Biochemical Sensing Application of Surface Plasmon Resonance Sensor Based on Flexible PDMS Substrate
Highlights
- A flexible surface plasmon resonance (SPR) sensor was developed with a PDMS substrate replacing conventional rigid glass, providing simple fabrication and low cost.
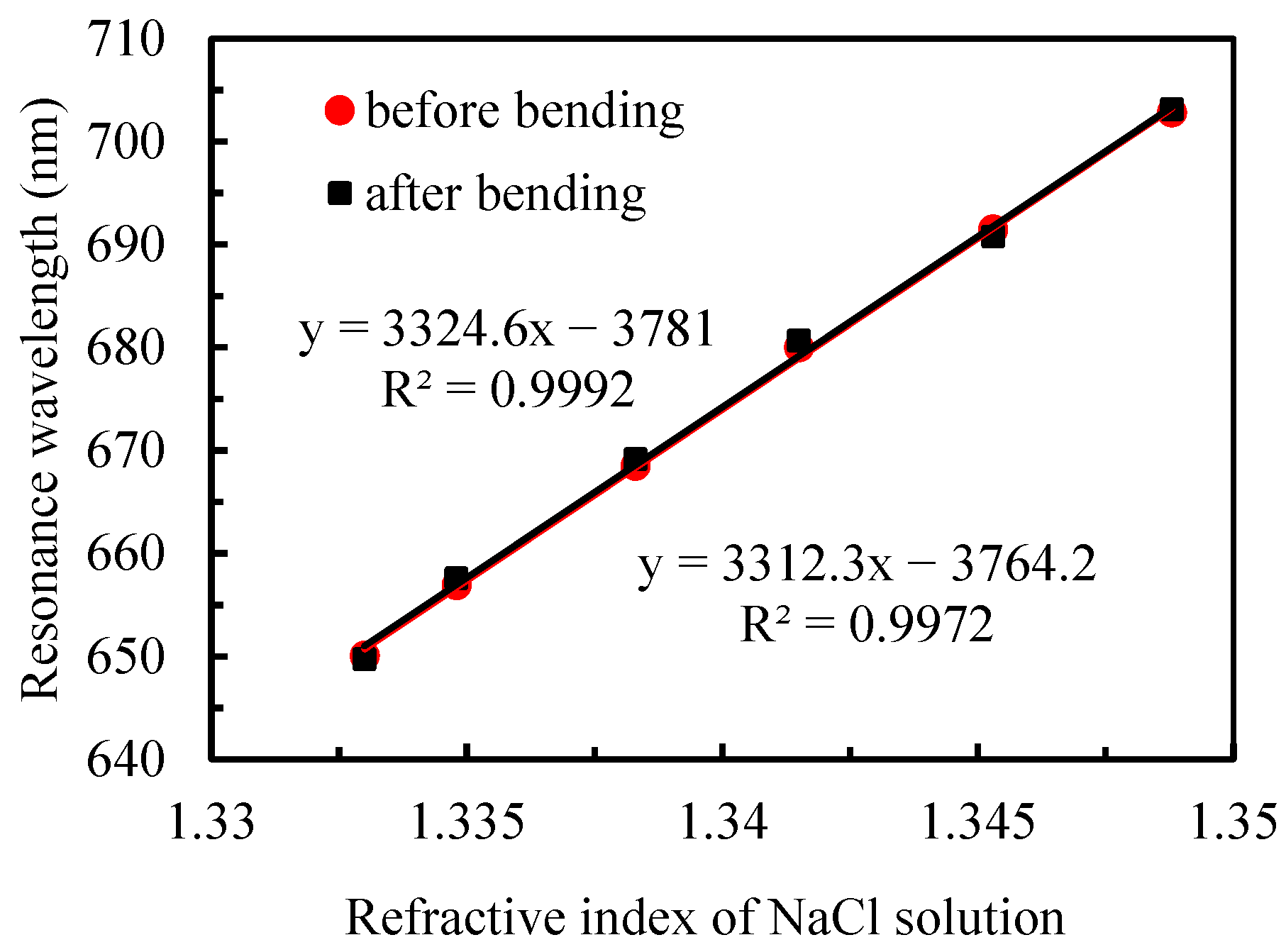
- The sensor exhibited remarkable stability, showing only 1% sensitivity variation after 50 bidirectional bending cycles.
- The sensor achieved rapid, stable detection of alcohol contents in three distinct Chinese Baijiu samples.
