Sensitivity Evaluation of a Dual-Finger Metamaterial Biosensor for Non-Invasive Glycemia Tracking on Multiple Substrates
Abstract
1. Introduction
2. Glucose-Dependent Dielectric Properties
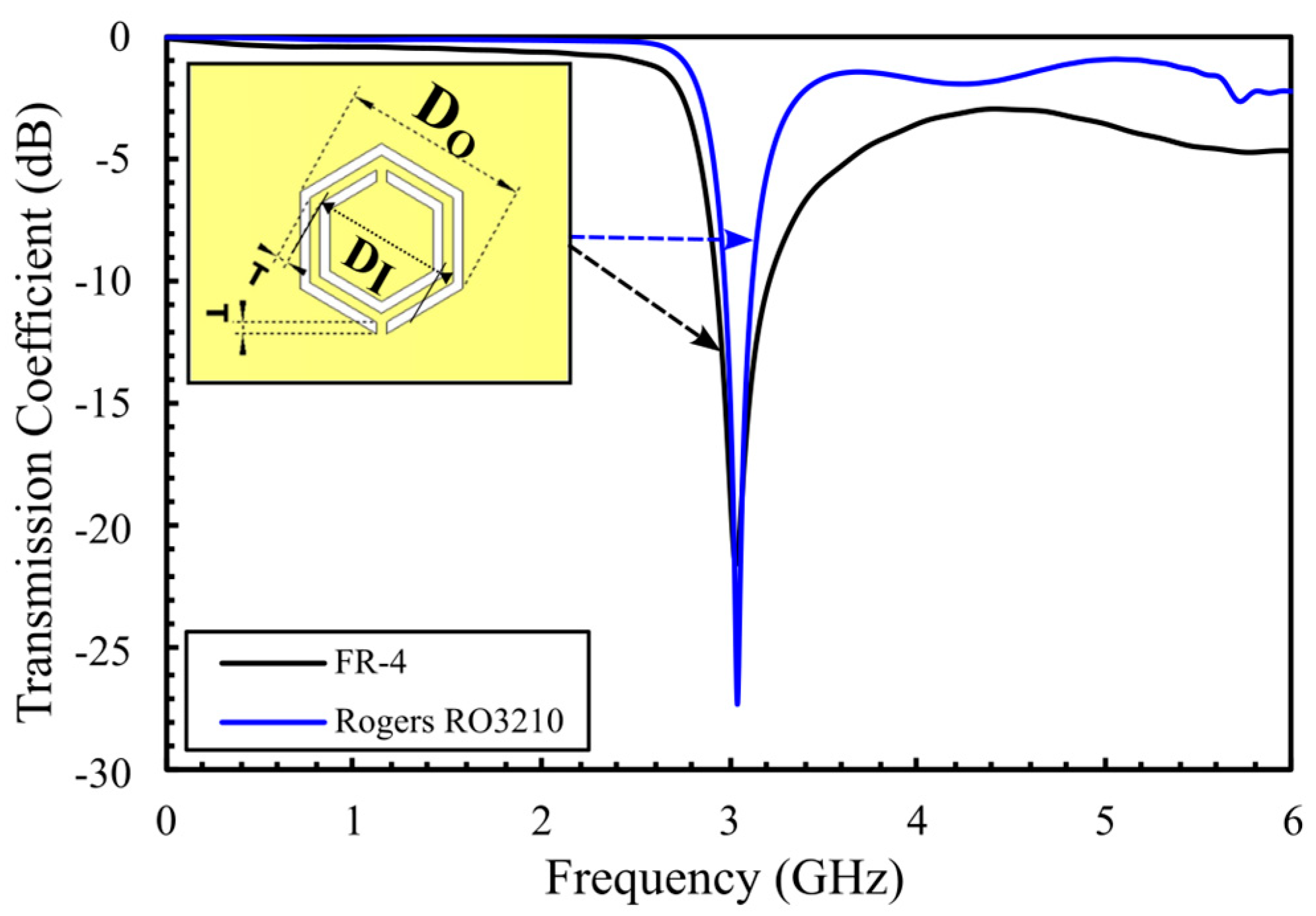
