An Overview of Stress Analysis Based on Physiological Signals: Systematic Review of Open Datasets and Current Trends
Abstract
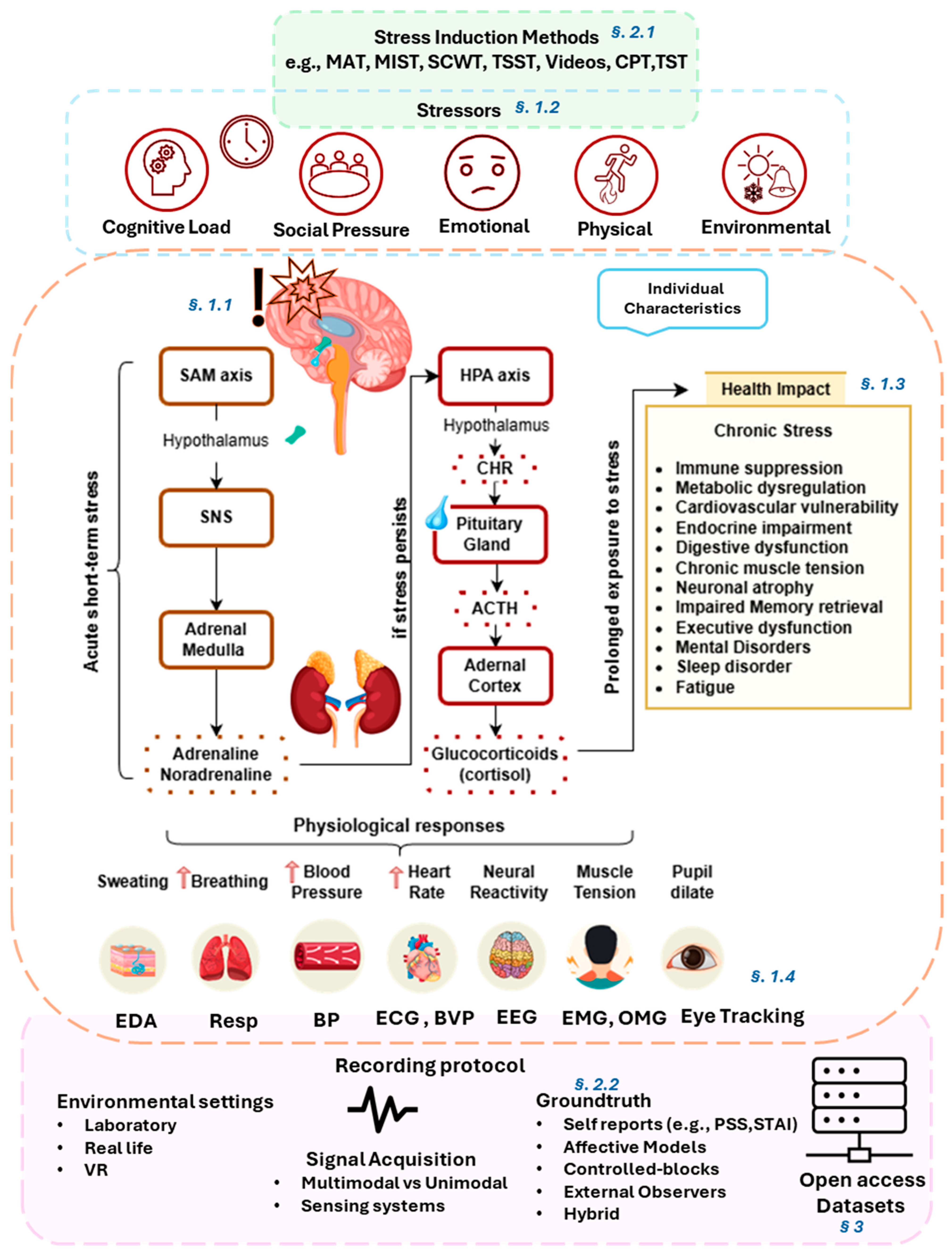
1. Introduction
1.1. Stress Mechanism
1.2. Types of Stress
1.3. Physiological and Psychological Effects
1.4. Biosignals in Stress Analysis
2. Experimental Framework for Stress Analysis
2.1. Stress Induction Methods
| Ref. | Stress Induction Method | Description | Related Stress Type |
|---|---|---|---|
| [102] | Mental Arithmetic Task (MAT) | Solving arithmetic problems under time constraints. Stress increases with difficulty and pressure. | Cognitive Load and Social (Task Performance) |
| [103] | Montreal Imaging Stress Task (MIST) | Mental arithmetic task with random failure feedback, even when correct, inducing frustration. | Cognitive Load and Social (Task Performance) |
| [104] | Paced Auditory Serial Addition Test (PASAT) | Listening to numbers and continuously summing the last two heard while time constraints increase. | Cognitive Load and Social (Task Performance) |
| [105,106] | Stroop Color Word Test (SCWT) | Naming the color of an incongruent word (e.g., “BLUE” written in red). Requires inhibitory control and attention. | Cognitive Load and Task Performance |
| [107] | Multitasking Challenge | Subjects are required to perform multiple simultaneous tasks to induce cognitive overload. | Cognitive Load and Social (Task Performance) |
| [108,109] | Multi-Attribute Task Battery -II | A computer-based set of tasks designed to evaluate simultaneous performance of monitoring, dynamic resource management, and tracking tasks (aircraft crewmembers, with freedom to use by non-pilot subjects). | Cognitive Load and Task Performance |
| [110,111] | Time Pressure Tasks | Participants must complete cognitive or motor tasks under strict time constraints. | Cognitive Load and Task Performance |
| [112] | Reading Span Task (RSPAN) | Memory span task exploring working memory, cognitive processing, and reading comprehension. | Memory, Cognitive Load, and Task Performance |
| [113,114] | Trier Social Stress Test (TSST) | 5 min of public speech, and 5 min of mental arithmetic task in front of a panel of evaluators (two to five) for 15 min. | Social and Cognitive Load (Task Performance) |
| [115] | Maastricht Acute Stress Test (MAST) | Combination of the Trier Social Stress Test and the Cold Pressor Test. | Social and Cognitive Load, Environmental and Physical |
| [116,117] | International Affective Digitized Sounds (IADS) and (IADS-E) | Listening to distressing sounds (screams, alarms) to evoke stress. | Acoustic and Emotional |
| [118] | International Affective Picture System (IAPS) | Exposure to emotionally charged images (negative, neutral, positive). | Emotional |
| [119] | Cyberball Social Exclusion Task | A virtual game in which participants are intentionally excluded, inducing social rejection stress. | Social |
| [120] | Public Speaking Task | Impromptu speech task with evaluation from peers or judges. | Social |
| [121] | Memory Recall of Traumatic Events | Participants recall past traumatic events, activating stress responses. | PTSD and Emotional |
| [122] | Cold Pressor Test (CPT) | Subjects immerse their hand in ice-cold water (0–4 °C) to induce a physiological stress response. | Environmental and Physical |
| [123] | Thermal Stress Test (TST) | Exposure to extreme heat or cold temperatures tests thermoregulation under stress. | Environmental and Physical |
| [124] | Exposure to Light (Photostimulation) | Sudden exposure to bright or flickering lights induces sensory processing stress. | Environmental and Physical (Visual Sensory Overload) |
| [125,126] | Hyperventilation Challenge | Subjects are asked to breathe rapidly to mimic anxiety-like symptoms and autonomic dysfunction. | Physical |
| [127] | Exposure to Noise | Subjects are exposed to loud, unpredictable noises such as alarms, construction sounds, or white noise. | Environmental and Physical (Acoustic Sensory Overload) |
2.2. Ground Truth of Affective States
| Ref. | Questionnaire | Description | Affect Condition |
|---|---|---|---|
| [128,129] | Perceived Stress Scale (PSS) and (PSS-10) | Measures perceived stress. | General Perceived Stress |
| [130,131] | Self-Assessment Manikin (SAM) | A visual scale for valence (emotion), arousal (activation), and dominance (control). | Positive/Negative Emotion |
| [132] | Positive and Negative Affect Schedule (PANAS) | Measures positive and negative emotional states separately to infer affective stress responses (Positive/Negative Affect). | Positive/Negative Emotion |
| [133] | State-Trait Anxiety Inventory (STAI) | Differentiates temporary (state) vs. long-term (trait) anxiety. | Anxiety (acute and chronic stress) |
| [134] | Depression Anxiety Stress Scales (DASS-21) | Evaluates the negative emotional states of depression, anxiety, and stress. | Anxiety, Depression, Stress |
| [135] | Beck Anxiety Inventory (BAI) | Assesses physical symptoms of anxiety. | Anxiety and PTSD |
| [136] | Hamilton Anxiety Rating Scale (HAM-A) | Evaluates clinical anxiety severity. | Anxiety and PTSD |
| [137,138] | Post Trauma Cognitions Inventory (PTCI) and (PTCI-9) | Evaluates negative trauma-related thoughts and beliefs. | PTSD |
| [139] | Post-traumatic Stress Disorder Checklist for DSM-5 (PCL-5) | Measures PTSD symptoms in line with DSM-5 diagnostic criteria. | PTSD |
| [140] | General Health Questionnaire-12 (GHQ-12) | Assesses mental health problems, specifically psychological distress such as anxiety, depression, and social dysfunction. | General mental and health psychological distress |
| [141] | Stress Response Inventory (SRI) | Assesses emotional, cognitive, somatic, and behavioral stress responses. | Stress response |
| [142] | Attentional Control Scale | Assesses an individual’s capacity for attentional control, including focusing attention, shifting attention, and flexibly controlling thoughts. | Cognitive and Attentional Control Under Stress |
| [143] | NASA Task Load Index (NASA-TLX) | Assesses cognitive workload in tasks under pressure. | Cognitive and Task-Related Stress |
| [144,145] | Rating Scale Mental Effort (RSME) | Measures subjective mental workload and effort during tasks. | Cognitive |
| [146] | Daily Stress Inventory (DSI) | Captures frequency and intensity of daily stressful events. | Daily Hassles and Minor Stressors |
| [147] | Cambridge Cognitive Assessment -Revised (CAMCOG-R) | Assesses cognitive function including memory, orientation, and attention. | Cognitive Function and Stress-Related Decline |
| [148] | Mini-Mental State Examination (MMSE) | Screen cognitive function, often used to rule out cognitive decline. | Cognitive Function and Mental Impairment |
| [149] | Cohen–Hoberman Inventory of Physical Symptoms (CHIPS) | Assesses physical symptoms commonly associated with stress. | Physical symptoms |
