Design and Validation of a High-Speed Miniaturized Thermocycler with Peltier Elements for Efficient PCR Thermal Cycling
Abstract
1. Introduction
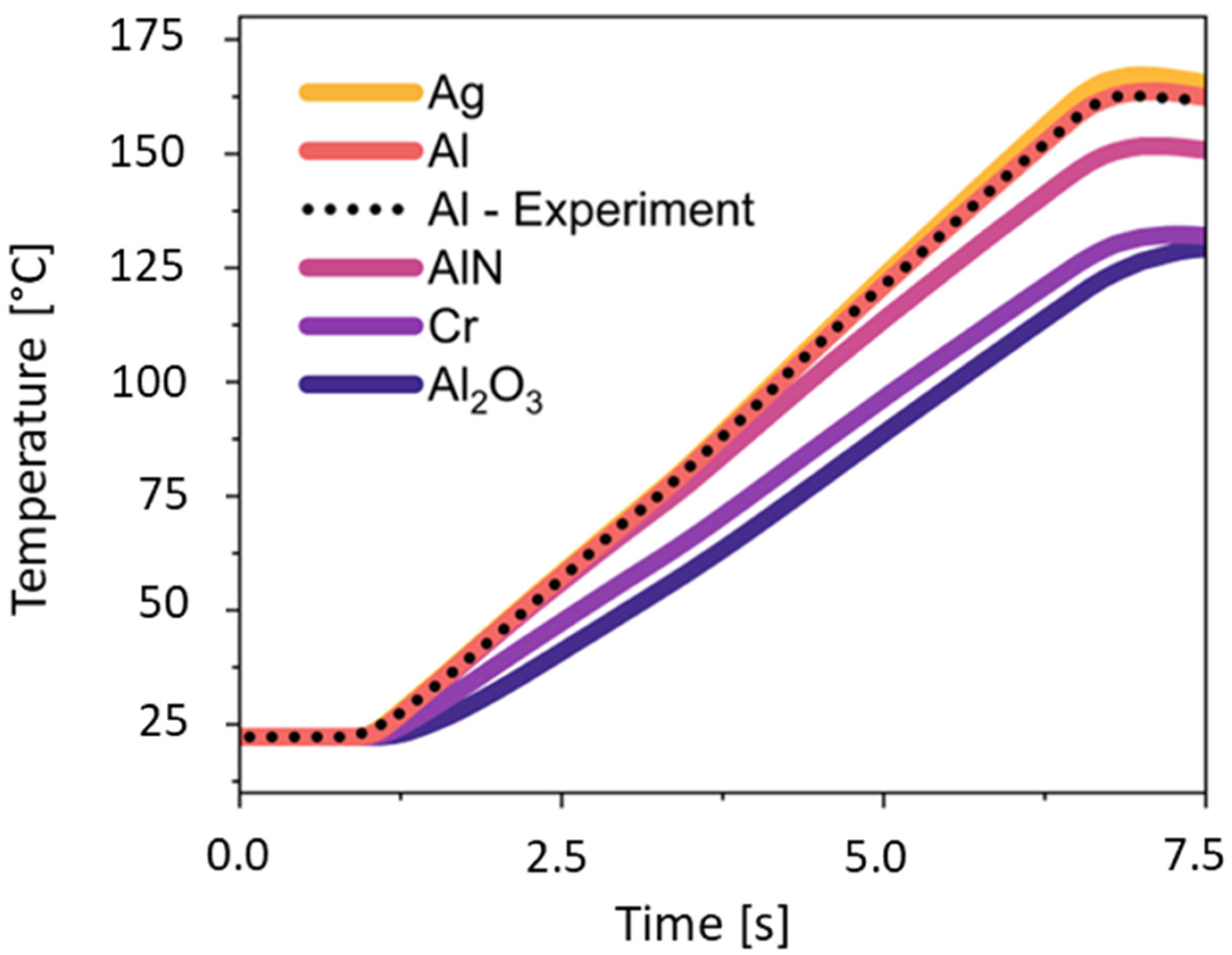
2. Thermal Properties and Material Selection
| Thermal Conductivity κ [W/m·K] | Specific Heat Capacity cp [J/kg·K] | Density ρ [kg/m3] | |
|---|---|---|---|
| Al2O3 | 35 [33] | 880 [30] | 3940 [33] |
| AlN | 170 [34] | 740 [35] | 3260 [36] |
| Al | 237 [25] | 900 [31] | 2700 [37] |
| Au | 315 [31] | 128 [31] | 19,320 [38] |
| Cr | 94 [37] | 448 [37] | 7190 [37] |
| Cu | 401 [25] | 386 [25] | 8940 [37] |
| Ag | 429 [25] | 235 [31] | 10,490 [37] |
3. Design and Performance
3.1. Materials and Equipment
3.1.1. Materials
3.1.2. Equipment
3.1.3. Cost Basis
3.2. Prototype Design and Assembly
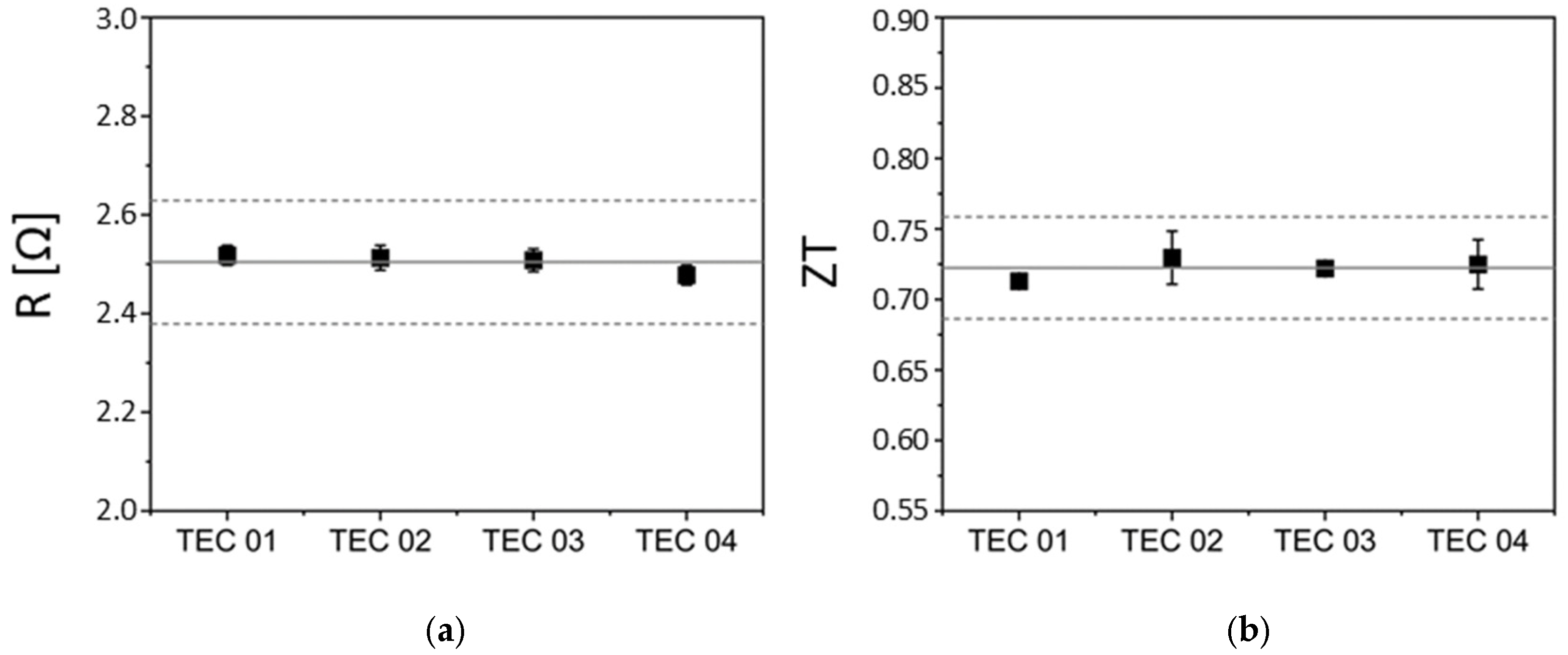
3.3. Electrical Characterization and Preliminary Tests
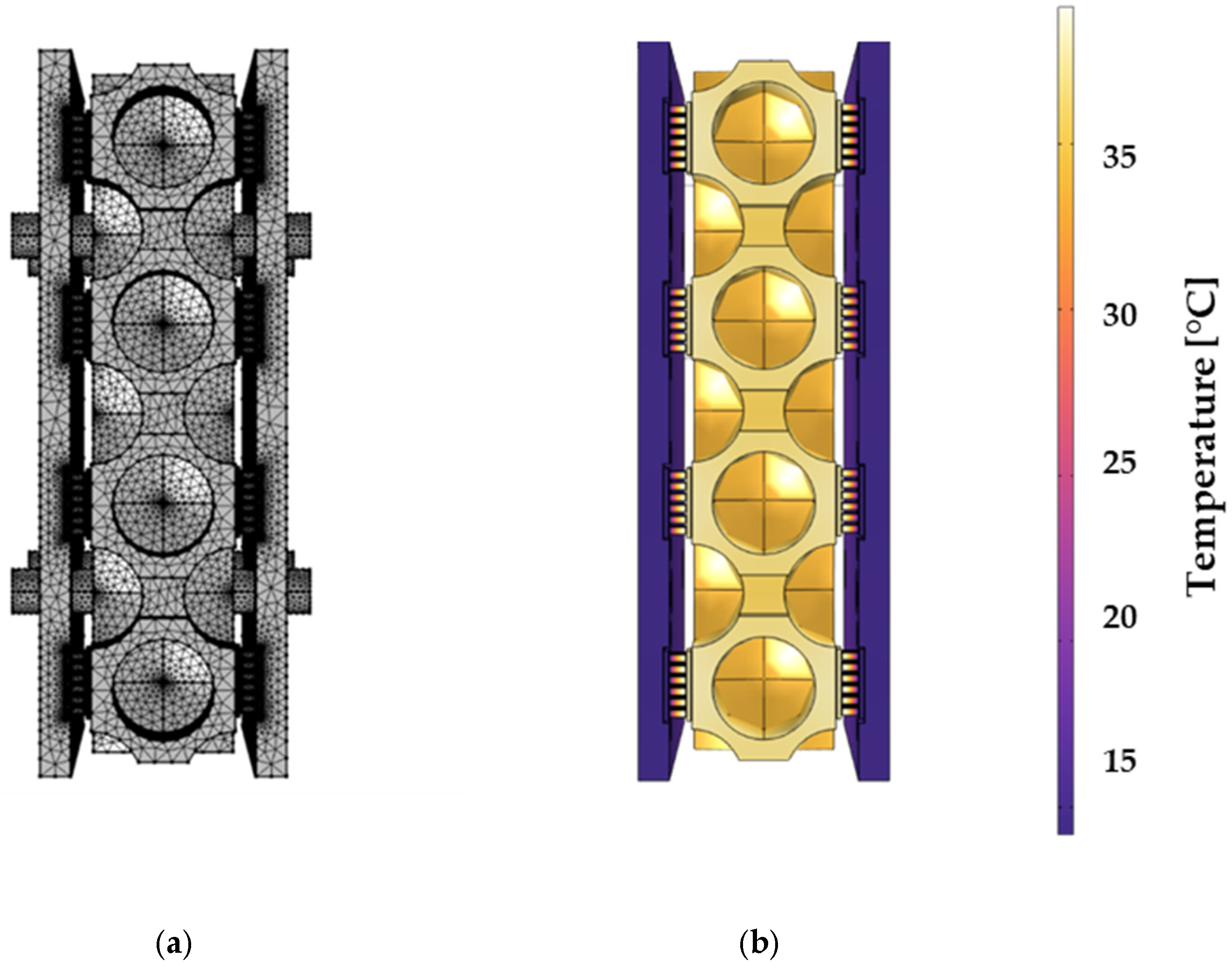
3.4. Numerical Simulation