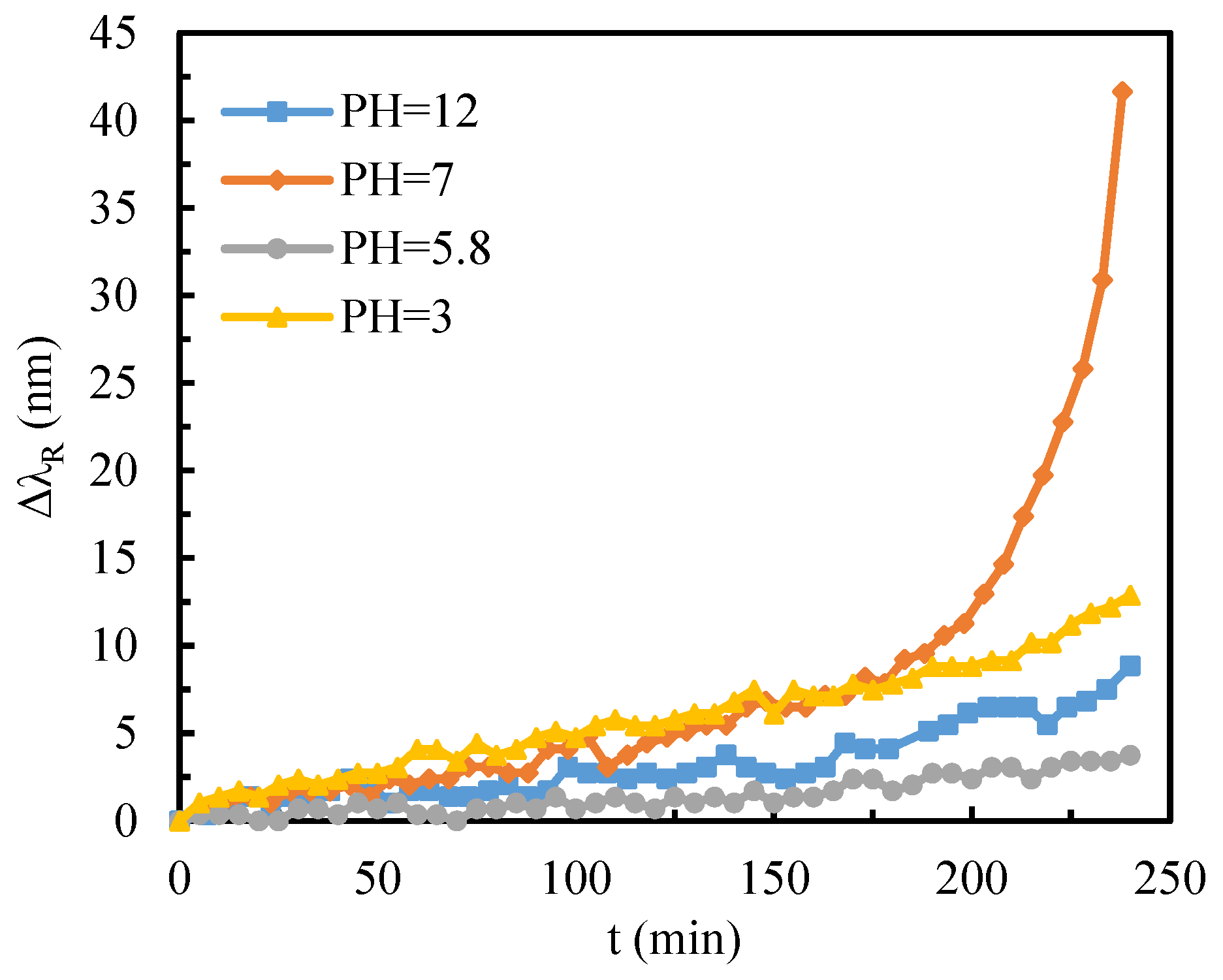
- Glutathione (GSH) adsorption on gold films at various pH was studied, elucidating GSH-metal interface mechanisms.
Abstract
1. Introduction
2. Experimental Sections
2.1. Experimental Materials
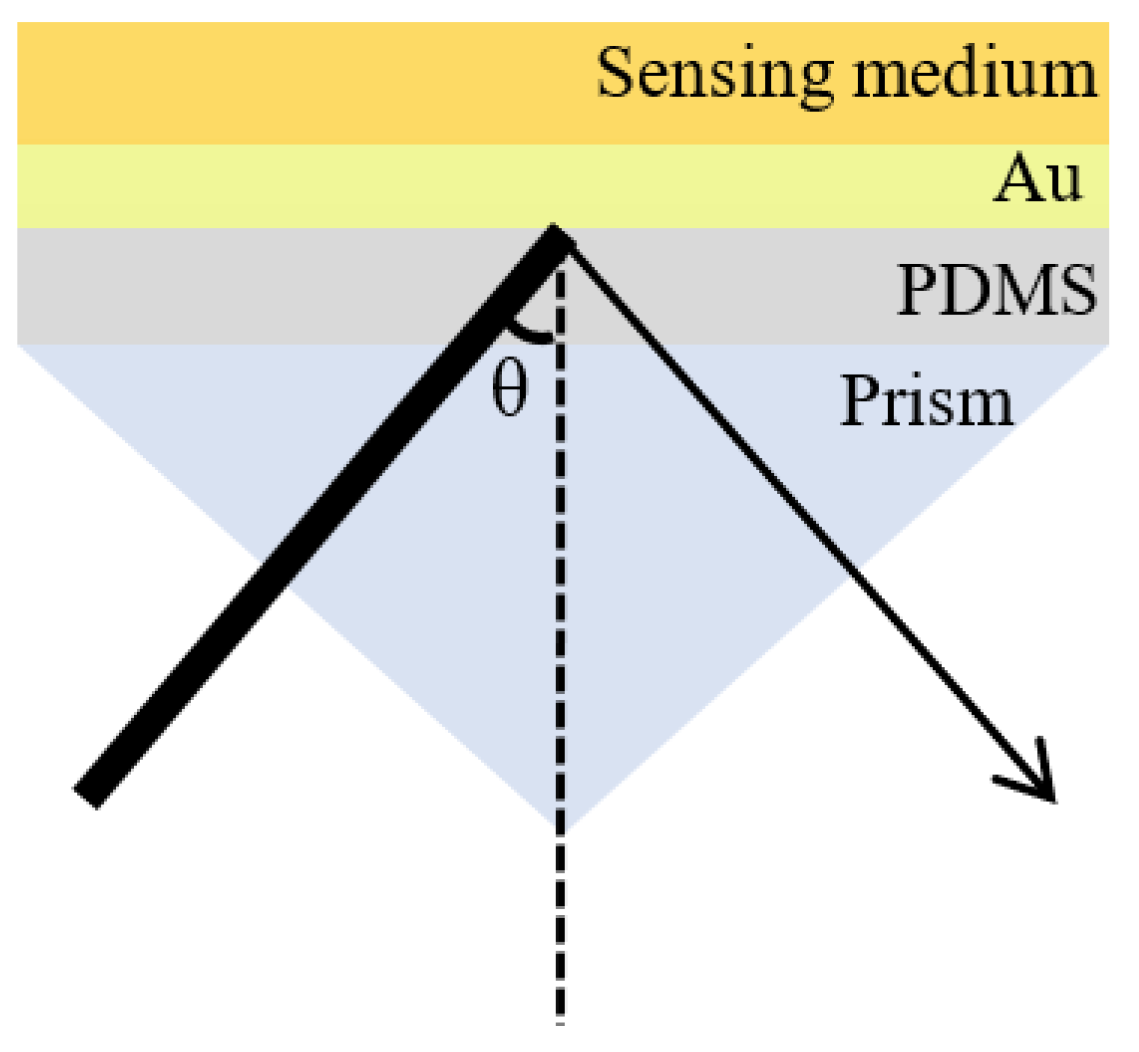
2.2. Chip Fabrication and Experimental Platform Construction
2.3. Experimental Methods
3. Results and Discussion
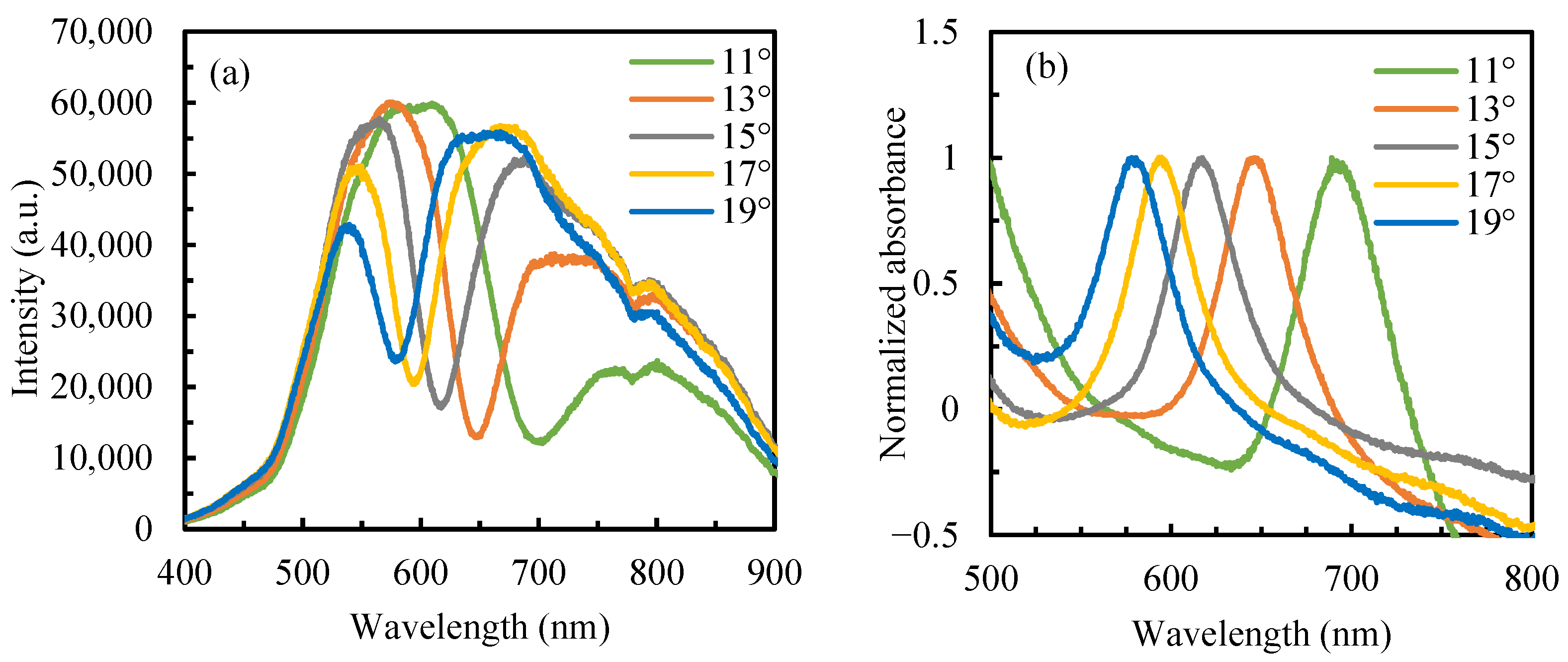
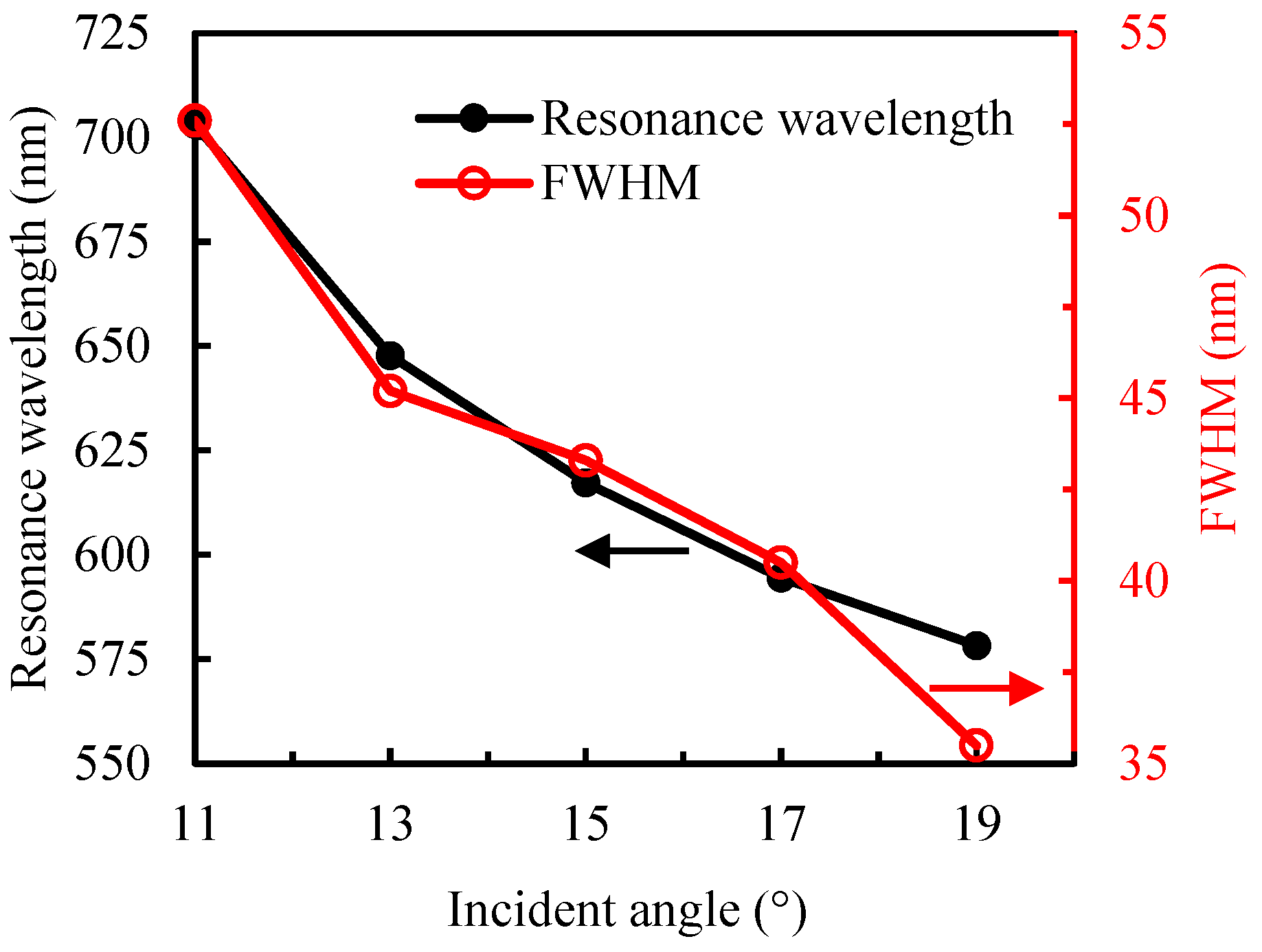
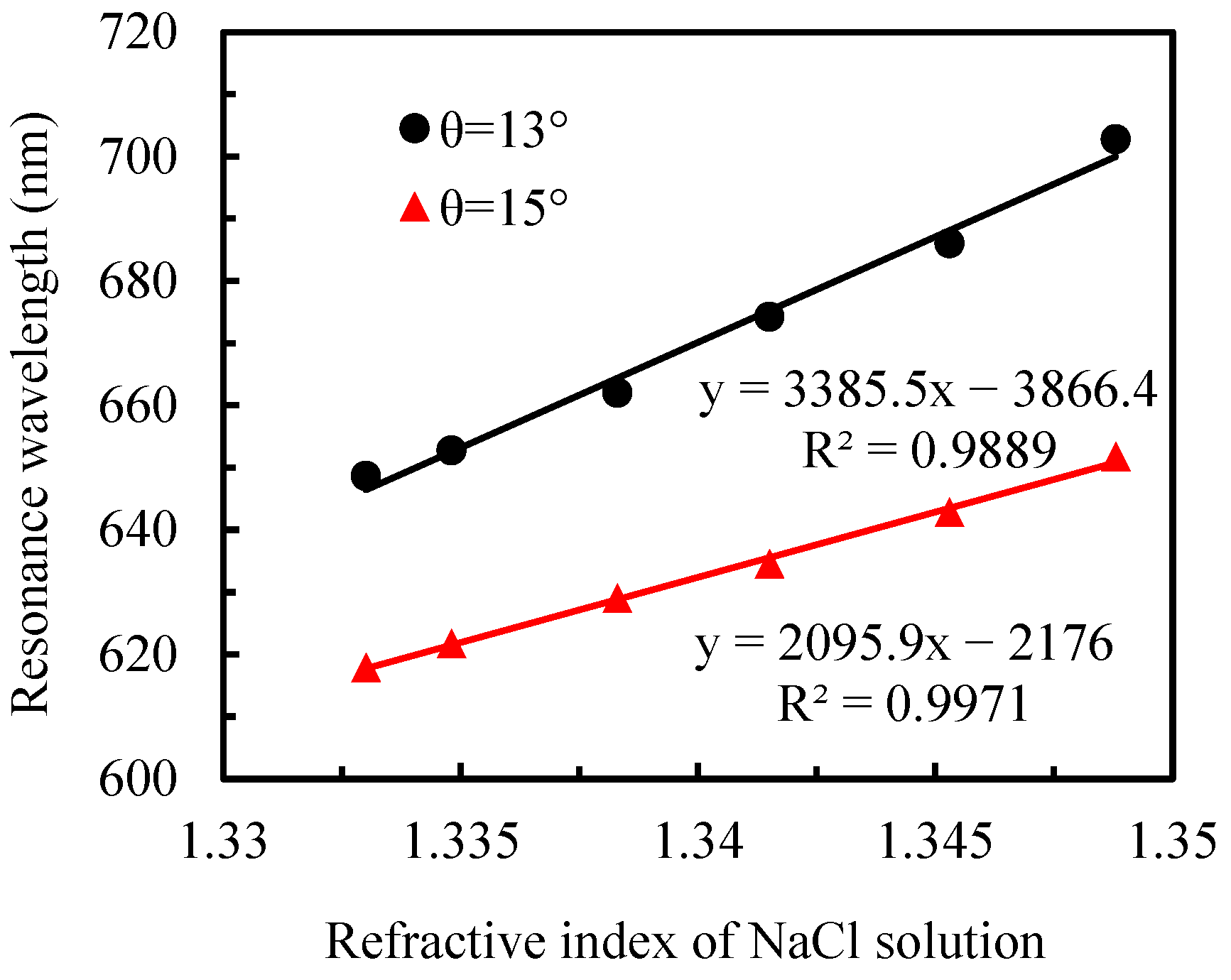
3.1. Refractive-Index Sensitivity of Sensor
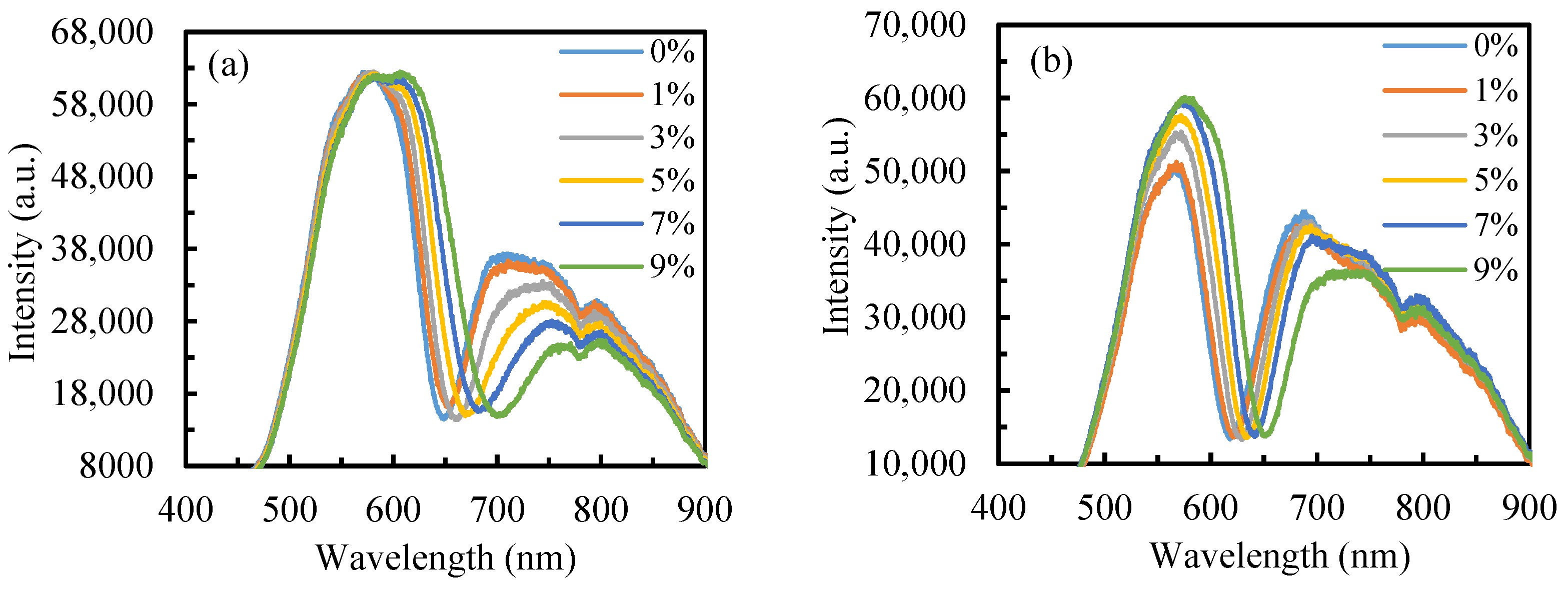
3.2. Sensor Performance in Alcohol Content Detection
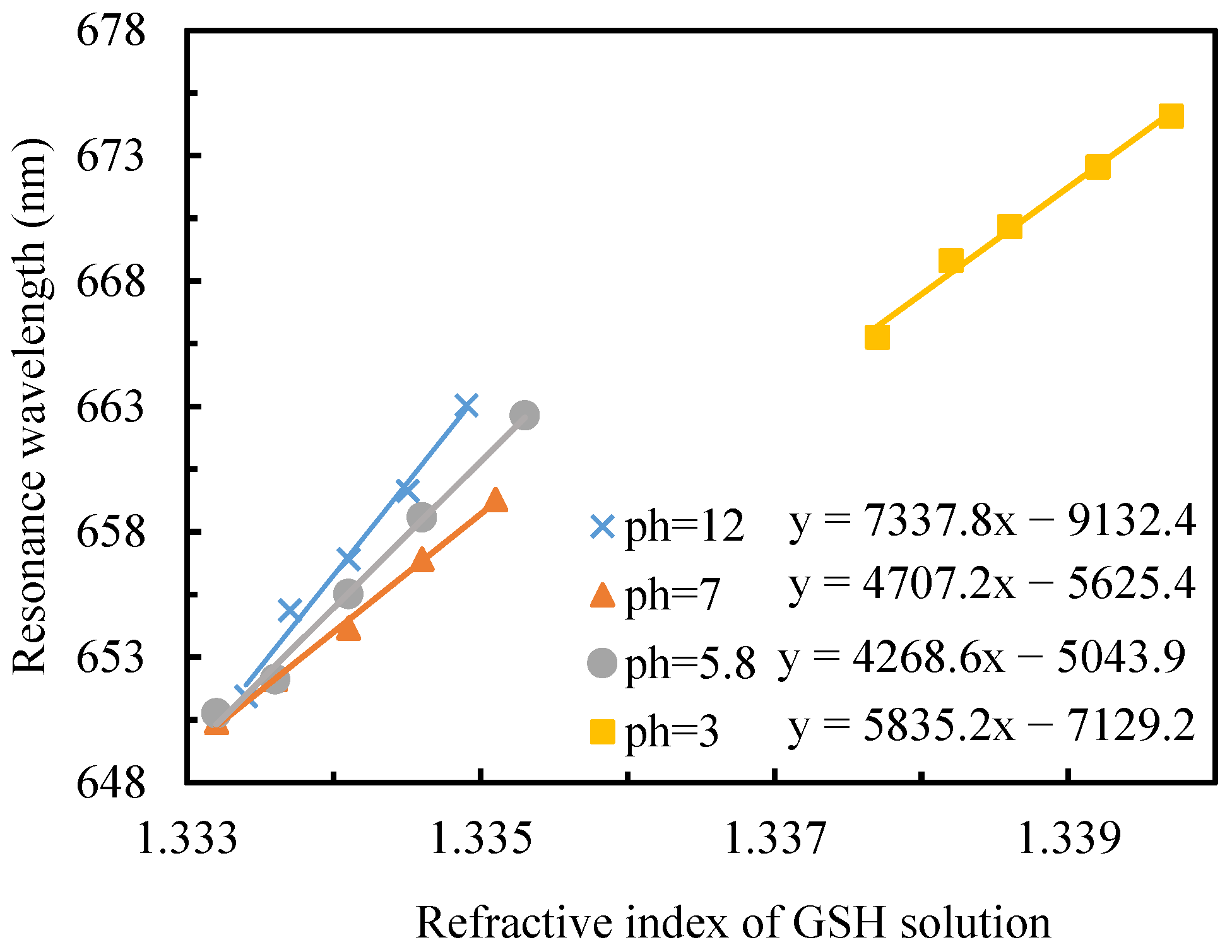
3.3. Adsorption Behavior of GSH Molecules at Different pH Conditions
3.3.1. Instantaneous Adsorption Characteristics of GSH
3.3.2. Kinetic Investigation of GSH Adsorption on Gold Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Xi, Y.; Yuan, Y.Q.; Dai, Z.C.; Liu, F.; Huang, J. Optical property and adsorption isotherm models of glucose sensitive membrane based on prism SPR sensor. Sens. Actuators B Chem. 2016, 237, 150–158. [Google Scholar] [CrossRef]
- Tabasi, O.; Falamaki, C. Recent advancements in the methodologies applied for the sensitivity enhancement of surface plasmon resonance sensors. Anal. Methods 2018, 10, 3906–3925. [Google Scholar] [CrossRef]
- Almawgani, A.H.; Taya, S.A.; Daher, M.G.; Colak, I.; Wu, F.; Patel, S.K. Detection of glucose concentration using a surface plasmon resonance biosensor based on barium titanate layers and molybdenum disulpHide sheets. Phys. Scr. 2022, 97, 065501. [Google Scholar] [CrossRef]