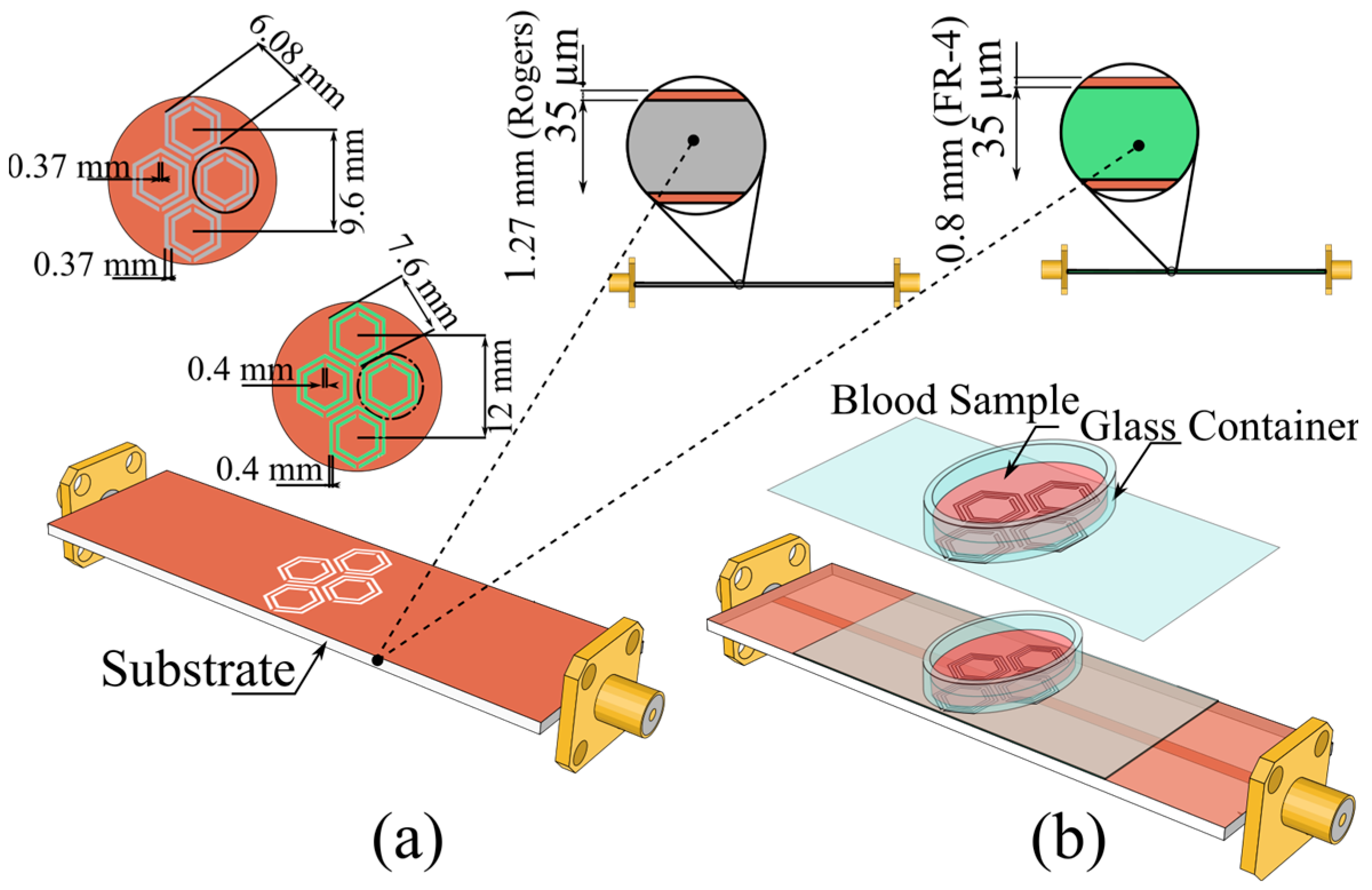
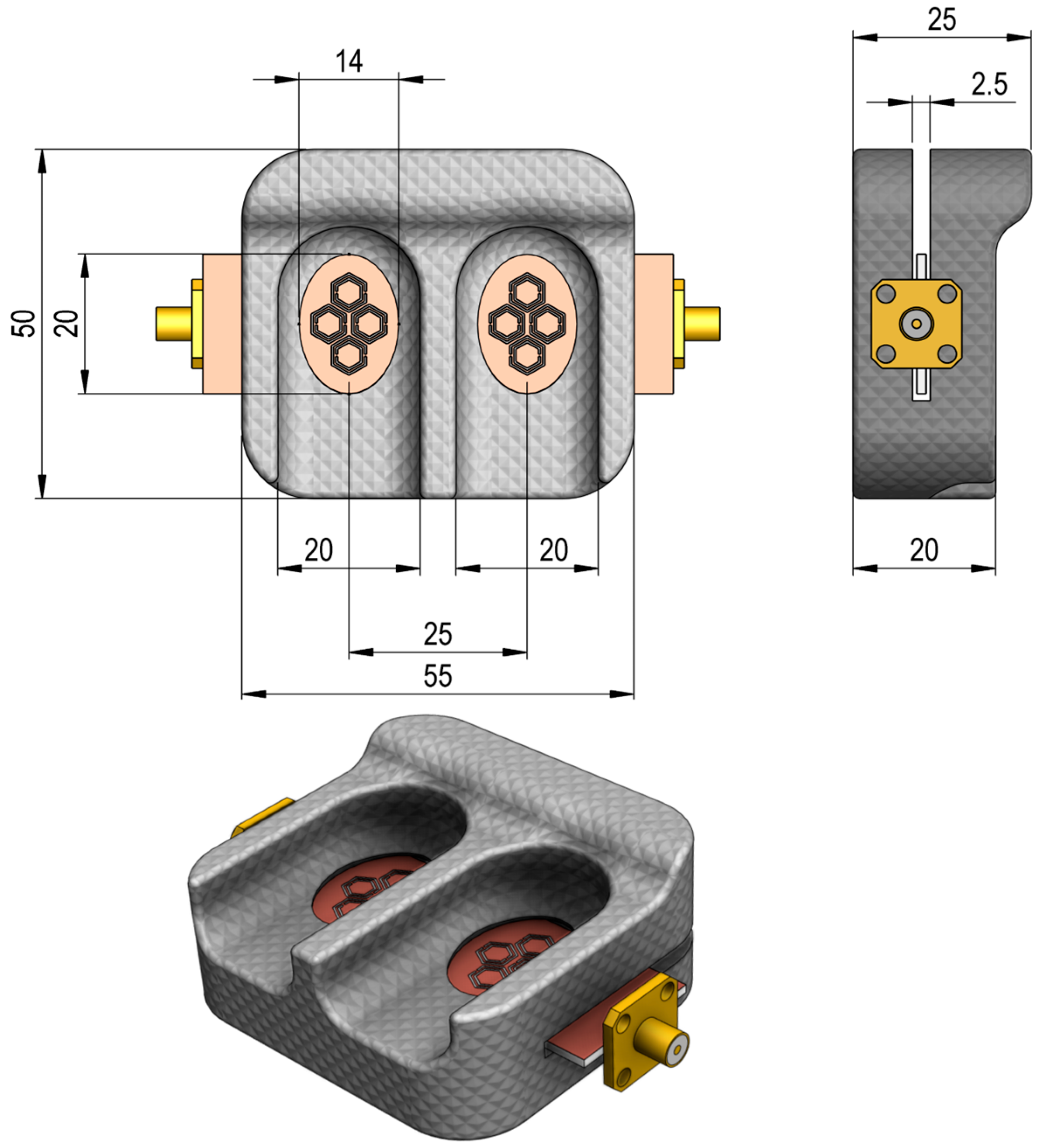
3. Proposed Sensor Design
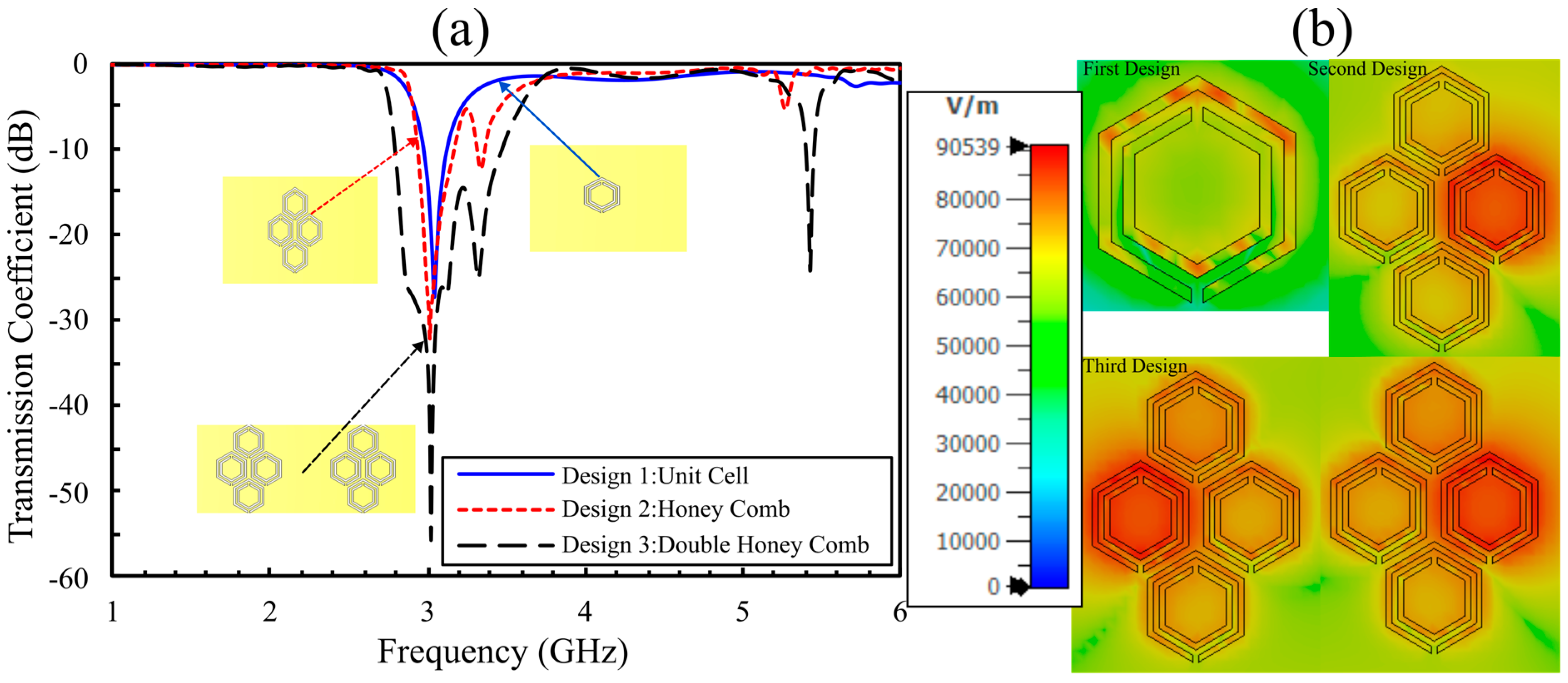
4. Simulation Results for Aqueous Glucose Concentrations: Comparison Between Eight- and Sixteen-Cell Hexagonal CSRR Configurations
4.1. Simulation Results for the 8-Cell Single-Honeycomb Configuration
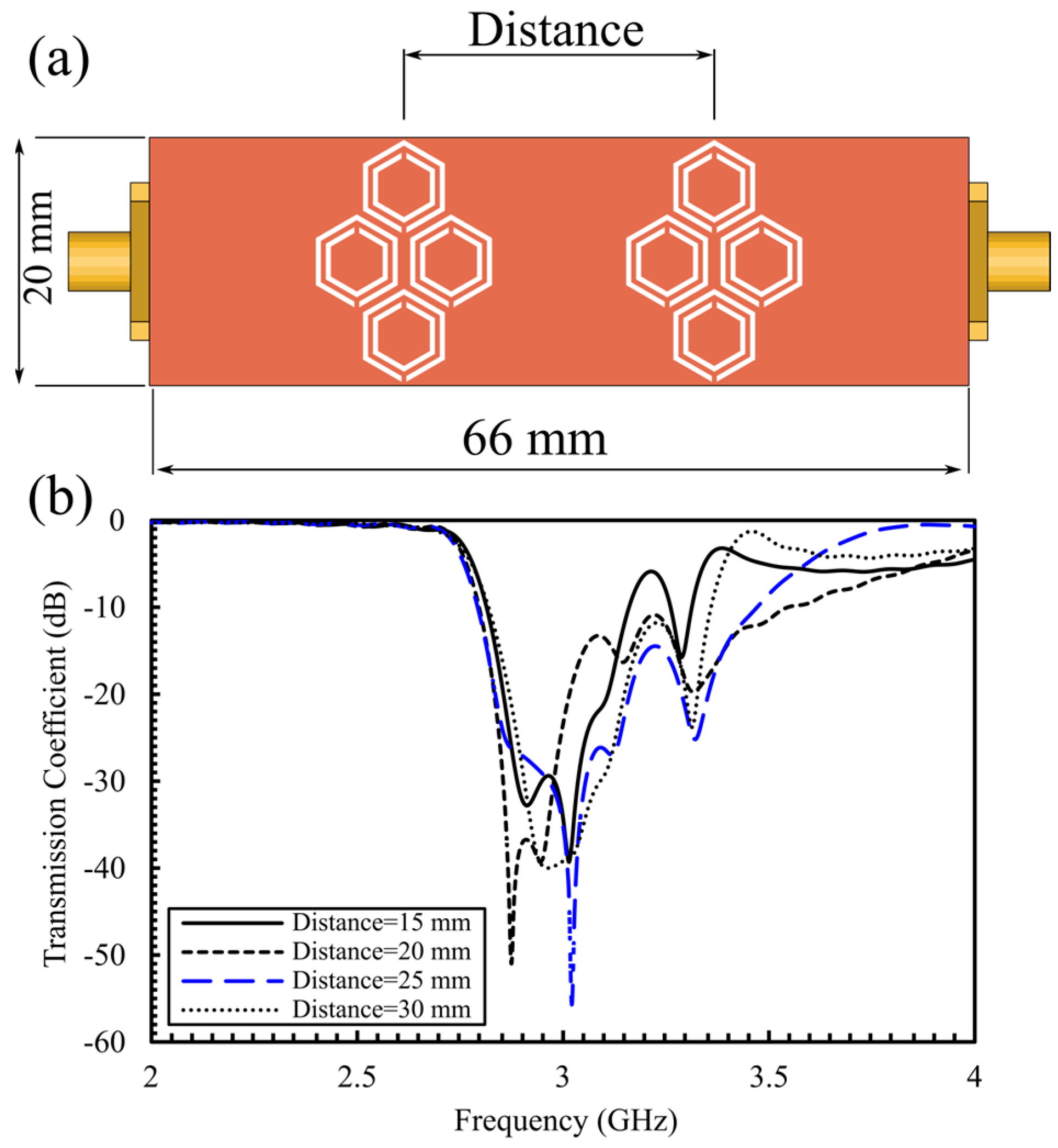
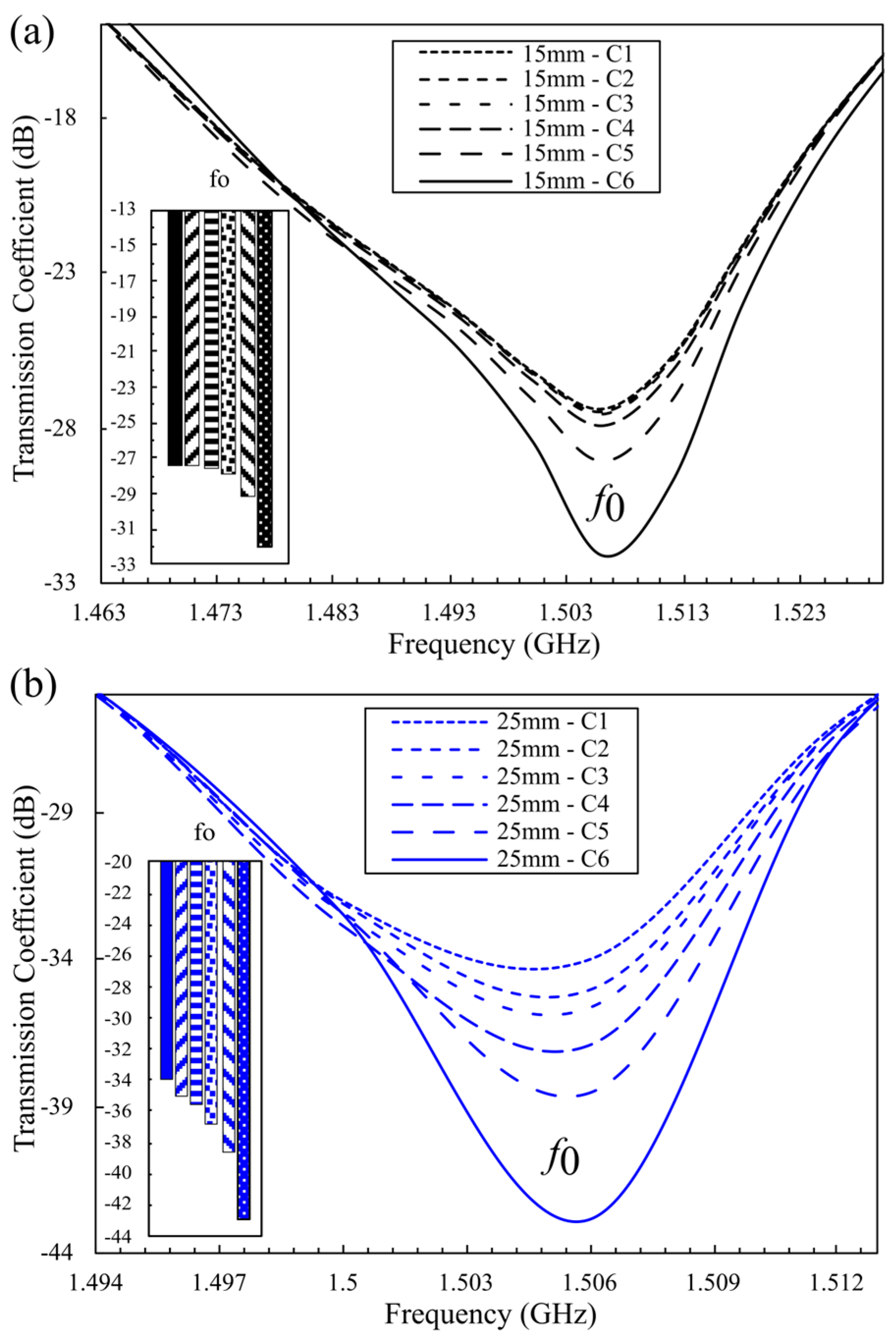
