2.1. Measurement Setup and Gas Characterization System
To experimentally evaluate the performance of the BME688 gas sensor in a controlled measurement environment, a BME688 Development Kit from Bosch Sensortec was employed [
10], and the board can be observed in
Figure 1. This evaluation platform integrates eight identical BME688 sensors on a single board, each featuring the same metal oxide sensing element, microheater, and integrated circuitry. The use of multiple sensors does not rely on different materials or custom designs; rather, it enables parallel measurements under identical environmental conditions. Indeed, the development kit architecture is designed to support AI-based air quality analysis through Bosch’s BME AI-Studio (v1.3) software and BSEC library [
6,
10]. The inclusion of multiple identical sensors facilitates the collection of richer datasets, which can be used to train and validate application-specific machine learning models for gas or air-quality recognition.
The development kit offers substantial flexibility in acquiring the raw chemical-resistance data necessary to accurately characterize the gas-sensing performance of each sensor sample in different measurement conditions. Users can define the sampling interval and adjust the sensor operating temperature at each sampling point. This functionality enables the implementation of dynamic temperature profiles; however, it is limited to a fixed sequence of up to ten temperature values per measurement cycle. While the system supports a broad range of gas-sensing scenarios, its constraints on temperature-profile length and sampling frequency should be considered when designing experiments.
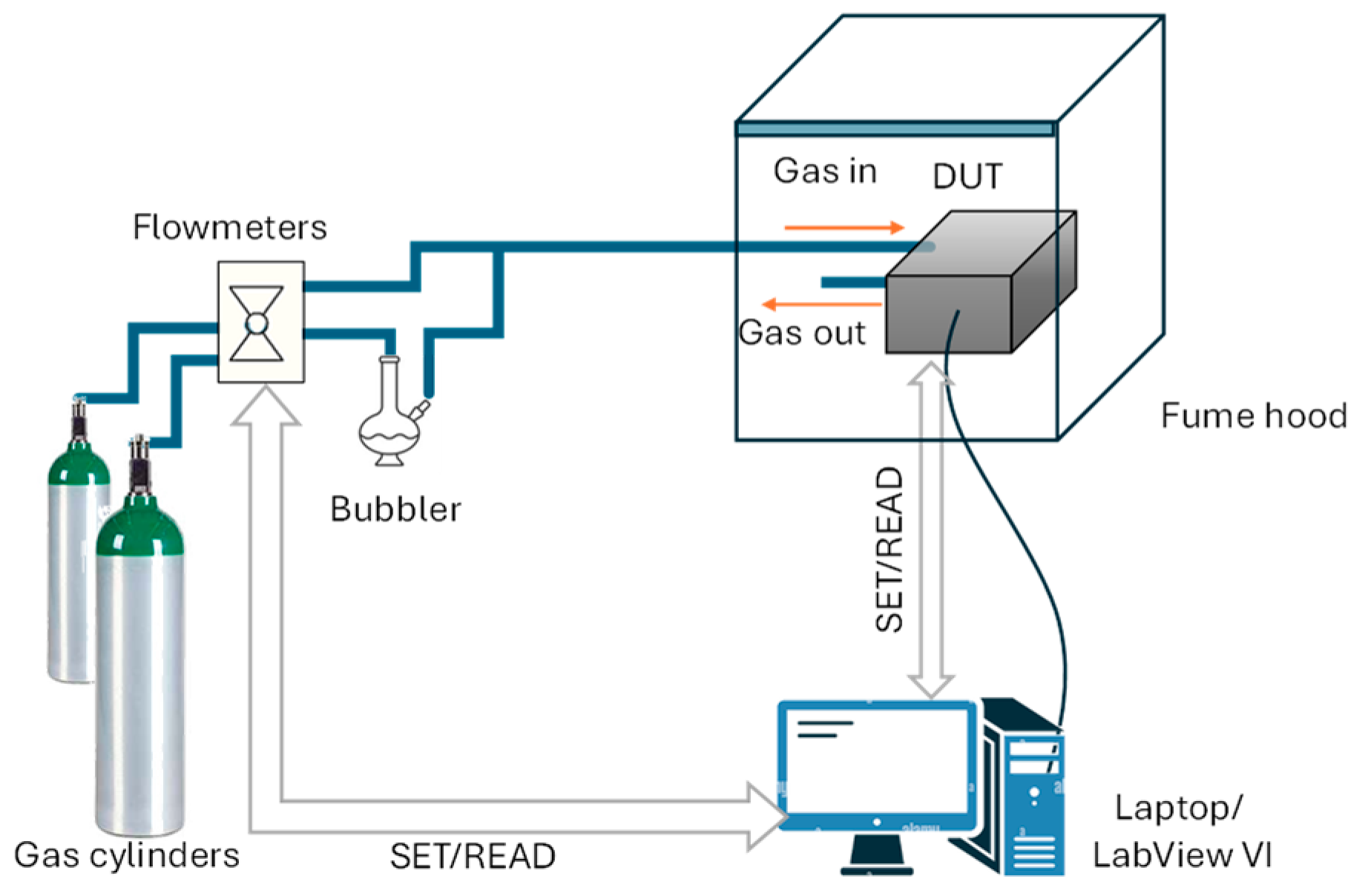
For the purposes of this study, the sensor temperature was kept constant during each measurement cycle, with values in the admissible range as desired for different tests. To facilitate the observation of the gas response and minimize the influence of fluid dynamics, a custom measurement chamber was specifically designed for the application [
11]. The test chamber was custom-designed and fabricated using 3D-printed ABS, and it is shown in
Figure 1. This chamber was engineered to enclose the Device Under Test (DUT), effectively minimizing leakage and ensuring a more direct flow of gas to the sensing area. By reducing the internal dead volume, the system allows for fast gas exchanges, enabling the evaluation of the chemical response/recovery time of the sensors to the different target gases.
The whole measurement process is remotely managed by a Virtual Instrument (VI) developed in the LabVIEW environment. This interface enables the configuration of the BME688 sensors’ operating conditions, such as the working temperature and sampling time, set as 140 ms in this work, and allows for the continuous acquisition as well as the visualization and saving of raw resistance data and other working parameters such as humidity, pressure, and heater current. Communication with the DUT is established via USB (virtual RS232).
In addition, the VI also controls the gas system that regulates the composition (i.e., the desired concentration of each target gas) and flow of the gas mixtures used for the tests. This includes the remote control of a mass flow controller system (Bronkhorst F-201C), which allows for the mixing of accurately regulated gas flows—up to four test gases—to obtain mixtures with known compositions and total flow. During all measurements reported in this paper, the total gas flow rate was maintained at 200 mL/min. The desired gas mixtures at known concentrations were generated by mixing different flow rates originated from two certified reference cylinders: one containing the target gas (e.g., NO2) at a certified concentration, and the other containing ultrapure dry air, used as the carrier gas. The concentration of all cylinders was guaranteed by the gas supplier to be within ±2% of the nominal value.
As an example, for the NO2 measurements, we used a certified cylinder containing 2 ppm NO2 in air and another cylinder of ultrapure air. By mixing 100 mL/min from the NO2 cylinder with 100 mL/min from the carrier gas cylinder, we obtained a final mixture with a NO2 concentration of 1 ppm while maintaining the total flow at 200 mL/min by exploiting the accurate dilution of the original cylinder. This approach enables the production of a wide range of concentrations from a limited set of certified reference cylinders, ensuring both flexibility and cost-efficiency. The accuracy of the mass flow controllers, specified as ±0.5% of the reading plus ±0.1% of the full scale, ensures that the generated concentrations remain within an acceptable uncertainty range. Ultrapure air was used as the carrier gas in order to ensure accurate and reproducible measurements because it provides a clean reference atmosphere, free from interfering species such as CO2, VOCs, NOx, PMx, or uncontrolled water vapor content, which could otherwise affect the baseline signal and lead to non-reproducible results.