| [150] | Stress Mindset Measure (SMM) | Assesses beliefs about the nature and the effects of stress (positive or negative). | Personality Traits: Stress Beliefs and Mindset Influence |
| [151] | Eysenck Personality Questionnaire (EPQ) | A brief measure of three broad personality dimensions: Psychoticism, Extraversion, and Neuroticism. | Personality Traits: Stress susceptibility |
| [152] | Big Five Inventory 10 Item Scale (BFI-10) | A brief measure of five broad personality dimensions: Openness, Conscientiousness, Extraversion, Agreeableness, and Neuroticism. | Personality Traits: Stress susceptibility |
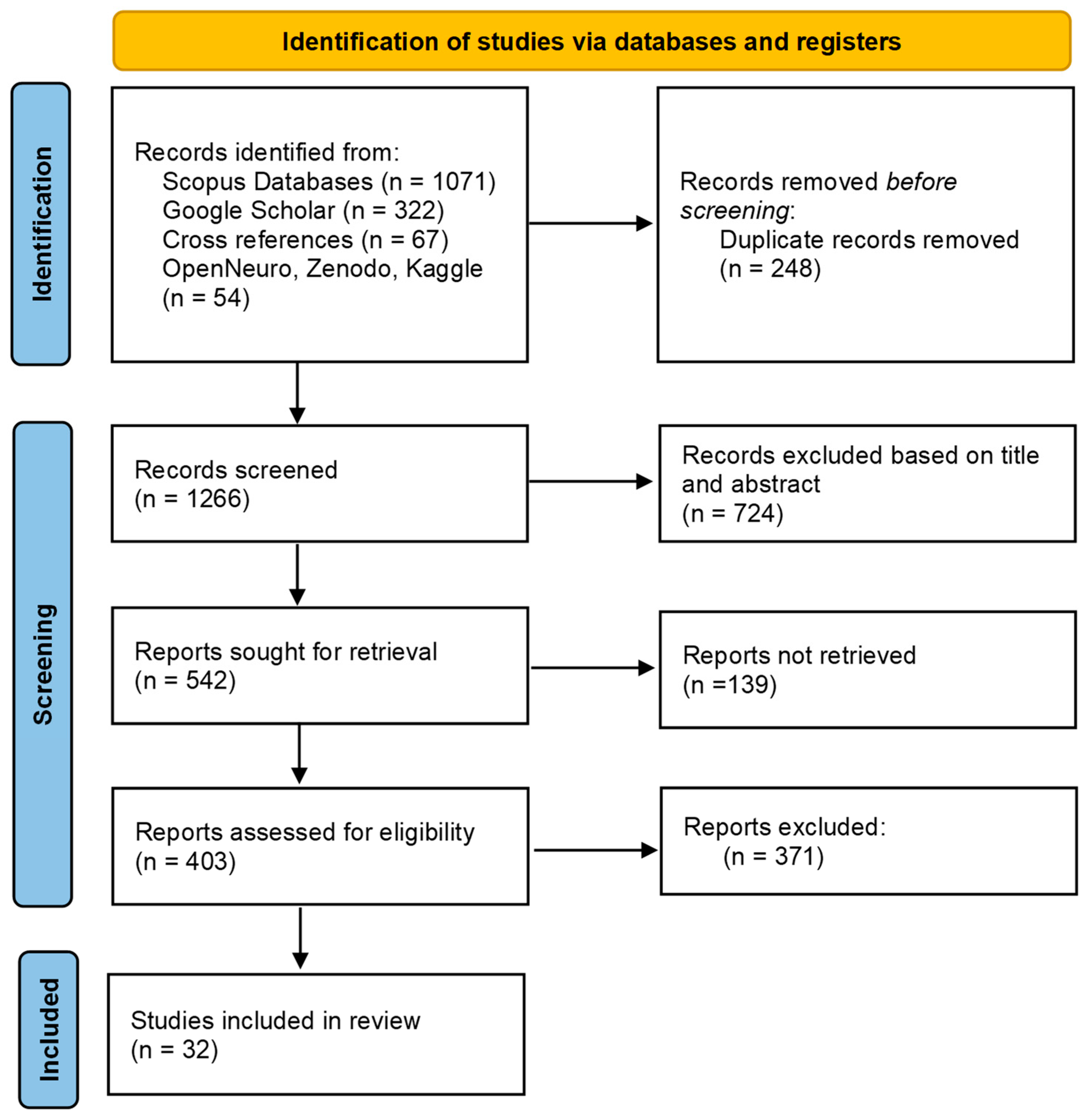
3. Open Datasets
- Search Strategy
- Inclusion and exclusion criteria
- Accessibility: Sufficient and clear description of the terms and conditions of dataset availability and accessibility for research and educational purposes.
- Physiological signals: Combination of at least two signal modalities (EEG, EDA/GSR, ECG/BVP/PPG, EMG/OMG, Resp), or single in the case of EEG.
- Experimental framework: Documentation of sufficient details of the experimental protocol, including data acquisition, stimulus/elicitation method, ground truth labeling, and baseline.
- Eligibility for stress analysis.
- Scientific relevance: Explicit focus on stress by experimental protocol design.
- Affective model: Comply to Russell’s Circumplex Model of Affect or the PAD framework for identifying valence/arousal states.
- Stimuli: Use of stimuli validated in stress-related studies.
- Ground truth: Inclusion of stress-relevant assessment questionnaires.
- Selection Process
- Results
| Ref. | Year | Dataset Name | Signal Modalities | Sensors/Equipment | Stimulus | Affective Condition | Questionnaires | No. Par. F/M, Age |
|---|---|---|---|---|---|---|---|---|
| [98] | 2011 | DEAP | EEG, GSR, ECG, BVP, EMG, EOG, Resp, ST, facial video | Biosemi Active II-32 active AgCl electrodes, peripheral sensors | 40 one-minute emotional music video clips | Valence, arousal, dominance, liking | SAM (valence, arousal, dominance, liking, familiarity) | 32, 16/16 |
| [159] | 2012 | MAHNOB-HCI | EEG, ECG, EDA, Resp, ST, eye gaze, facial video | Biosemi Active II-32 active AgCl electrodes, Tobii X120, AKG mics, multi-camera video | 20 emotional video clips, 28 images, implicit tagging trials | Valence, arousal, dominance, predictability, emotional labels of neutral, anxiety, amusement, sadness, joy, disgust, anger, surprise, and fear | SAM (valence, arousal, dominance), emotional keywords | 27, 17/13, 26 |
| [160] | 2014 | SWELL-KW | ECG, EDA, facial video, posture (Kinect), mouse/keyboard activity | TMSI Mobi, Kinect 3D, iDS FaceCam, Computer logging (uLog) | Office tasks with time pressure and email interruptions | Stress, valence, arousal, dominance, task load | NASA-TLX, SAM (valence, arousal, dominance), RSME, 1–10 scale (stress), internal control index | 25, 8/17, 25 |
| [161] | 2015 | SEED | EEG | ESI NeuroScan 62-electrode cap, 1000 Hz | 15 film clips (5/positive, neutral, negative) | Positive, neutral, negative | Self-assessment, EPQ | 15, 7/8, 23 |
| [162] | 2018 | DREAMER | EEG, ECG | Emotiv EPOC, Shimmer | 18 emotional video clips from films | Valence, arousal, dominance | SAM | 23, 14/9, 26 |
| [99] | 2018 | WESAD | ECG, EDA, EMG, Resp, ST, BVP, ACC | RespiBAN Professional (chest), Empatica E4 (wrist) | TSST, amusing videos, guided meditation | Neutral, stress, amusement | PANAS, STAI, SAM, SSSQ | 15, 3/12, 27 |
| [163] | 2018 | STEW | EEG | Emotiv EPOC (14 channels, 128 Hz) | SIMKAP multitasking test (visual and auditory tasks) | Mental workload (low, moderate, high) | 9-point workload rating scale | 48, 0/48, 22–30 |
| [164] | 2019 | CLAS | ECG, PPG, EDA, ACC | Shimmer3 EDA and ECG units | MAT, logic, SCWT, DEAP video, IAPS images | Arousal, valence, concentration, cognitive load | Self-assessment questionnaires (not defined) | 62, 17/45, 23 |
| [165] | 2019 | DASPS | EEG | Emotiv Epoc (14 channels) | Recall real-life anxiety inducing events (e.g., loss, trauma, financial stress) | Anxiety (four levels), arousal, valence | HAM-A (pre/post), SAM (valence, arousal) | 23, 13/10, 30 |
| [166] | 2019 | EEGMAT | EEG | Neurocom EEG (23 channels), 500 Hz | Serial subtraction task (4-digit–2-digit), 4 min | Cognitive load | Performance-based grouping | 36, 24/12, 18 |
| [167] | 2020 | EEG Emotion DB | EEG | EEG Clarity BrainTech 32+ CMEEG-01, 32 channels, 256/1024 Hz | 12 emotional video clips 2.5 min each | Happy, sad, fear, neutral | After each video, participants matched their feelings with one of the listed four | 44, 23/21, |
| [168] | 2020 | IDEA | EEG | RMS EEG System, 24 channels used, 256 Hz | Movie clips, songs, instrumental music, complex math tasks | Pleased, cheerful, zest, relaxed, distress, anger, restlessness, sadness | Experimental design-based | 14, 6/8, 20 |
| [169] | 2020 | PASS | EEG, ECG, Resp, BVP, ST, ACC, | Muse 2 4-channel EEG headset, BioHarness 3, Empatica E4 wristband | Stationary biking at three speeds while playing a clam and a survival video game | Neutral, stress | NASA-TLX, BORG | 48, N/A, 26 |
| [170] | 2021 | AMIGOS | EEG, ECG, EDA, RGB video, depth video | Emotiv EPOC 14 channel EEG headset, Shimmer 2R, Microsoft Kinect V1 | Emotional videos (short/individual, long/group) | Valence, arousal, dominance, liking, familiarity, Nine feelings: neutral, disgust, happiness, surprise, anger, fear, and sadness | SAM (valence, arousal, dominance, liking, and familiarity), BFI, PANAS | 40, 13/27, 28 |
| [171] | 2021 | DEAR-MULSEMEDIA | EEG, GSR, PPG | Muse 4-channel EEG headset, Shimmer GSR and PPG, fan, heater, olfaction dispenser, haptic vest | Movie clips enhanced with cold air, hot air, olfaction, haptics | Valence, arousal | SAM (valence, arousal) 9-point | 18, 9/9, 20 |
| [172] | 2021 | MuSe 2021/Ulm-TSST | EDA, ECG, Resp, HR, audio and video recordings, text | N/A for physiological signals; cameras, microphones | TSST: oral presentation | Valence, arousal | External raters, valence and arousal | 69, 49/20, 18–39 |
| [173] | 2021 | VREED | EDA, ECG, eye tracking data | FOVE-0 VR headset with eye tracking, Biopac MP150 | Immersive 360° video-based virtual environments | Valence, arousal, joy, anger, calmness, sadness, relaxation, happiness, fear, anxiousness, dizziness | SAM (valence, arousal), VAS (discrete emotions), Presence Questionnaire (immersion) | 34, 17/17, 25 |
| [174] | 2022 | Anxiety Dataset | ECG, Resp | Biopac MP45 | Anxiety inducing vs. relaxing video clips | Anxiety (pre/post-induction) | BAI, HAM-A | 19, 5/14, 26 |
| [175] | 2022 | NURSE | EDA, BVP, HR, ST, ACC | Empatica E4 | Nurses working in a hospital during the COVID-19 outbreak | Stress | Validation survey on COVID-19/medical stressor category | 15, 15/0, 30–55 |
| [176] | 2022 | Emognition | EEG, BVP, EDA, ST, HR, IBI, PPI, ACC, GYRO, facial expressions | Muse 2, Empatica E4, Samsung Galaxy Watch | Video clips in nine emotions categories | Amusement, awe, enthusiasm, liking, surprise, anger, disgust, fear, sadness, valence, arousal, motivation | Custom 5-point scale for nine emotions, SAM (valence, arousal, motivation) | 43, 21/22, 22 |
