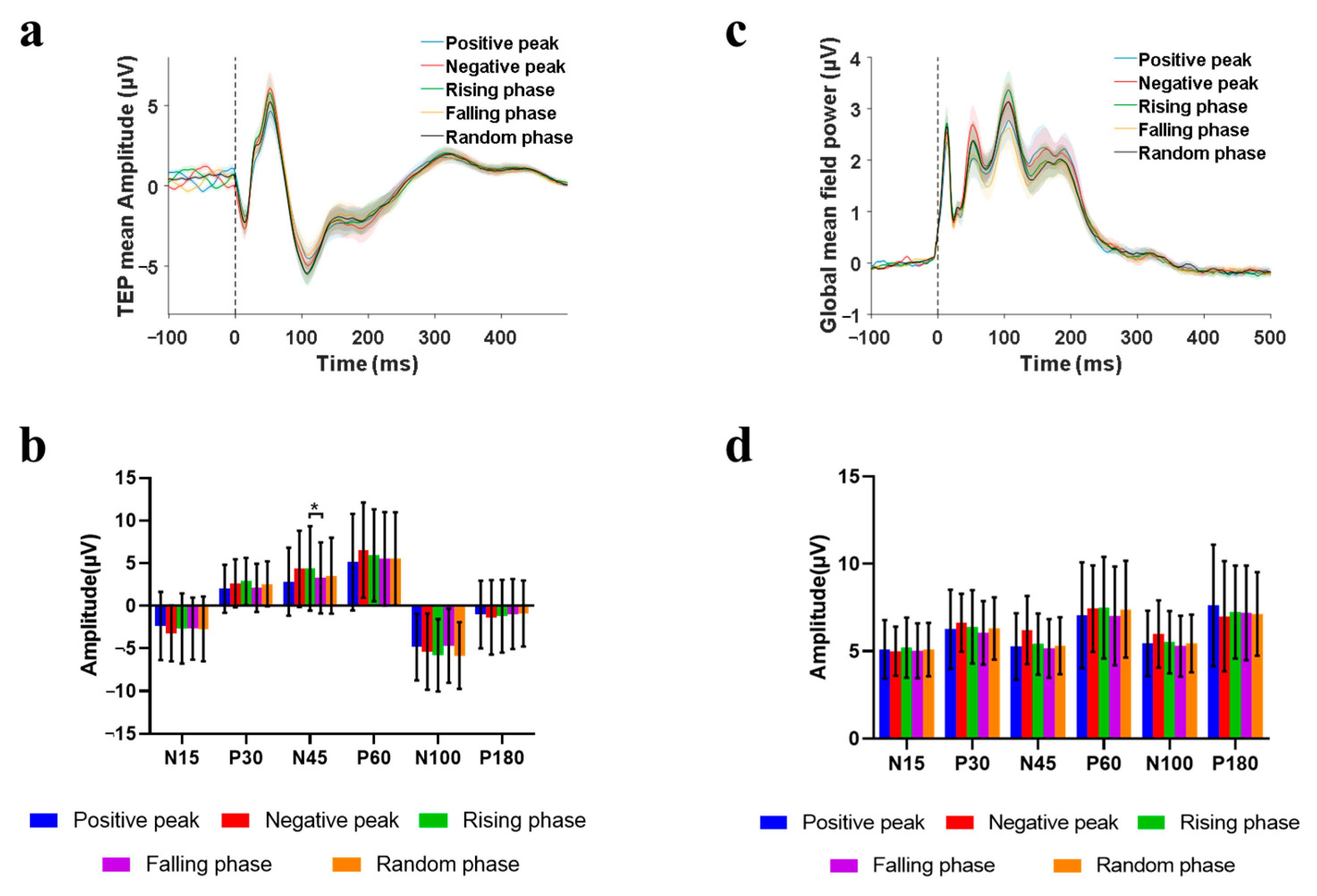
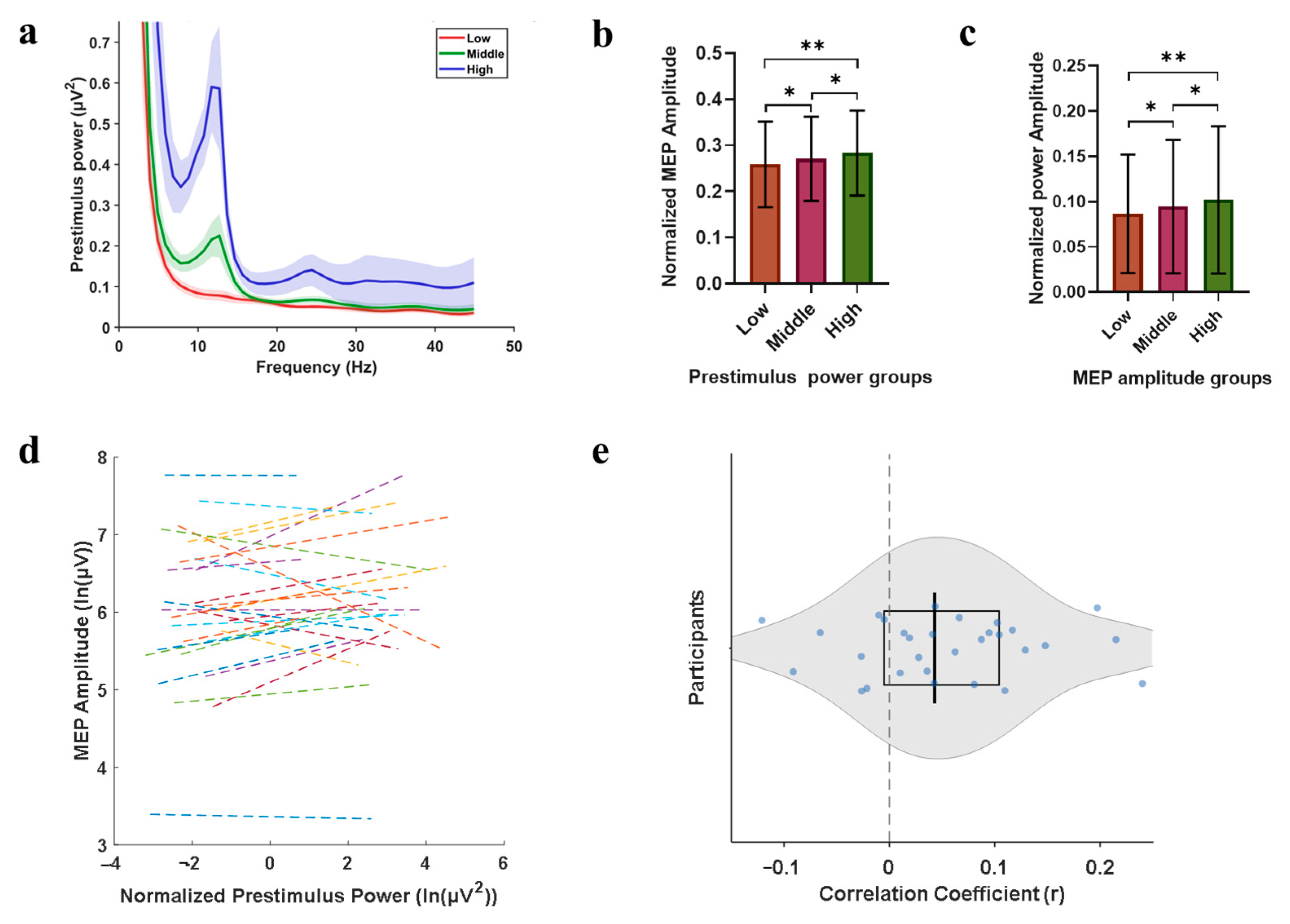
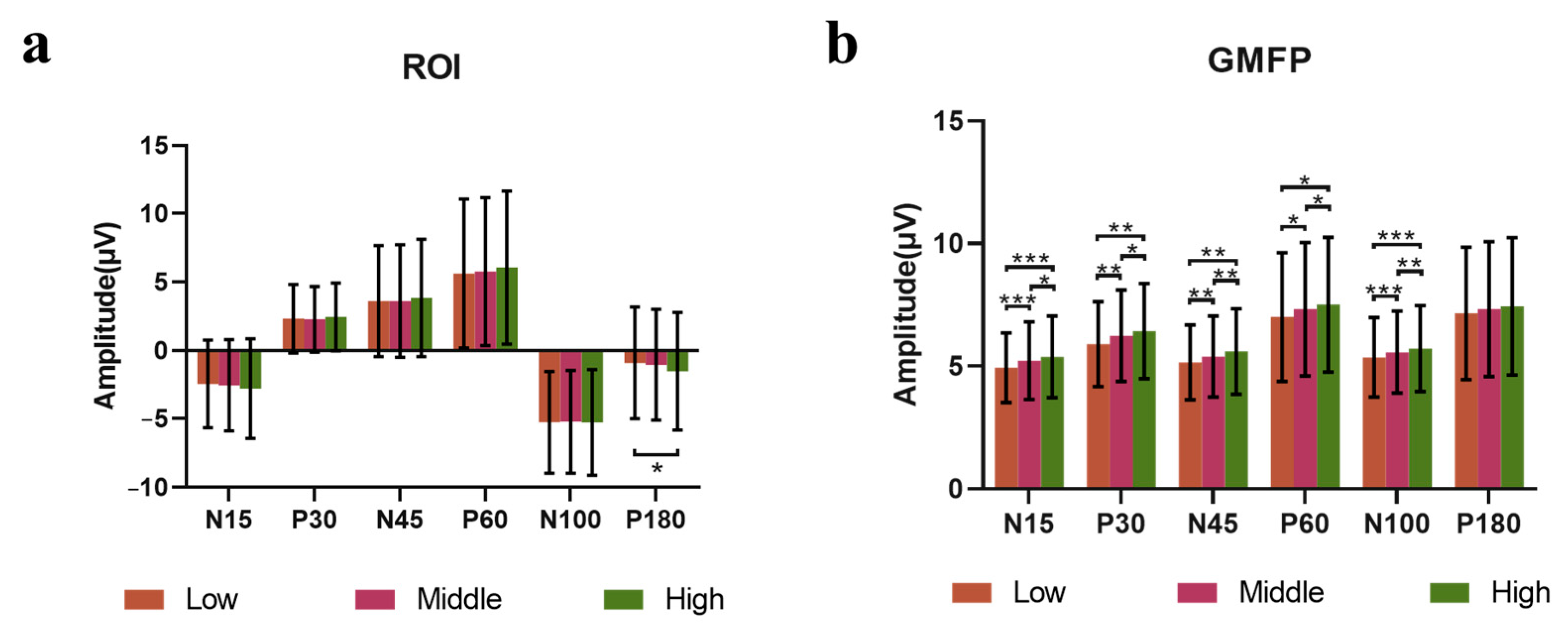
| Phase (°) | Target Error (mm) (mean ± std) | F Statistic | p Value | Angular Error (°) (mean ± std) | F Statistic | p Value |
|---|---|---|---|---|---|---|
| 0 | 0.955 ± 0.570 | 1.483 | 0.219 | 2.729 ± 1.801 | 1.833 | 0.153 |
| 180 | 0.965 ± 0.645 | 2.637 ± 1.842 | ||||
| −90 | 0.953 ± 0.604 | 2.787 ± 1.879 | ||||
| 90 | 0.982 ± 0.601 | 2.700 ± 1.799 | ||||