- Pal, A.; Jha, A. A theoretical analysis on sensitivity improvement of an SPR refractive index sensor with grapHene and barium titanate nanosheets. Optik 2021, 231, 166378. [Google Scholar] [CrossRef]
- Karki, B.; Ramya, K.C.; Sandhya Devi, R.S.; Srivastava, V.; Pal, A. Titanium dioxide, black phosphorus and bimetallic layer-based surface plasmon biosensor for formalin detection: Numerical analysis. Opt. Quant. Electron. 2022, 54, 451. [Google Scholar] [CrossRef]
- Yupapin, P.; Trabelsi, Y.; Vigneswaran, D.; Taya, S.A.; Daher, M.G.; Colak, I. Ultra-High-Sensitive Sensor Based on Surface Plasmon Resonance Structure Having Si and Graphene Layers for the Detection of Chikungunya Virus. Plasmonics 2022, 17, 1315–1321. [Google Scholar] [CrossRef]
- Hasler, R.; Mor, D.C.; Aktug, G.; Fossati, S.; Vu, V.T.; Tamayo, A.; Giordani, E.; Ricciardi, E.; Giacomini, P.; Perutka, J.; et al. Surface plasmon resonance biosensor with anti-crossing modulation readout. Sens. Actuators B Chem. 2024, 417, 136163. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Ahn, H.; Song, H.; Choi, J.R.; Kim, K. A localized surface plasmon resonance sensor using double-metal-complex nanostructures and a review of recent approaches. Sensors 2018, 18, 98. [Google Scholar] [CrossRef]