| [177] | 2022 | MMSD | ECG, PPG, EDA, EMG, GYRO | Shimmer sensing devices | SCWT, mental arithmetic, computer work, subtractions | Relaxation, stress, recovery | STAI-S, salivary cortisol (labeling) STAI-T, PSS4 (interpretation) | 74, 38/36, 34 |
| [178] | 2022 | SAM 40 | EEG | Emotiv Epoc Flex (32-channel EEG gel kit) | SCWT, mental arithmetic tasks, mirror image recognition task | Task-induced short-term stress, relaxation | Stress rating scale (1–10) | 40, 14/26, 21 |
| [179] | 2022 | VERBIO | EDA, BVP, ECG, ST, ACC, speech signals | Empatica E4, chest-based Actiwave Cardio Monitor, Creative lavalier microphone | Real-life and VR public speaking tasks | Public speaking anxiety | STAI-T, CAI, PRPSA, BFI, BFNE, RWTC | 55, 23/32, 22 |
| [180] | 2023 | XR4DRAMA Stress Dataset | ECG, RSP, IMU, simulated emergency dialogs | Smart vest (ECG, RSP, IMU) | SCWT, cold pressor, stair climb, mental arithmetic, relaxation tasks | Stress | Stress self-annotation (0–100 scale per task) | 5, 2/3, 22 |
| [181] | 2023 | StressID | ECG, EDA, Resp, facial video, audio | BioSignalsPlux, Logitech QuickCam Pro 9000 | Guided breathing, emotional videos, SCWT, MAT, public speaking | Stress, relaxation, neutral, arousal, valence | Self-assessment perceived stress (0–10) and relaxation, SAM (valence, arousal), two discrete labels | 65, 18/47, 29 |
| [182] | 2023 | EMAP | EEG, ECG, ResVP | BrainVision actiCHamp EEG amplifier-active Ag/AgCl 64-electrodeS, PowerLab 16/35 amplifier | Video Clips (rated on valence/arousal) | Positive, negative, discrete emotions | Valence, arousal, linking, engagement, discrete emotions: anger, sadness, happiness, disgust, fear | 145, 93/48, 22 |
| [183] | 2024 | BESST | ECG, EDA, ST, ACC, facial video, audio | Empatica E4, Faros 180, Zoom H4n recorder, Panasonic HC-VX9805 camcorder | Reading span task, hand immersion task | Labeling based on paradigm context and relaxation | PSS-14, STAI-Y1, NASA-TLX | 90, 21/69, 19–26 |
| [184] | 2024 | WorkStress3D | EDA, BVP, ST, ACC | Empatica E4, smartphone’s camera and microphone | Naturally occurring workplace stress (experience sampling) | Stress, mood, emotion | PANAS, general stress test, instant mood surveys | 20, 35%/65, 38 |
| [185] | 2024 | DSRP | EEG, HR | Neuroscan EEG cap, Huawei wrist HR monitor, PICO VR headset | Virtual reality scenarios (nature and animals) | Valence, arousal, dominance | SAM (arousal, valence, dominance), WHOQOL-BREF | 15, N//A |
| [186] | 2024 | EEVR | EDA, PPG | Biopac MP36 (SS57LA and SS4LA modules), Meta Quest Pro VR headset | 360° VR videos (eight from Russell’s circumplex quadrants) | Valence, arousal, dominance, PANAS emotions | SAM, PANAS, BFI-10, GHQ-12, qualitative self-reports | 37, 16/21, 23 |
| [187] | 2024 | EmoPairCompete | HR, EDA, BVP, ST, ACC | Empatica E4 wristband | Competitive tangram teamwork task (puzzle-solving in pairs) | Frustration, 10 PANAS emotions | I-PANAS-SF, visual analog scales (0–10) | 28, N/A, 20–42 |
| [188] | 2024 | MGEED | EEG, ECG, and OMG signals | Emotiv EPOC EEG 14 ch. Garmin HRM soft strap, Emteq smart glasses, Kinect | Emotional video clips | Positive, negative, discrete emotions | SAM (valence, arousal), Choice of happy, surprise, neutral, disgust, anxiety, sad, and fear | 17, N/A, 18–40 |
3.1. Experimental Environments: Laboratory, VR, and Real-World Settings
3.2. Type of Stimulus
3.3. Multimodal and EEG Focused Approaches
3.4. Data Synchronization
3.5. Ground Truth
3.6. Participant Demographics
3.7. Class Imbalance
3.8. Accessibility and FAIRification
3.9. Evaluation of Open Datasets
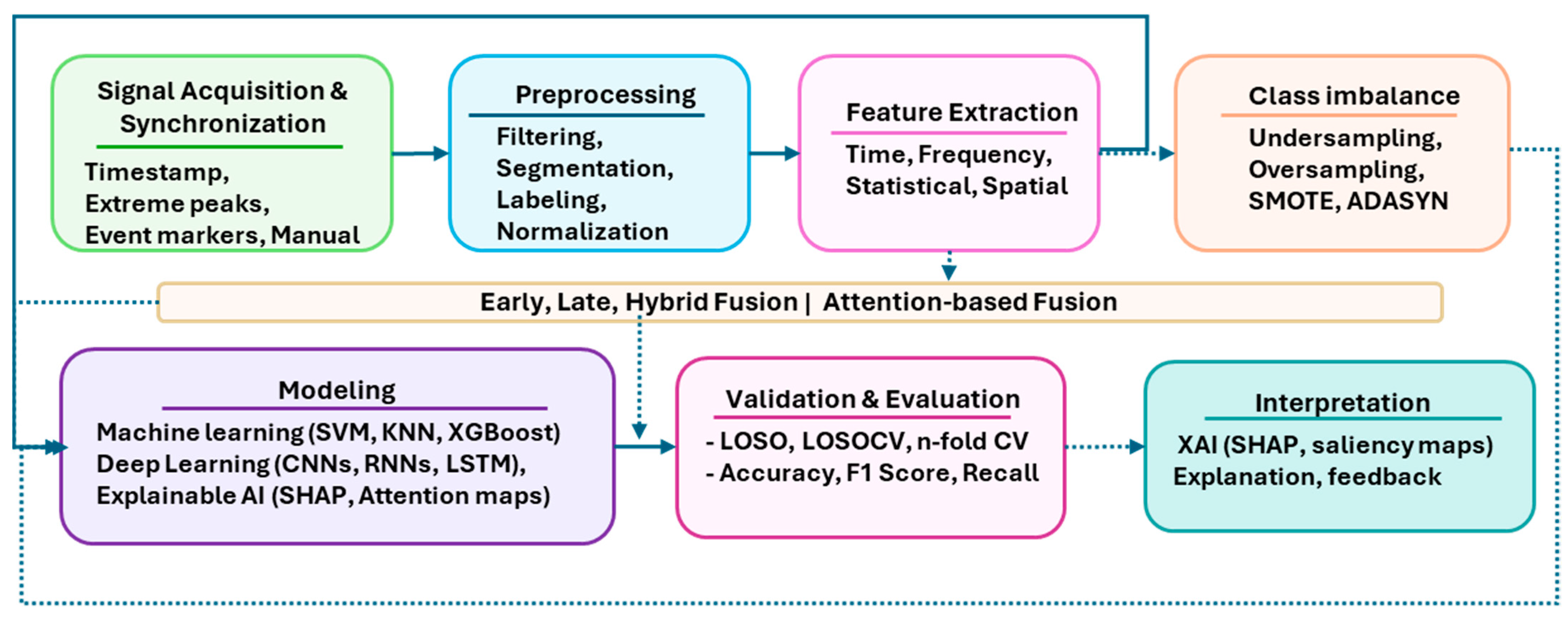
4. Current Trends in Stress Research
| Ref. (Year) | Dataset Used | Signal Modalities | Feature Extraction | Modeling Approach | Evaluation Protocol | Key Results (Accuracy %) |
|---|---|---|---|---|---|---|
| [205] (2024) | WESAD | ECG, EDA, and context features | ECG: time and statistical; EDA: SCR peak magnitude/duration and statistical; Context: BMI, caffeine, exercise, posture, etc. | DT, GBDT with multiple balancing methods | LOOCV with imbalanced data handling (SMOTE, ADASYN) | GBDT and LOOCV: 97.3% (ECG, EDA, context), ECG only: 89.8%, EDA only: 85.9% |
| [207] (2024) | Internal dataset (11 subjects, TSST, MIST) | EEG, PPG, HR, Temp, SpO2, EDA | Time, frequency, geometric domain (EDA, HRV, EEG power, and ratios), EEG artifact removal (ICA and DSWT) | LDA, SVM, KNN, DT, NB | 10-fold CV, windows (60, 30, 10, 4 s), within-subject classification | Stress type (LDA, 60 s): 99.8%; Stress level (DT, 4 s): 97.8%; EEG alone best at long windows, physio best at short |
| [201] (2023) | WorkStress3D and own dataset (daily workplace) | EDA, BVP, ST, ACC, audio, facial video | Biosignals: down sampling, normalization, polynomial transformation; Audio: Mel-spectrogram-MFCCs, Face: CNN preprocessing alignment | CNN-based early fusion model, modality-specific subnetworks, transfer learning ResNet/VGG16) | 80/20 split and 10-fold CV, (15 s, 30 s, 60 s) windows | Early fusion F1: 0.94, transfer learning F1: 0.93, best accuracy: 94% |
| [199] (2024) | DEAP, AMIGOS | EEG (14 channels) | FFT-based frequency features in 5 bands, segmented 2 s | Ensemble DL (CNN/LSTM, CNN/GRU, CNN), fuzzy Gompertz function | Subject-independent (60/20/20 split), subject-dependent (per-subject average) | DEAP: 95.97%, AMIGOS: 99.38% |
| [204] (2024) | DEAP, SEED | EEG | Differential entropy (DE) across frequency bands, time windowed | STLGCNN: Attention/BiLSTM/GCNN/LSTM | 10-fold CV, subject-dependent | DEAP: 94.16%, SEED: 96.78% |
| [208] (2024) | DREAMER, SEED, SEED-IV, | EEG | Time (Hjorth), frequency domain (α, β, δ), band ratios, frontal asymmetry, CSP maps | MLP (2 hidden layers), ReLU, dropout | LOTO (trial), LOSO (subject), 10-fold CV | DREAMER: 94%, SEED-IV: 44% SEED: 62% |
| [209] (2024) | SEED, SEED-V | EEG | STFT-DE features, sliding window composition (SWC), spatial and temporal encoding | Static spatial adapter, temporal causal network (GRU and MHA) | Subject-independent (8/2 split), ablation and cross-subject testing | SEED: 95.35% SEED-V: 94.28% |
| [210] (2024) | WESAD | ECG, EDA, EMG, BVP | Bandpass, normalized, windowed, attention maps/CNN/LSTM | CNN–LSTM with feature-level and semantic-level attention fusion | 5-fold cross-validation | Accuracy 83.88%, F1: 0.85 |
| [211] (2023) | Internal dataset (24 subjects, MAST-CPT, MAT) | ECG, EDA, EOG, EEG | Time, frequency, and statistical features, interpretable feature subset | Classical ML (SVM, RF, XGBoost, LDA) and SHAP XAI | Leave-three-out nested CV, SFFS feature selection, SHAP explanation | 86.5% balanced accuracy (XGBoost, 45 s, full fusion), 81.5% interpretable features |