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mullis, K.; Faloona, F. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; pp. 155–335. [Google Scholar] [CrossRef]
- Saiki, R.K.; Gelfand, D.H.; Stoffel, S.; Scharf, S.J.; Higuchi, R.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988, 239, 487–491. [Google Scholar] [CrossRef]
- Mackay, I.M. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 2004, 10, 190–212. [Google Scholar] [CrossRef]
- Higuchi, R.; Dollinger, G.; Walsh, P.S.; Griffith, R. Simultaneous amplification and detection of specific DNA sequences. Bio/Technology 1992, 10, 413–417. [Google Scholar] [CrossRef]
- Madadelahi, M.; Agarwal, R.; Martinez-Chapa, S.O.; Madou, M.J. A roadmap to high-speed polymerase chain reaction (PCR): COVID-19 as a technology accelerator. Biosens. Bioelectron. 2024, 246, 115830. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Q.; Chang, C.; Zhao, X.; Yong, H.; Ke, X.; Wu, Z. A Thermal Cycler Based on Magnetic Induction Heating and Anti-Freezing Water Cooling for Rapid PCR. Micromachines 2024, 15, 1462. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.; Yu, H.; Lee, C.Y.; Lee, J.U.; Choi, H.J.; Lee, J.H.; Lee, H.; Cheon, J. Publisher Correction: Fast detection of SARS-CoV-2 RNA via the integration of plasmonic thermocycling and fluorescence detection in a portable device. Nat. Biomed. Eng. 2021, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lim, J.; Kim, M.-Y.; Yeom, J.; Cho, H.; Lee, H.; Shin, Y.-B.; Lee, J.-H. Portable low-power thermal cycler with dual thin-film Pt heaters for a polymeric PCR chip. Biomed. Microdevices 2018, 20, 14. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Papadopoulos, V.; Loukas, C.-M.; Kokkoris, G.; Tserepi, A. Towards PCB-Based Miniaturized Thermocyclers for DNA Amplification. Micromachines 2020, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.K.; Camargo, B.D.; Alexandrino, F.; Morello, L.G.; Marchini, F.K.; Aoki, M.N.; Blanes, L. A low-cost PCR instrument for molecular disease diagnostics based on customized printed circuit board heaters. Biomed. Microdevices 2021, 23, 24. [Google Scholar] [CrossRef]
- Just, V.M.; Welzel, F.; Jacobs, H.; Gau, G.; Hauschultz, M.T.; Friedo, M.H.; Foitzik, A.H. Development of a Thermal Cycler for a Low-Cost Real-Time PCR Application. Key Eng. Mater. 2023, 968, 73–79. [Google Scholar] [CrossRef]
- Chong, K.S.; Gan, K.B.; Then, S.-M. Development of a portable low-cost real-time PCR system. In Proceedings of the 2017 International Conference on Robotics, Automation and Sciences (ICORAS), Melaka, Malaysia, 27–29 November 2017; pp. 1–5. [Google Scholar] [CrossRef]
- Sun, K.; Whiteside, B.; Hebda, M.; Fan, Y.; Zhang, Y.; Xie, Y.; Liang, K. A low-cost and hand-hold PCR microdevice based on water-cooling technology. Biomed. Microdevices 2023, 25, 12. [Google Scholar] [CrossRef]
- Sensoquest, Labcycler 48s, Small Size–Maximum Performance. 2024. Available online: https://www.sensoquest.de/wp-content/uploads/Labcycler_48s_WEB.pdf (accessed on 3 November 2025).