- Gunnarsson, A.s.; Stubbs, C.J.; Rawlins, P.B.; TaylorNewman, E.; Lee, W.; Geschwindner, S.; Hytönen, V.; Holdgate, G.; Jha, R.; Dahl, G. Regenerable Biosensors for Small-Molecule Kinetic Characterization Using SPR. Original Research 2021, 26, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Q.; Shi, W.C.; Chen, Y.; Yuan, J.M.; Xiong, X.; Liu, T.T.; Ding, S.L.; Xiao, W.; Chen, Y.F.; Liu, G.S.; et al. Universal and flexible design for high-sensitivity and wide-ranging surface plasmon resonance sensors based on a three-dimensional tuning hypersurface. Sens. Actuators B Chem. 2023, 380, 133284. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, F.L.; Ren, J.M.; Ren, J.M.; Wang, Y.S.; Li, Z.L.; Li, X.T.; Wang, Y.Q.; Zhao, W.B.; Peng, W. Excitation-detection integrated trapezoidal prism-coupled surface plasmon resonance based on a compact optical system. Sens. Actuators B Chem. 2025, 427, 137234. [Google Scholar] [CrossRef]
- Xia, S.X.; Zhang, D.; Zhai, X.; Wang, L.L.; Wen, S.C. Phase-controlled topological plasmons in 1D graphene nanoribbon array. Appl. Phys. Lett. 2023, 123, 101102. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, S.X.; Xu, W.; Zhai, X.; Li, H.; Wang, L. Dynamic reversal of Fano response of metagratings by rotation of linear polarization. Phys. Rev. B 2024, 110, 035415. [Google Scholar] [CrossRef]
- Cui, K.X.; Tang, Q.X.; Zhao, T.Y.; Qiao, M.; Peng, H.N.; Ding, L.P.; Fang, Y. Bifunctional mesoporous silica nanoparticles with Europium(III) and pyrene for visual detection and discriminative identification of six fluoroquinolone antibiotics. Sens. Actuators B Chem. 2025, 427, 137162. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lin, H.T.; Lai, M.S.; Shieh, T.Y.; Peng, C.C.; Shih, M.H.; Tung, Y.C. Flexible Localized Surface Plasmon Resonance Sensor with Metal Insulator–Metal Nanodisks on PDMS Substrate. Sci. Rep. 2018, 8, 11812. [Google Scholar] [CrossRef]
- Nan, M.H.; Darmawan, B.A.; Go, G.; Zheng, S.R.; Lee, J.; Kim, S.; Lee, T.; Choi, E.; Park, J.O.; Bang, D. Wearable Localized Surface Plasmon Resonance-Based Biosensor with Highly Sensitive and Direct Detection of Cortisol in Human Sweat. Biosensors 2023, 13, 184. [Google Scholar] [CrossRef]
- Tang, Z.l.; Wu, J.Y.; Yu, X.F.; Hong, R.; Zu, X.H.; Lim, X.F.; Luo, H.S.; Lin, W.J.; Yi, G.B. Fabrication of Au Nanoparticle Arrays on Flexible Substrate for Tunable Localized Surface Plasmon Resonance. ACS Appl. Mater. Interfaces 2021, 13, 9281–9288. [Google Scholar] [CrossRef]
- Peng, W.; Pan, X.; Yu, J.; Liao, L.L. A flexible nanostructured multimodal sensor based on surface plasmon resonance. OPT. Commun. 2024, 571, 130946. [Google Scholar] [CrossRef]
- Verma, S.K.; Singh, Y.; Subbaraman, H.; Kandadai, N. A Graphene Integrated Kapton Based Flexible and Highly Sensitive Plasmonic Sensor for Uric Acid Sensing. Sens. Actuators Rep. 2025, 9, 100337. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; He, J.; Liu, W.; Wang, Y.; Huang, Z.; Pang, H.; Chen, Y. Functional PDMS Elastomers: Bulk Composites, Surface Engineering, and Precision Fabrication. Adv. Sci. 2023, 10, e2304506. [Google Scholar] [CrossRef]