| [212] (2024) | Internal dataset (28 subjects, airhorn stimulus) | EDA, ST | Raw EDA and ST, preprocessed phasic signals (1 Hz, 16 s sliding windows) | LSTM ensemble, conditional GAN, integrated gradients | 3 train-test seeds, 5-fold CV grid search, time-windowed evaluation | LSTM-DGE: Recall: 76.3%, Precision: 35.9%, Accuracy: 98.1%, Rule-based: Recall 73.3%, Precision 32.3% |
| [213] (2025) | WESAD | EDA, PPG, Temp, ACC | Raw signals, human-engineered features, sliding window, normalization | Residual attention DNN with multi-head blocks, Guided Grad-CAM for explainability | LOSO CV | Stress: 96.57%, Emotion: 87.77% |
| [214] (2024) | SWEET (240 subjects, free-living context, wearables) | ECG, ST, skin conductance | Time and statistical features, 3-class and binary labels, SMOTE for balancing | Classical ML: RF, XGBoost, SVC, KNN, DT | 4 settings: binary and 3-class, with and without SMOTE | Binary: RF 98.29%, 3-class: XGBoost 98.98% |
| [215] (2025) | Nurse Stress Prediction | EDA, HR, skin temp, ACC | Time domain, statistical, FFT spectral features, sliding window, jittering, 60 s windows segments | Dual-branch CNN (time, frequency), FC classifier | Stratified split 80/20, hyperparameter tuning via Bayesian optimization | Own model: 91%, outperformed RF, SVM, XGBoost |
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABDT | AdaBoost Decision Tree |
| ACC | Accelerometer |
| ACTH | Adrenocorticotropic Hormone |
| AdaBoost | Adaptive Boosting |
| ADASYN | Adaptive Synthetic Sampling Approach For Imbalanced Learning |
| AgCl | Silver Chloride |
| AI | Artificial Intelligence |
| ANOVA | Analysis of Variance |
| ANS | Autonomic Nervous System |
| API | Application Programming Interface |
| AUs | Action Units |
| BAI | Beck Anxiety Inventory |
| BESST | Brno Extended Stress and Speech Test |
| BFI | Big Five Inventory |
| BFNE | Brief Fear of Negative Evaluation |
| BiLSTM | Bidirectional Long Short-Term Memory |
| BORG | Borg Rating of Perceived Exertion |
| BVP | Blood Volume Pulse |
| CAI | Communication Anxiety Inventory |
| CAMCOG-R | Cambridge Cognitive Assessment (Revised) |
| CHIPS | Cohen–Hoberman Inventory of Physical Symptoms |
| CLSP | Contrastive Language Signal Pre-training |
| CNN | Convolutional Neural Network |
| CPT | Cold Pressor Test |
| CRH | Corticotropin-Releasing Hormone |
| CSP | Common Spatial Patterns |
| CV | Cross-Validation |
| DASS-21 | Depression Anxiety Stress Scales |
| DBNs | Deep Belief Networks |
| DE | Differential Entropy |
| DGE | Deep Generative Ensemble |
| DL | Deep Learning |
| DNN | Deep Neural Network |
| DSI | Daily Stress Inventory |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| DSWT | Discrete Stationary Wavelet Transform |
| DT | Decision Tree |
| DWT | Discrete Wavelet Transform |
| ECG | Electrocardiography |
| EDA | Electrodermal Activity |
| EEG | Electroencephalography |
| ELM | Extreme Learning Machine |
| EMG | Electromyography |
| EOG | Electrooculogram |
| EPQ | Eysenck Personality Questionnaire |
| ES | Experience Sampling |
| FFT | Fast Fourier Transform |
| GAD | Generalized Anxiety Disorder |
| GAN | Generative Adversarial Network |
| GBDT | Gradient Boosted Decision Tree |
| GCNN | Graph Convolutional Neural Network |
| GHQ-12 | General Health Questionnaire-12 |
| Grad-CAM | Gradient-Weighted Class Activation Mapping |
| GRUs | Gated Recurrent Units |
| GSR | Galvanic Skin Response |
| HAM-A | Hamilton Anxiety Rating Scale |
| HPA | Hypothalamic–Pituitary–Adrenal |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| IADS | International Affective Digitized Sounds |
| IAPS | International Affective Picture System |
| IBI | Interbeat Interval |
| ICA | Independent Component Analysis |
| IG | Integrated Gradients |
| IMU | Inertial Measurement Unit |
| KNN | K-Nearest Neighbor |
| LDA | Linear Discriminant Analysis |
| LOOCV | Leave-One-Out Cross-Validation |
| LOSO | Leave-One-Subject-Out |
| LOTO | Leave-One-Trial-Out |
| LR | Logistic Regression |
| LSTM | Long Short-Term Memory |
| MAST | Maastricht Acute Stress Test |
| MAT | Mental Arithmetic Task |
| MCA | Multimedia Content Analysis |
| MD-DE | Modified Differential Entropy |
| MFCCs | Mel-Frequency Cepstral Coefficients |
| MHA | Multi-Head Attention |
| MIST | Montreal Imaging Stress Task |
| ML | Machine Learning |
| MLP | Multi-Layer Perceptron |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic Resonance Imaging |
| NASA-TLX | NASA Task Load Index |
| NB | Naïve Bayes |
| OMG | Optomyography |
| PANAS | Positive and Negative Affect Schedule |
| PASAT | Paced Auditory Serial Addition Test |
| PCL-5 | Post-Traumatic Stress Disorder Checklist |
| PFC | Prefrontal Cortex |
| PPG | Photoplethysmography |
| PQ | Presence Questionnaire |
| PRPSA | Personal Report of Public Speaking Anxiety |
| PSS | Perceived Stress Scale |
| PTCI | Post Trauma Cognitions Inventory |
| PTSD | Post-Traumatic Stress Disorder |
| QDA | Quadratic Discriminant Analysis |
| RBF | Radial Basis Function |
| ReLU | Rectified Linear unit |
| ResNet | Residual Network |
| Resp | Respiration |
| RF | Random Forest |
| RGB | Red, Green, and Blue |
| RSME | Rating Scale Mental Effort |
| RSPAN | Reading Span Task |
| RWTC | Reticence Willingness to Communicate |
| SAM axis | Sympathetic–Adreno–Medullary |
| SAM | Self-Assessment Manikin |
| SCR | Skin Conductance Response |
| SCWT | Stroop Color Word Test |
| SFFS | Sequential Forward Floating Search |
| SHAP | Shapley Additive Explanation |
| SIMKAP | Simultaneous Capacity |
| SMM | Stress Mindset Measure |
| SMOTE | Synthetic Minority Oversampling Technique |
| SRI | Stress Response Inventory |
| SSAE | Stacked Sparse Autoencoder |
| ST | Skin Temperature |
| STAI | State-Trait Anxiety Inventory |
| STFT | Short-Time Fourier Transform |
| STLGCNN | Spatio-Temporal Learning Graph Convolutional Neural Network |
| SVC | Support Vector Classification |
| SVM | Support Vector Machine |
| SWC | Sliding Window Composition |
| TSST | Trier Social Stress Test |
| TST | Thermal Stress Test |
| VAS | Visual Analog Scale |
| VR | Virtual Reality |
| WHOQOL-BREF | World Health Organization Quality of Life |
| XAI | Explainable Artificial Intelligence |
| XGBoost | Extreme Gradient Boosting |
References
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; Umeoka, E.H.L. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Oken, B.S.; Chamine, I.; Wakeland, W. A systems approach to stress, stressors and resilience in humans. Behav. Brain Res. 2015, 282, 144–154. [Google Scholar] [CrossRef]
- Doewes, R.I.; Gangadhar, L.; Subburaj, S. An overview on stress neurobiology: Fundamental concepts and its consequences. Neurosci. Inform. 2021, 3, 100011. [Google Scholar] [CrossRef]
- Almeida, D.M.; Rush, J.; Mogle, J.; Piazza, J.R.; Cerino, E.; Charles, S.T. Longitudinal Change in Daily Stress Across 20 Years of Adulthood: Results From the National Study of Daily Experiences. Dev. Psychol. 2022, 59, 515–523. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 1 July 2025).