- Analytic Jena, Biometra TAdvanced 96 S, 2020, Simply the Best in PCR, Die Biometra Thermocycler-Familie. Available online: https://www.analytik-jena.de/produkte/life-science/pcr-qpcr-thermocycler/thermocycler-pcr/biometra-tadvanced-serie/ (accessed on 3 November 2025).
- Qiagen, QIAquant 96 (2 plex and 5 plex), See qPCR in a New Light, QIAquant® 96 and 384 Thermal Cyclers to Boost Your qPCR Workflow. 2022. Available online: https://www.qiagen.com/-/media/project/qiagen/qiagen-home/content-worlds/pcr/cyclers-quantinova-and-mircury/qpro-2180_1130039_bro_pcr_qiaquant_1222--v1d.pdf/ (accessed on 3 November 2025).
- Eppendorf, Mastercycler® X50-PCR-Thermocycler, The Next Stage, Der neue Mastercycler X50. 2022. Available online: https://www.eppendorf.com/de-de/eShop-Produkte/PCR/Thermocycler/Mastercycler-X50-p-PF-217186 (accessed on 3 November 2025).
- OpenPCR, Open-Source PCR Thermocycler. Available online: https://openpcr.org/ (accessed on 3 November 2025).
- miniPCR® mini16 Thermal Cycler. Available online: https://www.minipcr.com/wp-content/uploads/mini16-Technical-Specs-221128.pdf (accessed on 3 November 2025).
- Luo, K.; Cheng, W.; Chen, Y.; Zhang, Q.; Liang, C.; Li, J.; Wang, W. A portable low-cost polymerase chain reaction device. HardwareX 2025, 21, e00635. [Google Scholar] [CrossRef]
- Yeom, D.; Kim, J.; Kim, S.; Ahn, S.; Choi, J.; Kim, Y.; Koo, C. A Thermocycler Using a Chip Resistor Heater and a Glass Microchip for a Portable and Rapid Microchip-Based PCR Device. Micromachines 2022, 13, 339. [Google Scholar] [CrossRef]
- Ling, W.; Zhou, W.; Cui, J.; Shen, Z.; Wei, Q.; Chu, X. Experimental study on the heating/cooling and temperature uniformity performance of the microchannel temperature control device for nucleic acid PCR amplification reaction of COVID-19. Appl. Therm. Eng. 2023, 226, 120342. [Google Scholar] [CrossRef]
- Mandal, P.; Dhakane, V.; Kulkarni, A.; Tallur, S. WELPCR: Low-cost polymerase chain reaction (PCR) thermal cycler for nucleic acid amplification and sensing. In Proceedings of the 2024 IEEE Applied Sensing Conference (APSCON), Goa, India, 22–24 January 2024. [Google Scholar]
- Jeff, S.; Eric, T. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Saunders College: Philadelphia, PA, USA, 1976. [Google Scholar]
- Terpe, K. Overview of thermostable DNA polymerases for classical PCR applications: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2013, 97, 10243–10254. [Google Scholar] [CrossRef]
- Elstner, M. Elektronenstruktur der Atome. In Physikalische Chemie II: Quantenmechanik und Spektroskopie; Springer Spektrum: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Periodensystem.info. Aluminium (Al)—Periodensystem der Elemente (PSE). 2024. Available online: https://www.periodensystem.info/elemente/aluminium/ (accessed on 10 July 2024).
- Periodensystem.info. Silber (Ag)—Periodensystem der Elemente (PSE). 2024. Available online: https://www.periodensystem.info/elemente/silber/ (accessed on 10 July 2024).
- MatWeb, LLC. Alumina, Alpha Al2O3, 99.5%; Thermal Conductivity: 30 W/m·K. Available online: https://www.matweb.com/search/datasheettext.aspx?matid=143MatWeb (accessed on 5 July 2024).