- Maher, P. The Effects of Stress and Aging on Glutathione Metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef]
- Sun, M.; Wu, H.Y.; Song, Y.T.; Wang, Q. Surface Plasmon Resonance Alcohol Sensor with Ni(OH)2 Nanoflowers/Au Structure. Meas. 2023, 210, 112564. [Google Scholar] [CrossRef]
- Takehara, K.; Ide, Y.; Aihara, M.; Obuchi, E. An Ion-Gate Response of the Glutathione Monolayer Assembly Formed on a Gold Electrode. Bioelectrochem. Bioenerg. 1992, 29, 103–111. [Google Scholar] [CrossRef]
- Takehara, K.; Aihara, M.; Ueda, N. An Ion-Gate Response of a Glutathione Monolayer Assembly Highly Sensitive to Lanthanide Ions. Electroanalysis 1994, 6, 1083–1086. [Google Scholar] [CrossRef]
- Hepel, M.; Tewksbury, E. Ion-Gating p. Henomena of Self-Assembling Glutathione Films on Gold Piezoelectrodes. J. Electroanal. Chem. 2003, 552, 291–305. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, B.Z.; Fang, C.; Zhou, X.Y. Ion gate response of glutathione monomembrane under surfactant. Chem. J. Chinese Universities 2000, 21, 1552–1554. [Google Scholar]
- Qi, Z.; Xia, S.; Wei, M.; Matsuda, H.; Zhou, H. Systematic characterization of spectral surface plasmon resonance sensors with absorbance measurement. Appl. Opt. 2007, 46, 7963–7969. [Google Scholar] [CrossRef]
- Zeng, S.H.; Ruan, X.Y.; Cai, M.Z. Research Progress on the Interaction between Glutathione and Metal Ions. Chin. J. Public Health 2009, 25, 882–884. [Google Scholar] [CrossRef]