| random | 1.001 ± 0.625 | 2.701 ± 1.891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, W.; Zhao, X.; Chen, P.; Zhao, Y.; Wang, H.; Wang, X.; Liu, Z.; Jin, J.; Yin, T. The Association of EEG μ Rhythm Phase and Power with TMS-Assessed Cortical Excitability States. Sensors 2025, 25, 7187. https://doi.org/10.3390/s25237187
Mai W, Zhao X, Chen P, Zhao Y, Wang H, Wang X, Liu Z, Jin J, Yin T. The Association of EEG μ Rhythm Phase and Power with TMS-Assessed Cortical Excitability States. Sensors. 2025; 25(23):7187. https://doi.org/10.3390/s25237187
Chicago/Turabian StyleMai, Wenshu, Xinyu Zhao, Panli Chen, Yuezhuo Zhao, He Wang, Xin Wang, Zhipeng Liu, Jingna Jin, and Tao Yin. 2025. "The Association of EEG μ Rhythm Phase and Power with TMS-Assessed Cortical Excitability States" Sensors 25, no. 23: 7187. https://doi.org/10.3390/s25237187
APA StyleMai, W., Zhao, X., Chen, P., Zhao, Y., Wang, H., Wang, X., Liu, Z., Jin, J., & Yin, T. (2025). The Association of EEG μ Rhythm Phase and Power with TMS-Assessed Cortical Excitability States. Sensors, 25(23), 7187. https://doi.org/10.3390/s25237187