- Piao, X.; Xie, J.; Managi, S. Continuous worsening of population emotional stress globally: Universality and variations. BMC Public Health 2024, 24, 3576. [Google Scholar] [CrossRef]
- Anderson, T.L.; Valiauga, R.; Tallo, C.; Hong, C.B.; Manoranjithan, S.; Domingo, C.; Paudel, M.; Untaroiu, A.; Barr, S.; Goldhaber, K. Contributing Factors to the Rise in Adolescent Anxiety and Associated Mental Health Disorders: A Narrative Review of Current Literature. J. Child Adolesc. Psychiatr. Nurs. 2025, 38, e70009. [Google Scholar] [CrossRef]
- Smith, M.D.; Wesselbaum, D. Global evidence on the prevalence of and risk factors associated with Stress. J. Affect. Disord. 2025, 374, 179–183. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, N. A Survey on Physiological Signal-Based Emotion Recognition. Bioengineering 2022, 9, 688. [Google Scholar] [CrossRef]
- Giannakakis, G.; Grigoriadis, D.; Giannakaki, K.; Simantiraki, O.; Roniotis, A.; Tsiknakis, M. Review on Psychological Stress Detection Using Biosignals. IEEE Trans. Affect. Comput. 2022, 13, 440–460. [Google Scholar] [CrossRef]
- Subhani, A.R.; Kamel, N.; Saad, M.N.M.; Nandagopal, N.; Kang, K.; Malik, A.S. Mitigation of stress: New treatment alternatives. Cogn. Neurodynamics 2017, 12, 1–20. [Google Scholar] [CrossRef]
- Gedam, S.; Paul, S. A Review on Mental Stress Detection Using Wearable Sensors and Machine Learning Techniques. IEEE Access 2021, 9, 84045–84066. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Kyrou, I.; Tsigos, C. Stress hormones: Physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef]
- Alotiby, A. Immunology of Stress: A Review Article. J. Clin. Med. 2024, 13, 6394. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Mcewen, B.S.; Gianaros, P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef]
- Oei, N.Y.L.; Veer, I.M.; Wolf, O.T.; Spinhoven, P.; Rombouts, S.A.R.B.; Elzinga, B.M. Stress shifts brain activation towards ventral ‘affective’ areas during emotional distraction. Soc. Cogn. Affect. Neurosci. 2012, 7, 403–412. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Albayrak, Z.S.; Vaz, A.; Bordes, J.; Ünlü, S.; Sep, M.S.C.; Vinkers, C.H.; Pinto, L.; Yapici-Eser, H. Translational models of stress and resilience: An applied neuroscience methodology review. Neurosci. Appl. 2024, 3, 104064. [Google Scholar] [CrossRef]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Michopoulos, V.; Norrholm, S.D.; Jovanovic, T. Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol. Psychiatry 2015, 78, 344–353. [Google Scholar] [CrossRef]
- Bienertova-Vasku, J.; Lenart, P.; Scheringer, M. Eustress and Distress: Neither Good Nor Bad, but Rather the Same? BioEssays 2020, 42, e1900238. [Google Scholar] [CrossRef]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- Hermans, E.J.; Henckens, M.J.A.G.; Joëls, M.; Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases 11th Revision; World Health Organization (WHO): Geneva, Switzerland, 2022. [Google Scholar]
- Maercker, A.; Eberle, D.J. Disorders Specifically Associated with Stress in ICD-11. Clinical Psychology in Europe. 2022, 4, 1–16. [Google Scholar] [CrossRef]
- Buenrostro-Jáuregui, M.H.; Muñóz-Sánchez, S.; Rojas-Hernández, J.; Alonso-Orozco, A.I.; Vega-Flores, G.; Tapia-de-Jesús, A.; Leal-Galicia, P. A Comprehensive Overview of Stress, Resilience, and Neuroplasticity Mechanisms. Int. J. Mol. Sci. 2025, 26, 3028. [Google Scholar] [CrossRef]
- Hermans, E.J.; Hendler, T.; Kalisch, R. Building Resilience: The Stress Response as a Driving Force for Neuroplasticity and Adaptation. Biol. Psychiatry 2024, 97, 330–338. [Google Scholar] [CrossRef]
- Benson, H.; Beary, J.F.; Carol, M.P. The Relaxation Response. Psychiatry 1974, 37, 37–46. [Google Scholar] [CrossRef]
- Barrows, K.A.; Jacobs, B.P. Mind-body medicine: An introduction and review of the literature. Med. Clin. 2002, 86, 11–31. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B.; Head, M.D. The Neurobiology of Stress Management. Neuro Endocrinol. Lett. 2010, 31, 19–39. [Google Scholar] [PubMed]
- Esch, T.; Stefano, G.B. The BERN Framework of Mind-Body Medicine: Integrating Self-Care, Health Promotion, Resilience, and Applied Neuroscience. Front. Integr. Neurosci. 2022, 16, 913573. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, O.; Wilding, S.; Prudenzi, A.; O’Connor, D.B. Effectiveness of stress management interventions to change cortisol levels: A systematic review and meta-analysis. Psychoneuroendocrinology 2023, 159, 106415. [Google Scholar] [CrossRef] [PubMed]
- Van Hedger, K.; Bershad, A.K.; de Wit, H. Pharmacological challenge studies with acute psychosocial Stress. Psychoneuroendocrinology 2017, 85, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Impact of Mental Stress on Cardiovascular Health—Part II. J. Clin. Med. 2022, 11, 4405. [Google Scholar] [CrossRef]
- Sharma, K.; Akre, S.; Chakole, S.; Wanjari, M.B. Stress-Induced Diabetes: A Review. Cureus 2022, 14, e29142. [Google Scholar] [CrossRef]
- Qin, H.Y.; Cheng, C.W.; Tang, X.D.; Bian, Z.X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef]
- Shah, N.; Asuncion, S.; Ria Monica, D.; Hameed, S. Muscle Contraction Tension Headache. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef]
- Kokubun, K.; Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y.; Watanabe, Y. Association of fatigue and stress with gray matter volume. Front. Behav. Neurosci. 2018, 12, 154. [Google Scholar] [CrossRef]
- Mcewen, B.S. The Brain is the Central Organ of Stress and Adaptation. Neuroimage 2009, 47, 911–913. [Google Scholar] [CrossRef]
- Kalmbach, D.A.; Anderson, J.R.; Drake, C.L.; Hospital, H.F. Vulnerability To Insomnia and Circadian Disorders. J. Sleep. Res. 2018, 27, 1–39. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Oyola, M.G.; Handa, R.J. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Domes, G.; Linnig, K.; von Dawans, B. Gonads under stress: A systematic review and meta-analysis on the effects of acute psychosocial stress on gonadal steroids secretion in humans. Psychoneuroendocrinology 2024, 164, 107004. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.; Monteiro, S.; Roque, S.; Correia-Neves, M.; Sousa, N. How age, sex and genotype shape the stress response. Neurobiol. Stress 2017, 6, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Mikneviciute, G.; Pulopulos, M.M.; Allaert, J.; Armellini, A.; Rimmele, U.; Kliegel, M.; Ballhausen, N. Adult age differences in the psychophysiological response to acute Stress. Psychoneuroendocrinology 2023, 153, 106111. [Google Scholar] [CrossRef]
- Starcke, K.; Brand, M. Effects of Stress on Decisions Under Uncertainty: A Meta-Analysis. Psychol. Bull. 2016, 142, 909–933. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Pruessner, J.; Sousa, N.; Almeida, O.F.X.; Van Dam, A.M.; Rajkowska, G.; Swaab, D.F. Neuropathology of Stress. Acta Neuropathol. 2014, 127, 109–135. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef]
- Gkikas, S.; Chatzaki, C.; Tsiknakis, M. Multi-task Neural Networks for Pain Intensity Estimation Using Electrocardiogram and Demographic Factors. In Information and Communication Technologies for Ageing Well and E-Health; Maciaszek, L.A., Mulvenna, M.D., Ziefle, M., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 324–337. [Google Scholar]
- Khurade, S.T.; Gowali, S.C.M.C.; Shivaprakasha, K.S. Stress Detection Indicators: A Review. J. Electron. Commun. Syst. 2019, 4, 12–17. [Google Scholar]
- Medtronic. Zephyr BioHarness. Available online: https://www.zephyranywhere.com/ (accessed on 1 September 2025).
- Shimmer Research Ltd. Shimmer3R ECG Unit. Available online: https://www.shimmersensing.com/ (accessed on 1 September 2025).
- Sacha, J. Interaction between Heart Rate and Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2014, 19, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rudics, E.; Buzás, A.; Pálfi, A.; Szabó, Z.; Nagy, Á.; Hompoth, E.A.; Dombi, J.; Bilicki, V.; Szendi, I.; Dér, A. Quantifying Stress and Relaxation: A New Measure of Heart Rate Variability as a Reliable Biomarker. Biomedicines 2025, 13, 81. [Google Scholar] [CrossRef]
- Gullett, N.; Zajkowska, Z.; Walsh, A.; Harper, R.; Mondelli, V. Heart rate variability (HRV) as a way to understand associations between the autonomic nervous system (ANS) and affective states: A critical review of the literature. Int. J. Psychophysiol. 2023, 192, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Scardulla, F.; Cosoli, G.; Spinsante, S.; Poli, A.; Iadarola, G.; Pernice, R.; Busacca, A.; Pasta, S.; Scalise, L.; D’Acquisto, L. Photoplethysmograhic sensors, potential and limitations: Is it time for regulation? A comprehensive review. Measurement 2023, 218, 113150. [Google Scholar] [CrossRef]
- Ghamari, M. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef]
- Akl, T.J.; Wilson, M.A.; Ericson, M.N.; Coté, G.L. Quantifying tissue mechanical properties using photoplethysmography. Biomed. Opt. Express 2014, 5, 2362. [Google Scholar] [CrossRef]
- Peper, E.; Harvey, R.; Lin, I.-M.; Moss, D. Is There More to Blood Volume Pulse Than Heart Rate Variability, Respiratory Sinus Arrhythmia, and Cardiorespiratory Synchrony? Biofeedback 2007, 35, 54–61. [Google Scholar]
- Kim, K.B.; Baek, H.J. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics 2023, 12, 2923. [Google Scholar] [CrossRef]
- Empatica. Empatica E4. Available online: https://www.empatica.com/ (accessed on 1 September 2025).
- Seo, S.-H.; Lee, J.-T. Stress and EEG. In Convergence and Hybrid Information Technologies; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef]
- Pomer-Escher, A.G.; de Souza, M.D.P.; Filho, T.F.B. Methodology for analysis of stress level based on asymmetry patterns of alpha rhythms in EEG signals. In Proceedings of the 5th ISSNIP-IEEE Biosignals and Biorobotics Conference (2014): Biosignals and Robotics for Better and Safer Living (BRC), Bahia, Brazil, 26–28 May 2014; IEEE: New York, NY, USA; pp. 1–5. [Google Scholar] [CrossRef]
- Peng, H.; Hu, B.; Zheng, F.; Fan, D.; Zhao, W.; Chen, X.; Yang, Y.; Cai, Q. A method of identifying chronic stress by EEG. Pers. Ubiquitous Comput. 2013, 17, 1341–1347. [Google Scholar] [CrossRef]
- Putman, P.; Verkuil, B.; Arias-Garcia, E.; Pantazi, I.; van Schie, C. EEG theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention. Cogn. Affect. Behav. Neurosci. 2014, 14, 782–791. [Google Scholar] [CrossRef]
- Zheng, W.-L.; Zhu, J.-Y.; Lu, B.-L. Identifying Stable Patterns over Time for Emotion Recognition from EEG. IEEE Trans. Affect. Comput. 2019, 10, 417–429. [Google Scholar] [CrossRef]
- Siuly, S.; Li, Y.; Zhang, Y. EEG Signal Analysis and Classification. In Health Information Science; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- InteraXon Inc. Muse. Available online: https://choosemuse.com/ (accessed on 1 September 2025).
- NeuroSky. NeuroSky. Available online: https://neurosky.com/ (accessed on 1 September 2025).
- EMOTIV. Emotiv. Available online: https://www.emotiv.com/ (accessed on 1 September 2025).
- BIOSEMI. biosemi. Available online: https://www.biosemi.com/ (accessed on 1 September 2025).
- g.tec Medical Engineering GmbH. Available online: https://www.gtec.at/ (accessed on 1 September 2025).
- BIOPAC Systems. Biopac. Available online: https://www.biopac.com/ (accessed on 1 September 2025).