4.2. Simulation Results for the Optimized 16-Cell Double-Honeycomb Configuration and Sensitivity Comparison at 15 mm and 25 mm Spacing
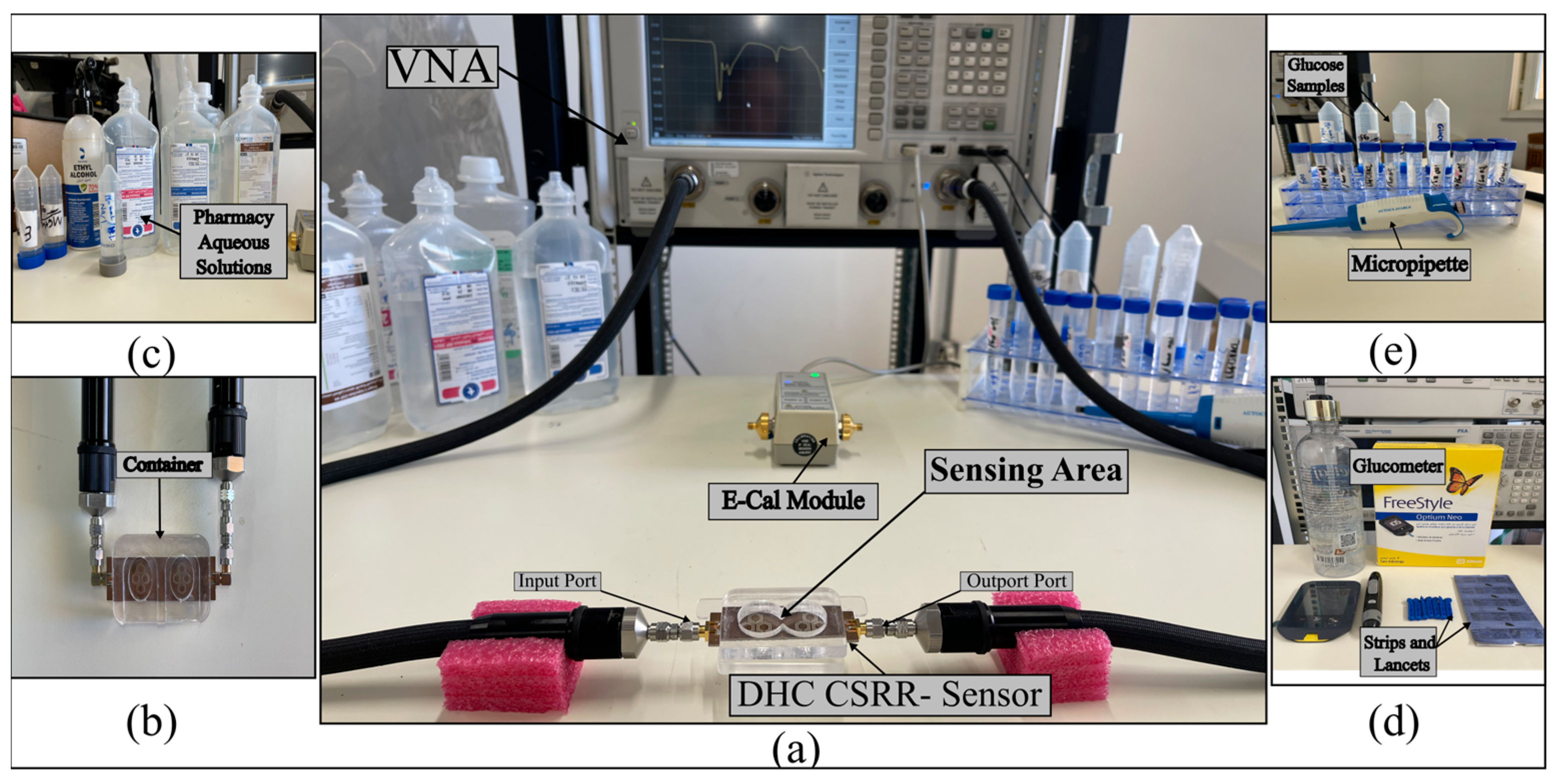
5. Experimental Measurements