The tests reported in this work evaluate the sensor response to nitrogen dioxide (NO
2; original cylinder of 2 ppm concentration in air), isobutylene (C
4H
8; original cylinder of 2 ppm concentration in air), carbon monoxide (CO; original cylinder of 20 ppm concentration in air), and sulfur dioxide (SO
2; original cylinder of 40 ppm concentration in air). These compounds were selected as representative outdoor air pollutants. In particular, NO
2, CO, and sulfur dioxide (SO
2) are classified by the U.S. Environmental Protection Agency (EPA) as criteria air pollutants, ubiquitous components of ambient air that are stringently regulated due to their documented adverse effects on human health and the environment [
12], whereas isobutylene is a gas representative of VOCs commonly emitted in urban and industrial environments, contributing to atmospheric photochemical reactions and serving as a typical tracer of industrial hydrocarbon emissions [
13].
The humidity levels inside the test chamber were adjusted using a bubbler system filled with ultrapure water. A dry synthetic air (or nitrogen) stream was passed through the bubbler to achieve saturation, and the relative humidity (RH) levels were modulated by mixing this saturated airflow with the dry mixtures in varying proportions. All tests were performed at 50% RH, a representative and easily controllable condition corresponding to typical ambient humidity levels.
In
Figure 2, a schematic diagram of the measurement and characterization setup is reported.
The experimental dataset was collected over a measurement campaign lasting several months, during which repeated exposures were performed under controlled conditions of temperature, humidity, and gas composition. It should be underlined that, although the BME688 sensors were characterized over a wide temperature range (200–400 °C), only the responses obtained above approximately 240 °C are reported in the following sections. Indeed, at lower temperatures, the response and recovery kinetics were extremely slow, requiring several tens of minutes to approach steady state. Because such conditions are not compatible with dynamic sensing applications (e.g., mobile or wearable systems), these data were excluded from the investigation of the sensors’ responses to the tested gases. Therefore, the temperature range shown corresponds to the region of practical operation, where the sensors display measurable and reliable gas differentiation.
2.2. Experimental Results: Gas Response
Taking into account the structure of the sensing film [
7], which consists of a loosely connected micro-grained layer, the sensing mechanism is primarily related to the formation of a potential barrier at the grain surface, which determines the conductivity. The sensing material is SnO
2, which behaves as an n-type semiconductor.
In some more detail, for this kind of sensing film, the height of the potential barrier, VS, can be obtained from a 1D description of the depleted region beneath the grain surface. This region is typically formed due to the adsorption of negatively charged species, such as oxygen ions (e.g., O−, O2−), on the grain surface of the n-type semiconductor. These species extract electrons from the conduction band, creating a space charge region (also called the depletion layer) near the surface.
The potential barrier V
S represents the energy barrier that electrons must overcome to move from one grain to another; thus, it determines the electrical conductivity of the film. Under the assumption of large grains and a fully depleted surface, the barrier height depends directly on the surface density of the negatively charged species, hereafter denoted as N
s, as follows [
14]:
where n is the bulk donor volume density,
is the electrical permittivity of the bulk material, and q is the electron charge.
The resistance of the film is then derived from the density of the carriers able to overcome the potential barrier through thermal energy, and it can be expressed as
where
R0 is the baseline resistive value of the film, a parameter that depends on the film geometry and the material bulk properties;
k is the Boltzmann constant; and
T is the absolute temperature.
The charged species surface density can be written as follows:
where N
i indicates the density of negatively ionized intrinsic surface defects (considered to be the only type present) and
X and
Y represent species adsorbed from the gas phase onto specific surface sites and subsequently ionized, where the squared brackets [ ⋅ ] indicate the surface density of the given species.
From Equations (1) to (3), the overall behavior of the sensor can be understood: Chemisorbed species either increase the barrier height (oxidizing gases) or decrease it (reducing gases), thereby producing a highly nonlinear effect on the film resistance. This simple model clarifies that the sensor response to a gas depends on the chemisorption reaction, on the baseline value of
Ns (ionized intrinsic defects), and on the bulk donor density. Importantly, temperature influences resistance both explicitly (through the exponential factor in (2)) and indirectly (by affecting adsorption equilibria and the ionization of surface species) [
14,
15].
It should be noted that, although the simplified model of Equations (1)–(3) can be applied to the material of the BME688, neither the magnitude of the response nor its dynamics can be predicted, because the reaction rate constants, the baseline values of Ns and n, and their variations all depend on material defects (both bulk and surface), not solely on the intrinsic properties of the pure material. The defect chemistry ultimately depends on the specific preparation route. Therefore, the actual behavior cannot be predicted a priori and must be determined experimentally.
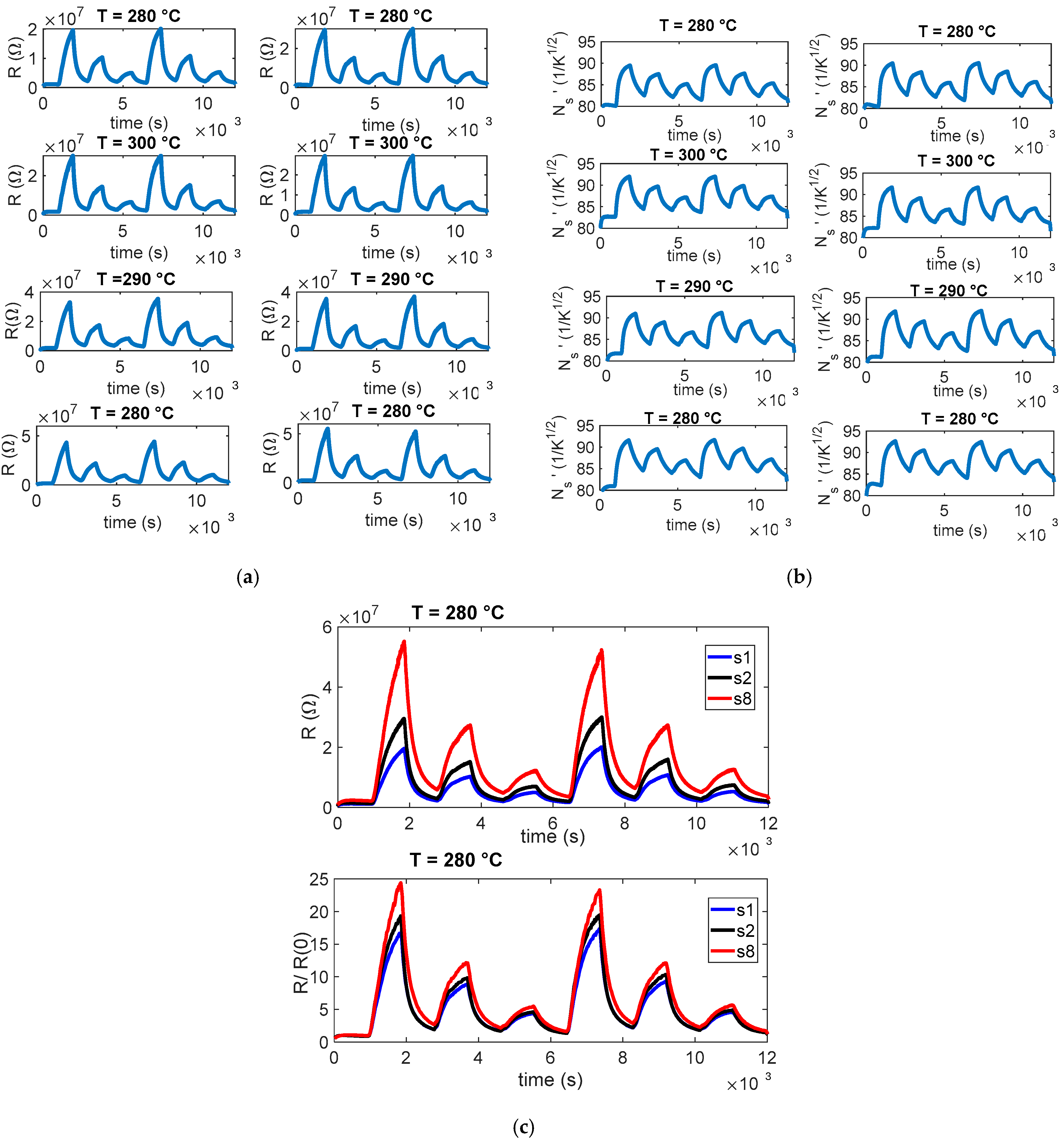
The planning and execution of the tests must be accurate, as they are often lengthy and time-consuming. In this work, we evaluate the sensor response by exposing it for a fixed duration (15 min) to gas mixtures containing different concentrations of the target gas, diluted in synthetic air under either dry conditions or in the presence of water vapor. Each exposure phase is followed by a recovery phase in clean air, which allows the sensor to approach its baseline resistance. An example of the characterization results obtained following this protocol is shown in
Figure 3. Each test was repeated at least twice, and pairs of the eight sensors were always tested under the same operating conditions (same temperature).