- Muhammed, H.H.; Raghavendra, J. Optomyography (OMG): A Novel Technique for the Detection of Muscle Surface Displacement Using Photoelectric Sensors. In Measurements, Proceedings of the 10th International Conference on Bioelectromagnetism, Tallinn, Estonia, 16–18 June 2015; International Society for Bioelectromagnetism: Tallinn, Estonia, 2015. [Google Scholar]
- Muhammed, H.H.; Jammalamadaka, R. A new approach for rehabilitation and upper-limb prosthesis control using optomyography (OMG). In Proceedings of the 2016 1st International Conference on Biomedical Engineering (IBIOMED), Yogyakarta, Indonesia, 5–6 October 2016; IEEE: New York, NY, USA, 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Broulidakis, M.J.; Kiprijanovska, I.; Severs, L.; Stankoski, S.; Gjoreski, M.; Mavridou, I.; Gjoreski, H.; Cox, S.; Bradwell, D.; Stone, J.M.; et al. Optomyography-based sensing of facial expression derived arousal and valence in adults with depression. Front. Psychiatry 2023, 14, 1232433. [Google Scholar] [CrossRef]
- Kiprijanovska, I.; Stankoski, S.; Broulidakis, M.J.; Archer, J.; Fatoorechi, M.; Gjoreski, M.; Nduka, C.; Gjoreski, H. Towards smart glasses for facial expression recognition using OMG and machine learning. Sci. Rep. 2023, 13, 16043. [Google Scholar] [CrossRef] [PubMed]
- Arsalan, A.; Majid, M.; Nizami, I.F.; Manzoor, W.; Anwar, S.M.; Ryu, J. Human Stress Assessment: A Comprehensive Review of Methods Using Wearable Sensors and Non-wearable Techniques. arXiv 2023, arXiv:2202.03033. [Google Scholar] [CrossRef]
- Katmah, R.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. A review on mental stress assessment methods using EEG signals. Sensors 2021, 21, 5043. [Google Scholar] [CrossRef]
- Roos, L.G.; Slavich, G.M. Wearable technologies for health research: Opportunities, limitations, and practical and conceptual considerations. Brain Behav. Immun. 2023, 113, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Gómez, M.; Fernandez-Carmona, M. Trends and Challenges in Real-Time Stress Detection and Modulation: The Role of the IoT and Artificial Intelligence. Electronics 2025, 14, 2581. [Google Scholar] [CrossRef]
- Behi, R.; Nolan, M. The basic experimental design. Br. J. Nurs. 1996, 5, 563–566. [Google Scholar] [CrossRef]
- Martínez-Mesa, J.; González-Chica, D.A.; Bastos, J.L.; Bonamigo, R.R.; Duquia, R.P. Sample size: How many participants do i need in my research? An. Bras. Dermatol. 2014, 89, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Althubaiti, A. Sample Size Determination: A Practical Guide for Health Researchers; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Vozzi, A.; Ronca, V.; Aricò, P.; Borghini, G.; Sciaraffa, N.; Cherubino, P.; Trettel, A.; Babiloni, F.; Di Flumeri, G. The sample size matters: To what extent the participant reduction affects the outcomes of a neuroscientific research. A case-study in neuromarketing field. Sensors 2021, 21, 6088. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, C. Review of Studies on Emotion Recognition and Judgment Based on Physiological hysiological Signals. Appl. Sci. 2023, 13, 2573. [Google Scholar] [CrossRef]
- Reis, S.; Pinto-Coelho, L.; Sousa, M.; Neto, M.; Silva, M. Advancing Emotion Recognition: EEG Analysis and Machine Learning for Biomedical Human–Machine Interaction. BioMedInformatics 2025, 5, 5. [Google Scholar] [CrossRef]
- Sedehi, J.F.; Dabanloo, N.J.; Maghooli, K.; Sheikhani, A. Develop an emotion recognition system using jointly connectivity between electroencephalogram and electrocardiogram signals. Heliyon 2025, 11, e41767. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhou, T.; Liang, W.; Shan, M. Multidimensional emotion recognition based on semantic analysis of biomedical eeg signal for knowledge discovery in psychological healthcare. Appl. Sci. 2021, 11, 1338. [Google Scholar] [CrossRef]
- Koelstra, S.; Mühl, C.; Soleymani, M.; Lee, J.S.; Yazdani, A.; Ebrahimi, T.; Pun, T.; Nijholt, A.; Patras, I. DEAP: A Database for Emotion Analysis; Using Physiological Signals. IEEE Trans. Affect. Comput. 2012, 3, 18–31. [Google Scholar] [CrossRef]
- Schmidt, P.; Reiss, A.; Duerichen, R.; Van Laerhoven, K. Introducing WESAD, a multimodal dataset for wearable stress and affect detection. In Proceedings of the ICMI 2018 International Conference on Multimodal Interaction, Boulder, CO, USA, 16–20 October 2018; pp. 400–408. [Google Scholar] [CrossRef]
- Waugh, C.E.; Running, K.E.; Reynolds, O.C.; Gotlib, I.H. People are better at maintaining positive than negative emotional states. Emotion 2019, 19, 132–145. [Google Scholar] [CrossRef]
- Roldan-Rojo, T.A.; Rendon-Velez, E.; Carrizosa, S. Stressors and Algorithms Used for Stress Detection: A Review. In Proceedings of the 2021 9th International Conference on Affective Computing and Intelligent Interaction (ACII), Nara, Japan, 28 September–1 October 2021; IEEE: New York, NY, USA, 2024; pp. 1–8. [Google Scholar] [CrossRef]
- Morell, M.A.; Myers, H.F.; Shapiro, D.; Goldstein, I.; Armstrong, M. Psychophysiological reactivity to mental arithmetic stress in Black and White normotensive men. Health Psychol. 1988, 7, 479–496. [Google Scholar] [CrossRef]
- Dedovic, K.; Renwick, R.; Khalili Mahani, N.; Engert, V.; Lupien, S.J.; Pruessner, J.C.; Mahani, K.; Douglas, P. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005, 30, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.B.; Giraud, V.O.; Saleh, Y.J.; Rodrigues, S.J.; Daia, L.A.; Fragoso, Y.D. Paced auditory serial addition test (PASAT) A very difficult test even for individuals with high intellectual capability. Arq. Neuropsiquiatr. 2011, 69, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Wetherell, M.A.; Carter, K. The multitasking framework: The effects of increasing workload on acute psychobiological stress reactivity. Stress Health 2014, 30, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Comstock, J.R.; Arnegard, R.J. The Multi-Attribute Task Battery for Human Operator Workload and Strategic Behavior Research; NASA Langley Research Center: Hampton, VA, USA, 1992. Available online: https://ntrs.nasa.gov/citations/19920007912 (accessed on 8 September 2025).
- Santiago-Espada, Y.; Myer, R.R.; Latorella, K.A.; Comstock, J.R. The Multi-Attribute Task Battery II (MATB-II) Software for Human Performance and Workload Research: A User’s Guide; NASA Langley Research Center: Hampton, VA, USA, 2011. Available online: http://www.sti.nasa.gov (accessed on 8 September 2025).
- Hockey, R. Stress and fatigue in human performance. Am. J. Psychol. 1984, 97, 630. [Google Scholar] [CrossRef]
- Maule, A.J.; Hockey, G.R.J. State, Stress, and Time Pressure. In Time Pressure and Stress in Human Judgment and Decision Making; Springer: Boston, MA, USA, 1993; pp. 83–101. [Google Scholar] [CrossRef]
- Daneman, M.; Carpenter, P.A. Individual Differences in Working Memory and Reading. J. Verbal Learn. Verbal Behav. 1980, 19, 450–466. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test’—A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Allen, A.P.; Kennedy, P.J.; Dockray, S.; Cryan, J.F.; Dinan, T.G.; Clarke, G. The Trier Social Stress Test: Principles and practice. Neurobiol. Stress 2017, 6, 113–126. [Google Scholar] [CrossRef]
- Shilton, A.L.; Laycock, R.; Crewther, S.G. The Maastricht Acute Stress Test (MAST): Physiological and Subjective Responses in Anticipation, and Post-Stress. Front. Psychol. 2017, 8, 567. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. International Affective Digitized Sounds (IADS-1): Stimuli, Instruction Manual, and Affective Ratings (Technical Report B-2); University of Florida: Gainesville, FL, USA, 1999. [Google Scholar]
- Yang, W.; Makita, K.; Nakao, T.; Kanayama, N.; Machizawa, M.G.; Sasaoka, T.; Sugata, A.; Kobayashi, R.; Hiramoto, R.; Yamawaki, S.; et al. Affective auditory stimulus database: An expanded version of the International Affective Digitized Sounds (IADS-E). Behav. Res. Methods 2018, 50, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; University of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Williams, K.D.; Jarvis, B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behav. Res. Methods 2006, 38, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Geer, J.H.; Davison, G.C.; Gatchel, R.I. Reduction of stress in humans through nonveridical perceived control of aversive stimulation. J. Pers. Soc. Psychol. 1970, 16, 731–738. [Google Scholar] [CrossRef]
- Pitman, R.K. Psychophysiologic Assessment of Posttraumatic Stress Disorder Imagery in Vietnam Combat Veterans. Arch. Gen. Psychiatry 1987, 44, 970. [Google Scholar] [CrossRef]
- Hines, E.A.; Brown, G.E. The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. Am. Heart J. 1936, 11, 1–9. [Google Scholar] [CrossRef]
- Fealey, R.D.; Low, P.A.; Thomas, J.E. Thermoregulatory Sweating Abnormalities in Diabetes Mellitus. Mayo Clin. Proc. 1989, 64, 617–628. [Google Scholar] [CrossRef]
- Walter, V.J.; Walter, W.G. The central effects of rhythmic sensory stimulation. Electroencephalogr. Clin. Neurophysiol. 1949, 1, 57–86. [Google Scholar] [CrossRef]
- Suess, W.M.; Alexander, A.B.; Smith, D.D.; Sweeney, H.W.; Marion, R.J. The Effects of Psychological Stress on Respiration: A Preliminary Study of Anxiety and Hyperventilation. Psychophysiology 1980, 17, 535–540. [Google Scholar] [CrossRef]
- Clark, D.M. A cognitive approach to panic. Behav. Res. Ther. 1986, 24, 461–470. [Google Scholar] [CrossRef]
- Westman, J.C.; Walters, J.R. Noise and stress: A comprehensive approach. Environ. Health Perspect. 1981, 41, 291–309. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived Stress. J. Health Soc. Behav. 1983, 24, 385. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Williamson, G. Perceived stress in a probability sample of the United States. In The Social Psychology of Health—The Claremont Symposium on Applied Social Psychology; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1988; pp. 31–67. [Google Scholar]
- Hodes, R.L.; Cook, E.W.; Lang, P.J. Individual Differences in Autonomic Response: Conditioned Association or Conditioned Fear? Psychophysiology 1985, 22, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory for Adults; APA PsycTests: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety & Stress Scales, 2nd ed.; Psychology Foundation: Sydney, Australia, 1995. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Hamilton, M. The Assessment of Anxiety States by Rating. British Journal of Medical Psychology 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Foa, E.B.; Ehlers, A.; Clark, D.M.; Tolin, D.F.; Orsillo, S.M. The Posttraumatic Cognitions Inventory (PTCI): Development and validation. Psychol. Assess. 1999, 11, 303–314. [Google Scholar] [CrossRef]
- Wells, S.Y.; Morland, L.A.; Torres, E.M.; Kloezeman, K.; Mackintosh, M.-A.; Aarons, G.A. The Development of a Brief Version of the Posttraumatic Cognitions Inventory (PTCI-9). Assessment 2019, 26, 193–208. [Google Scholar] [CrossRef]
- Weathers, F.W.; Litz, B.T.; Keane, T.M.; Palmieri, P.A.; Marx, B.P.; Schnurr, P.P. The PTSD Checklist for DSM-5 (PCL-5); National Center for PTSD, U.S. Department of Veterans Affairs: Washington, DC, USA, 2013. Available online: www.ptsd.va.gov (accessed on 7 September 2025).