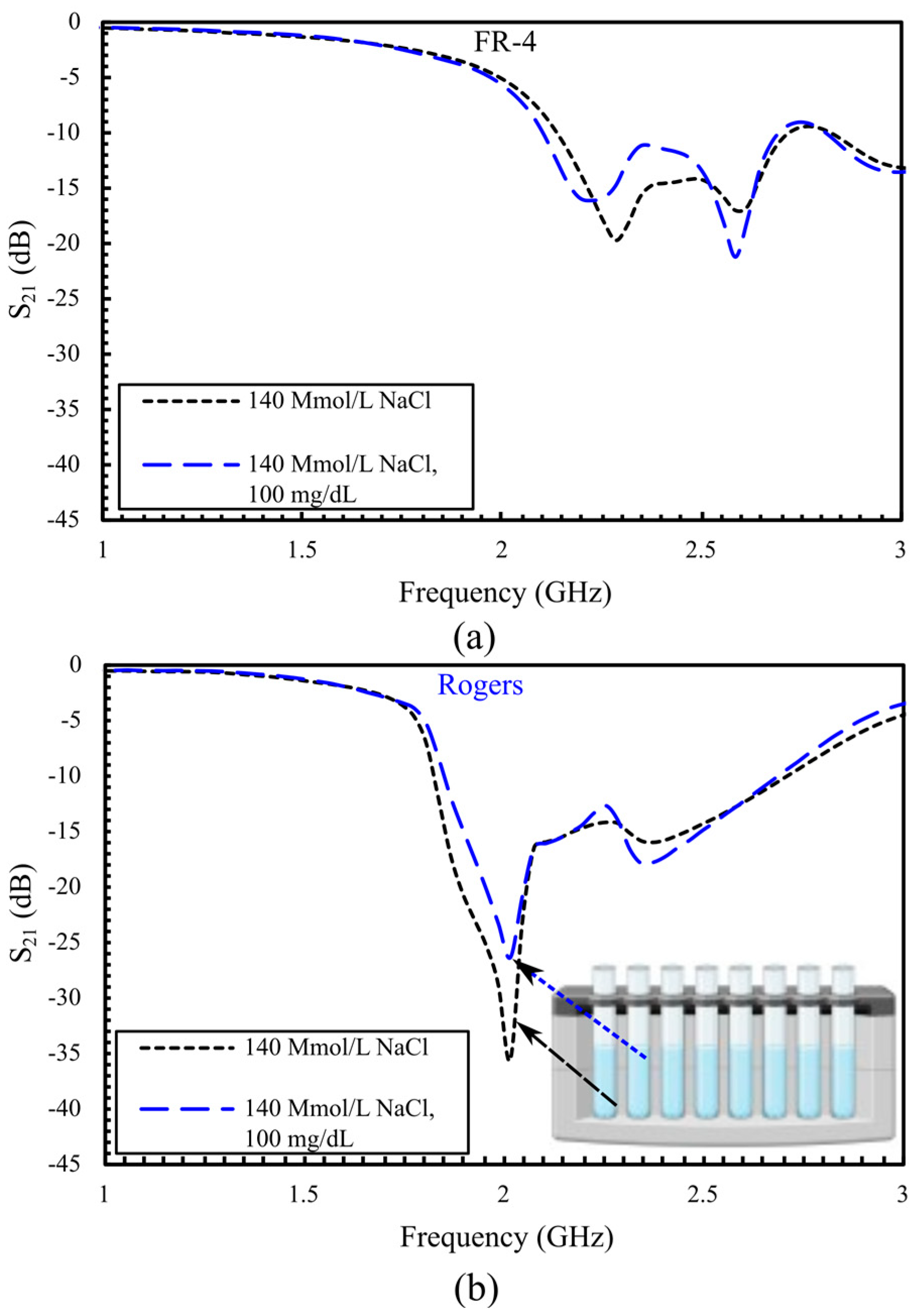
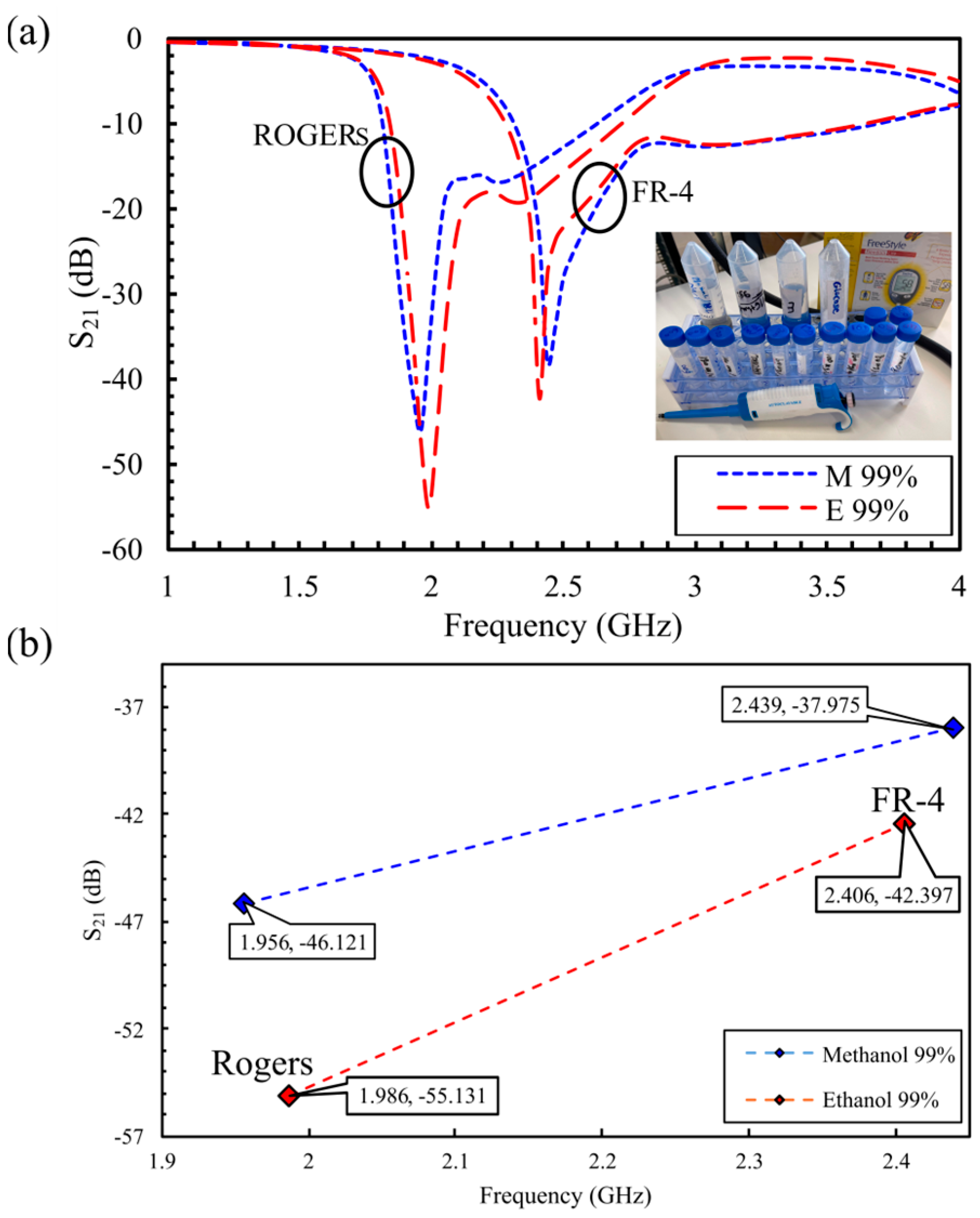
5.1. S21-Based In Vitro Detection of Glucose in Deionized Water, and Evaluation Across Saline
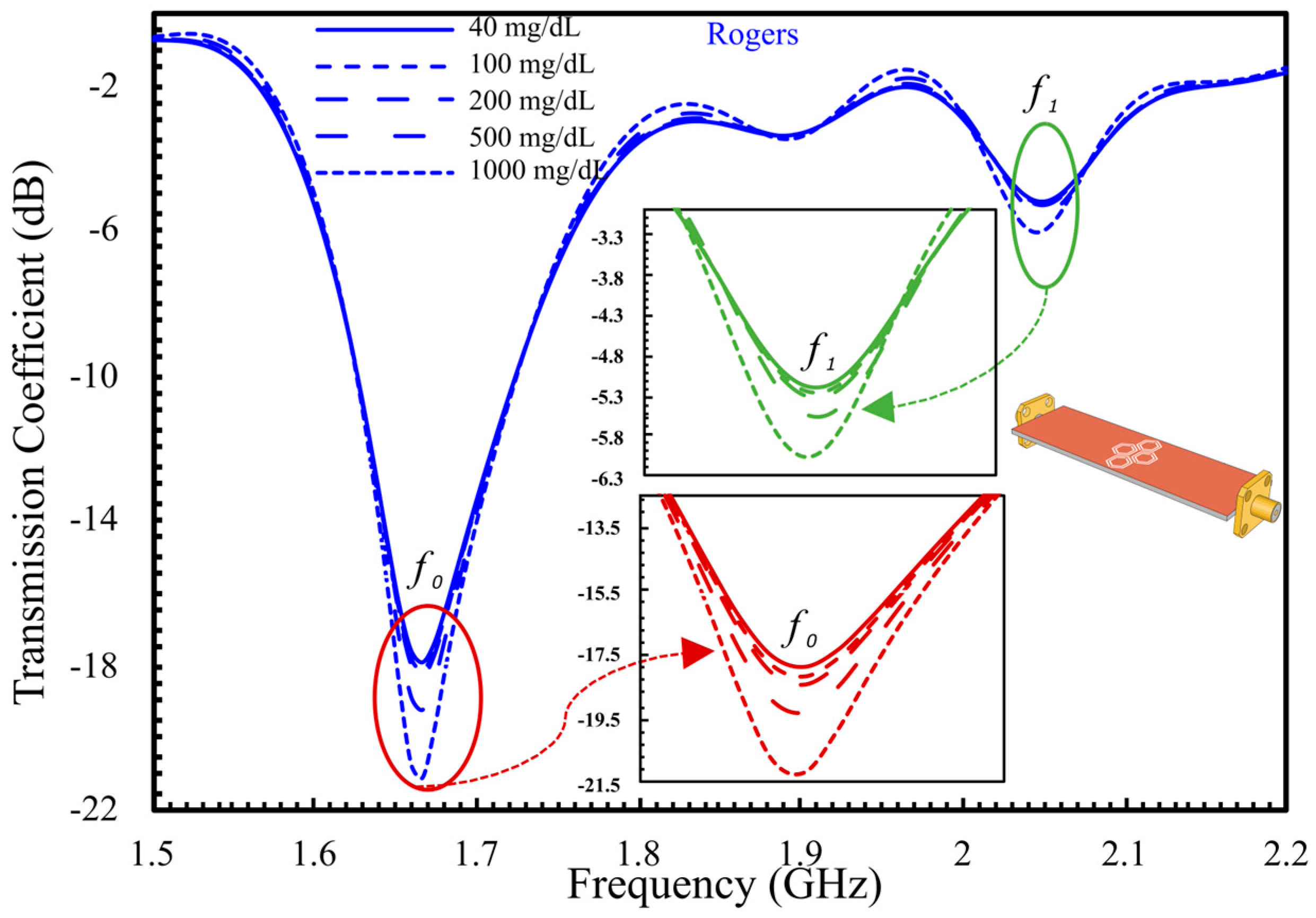