The normalized sensor response is quantified using the following parameter:
Here, by denoting with the time-dependent resistance of the sensor, the normalized response parameter Resp represents the relative variation in resistance between the beginning and the end of the gas-exposure phase, where is the resistance at the end of a 15 min exposure phase to the target-gas mixture and R(0) is the resistance at the beginning of the test (i.e., its baseline reference value in carrier gas).
This formulation captures the fractional resistance change induced by the target gas. For reducing gases, Resp values range between 0 and 1 (decreasing resistance), whereas for oxidizing gases, they are greater than 1 (increasing resistance), in accordance with the expected behavior of SnO2-based resistive sensors.
It is worth noting that, especially at low temperatures, the resistance may not reach a steady-state value within the 15 min exposure window. As a result, the calculated response may underestimate the true steady-state response. However, in practice, when the response times at low temperatures are significantly longer than 15 min, the exact steady-state value is of limited relevance. This is because such low temperatures are outside the intended operational range for mobile or real-time applications, where long stabilization times are incompatible with requirements. Therefore, performance at these low temperatures is not considered interesting for practical use.
We emphasize that the same definition of sensor response was maintained for both oxidizing and reducing gases. Naturally, with this definition, the maximum response to a reducing gas is bounded at −1, whereas the response to an oxidizing gas is not upper-limited. For a fairer comparison between the two cases, resistance should ideally be used for oxidizing gases and conductivity for reducing ones. However, this would introduce additional complexity in data presentation, because some gases may switch behavior depending on the operating temperature. In any case, the artifacts introduced by adopting a single unified definition are effectively resolved by examining the response plots, which clearly illustrate the underlying sensor behavior.
As already observed in [
11], the eight sensors have very different baseline values (in some conditions, differences larger than 100%), but the normalized resistance shows consistent behaviors (difference within 30%). Such behavior can be seen in the examples reported in
Figure 3. Specifically, panel (a) reports the measured resistance values R for the eight sensors; panel (b) shows an estimation of the normalized surface charge density, N
s’=
; and panel (c) compares three representative sensors, those with the highest, intermediate, and lowest baseline values, highlighting how normalization with respect to the baseline reduces variability across devices. In our procedure,
is retrieved in two steps. First,
is estimated by inverting (2) during rapid temperature steps performed at low temperature, where the density of surface states
can be treated as constant because the thermal response of the sensing micro-membrane (milliseconds) is much faster than the surface kinetics (minutes). Using
just before and just after this temperature step, we solve for
from (2). Second, with
known,
is obtained over the full trajectory by rearranging (2) and solving for
from the measured
and the known
. This time-scale-separation approach yields a robust and physically consistent estimation of the surface-state density.
As already stated, after normalization, the variability among the eight tested sensors is significantly reduced and confined to levels that are acceptable for detection tasks, even though the absolute accuracy of the concentration measurements remains limited. This indicates that the BME688, when properly normalized, can provide reliable qualitative information on the presence of and relative variation in target gases across a sensor network. However, to ensure comparability among devices, it is essential to establish the baseline resistance of each sensor under standard reference conditions. This step represents a form of individual calibration that cannot be avoided, because baseline values are strongly influenced by intrinsic material defects and manufacturing variability. Defining and standardizing such a calibration procedure by specifying the reference environment, temperature, and humidity conditions is, therefore, a necessary prerequisite for large-scale deployments. By implementing this individual calibration step, the reduced variability achieved through normalization can be effectively exploited, enabling the use of low-cost MOX sensors for distributed monitoring applications. In more detail, before gas exposure, the dynamic resistance trend in synthetic air was analyzed to evaluate the baseline stability and transient effects associated with the heating cycles. The results show gradual stabilization of the baseline resistance over time, mainly attributed to the thermal equilibration of the sensing layer and the desorption of residual surface species. Minor fluctuations observed during steady-state operation reflect the intrinsic dynamic balance between adsorption and desorption processes under ambient conditions. This behavior is consistent with the expected thermal activation of the SnO2-based sensing layer and confirms the stable operation of the sensor in air before exposure to target gases.
Because the behavior of the eight sensors in terms of normalized response is acceptably similar and the trend as a function of temperature or of target-gas concentrations is similar and consistent, from now on, the results of a single sensor are presented, but the comments and conclusions drawn can be extended to all the sensors in the batch and, ultimately, to the whole sensor family. In this context, concerning the transient gas test results, the sensor response was expressed as the normalized ratio / (RCHIM/) to minimize the effect of baseline differences among the eight sensors. Because each device may exhibit a different initial resistance value, normalization allows researchers to focus on the relative variation induced by gas exposure rather than the absolute resistance, enabling a direct comparison of the dynamic behavior across sensors. Although the normalized responses start approximately from unity, small deviations are observed before gas injection. These deviations arise because the baseline resistance in air is not perfectly stable during the initial phase of each measurement and because normalization with respect to a single reference point can slightly shift the starting level.
2.2.1. NO2 Gas Sensor Response
The reaction path usually assumed for NO
2 sensing is the following [
15]:
where
indicates an adsorbed
molecule and
a free electron.
So, NO2 behaves as an oxidizing gas and causes an increase in resistance. In fact, considering dry mixtures of air and NO2, we can assume for the charge surface density that Therefore, the presence of NO2 contributes to the increase in .
Note that represents the concentration of chemisorbed oxygen from air.