- Callister, W.D. Materials Science and Engineering: An Introduction, 7th ed.; John Wiley & Sons: New York, NY, USA, 2007; pp. 483–486. [Google Scholar]
- Jain, A.; McGaughey, A.J.H. Thermal Transport by Phonons and Electrons in Aluminum, Silver, and Gold from First Principles. Phys. Rev. B 2016, 93, 081206. [Google Scholar] [CrossRef]
- Engineering Toolbox. Density of Solids. Available online: https://www.engineeringtoolbox.com/density-solids-d_1265.html (accessed on 27 August 2025).
- Lee, W.E.; Rainforth, W.M. Ceramic Microstructures: Property Control by Processing; Chapman & Hall: London, UK, 1994; p. 504. [Google Scholar]
- MatWeb. Aluminum Nitride (AlN)-Material Property Data; Specific Heat Capacity: 740 J/kg·K. Available online: https://www.matweb.com/search/datasheet.aspx?matguid=1faaabab5cac40c08a823d1b0083e2d1 (accessed on 5 July 2024).
- Hull, R.; Kasap, S.O. (Eds.) Springer Handbook of Electronic and Photonic Materials, 2nd ed.; Springer: Berlin/Heidelberg, Ger-many, 2017; p. 204. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 86th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- The Engineering Mindset. Density of Metals. 2019. Available online: https://theengineeringmindset.com/density-of-metals/ (accessed on 27 August 2025).
- Rattanapoltee, P.; Thongnopkun, P. Tarnish Resistance of Silver by Gold Microplates Coating. J. Phys. Conf. Ser. 2019, 1380, 012006. [Google Scholar] [CrossRef]
- Quick-Ohm, Grafit-wärmeleitende-Montagefolie, QGF-G02AA. 2024. Available online: https://www.quick-ohm.de/graphitfolie-selbstklebend-200x300/qgf-g02aa/ (accessed on 3 November 2025).
- Hao, X.; Peng, B.; Xie, G.; Chen, Y. Efficient on-chip hotspot removal combined solution of thermoelectric cooler and mini-channel heat sink. Appl. Therm. Eng. 2016, 100, 170–178. [Google Scholar] [CrossRef]
- Marco, W. Bauelemente der Elektronik; Elektronik für Entscheider: Grundwissen für Wirtschaft und Technik; Springer: Berlin, Germany, 2008; pp. 13–22. [Google Scholar]
- Harman, T.C. Special Techniques for Measurement of Thermoelectric Properties. J. Appl. Phys. 1958, 29, 1373–1374. [Google Scholar] [CrossRef]