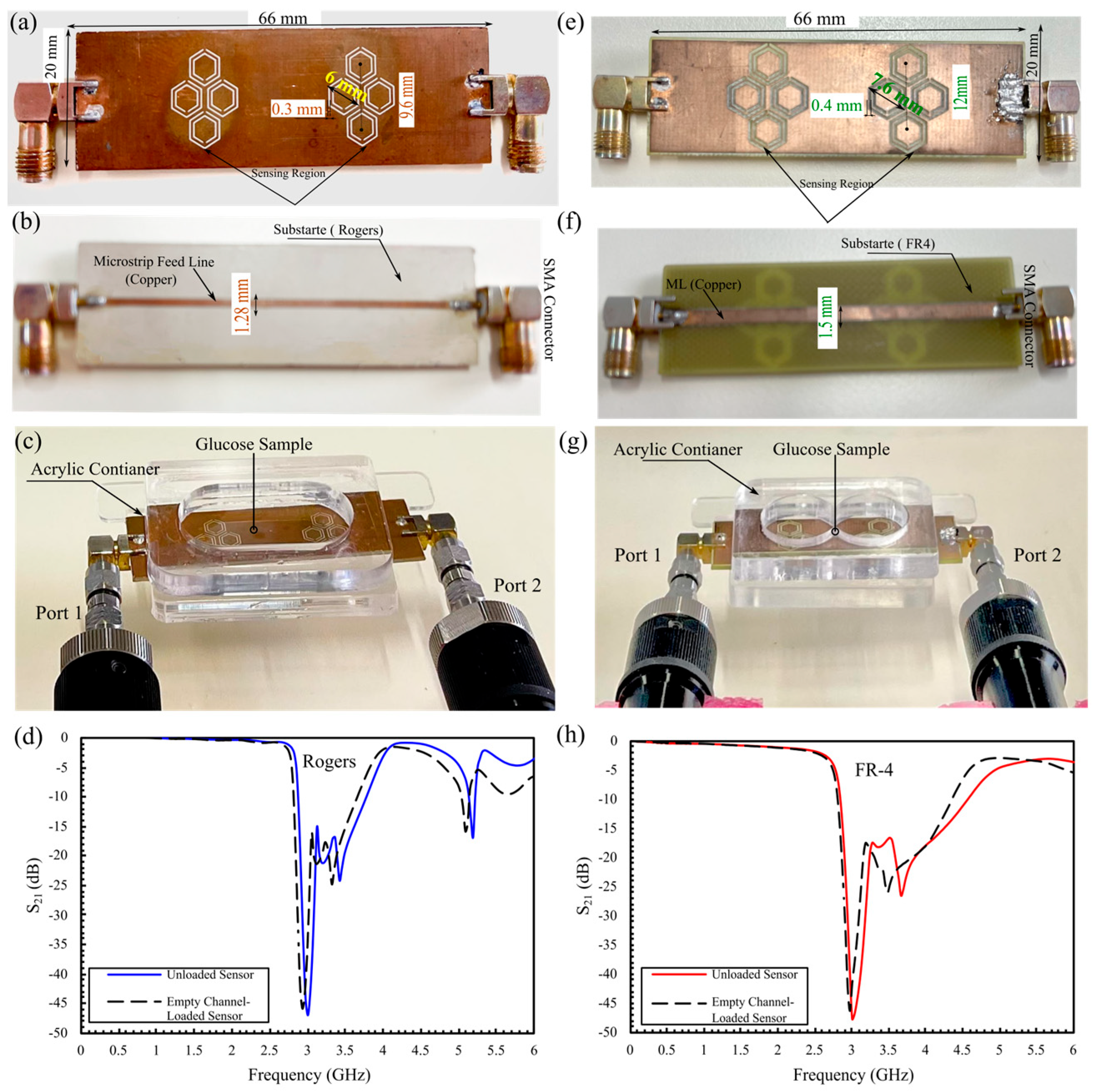
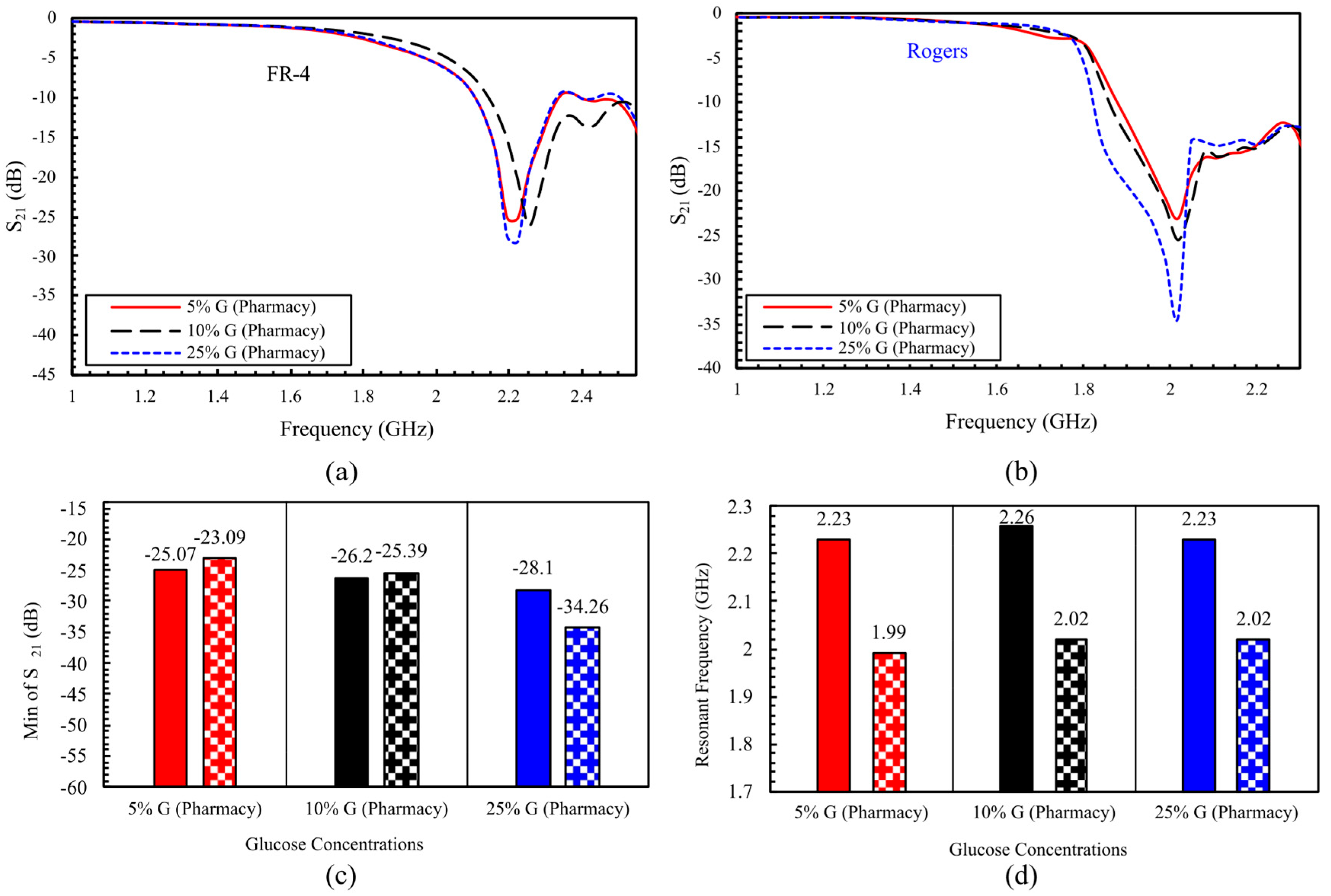
5.2. Comprehensive Evaluation of Glucose Sensing Performance Using FR-4 and Rogers Substrates in One- and Two-Finger Configurations