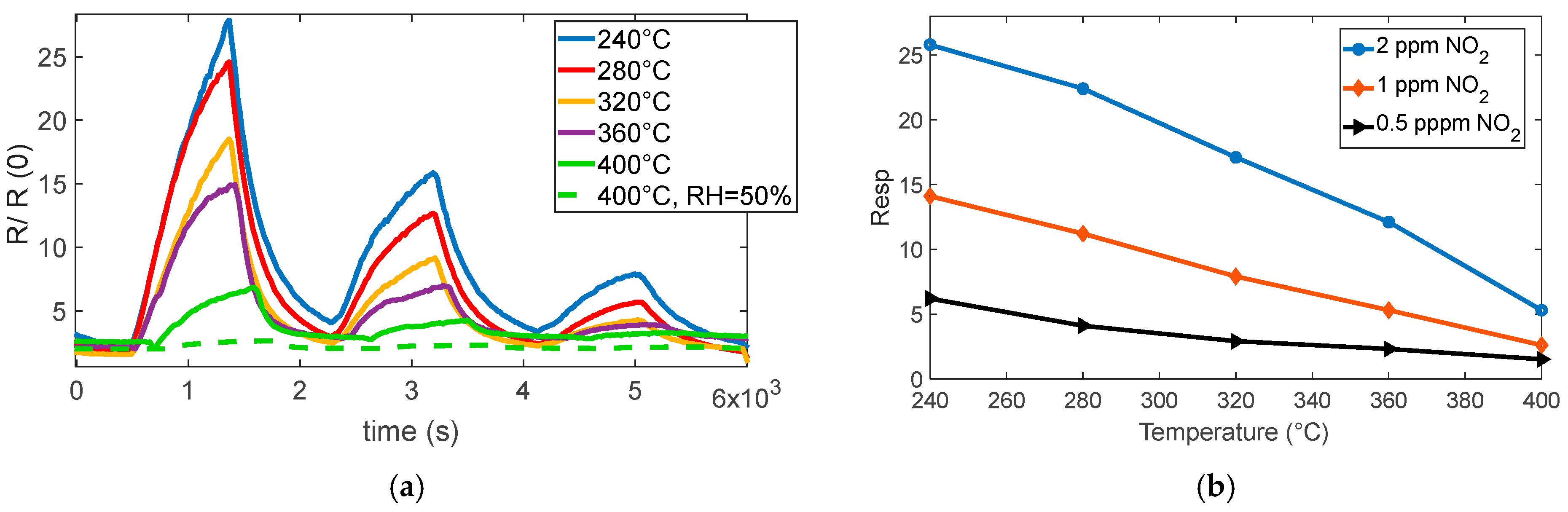
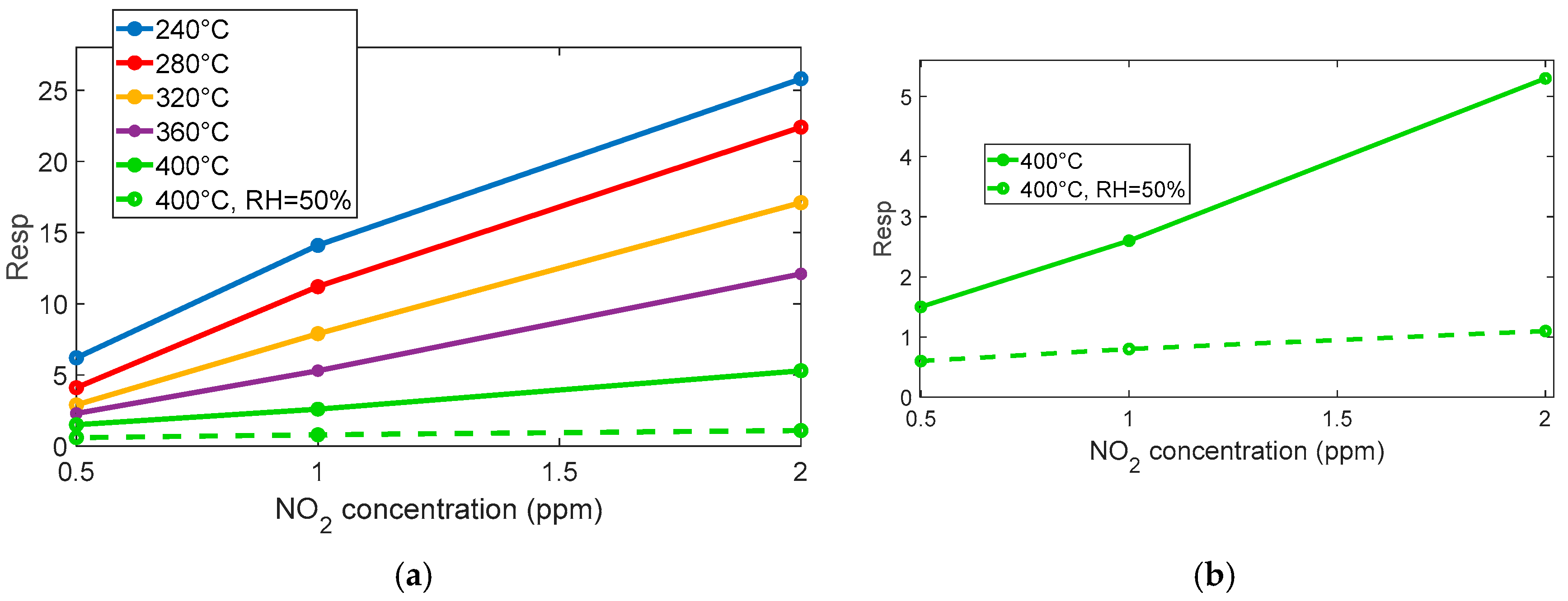
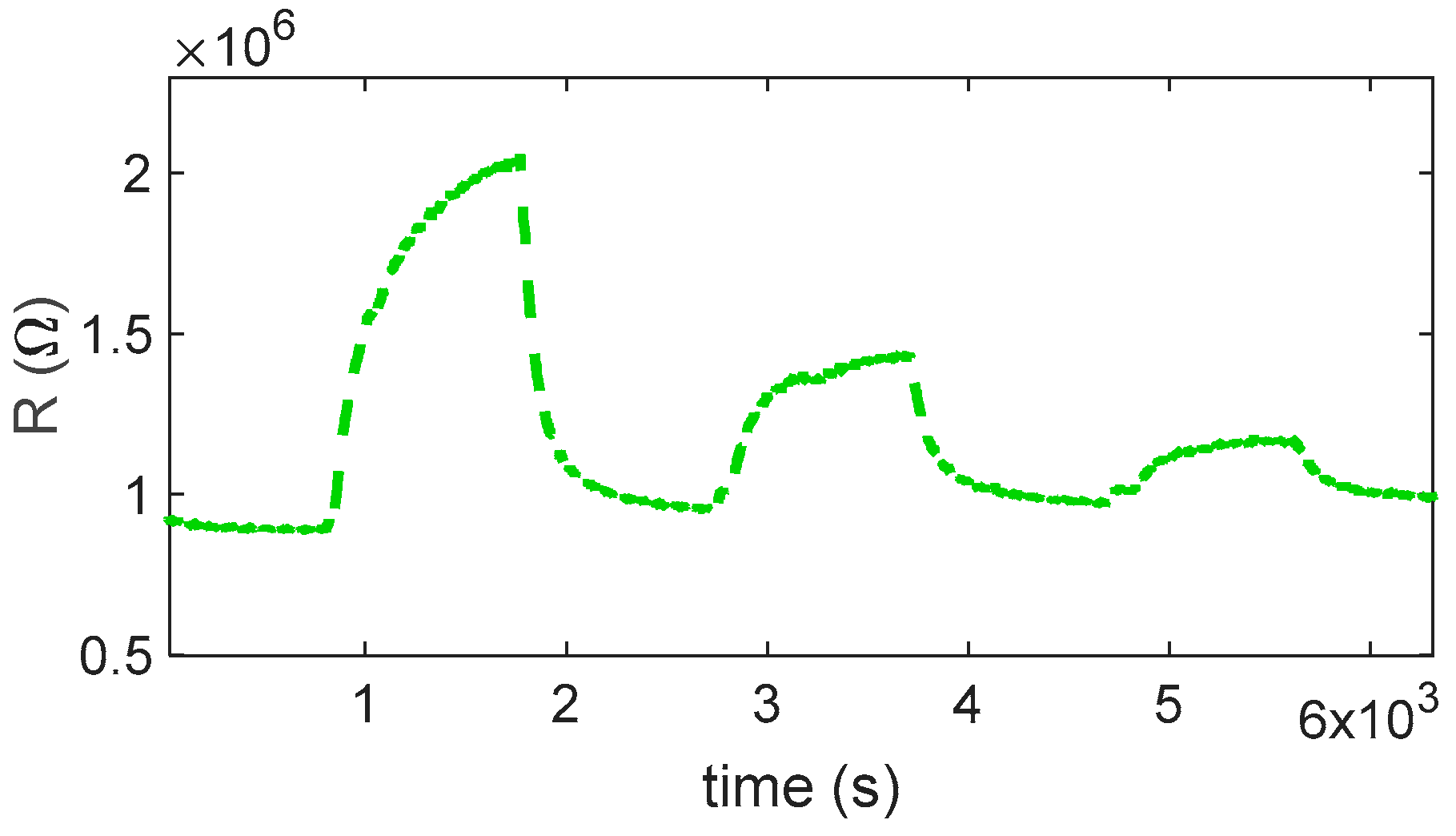
The reaction is very favored at the lower temperature, below 360° for instance; at 280 °C, a very large response (as per (4)) of about 8 is found for 0.5 ppm NO
2. Nevertheless, at this temperature, the reaction kinetics are very slow, as can be seen in
Figure 4a.