- Gureje, O.; Obikoya, B. The GHQ-12 as a screening tool in a primary care setting. Soc. Psychiatry Psychiatr. Epidemiol. 1990, 25, 276–280. [Google Scholar] [CrossRef]
- Koh, K.B.; Park, J.K.; Kim, C.H.; Cho, S. Development of the Stress Response Inventory and Its Application in Clinical Practice. Psychosom. Med. 2001, 63, 668–678. [Google Scholar] [CrossRef]
- Derryberry, D.; Reed, M.A. Anxiety-related attentional biases and their regulation by attentional control. J. Abnorm. Psychol. 2002, 111, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research. In Advances in Psychology; North-Holland: Amsterdam, The Netherlands, 1988; pp. 139–183. [Google Scholar] [CrossRef]
- Zijlstra, F.R.H.; van Doorn, L. The Construction of a Scale to Measure Perceived Effort; Delft University of Technology: Delft, The Netherlands, 1985. [Google Scholar]
- Zijlstra, F.R.H. Efficiency in Work Behaviour: A Design Approach for Modern Tools. Delft University Press: Delf, The Netherlands, 1993. [Google Scholar]
- Brantleyr, J.; Waggoner, C.D.; Jones, G.N.; Rappaport, N.B. Daily Stress Inventory; APA PsycTests: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Verhey, F.R.J.; Huppert, F.A.; Korten, E.C.C.M.; Houx, P.; de Vugt, M.; van Lang, N.; DeDeyn, P.P.; Saerens, J.; Neri, M.; de Vreese, L.; et al. Cross-national comparisons of the Cambridge Cognitive Examination-revised: The CAMCOG-R: Results from the European Harmonization Project for Instruments in Dementia. Age Ageing 2003, 32, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State Examination; APA PsycTests: Washington, DC, USA, 1975. [Google Scholar] [CrossRef]
- Cohen, S.; Hoberman, H.M. Positive events and social supports as buffers of life change Stress. J. Appl. Soc. Psychol. 1983, 13, 99–125. [Google Scholar] [CrossRef]
- Crum, A.J.; Salovey, P.; Achor, S. Rethinking stress: The role of mindsets in determining the stress response. J. Pers. Soc. Psychol. 2013, 104, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, S.B.G.; Eysenck, H.J.; Barrett, P. A revised version of the psychoticism scale. Pers. Individ. Dif. 1985, 6, 21–29. [Google Scholar] [CrossRef]
- Rammstedt, B.; John, O.P. Big Five Inventory-10; APA PsycTests: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Hofmann, S.G. The subjective experience of emotion: A fearful view. Curr. Opin. Behav. Sci. 2018, 19, 67–72. [Google Scholar] [CrossRef]
- Russell, J.A.; Weiss, A.; Mendelsohn, G.A. Affect Grid: A Single-Item Scale of Pleasure and Arousal. J. Pers. Soc. Psychol. 1989, 57, 493–502. [Google Scholar] [CrossRef]
- Mehrabian, A.; Russell, J.A. An Approach to Environmental Psychology; MIT Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Smets, E.; Rios Velazquez, E.; Schiavone, G.; Chakroun, I.; D’Hondt, E.; De Raedt, W.; Cornelis, J.; Janssens, O.; Van Hoecke, S.; Claes, S.; et al. Large-scale wearable data reveal digital phenotypes for daily-life stress detection. npj Digit. Med. 2018, 1, 67. [Google Scholar] [CrossRef]
- Cai, H.; Yuan, Z.; Gao, Y.; Sun, S.; Li, N.; Tian, F.; Xiao, H.; Li, J.; Yang, Z.; Li, X.; et al. A multi-modal open dataset for mental-disorder analysis. Sci. Data 2022, 9, 178. [Google Scholar] [CrossRef]
- Babayan, A.; Erbey, M.; Kumral, D.; Reinelt, J.D.; Reiter, A.M.F.; Röbbig, J.; Lina Schaare, H.; Uhlig, M.; Anwander, A.; Bazin, P.L.; et al. Data descriptor: A mind-brain-body dataset of MRI, EEG, cognition, emotion, and peripheral physiology in young and old adults. Sci. Data 2019, 6, 180308. [Google Scholar] [CrossRef]
- Soleymani, M.; Lichtenauer, J.; Pun, T.; Pantic, M. A multimodal database for affect recognition and implicit tagging. IEEE Trans. Affect. Comput. 2012, 3, 42–55. [Google Scholar] [CrossRef]
- Koldijk, S.; Sappelli, M.; Verberne, S.; Neerincx, M.A.; Kraaij, W. The SWELL knowledge work dataset for stress and user modeling research. In Proceedings of the ICMI 2014 International Conference on Multimodal Interaction, Istanbul, Turkey, 12–16 November 2014; pp. 291–298. [Google Scholar] [CrossRef]
- Zheng, W.L.; Lu, B.L. Investigating Critical Frequency Bands and Channels for EEG-Based Emotion Recognition with Deep Neural Networks. IEEE Trans. Auton. Ment. Dev. 2015, 7, 162–175. [Google Scholar] [CrossRef]
- Katsigiannis, S.; Ramzan, N. DREAMER: A Database for Emotion Recognition Through EEG and ECG Signals from Wireless Low-cost Off-the-Shelf Devices. IEEE J. Biomed. Health Inf. Inform. 2018, 22, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.L.; Sourina, O.; Wang, L.P. STEW: Simultaneous task EEG workload data set. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2106–2114. [Google Scholar] [CrossRef]
- Markova, V.; Ganchev, T.; Kalinkov, K. CLAS: A Database for Cognitive Load, Affect and Stress Recognition. Proc. Int. Conf. Biomed. Innov. Appl. BIA 2019, 2019, 2019–2022. [Google Scholar] [CrossRef]
- Baghdadi, A.; Aribi, Y.; Fourati, R.; Halouani, N.; Siarry, P.; Alimi, A.M. DASPS: A Database for Anxious States based on a Psychological Stimulation. arXiv 2019, arXiv:1901.02942. [Google Scholar] [CrossRef]
- Zyma, I.; Tukaev, S.; Seleznov, I.; Kiyono, K.; Popov, A.; Chernykh, M.; Shpenkov, O. Electroencephalograms during mental arithmetic task Performance. Data 2019, 4, 14. [Google Scholar] [CrossRef]
- Seal, A.; Reddy, P.P.N.; Chaithanya, P.; Meghana, A.; Jahnavi, K.; Krejcar, O.; Hudak, R.; Jiang, Y.Z. An EEG Database and Its Initial Benchmark Emotion Classification Performance. Comput. Math. Methods Med. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Joshi, V.M.; Ghongade, R.B. IDEA: Intellect database for emotion analysis using EEG signal. J. King Saud. Univ. Comput. Inf. Sci. 2020, 34, 4433–4447. [Google Scholar] [CrossRef]
- Parent, M.; Albuquerque, I.; Tiwari, A.; Cassani, R.; Gagnon, J.-F.; Lafond, D.; Tremblay, S.; Falk, T.H. PASS: A Multimodal Database of Physical Activity and Stress for Mobile Passive Body/Brain-Computer Interface Research. Front. Neurosci. 2020, 14, 542934. [Google Scholar] [CrossRef]
- Miranda-Correa, J.A.; Abadi, M.K.; Sebe, N.; Patras, I. AMIGOS: A Dataset for Affect, Personality and Mood Research on Individuals and Groups. IEEE Trans. Affect. Comput. 2021, 12, 479–493. [Google Scholar] [CrossRef]
- Raheel, A.; Majid, M.; Anwar, S.M. DEAR-MULSEMEDIA: Dataset for emotion analysis and recognition in response to multiple sensorial media. Inf. Fusion 2021, 65, 37–49. [Google Scholar] [CrossRef]
- Stappen, L.; Baird, A.; Christ, L.; Schumann, L.; Sertolli, B.; Meßner, E.-M.; Cambria, E.; Zhao, G.; Schuller, B.W. The MuSe 2021 Multimodal Sentiment Analysis Challenge. In Proceedings of the 2nd on Multimodal Sentiment Analysis Challenge, New York, NY, USA, 20 October 2021; pp. 5–14. [Google Scholar] [CrossRef]
- Tabbaa, L.; Searle, R.; Mirzaee Bafti, S.; Hossain, M.M.; Intarasisrisawat, J.; Glancy, M.; Ang, C.S. VREED: Virtual Reality Emotion Recognition Dataset Using Eye Tracking and Physiological Measures. Proc. Acm Interact. Mob. Wearable Ubiquitous Technol. 2021, 5, 4. [Google Scholar] [CrossRef]
- Elgendi, M.; Galli, V.; Ahmadizadeh, C.; Menon, C. Dataset of Psychological Scales and Physiological Signals Collected for Anxiety Assessment Using a Portable Device. Data 2022, 7, 132. [Google Scholar] [CrossRef]
- Hosseini, S.; Gottumukkala, R.; Katragadda, S.; Bhupatiraju, R.T.; Ashkar, Z.; Borst, C.W.; Cochran, K. A multimodal sensor dataset for continuous stress detection of nurses in a hospital. Sci. Data 2022, 9, 255. [Google Scholar] [CrossRef]
- Saganowski, S.; Komoszyńska, J.; Behnke, M.; Perz, B.; Kunc, D.; Klich, B.; Kaczmarek, Ł.D.; Kazienko, P. Emognition dataset: Emotion recognition with self-reports, facial expressions, and physiology using wearables. Sci. Data 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Benchekroun, M.; Istrate, D.; Zalc, V.; Lenne, D. Mmsd: A Multi-modal Dataset for Real-time, Continuous Stress Detection from Physiological Signals. In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies, Virtual, 9–11 February 2022; SCITEPRESS—Science and Technology Publications: Setúbal, Portugal, 2022; pp. 240–248. [Google Scholar] [CrossRef]
- Ghosh, R.; Deb, N.; Sengupta, K.; Phukan, A.; Choudhury, N.; Kashyap, S.; Phadikar, S.; Saha, R.; Das, P.; Sinha, N.; et al. SAM 40: Dataset of 40 subject EEG recordings to monitor the induced-stress while performing Stroop color-word test, arithmetic task, and mirror image recognition task. Data Brief 2022, 40, 107772. [Google Scholar] [CrossRef]
- Yadav, M.; Sakib, M.N.; Nirjhar, E.H.; Feng, K.; Behzadan, A.H.; Chaspari, T. Exploring Individual Differences of Public Speaking Anxiety in Real-Life and Virtual Presentations. IEEE Trans. Affect. Comput. 2022, 13, 1168–1182. [Google Scholar] [CrossRef]
- Xefteris, V.-R.; Dominguez, M.; Grivolla, J.; Tsanousa, A.; Zaffanela, F.; Monego, M.; Symeonidis, S.; Diplaris, S.; Wanner, L.; Vrochidis, S.; et al. A Multimodal Late Fusion Framework for Physiological Sensor and Audio-Signal-Based Stress Detection: An Experimental Study and Public Dataset. Electronics 2023, 12, 4871. [Google Scholar] [CrossRef]
- Chaptoukaev, H.; Strizhkova, V.; Panariello, M.; D’Alpaos, B.; Reka, A.; Manera, V.; Thümmler, S.; Ismailova, E.; Evans, N.; Bremond, F.; et al. StressID: A Multimodal Dataset for Stress Identification. In Proceedings of the 37th Conference on Neural Information Processing Systems (NeurIPS 2023), New Orleans, LA, USA, 10–16 December 2023; Curran Associates, Inc.: Red Hook, NY, USA, 2023. [Google Scholar]
- Eisenbarth, H.; Oxner, M.; Shehu, H.A.; Gastrell, T.; Walsh, A.; Browne, W.N.; Xue, B. Emotional arousal pattern (EMAP): A new database for modeling momentary subjective and psychophysiological responding to affective stimuli. Psychophysiology 2023, 61, e14446. [Google Scholar] [CrossRef]