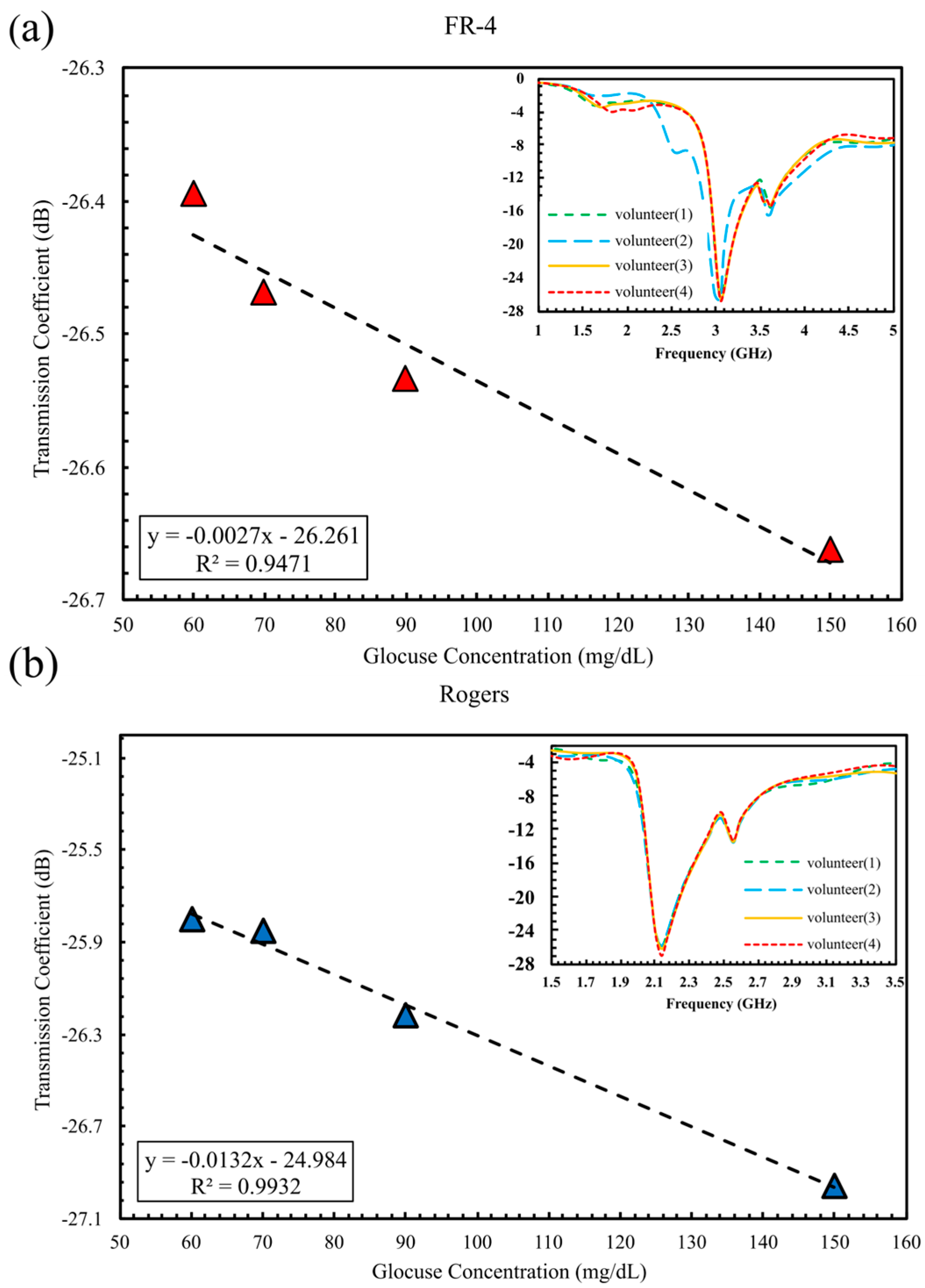
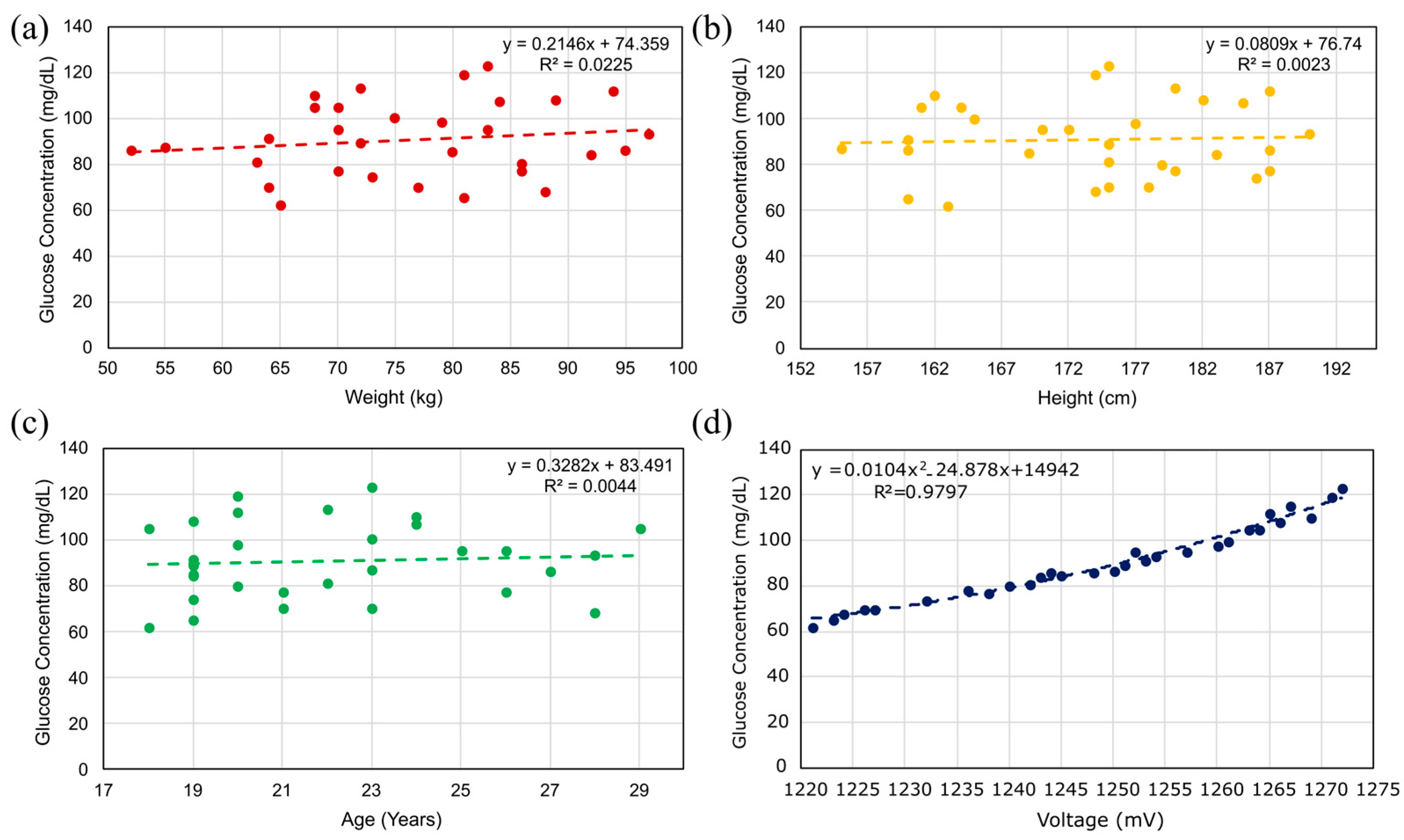
5.2.1. In Vivo Glucose Detection in Humans Across 60–150 mg/dL (Single-Finger Configuration Using FR-4 and Rogers Substrates)