At temperatures above 360 °C, both the response and recovery times improve significantly, reaching reasonable durations of the order of a few minutes. Although the magnitude of the response decreases (approximately 1 at 0.5 ppm), it remains adequate for detection purposes.
The presence of water vapor reduces the overall sensor response but simultaneously improves the response speed, as shown in
Figure 4a,
Figure 5a,b, and
Figure 6. Despite the reduced sensitivity, the sensor maintains a detection limit below 0.1 ppm even in the presence of high relative humidity (50%), which confirms its suitability for outdoor air quality monitoring.
In summary, operating the sensor at temperatures above 360 °C and under relative humidity levels exceeding 20% is appropriate and effective for the intended application.
2.2.2. Isobutylene Gas Sensor Response
According to the literature, the sensing mechanism relies on the oxidation of isobutylene by oxygen species that are chemisorbed on the surface of the SnO
2. This reaction releases electrons back into the conduction band, which, in turn, reduces the height of the grain boundary potential barrier. The reaction route assumed is the following [
16]:
The preadsorbed oxygen that can intermediate such reaction is that present in the intermediate temperature range [
16]:
As a result, the electrical resistance of the material decreases, with reaction (6) contributing to the decrease in , as per Equation (3).
At lower temperature, different oxygen species are expected [
16]:
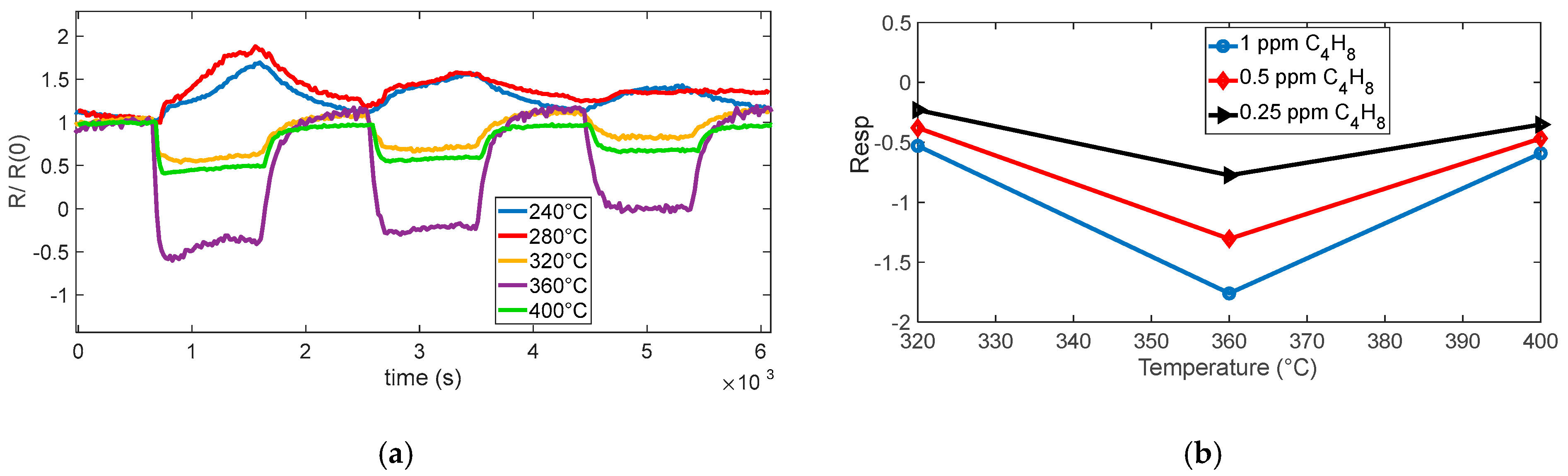
So, at low temperature, Equation (7) is not the expected reaction. Note that the temperature ranges are approximately known; therefore, for the sensing material used in BME688, the ranges can be different. Specifically, from the measurements (as can be seen in
Figure 7 and
Figure 8), the expected reducing behavior starts above 300 °C; below, a very slow opposite behavior is registered. Below this temperature, indeed, the ionization kinetics of chemisorbed oxygen species (O
2−, O
−) and the adsorption–desorption processes of target gases become extremely slow. The surface potential and charge density, therefore, remain nearly constant, resulting in an unstable or negligible resistance variation during gas exposure. Under these conditions, the sensor does not reach steady state within the time of measurement, and the response becomes impractically slow for real-world operation. Above 300 °C, the activation of surface reactions and charge transfer accelerates, producing a stable and reversible conductance change consistent with the typical behavior of SnO
2-based sensors.
In this regard, the responses evaluated at 240 °C and 280 °C were excluded from
Figure 7b because at these temperatures, the sensor response exhibits an inversion of the expected reducing-gas trend and unstable signal behavior, as visible in
Figure 7a. These responses are not considered reliable and were, therefore, omitted to provide a more representative comparison across the effective operating temperatures.
To the best of the authors’ knowledge, no reliable models or scientific publications exist in the current literature that describe aliphatic VOCs (such as isobutylene) as exhibiting oxidizing behavior (i.e., acting as electron acceptors) on SnO2 or other metal oxides at low temperatures.
At low temperatures, aliphatic VOCs such as isobutylene can undergo weak adsorption onto the SnO2 surface, primarily through physisorption mechanisms. However, this direct adsorption does not result in significant charge transfer between the gas molecules and the sensing material and, therefore, does not produce a measurable change in conductivity. It is worth noting that the slight increase in resistance observed at low temperatures upon exposure to isobutylene could be tentatively attributed to a weak competitive adsorption effect between the VOC molecules and atmospheric oxygen. Although the reactivity of isobutylene to adsorbed oxygen species is negligible under these conditions, the physisorption of VOCs may marginally influence the equilibrium coverage of oxygen ions, thereby affecting the resistance value. In any case, it is necessary to exceed a temperature of 300 °C to obtain a response to isobutylene fast enough to be exploited for measurement in the application of interest. Below this threshold, the sensor shows very slow behavior. For this gas, there is an optimum temperature around 360 °C; where a very large response is observed, the large response to the tested mixture with a concentration of 0.25 ppm allows for the estimation of a limit of detection (LOD) in the range of tens of ppb.
As expected, for reaction (6), the response time also depends on the concentration of the target gas, whereas the recovery time is independent.