- Beltrán-Pitarch, B.; Dunstan, D.J.; Muñoz Rojo, M. Experimental conditions required for accurate measurements of electrical resistivity, thermal conductivity and figure of merit (ZT) using Harman and impedance spectroscopy methods. Rev. Sci. Instrum. 2019, 90, 015112. [Google Scholar] [CrossRef]
- Sun, X.; Yan, Y.; Kang, M.; Zhao, W.; Yan, K.; Wang, H.; Li, R.; Zhao, S.; Hua, X.; Wang, B.; et al. General strategy for developing thick-film micro-thermoelectric coolers from material fabrication to device integration. Nat. Commun. 2024, 15, 3870. [Google Scholar] [CrossRef]
- Chew, L.W.; Chen, C.; Yuan, C.; Gorlé, C. Quantifying Convective Heat Transfer Coefficients During Natural Ventilation in a Full-Scale Operational Building. In Proceedings of the 5th International Conference on Building Energy and Environment, COBEE 2022, Montréal, QC, Canada, 25–29 July 2022; Environmental Science and Engineering. Wang, L.L., Ge, H., Zhai, Z.J., Qi, D., Ouf, M., Sun, C., Wang, D., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Preney, T.; Namy, P.; Wheeler, J.D. Adaptive mesh refinement: Quantitative computation of a rising bubble using COMSOL Multiphysics®. In Proceedings of the COMSOL Conference, Newton, MA, USA, 5–7 October 2016. [Google Scholar]
- Bergman, T.L.; Lavine, A.S.; Incropera, F.P. *Fundamentals of Heat and Mass Transfer, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9781118137253. [Google Scholar]
- Malhotra, K.; Foltz, L.; Mahoney, W.C.; Schueler, P.A. Interaction and effect of annealing temperature on primers used in differential display RT-PCR. Nucleic Acids Res. 1998, 26, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, N.; Takada, K. Applications of Aluminum Nitride (AlN) Ceramics. Key Eng. Mater. 2003, 247, 467. [Google Scholar] [CrossRef]
- Nora, G.; Berg, T.P.; Ivanisevic, A. Tuning the biocompatibility of aluminum nitride. Mater. Lett. 2017, 189, 1–4. [Google Scholar] [CrossRef]



| PCR Device | Heating Rate [°C/s] | Cooling Rate [°C/s] | Cost [USD] | Source |
|---|---|---|---|---|
| Xie et al. (2024) | 14.9 | 13.4 | -- | [6] |
| Cheong et al. (2020) | 13.1 | 4.9 | -- | [7] |
| Jeong et al. (2018) | 1.0 | 0.9 | -- | [8] |