- Dawson, R.M.C.; Elliott, D.C.; Elliott, W.H.; Jones, K.M. Data for Biochemical Research, 3rd ed.; Oxford University Press: New York, NY, USA, 1986; ISBN 9780198553588. [Google Scholar]
- Leitz, M.; Tamachkiarow, A.; Franke, H.; Grattan, K.T.V. Monitoring of biofilm growth using ATR-leaky mode spectroscopy. J. Phys. D. 2002, 35, 55–60. [Google Scholar] [CrossRef]
- Mohamed, H.A.A.; Paul, N.; Rao, B. Bacteriological quality of individually quick-frozen (IQF) raw and cooked ready-to-eat shrimp produced from farm raised black tiger shrimp (Penaeus monodon). Food Microbiol. 1998, 15, 177–183. [Google Scholar] [CrossRef]
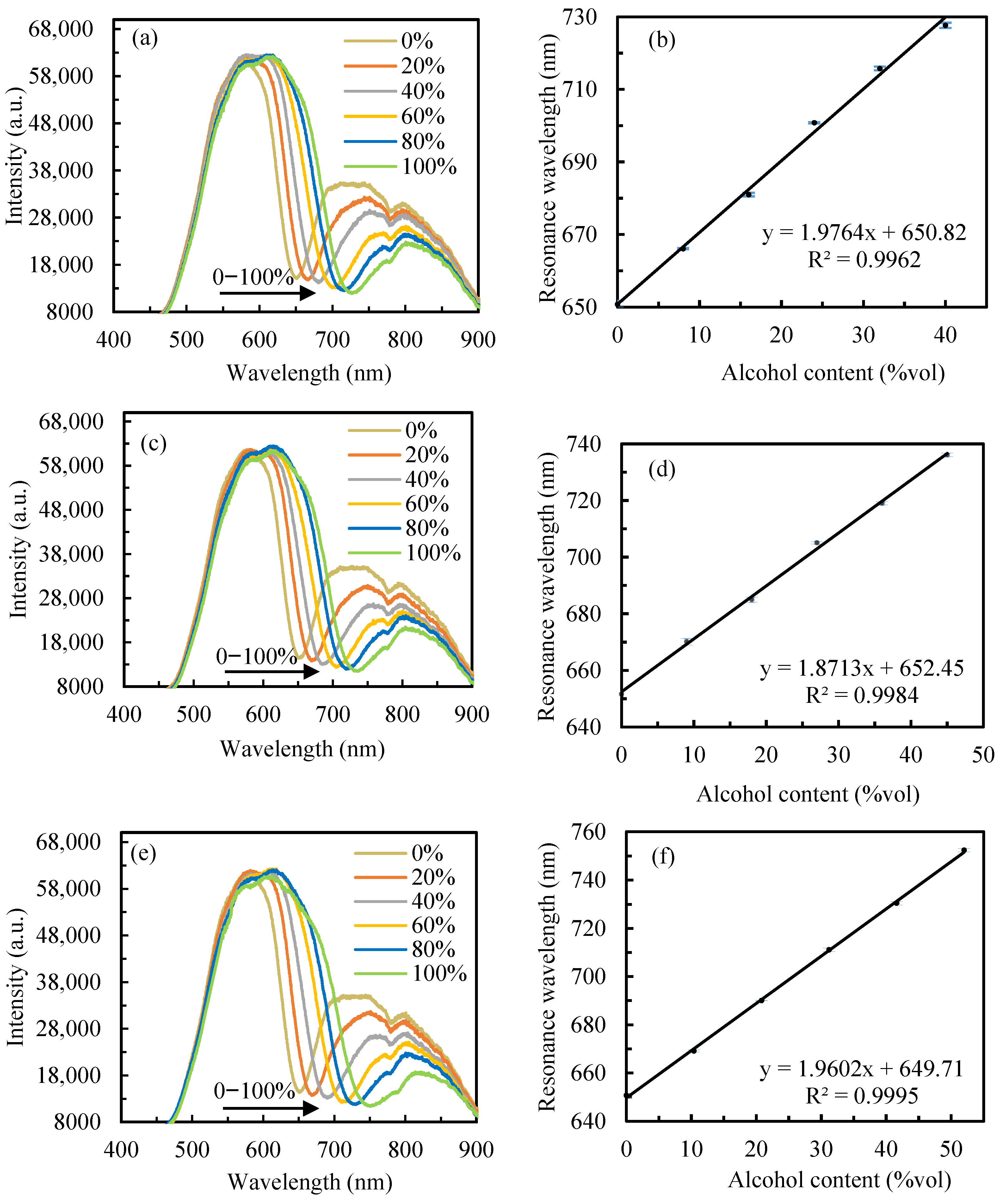
| Sample Dilution Factor (%) | JiangXiaobai | Little Lang Liquor | Zhusun Liquor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Alcohol Content (%vol) | Measured λR (nm) | Standard Deviation | Alcohol Content (%vol) | Measured λR (nm) | Standard Deviation | Alcohol Content (%vol) | Measured λR (nm) | Standard Deviation | |
| 10 | 4 | 658.612 | 0.425 | 4.5 | 660.658 | 0.482 | 5.2 | 660.317 | 0.425 |
| 30 | 12 | 674.584 | 0.575 | 13.5 | 678.304 | 0.552 | 15.6 | 679.317 | 0.276 |
| 50 | 20 | 690.093 | 0.419 | 22.5 | 695.458 | 0.273 | 26 | 701.142 | 0.417 |
| 70 | 28 | 706.476 | 0.627 | 31.5 | 712.123 | 0.157 | 36.4 | 721.728 | 0.947 |
| 90 | 36 | 722.718 | 0.539 | 40.5 | 728.978 | 0.411 | 46.8 | 741.761 | 0.8007 |
| Sample Dilution Factor (%) | Actual Alcohol Content (%vol) | Calculated Alcohol Content (%vol) | Relative Error (%) | |