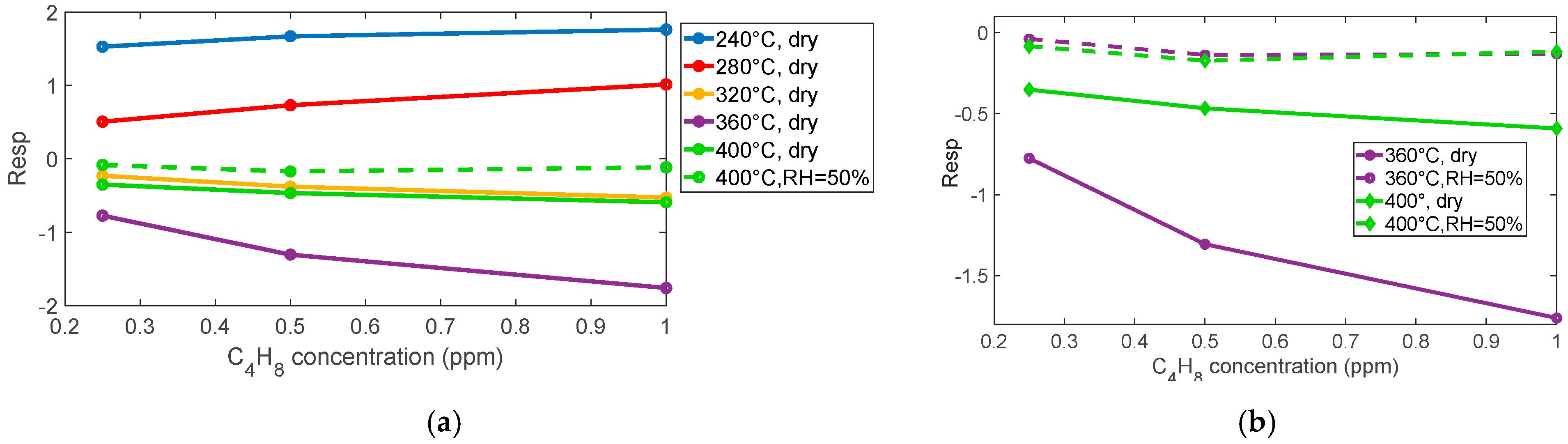
As observed also for NO
2, the presence of humidity affects the magnitude of the sensor response, which drops by about an order of magnitude (see
Figure 8a and
Figure 9). Nevertheless, the estimated limit of detection remains about 0.1 ppm, making this sensor suitable for the application.
2.2.3. SO2 Gas Sensor Response
Upon exposure to SO
2, the typical reactions occurring on the SnO
2 surface can be described as follows [
17,
18]:
In this mechanism, SO
2 acts as a reducing gas: it reacts with chemisorbed oxygen ions, releasing electrons back to the conduction band of SnO
2, decreasing
as per Equation (3), and, thus, lowering the sensor resistance. The process is thermally activated, with appreciable sensitivity generally observed above 350 °C, where the catalytic oxidation of SO
2 to SO
3 occurs and the reaction rate and recovery remain reasonably fast as to be used for detection applications [
19].
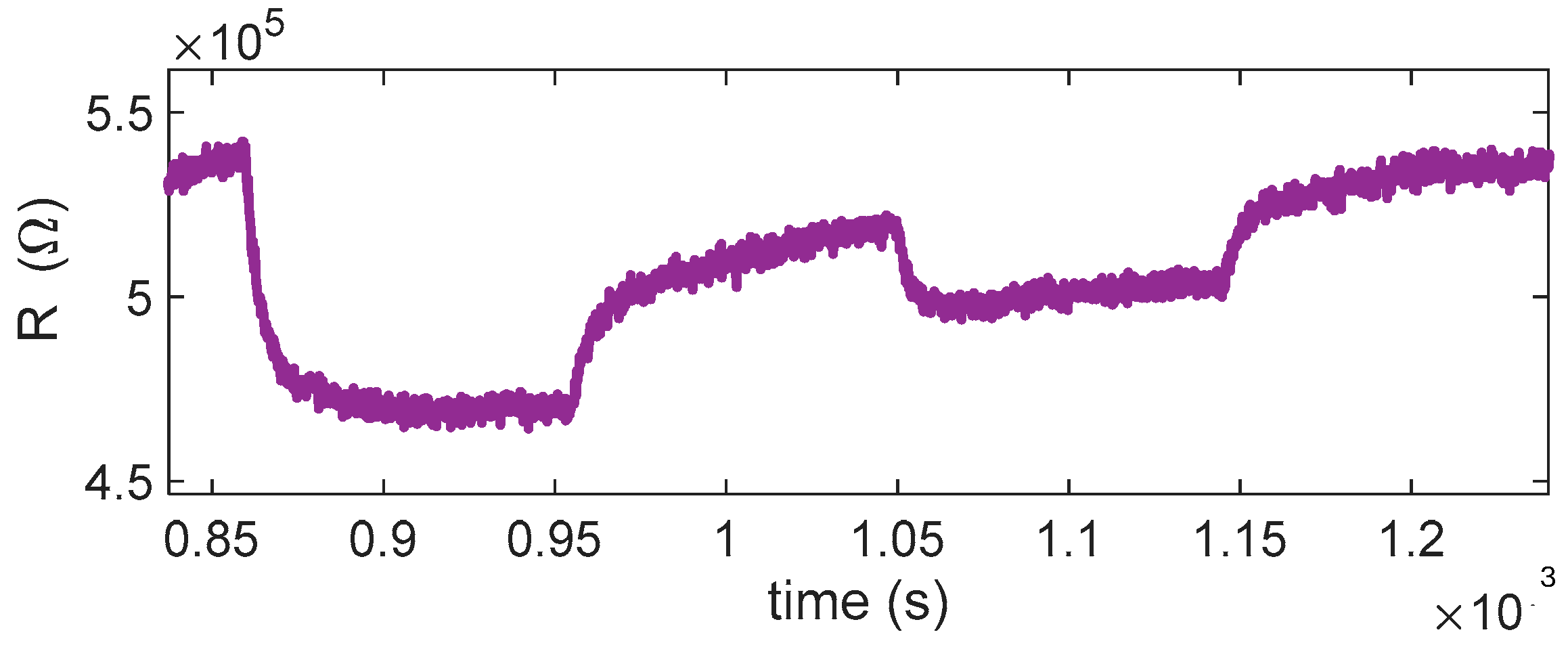
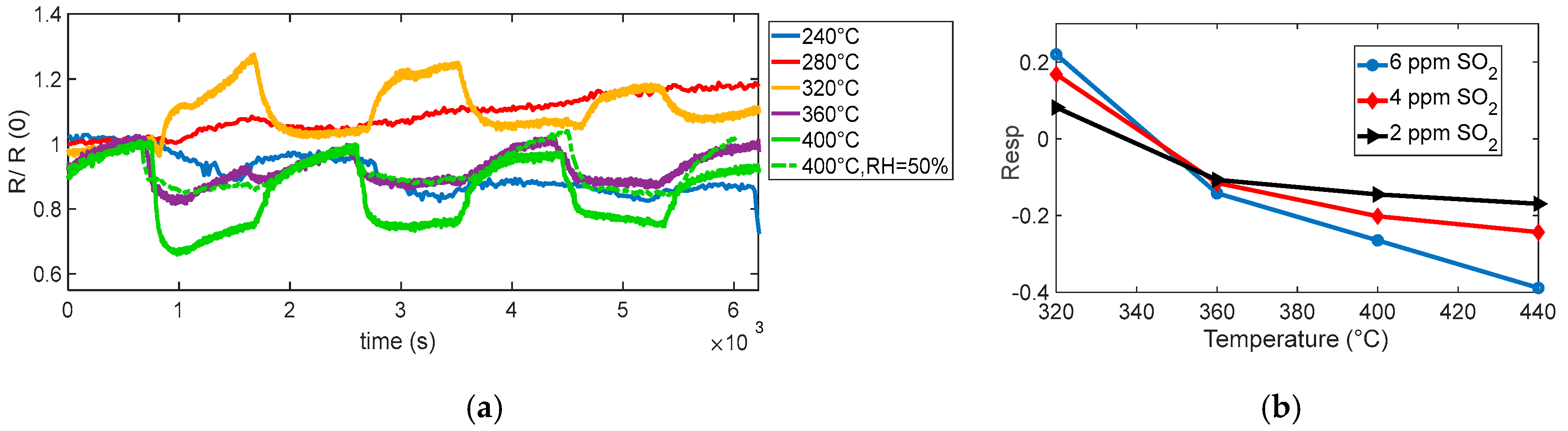
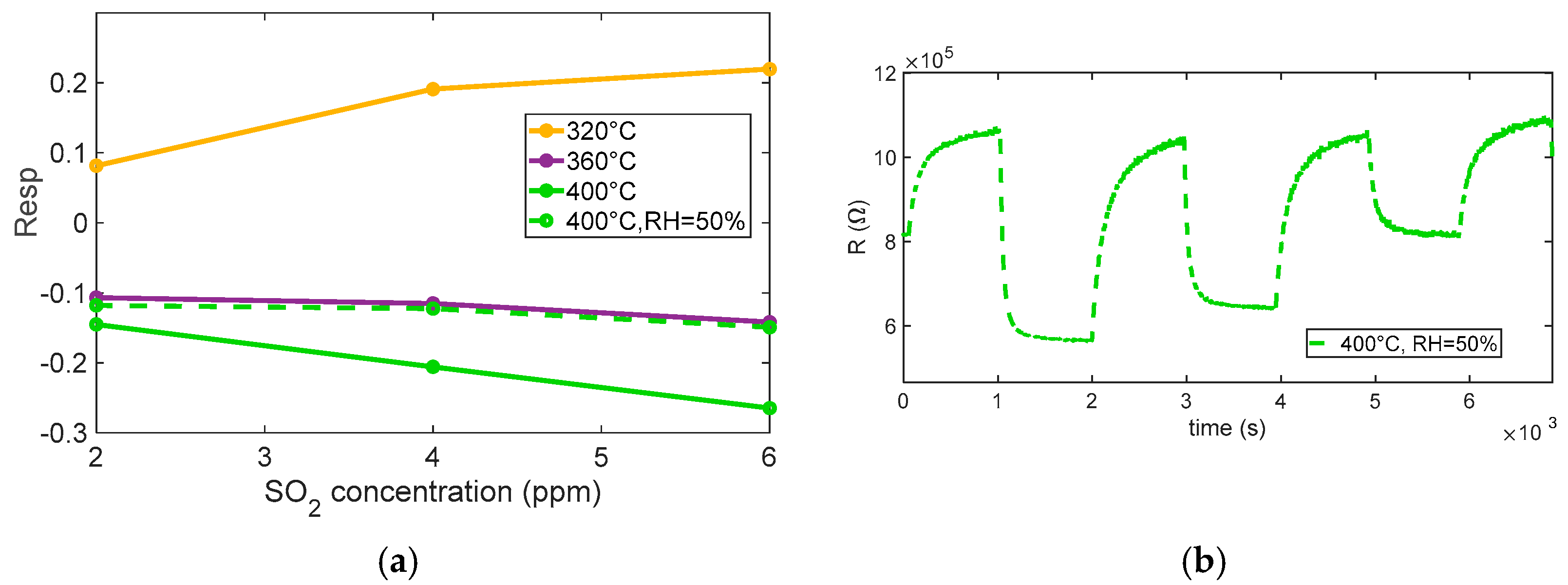
The behavior observed in the performed tests (see
Figure 10 and
Figure 11) shows a reducing-type response at the highest temperature considered, with an appreciable response becoming evident for temperatures above ~360 °C, as seen in
Figure 10. At lower temperatures (below ~320 °C), no considerable response is observed; interestingly, at 320 °C, the response even shows an apparent inversion: the sensor initially behaves as if SO
2 were oxidizing, increasing the resistance. This inversion effect, also reported for other MOX sensors, can be, as in the case of isobutylene, attributed to competing surface processes and also to the formation of strongly adsorbed species that trap conduction electrons instead of releasing them. At higher temperature, these species tend to desorb or further react, restoring the typical reducing behavior of SO
2.
In particular, the dynamic behavior visible at 360 °C and especially at 400 °C confirms the enhanced sensitivity of the sensor under these conditions, where the kinetics of surface reactions are accelerated [
17].
The test performed at 400 °C under 50% relative humidity shows that humidity slightly affects the response profile, the response appears to be somewhat modified, the baseline resistance value is greatly reduced, and the response appears to be slightly slowed down by the effect of humidity, but no appreciable reduction in SO2 sensitivity is observed.