| Kaprou et al. (2020) | 1.4 | 0.6 | -- | [9] |
| Oliveira et al. (2021) | 2.0 | 2.0 | -- | [10] |
| Just et al. (2023) | 2.8 | -- | -- | [11] |
| Chong et al. (2017) | 3.0 | -- | 2665 | [12] |
| Sun et al. (2023) | 4.0 | 8.1 | 170 | [13] |
| Labcycler 48s | 5.0 | 5.0 | 4790 | [14] |
| Biometra TAdvanced 96 S | 8.0 | 5.5 | 11,200 | [15] |
| QIAquant 96 | 8.0 | 5.5 | 10,500 | [16] |
| Mastercycler® X50 | 10.0 | 5.0 | 9800 | [17] |
| Open PCR | 1.0 | 1.0 | 499 | [18] |
| miniPCRTM mini 16 | 2.4 | 1.7 | 749 | [19] |
| Luo et al. (2025) | 2.8 | 2.2 | 120 | [20] |
| Yeom et al. (2022) | 21.9 | 1.4 | -- | [21] |
| Ling et al. (2023) | 6.1 | 5.3 | -- | [22] |
| Mandal et al. (2024) | 2.0 | -- | 120 | [23] |
| This work | 22.25 | 5.3 | 161 | -- |
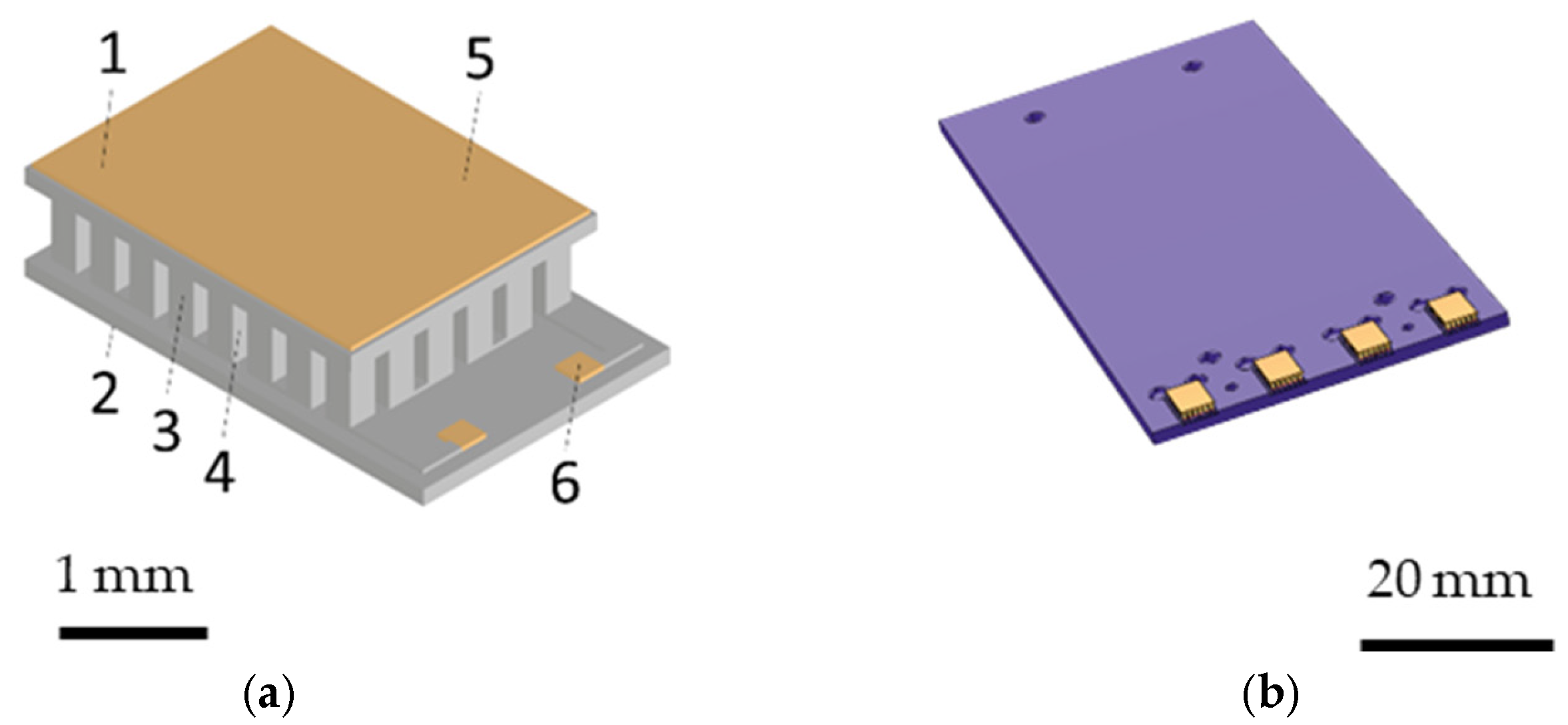
| Number | Component | Material | Quantity |
|---|---|---|---|
| 1 | hot side substrate | Al2O3 | 1 |
| 2 | cold side substrate | Al2O3 | 1 |
| 3 | n-type dice | Bi2Te3 | 24 |
| 4 | p-type dice | Bi2Te3 | 24 |
| 5 | metallization | Cu & Ni | 2 |
| 6 | metallization | Au | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamerni, P.; König, J.; Mistry, L.-A.; Schmitt, K.; Wöllenstein, J. Design and Validation of a High-Speed Miniaturized Thermocycler with Peltier Elements for Efficient PCR Thermal Cycling. Sensors 2025, 25, 7046. https://doi.org/10.3390/s25227046
Bamerni P, König J, Mistry L-A, Schmitt K, Wöllenstein J. Design and Validation of a High-Speed Miniaturized Thermocycler with Peltier Elements for Efficient PCR Thermal Cycling. Sensors. 2025; 25(22):7046. https://doi.org/10.3390/s25227046
Chicago/Turabian StyleBamerni, Passar, Jan König, Lee-Ann Mistry, Katrin Schmitt, and Jürgen Wöllenstein. 2025. "Design and Validation of a High-Speed Miniaturized Thermocycler with Peltier Elements for Efficient PCR Thermal Cycling" Sensors 25, no. 22: 7046. https://doi.org/10.3390/s25227046
APA StyleBamerni, P., König, J., Mistry, L.-A., Schmitt, K., & Wöllenstein, J. (2025). Design and Validation of a High-Speed Miniaturized Thermocycler with Peltier Elements for Efficient PCR Thermal Cycling. Sensors, 25(22), 7046. https://doi.org/10.3390/s25227046