|---|---|---|---|---|
| JiangXiaobai | 10 | 4 | 3.94 | 1.50 |
| 30 | 12 | 12.02 | 0.17 | |
| 50 | 20 | 19.87 | 0.65 | |
| 70 | 28 | 28.16 | 0.57 | |
| 90 | 36 | 36.37 | 1.03 | |
| Little Lang Liquor | 10 | 4.5 | 4.39 | 2.44 |
| 30 | 13.5 | 13.82 | 2.37 | |
| 50 | 22.5 | 22.96 | 2.04 | |
| 70 | 31.5 | 31.9 | 1.24 | |
| 90 | 40.5 | 40.89 | 0.99 | |
| Zhusun Liquor | 10 | 5.2 | 5.41 | 4.04 |
| 30 | 15.6 | 15.12 | 3.21 | |
| 50 | 26 | 26.22 | 0.92 | |
| 70 | 36.4 | 36.74 | 0.93 | |
| 90 | 46.8 | 46.95 | 0.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Li, M.; Yang, C.; Chen, L.; Wang, M.; Cao, C. Biochemical Sensing Application of Surface Plasmon Resonance Sensor Based on Flexible PDMS Substrate. Sensors 2025, 25, 7087. https://doi.org/10.3390/s25227087
Lu D, Li M, Yang C, Chen L, Wang M, Cao C. Biochemical Sensing Application of Surface Plasmon Resonance Sensor Based on Flexible PDMS Substrate. Sensors. 2025; 25(22):7087. https://doi.org/10.3390/s25227087
Chicago/Turabian StyleLu, Danfeng, Mingyue Li, Chenxi Yang, Luyang Chen, Minghui Wang, and Congjun Cao. 2025. "Biochemical Sensing Application of Surface Plasmon Resonance Sensor Based on Flexible PDMS Substrate" Sensors 25, no. 22: 7087. https://doi.org/10.3390/s25227087
APA StyleLu, D., Li, M., Yang, C., Chen, L., Wang, M., & Cao, C. (2025). Biochemical Sensing Application of Surface Plasmon Resonance Sensor Based on Flexible PDMS Substrate. Sensors, 25(22), 7087. https://doi.org/10.3390/s25227087