These observations suggest that the optimal operating temperature for achieving a clear and stable reducing response to SO2 lies around 400 °C, where surface processes are sufficiently activated to ensure a rapid and reversible sensor response, although care must be taken to account for possible interference from ambient humidity. Notably, at this temperature, the sensor exhibits adequate sensitivity to detect SO2 concentrations close to typical outdoor exposure limits (around 4 ppm), making the response time and recovery behavior suitable for practical environmental monitoring applications.
2.2.4. CO Gas Sensor Response
Carbon monoxide is a typical reducing gas whose surface reactions with n-type metal oxide semiconductors, such as SnO
2, are expected to reduce the film total resistance. The sensing process occurs via electron exchange between the gas molecules and the surface of the oxide, typically in a temperature range starting from 200 °C up to even 430 °C [
20]. As mentioned before, in air, molecular oxygen is adsorbed onto the SnO
2 surface and ionized by capturing electrons from the conduction band according to reaction (7) [
21,
22].
Upon CO exposure, the expected film behavior involves a combination of reversible chemisorption and irreversible oxidation on the surface, depending on the type of adsorbed oxygen species and operating temperature. We can expect a direct chemisorption of CO [
21] according to the following equation:
This is equivalent, in charge-transfer terms, to the adsorption of CO followed by electron release to the semiconductor surface [
14,
22], representing the direct adsorption and oxidation process of CO on SnO
2 surface sites, S
CO, involving interaction with surface oxygen vacancies or weakly bonded lattice oxygen. Although not the dominant mechanism, this pathway has been hypothesized and discussed in the literature [
23,
24] and supported by previous modeling and experimental works of the authors in [
25,
26]. Conversely, the reaction in the following Equation (12) represents the canonical oxygen-mediated route [
21], widely accepted as the principal mechanism governing CO sensing on SnO
2 surfaces at operating temperatures above ~300 °C, which can be written as
This reaction involves a more stable, irreversible oxidation of CO with surface-adsorbed oxygen species (e.g., O−), forming CO2 and releasing an electron. Both mechanisms contribute to a decrease in the negative charge trapped at the surface, resulting in a decrease in electrical resistance. In detail, according to reactions (11) and (12), the surface charge density is described by ; therefore, reaction (11) describes the formation of positive surface charge and reaction (12) the consumption of negative ones. The operating temperatures in this work (≤400 °C) are consistent with the activation range of these surface reactions. From measurements performed in the absence of oxygen, i.e., using pure nitrogen as the carrier gas, we observed a very low response to CO, which indicates that the prevailing reaction is (12).