- Pešán, J.; Juřík, V.; Karafiát, M.; Černocký, J. BESST Dataset: A Multimodal Resource for Speech-based Stress Detection and Analysis. In Interspeech 2024; ISCA: Kos, Greece, 2024; pp. 1355–1359. [Google Scholar] [CrossRef]
- Dogan, G.; Akbulut, F.P.; Catal, C. Biosignals, facial expressions, and speech as measures of workplace stress: Workstress3d dataset. Data Brief 2024, 54, 110303. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, W.; Zhang, J.; Li, Y.; Wang, L.; Hao, Y.; Li, X. DSRP: A Database for Stress Reduction Using Physiological Signals. IEEE Access 2024, 12, 135089–135102. [Google Scholar] [CrossRef]
- Singh, P.; Budhiraja, R.; Gupta, A.; Goswami, A.; Kumar, M.; Singh, P. EEVR: A Dataset of Paired Physiological Signals and Textual Descriptions for Joint Emotion Representation Learning. In Proceedings of the 38th International Conference on Neural Information Processing Systems, Vancouver, BC, Canada, 10–15 December 2024; Curran Associates Inc.: Red Hook, NY, USA, 2024; pp. 15765–15778. [Google Scholar]
- Das, S.; Leander, N.; González, C.R.; Lønfeldt, N.N. EmoPairCompete—Physiological signals dataset for emotion and frustration assessment under team and competitive behaviors. In Proceedings of the ICLR 2024 Workshop on Learning from Time Series For Health, Vienna, Austria, 7–11 May 2024; pp. 1–10. [Google Scholar]
- Wang, Y.; Yu, H.; Gao, W.; Xia, Y.; Nduka, C. MGEED: A Multimodal Genuine Emotion and Expression Detection Database. IEEE Trans. Affect. Comput. 2024, 15, 606–619. [Google Scholar] [CrossRef]
- Muhammad, S.; Mousavi, H.; Khaertdinov, B.; Jeuris, P.; Hortal, E.; Andreoletti, D.; Giordano, S. Emotion Recognition in Adaptive Virtual Reality Settings: Challenges and Opportunities. In Proceedings of the CEUR Workshop on Advances of Mobile and Wearable Biometrics, MobileHCI’23, Athens, Greece, 26 September 2023. [Google Scholar]
- Uhrig, M.K.; Trautmann, N.; Baumgärtner, U.; Treede, R.D.; Henrich, F.; Hiller, W.; Marschall, S. Emotion Elicitation: A Comparison of Pictures and Films. Front. Psychol. 2016, 7, 180. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [PubMed]
- Raufi, B.; Longo, L. An Evaluation of the EEG Alpha-to-Theta and Theta-to-Alpha Band Ratios as Indexes of Mental Workload. Front. Neurosci. 2022, 16, 861967. [Google Scholar] [CrossRef]
- Saeed, S.M.U.; Anwar, S.M.; Khalid, H.; Majid, M.; Bagci, U. EEG Based Classification of Long-Term Stress Using Psychological Labeling. Sensors 2020, 20, 1886. [Google Scholar] [CrossRef]
- Kelly, M.M.; Tyrka, A.R.; Anderson, G.M.; Price, L.H.; Carpenter, L.L. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J. Behav. Ther. Exp. Psychiatry 2008, 39, 87–98. [Google Scholar] [CrossRef]
- Verma, R.; Balhara, Y.S.; Gupta, C. Gender differences in stress response: Role of developmental and biological determinants. Ind. Psychiatry J. 2011, 20, 4–10. [Google Scholar] [CrossRef]
- Stando, A.; Moniz, N.; Branco, P.; Torgo, L.; Japkowicz, N.; Wozniak, M.L.; Wang, S. The Effect of Balancing Methods on Model Behavior in Imbalanced Classification Problems. arXiv 2023, arXiv:2307.00157. [Google Scholar] [CrossRef]
- Wilkinson, M.D.; Dumontier, M.; Jan Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef]
- Shen, J.; Wu, J.; Liang, H.; Zhao, Z.; Li, K.; Zhu, K.; Wang, K.; Ma, Y.; Hu, W.; Guo, C.; et al. Physiological signal analysis using explainable artificial intelligence: A systematic review. Neurocomputing 2025, 618, 128920. [Google Scholar] [CrossRef]
- Dhara, T.; Singh, P.K.; Mahmud, M. A Fuzzy Ensemble-Based Deep learning Model for EEG-Based Emotion Recognition. Cogn. Comput. 2024, 16, 1364–1378. [Google Scholar] [CrossRef]
- Karani, R.; Desai, S. Review on Multimodal Fusion Techniques for Human Emotion Recognition. Int. J. Adv. Comput. Sci. Appl. 2022, 13, 287–296. [Google Scholar] [CrossRef]
- Dogan, G.; Akbulut, F.P. Multi-modal fusion learning through biosignal, audio, and visual content for detection of mental Stress. Neural Comput. Appl. 2023, 35, 24435–24454. [Google Scholar] [CrossRef]
- Khandelwal, M.; Sharma, A. Machine Learning and Deep Learning Techniques to Detect Mental Stress Using Various Physiological Signals: A Critical Insight. WIREs Data Min. Knowl. Discov. 2025, 15, e70035. [Google Scholar] [CrossRef]
- Apicella, A.; Arpaia, P.; D’Errico, G.; Marocco, D.; Mastrati, G.; Moccaldi, N.; Prevete, R. Toward cross-subject and cross-session generalization in EEG-based emotion recognition: Systematic review, taxonomy, and methods. Neurocomputing 2024, 604, 128354. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, X.; Zhang, X.; Sun, B.; Wang, Y. An improved graph convolutional neural network for EEG emotion recognition. Neural Comput. Appl. 2024, 36, 23049–23060. [Google Scholar] [CrossRef]
- Jain, A.; Kumar, R. Machine Learning based Anxiety Detection using Physiological Signals and Context Features. In Proceedings of the 2024 2nd International Conference on Advancement in Computation & Computer Technologies (InCACCT), Gharuan, India, 2–3 May 2024; pp. 116–121. [Google Scholar] [CrossRef]
- Rajendran, V.G.; Jayalalitha, S.; Adalarasu, K.; Mathi, R. Machine learning based human mental state classification using wavelet packet decomposition-an EEG study. Multimed. Tools Appl. 2024, 83, 83093–83112. [Google Scholar] [CrossRef]
- Pei, D.; Tirumala, S.; Tun, K.T.; Ajendla, A.; Vinjamuri, R. Identifying neurophysiological correlates of Stress. Front. Med Eng. 2024, 2, 1434753. [Google Scholar] [CrossRef]
- Giannakakis, G.; Karasmanoglou, A.; Antonakakis, M.; Vorgia, P.; Zervakis, M. Emotion Recognition Based on EEG Signals and Deep Neural Networks Architectures. In Proceedings of the 12th International Conference on Affective Computing and Intelligent Interaction Workshops and Demos (ACIIW), Glasgow, UK, 15–18 September 2024; IEEE: New York, NY, USA, 2024; pp. 189–192. [Google Scholar] [CrossRef]
- Dong, Y.; Jing, C.; Mahmud, M.; Ng, M.K.-P.; Wang, S. Enhancing cross-subject emotion recognition precision through unimodal EEG: A novel emotion preceptor model. Brain Inform. 2024, 11, 31. [Google Scholar] [CrossRef]
- Zou, C.; Deng, Z.; He, B.; Yan, M.; Wu, J.; Zhu, Z. Emotion classification with multi-modal physiological signals using multi-attention-based neural network. Cogn. Comput. Syst. 2024, 6, 1–11. [Google Scholar] [CrossRef]
- Tervonen, J.; Närväinen, J.; Mäntyjärvi, J.; Pettersson, K. Explainable stress type classification captures physiologically relevant responses in the Maastricht Acute Stress Test. Front. Neuroergonomics 2023, 4, 1294286. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.K.; Ehrhart, M.; Resch, B. An Explainable Deep Learning Approach for Stress Detection in Wearable Sensor Measurements. Sensors 2024, 24, 5085. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Han, B.; Park, S.M.; Chang, J. Developing an explainable Deep Neural Network for stress detection using biosignals and human-engineered features. Biomed. Signal Process. Control 2025, 109, 107960. [Google Scholar] [CrossRef]
- Al-Alim, M.A.; Mubarak, R.; Salem, N.M.; Sadek, I. A machine-learning approach for stress detection using wearable sensors in free-living environments. Comput. Biol. Med. 2024, 179, 108918. [Google Scholar] [CrossRef]
- Xiang, J.-Z.; Wang, Q.-Y.; Fang, Z.-B.; Esquivel, J.A.; Su, Z.-X. A multi-modal deep learning approach for stress detection using physiological signals: Integrating time and frequency domain features. Front. Physiol. 2025, 16, 1584299. [Google Scholar] [CrossRef]
- Gao, H.; Cai, Z.; Wang, X.; Wu, M.; Liu, C. Multimodal Fusion of Behavioral and Physiological Signals for Enhanced Emotion Recognition Via Feature Decoupling and Knowledge Transfer. IEEE J. Biomed. Health Inform. 2025, 1–11. [Google Scholar] [CrossRef]
- Uribe, Y.F.; Alvarez-Uribe, K.C.; Peluffo-Ordoñez, D.H.; Becerra, M. Physiological Signals Fusion Oriented to Diagnosis—A Review. Commun. Comput. Inf. Sci. 2018, 885, 1–15. [Google Scholar] [CrossRef]
- Khoei, T.T.; Slimane, H.O.; Kaabouch, N. Deep learning: Systematic review, models, challenges, and research directions. Neural Comput. Appl. 2023, 35, 23103–23124. [Google Scholar] [CrossRef]
- Nguyen, D.; Nguyen, D.T.; Sridharan, S.; Denman, S.; Nguyen, T.T.; Dean, D.; Fookes, C. Meta-transfer learning for emotion recognition. Neural Comput. Appl. 2023, 35, 10535–10549. [Google Scholar] [CrossRef]
- Yurdem, B.; Kuzlu, M.; Gullu, M.K.; Catak, F.O.; Tabassum, M. Federated learning: Overview, strategies, applications, tools and future directions. Heliyon 2024, 10, e38137. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, L.; Miadzvetskaya, Y.; Ofe, H.; Barnett, A.; Helminger, L.; Lindstaedt, S.; Trügler, A. Privacy-Preserving Techniques for Trustworthy Data Sharing: Opportunities and Challenges for Future Research. In Data Spaces; Curry, E., Scerri, S., Tuikka, T., Eds.; Springer: Cham, Switzerland, 2022; pp. 319–335. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzaki, C.; Tsiknakis, M. An Overview of Stress Analysis Based on Physiological Signals: Systematic Review of Open Datasets and Current Trends. Sensors 2025, 25, 7108. https://doi.org/10.3390/s25237108
Chatzaki C, Tsiknakis M. An Overview of Stress Analysis Based on Physiological Signals: Systematic Review of Open Datasets and Current Trends. Sensors. 2025; 25(23):7108. https://doi.org/10.3390/s25237108
Chicago/Turabian StyleChatzaki, Chariklia, and Manolis Tsiknakis. 2025. "An Overview of Stress Analysis Based on Physiological Signals: Systematic Review of Open Datasets and Current Trends" Sensors 25, no. 23: 7108. https://doi.org/10.3390/s25237108
APA StyleChatzaki, C., & Tsiknakis, M. (2025). An Overview of Stress Analysis Based on Physiological Signals: Systematic Review of Open Datasets and Current Trends. Sensors, 25(23), 7108. https://doi.org/10.3390/s25237108