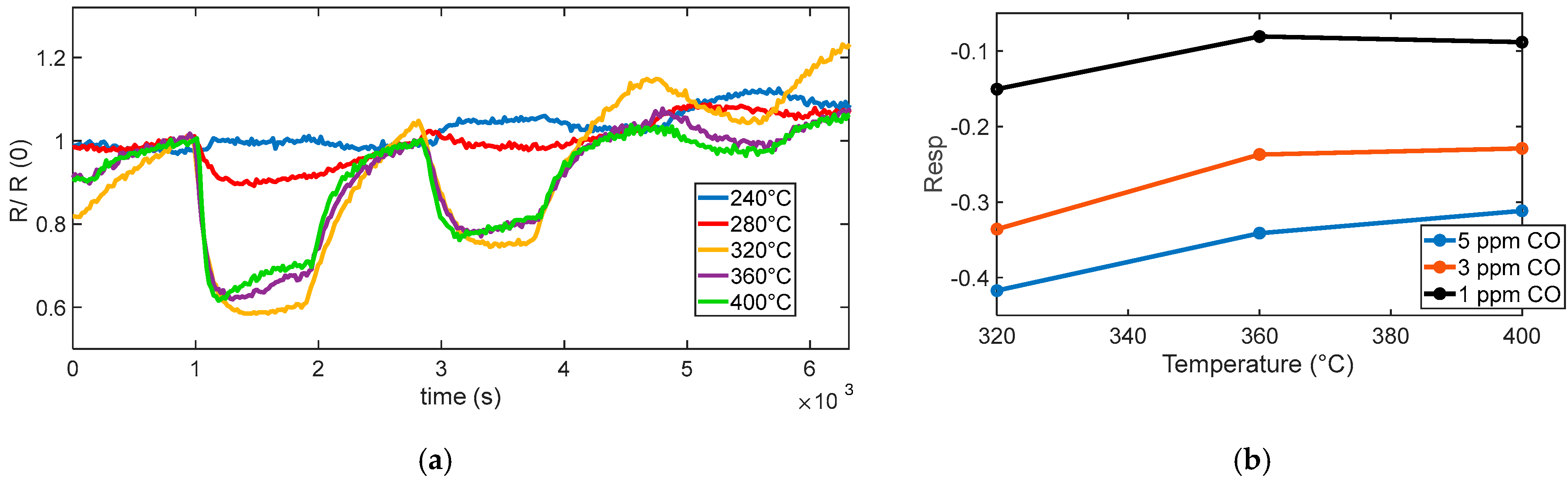
Figure 12a shows the measured sensor response to various CO concentrations (5 ppm, 3 ppm, and 1 ppm) at different operating temperatures. At low temperatures (below 300 °C in our tests), the sensor exhibited a slow or negligible response, which indicates that the oxygen species are weakly bound and predominantly present in molecular form. A noticeable and consistent response begins to emerge at 320 °C, which corresponds to the activation of chemisorbed oxygen species, primarily O
−. This temperature range supports fast reactions and ensures high sensitivity, although a significant response drift is observed. It is worth noting that all surface oxidation reactions require activation energy. The discussion in this section refers not to a barrier-free process but rather to the predominance of O
2− ions as the active oxygen species at low temperatures. According to previous studies [
25,
26,
27], O
2− species dominate below ~350 °C, while O
− ions become prevalent at higher temperatures, which explains the observed temperature-dependent changes in the CO response.
As the temperature increases, the sensor response becomes more stable, and the drift observed at 320 °C is progressively reduced.
Above 360 °C, no substantial further increase in sensitivity is observed; instead, the response tends to plateau and remain stable, suggesting an optimal operating temperature around 400 °C, as summarized in
Figure 12b.
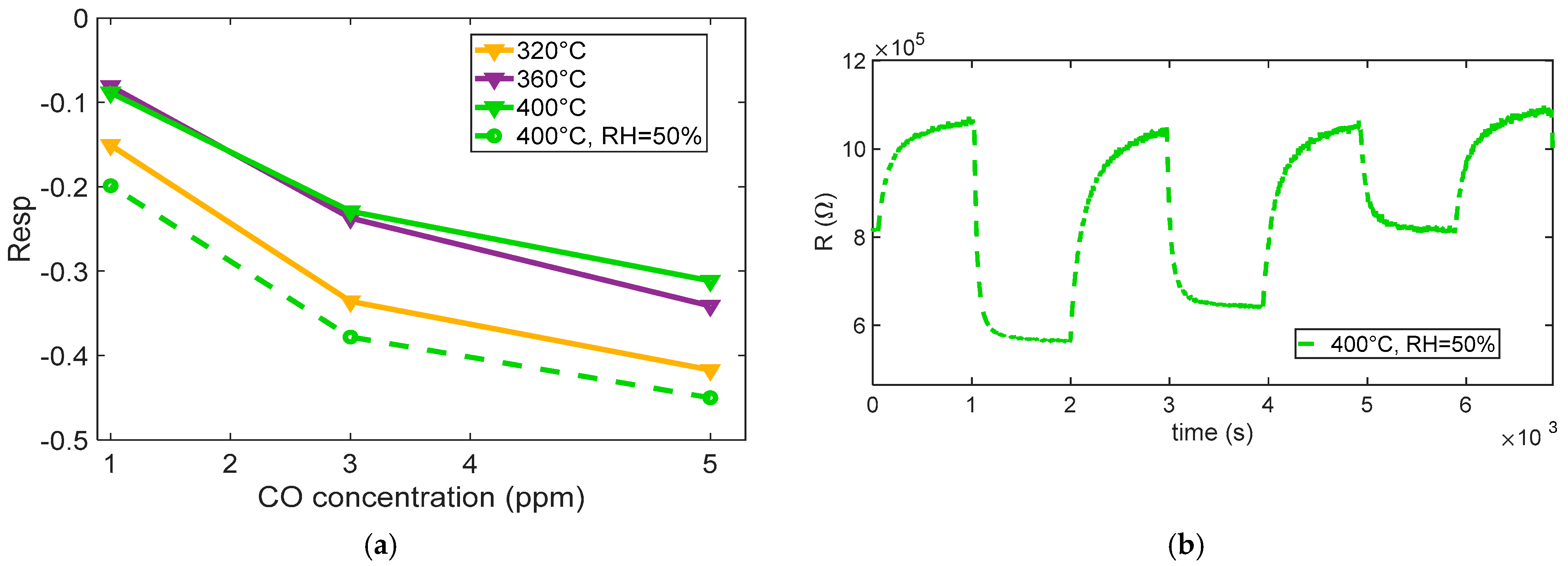
Figure 13 summarizes the measured response to the different CO concentrations. The results indicate that, in the presence of humidity, water vapor can significantly influence the CO sensing mechanism on SnO
2-based MOX sensors. Water molecules adsorb onto the sensor surface and may dissociate to form hydroxyl groups (–OH). These hydroxyl species can either serve as additional reactive sites or modify the surface electronic structure, thereby enhancing the interaction between CO and the sensing layer.
One proposed reaction pathway involves CO reacting with surface hydroxyls as follows [
28]:
This reaction contributes to the release of electrons into the conduction band, resulting in a larger decrease in resistance and, consequently, a stronger sensing signal. An additional explanation is that the presence of water vapor can enhance the surface mobility of adsorbed species and improve the kinetics of CO oxidation, thereby increasing both sensitivity and response speed [
28].
The enhancing effect of adsorbed water is clearly observed by comparing the curves in
Figure 12a with the results in
Figure 13b, which show the sensor’s transient response to CO under humid conditions at 400 °C. The response is notably faster than in dry environments and lower-temperature scenarios, with an enhanced gas response that confirms the positive influence of humidity on sensing dynamics.
For CO, the response times appear to be relatively independent of the gas concentration and shorter than the recovery times across all tested levels, indicating that desorption or re-equilibration processes are slower and may represent the rate-limiting step in sensor operation.