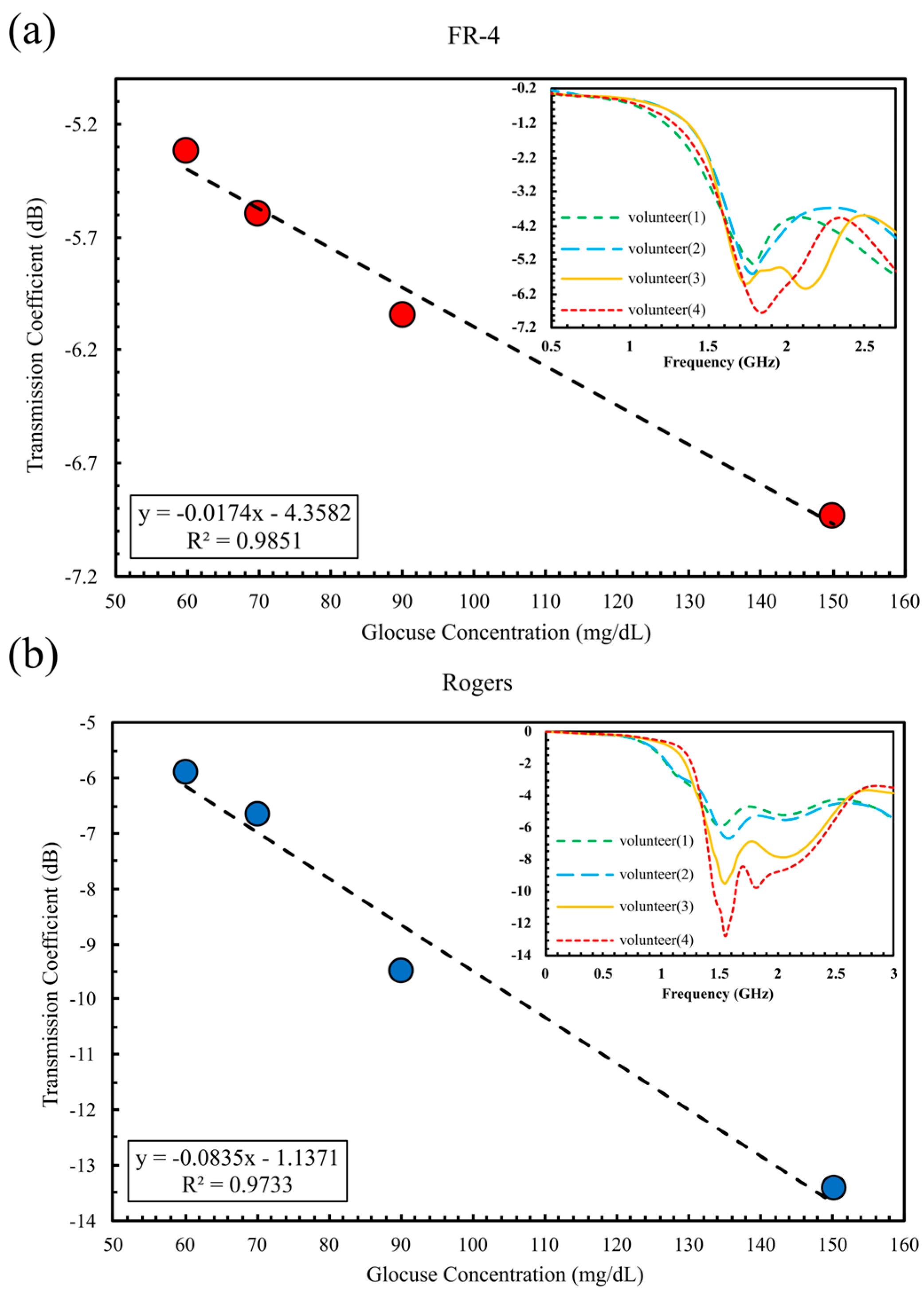
5.2.2. In Vivo Glucose Detection Utilizing DHC-CSRR on Rogers and FR-4 Substrates (Dual-Finger Configuration)
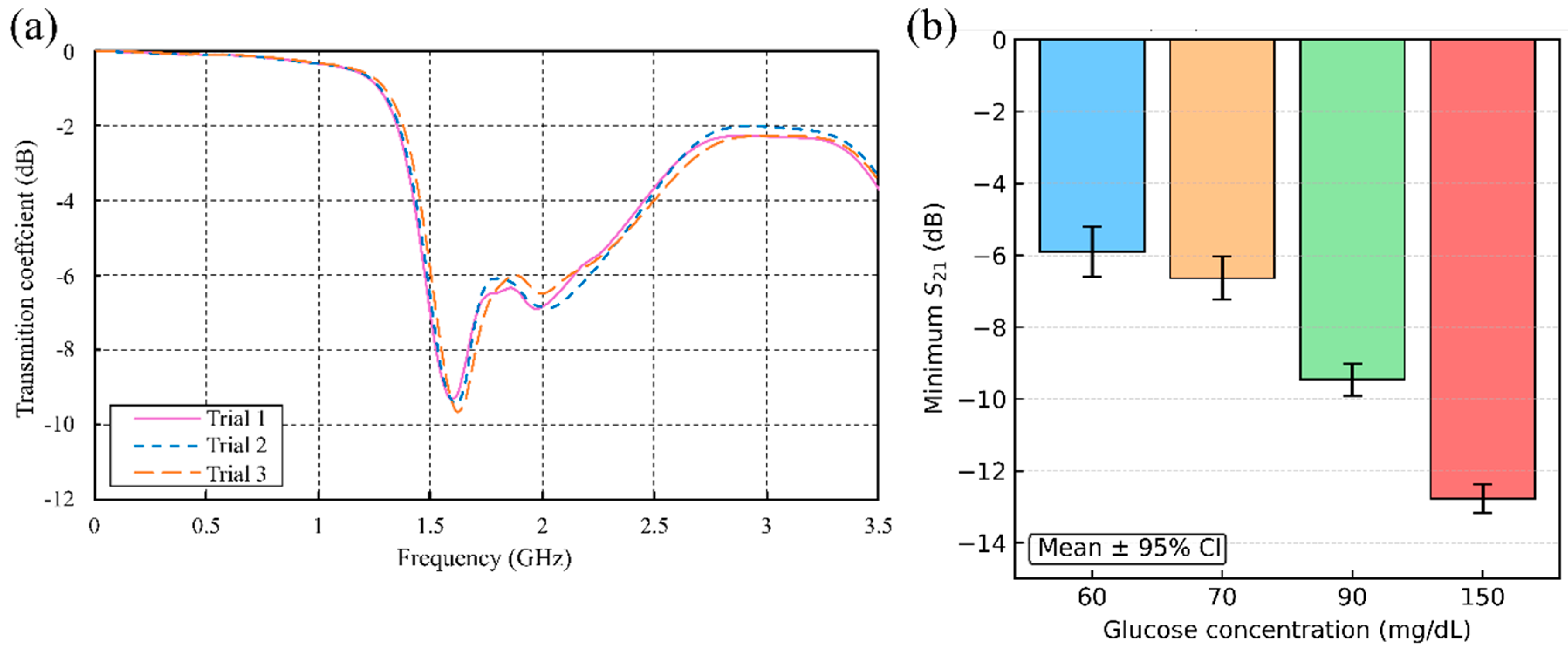
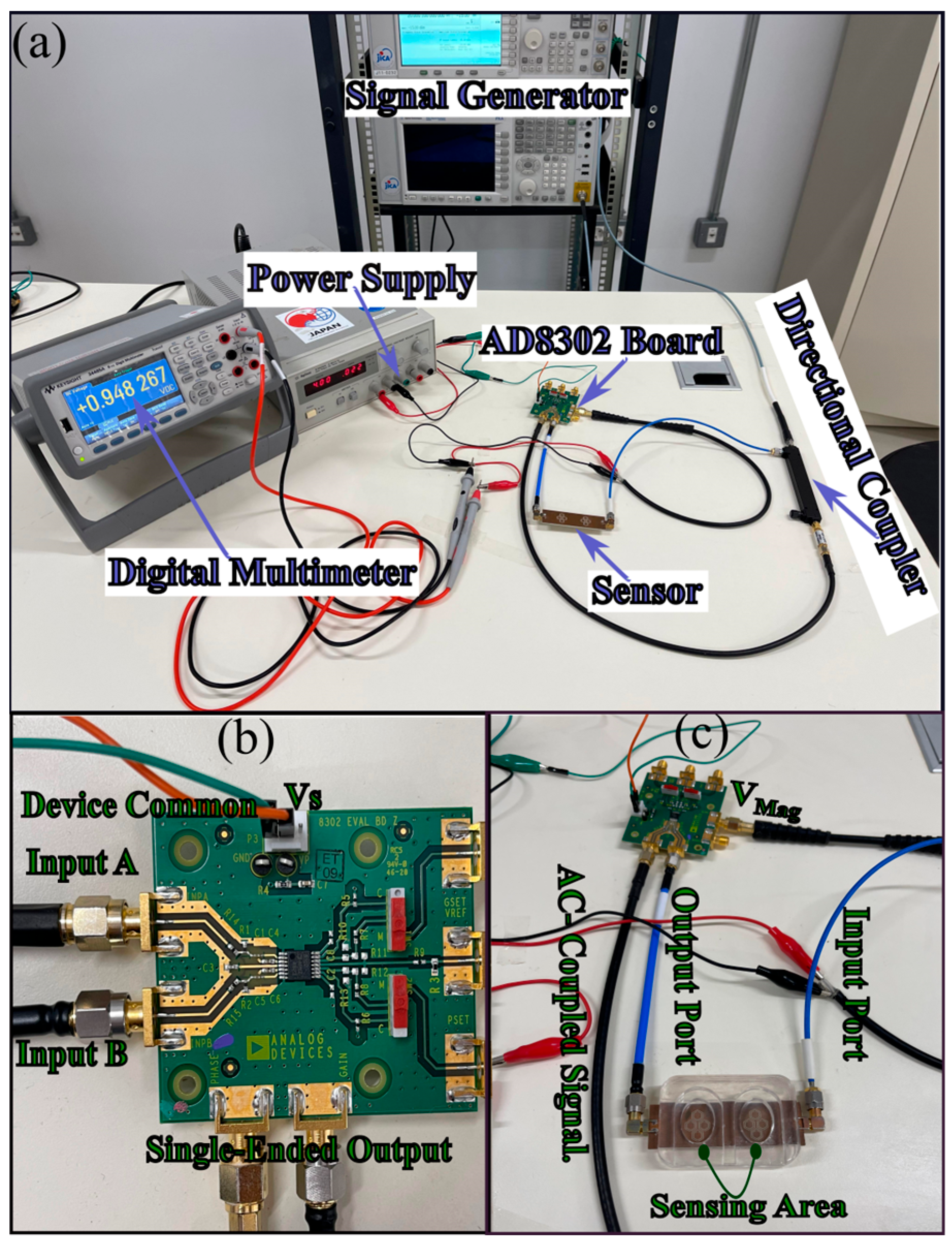
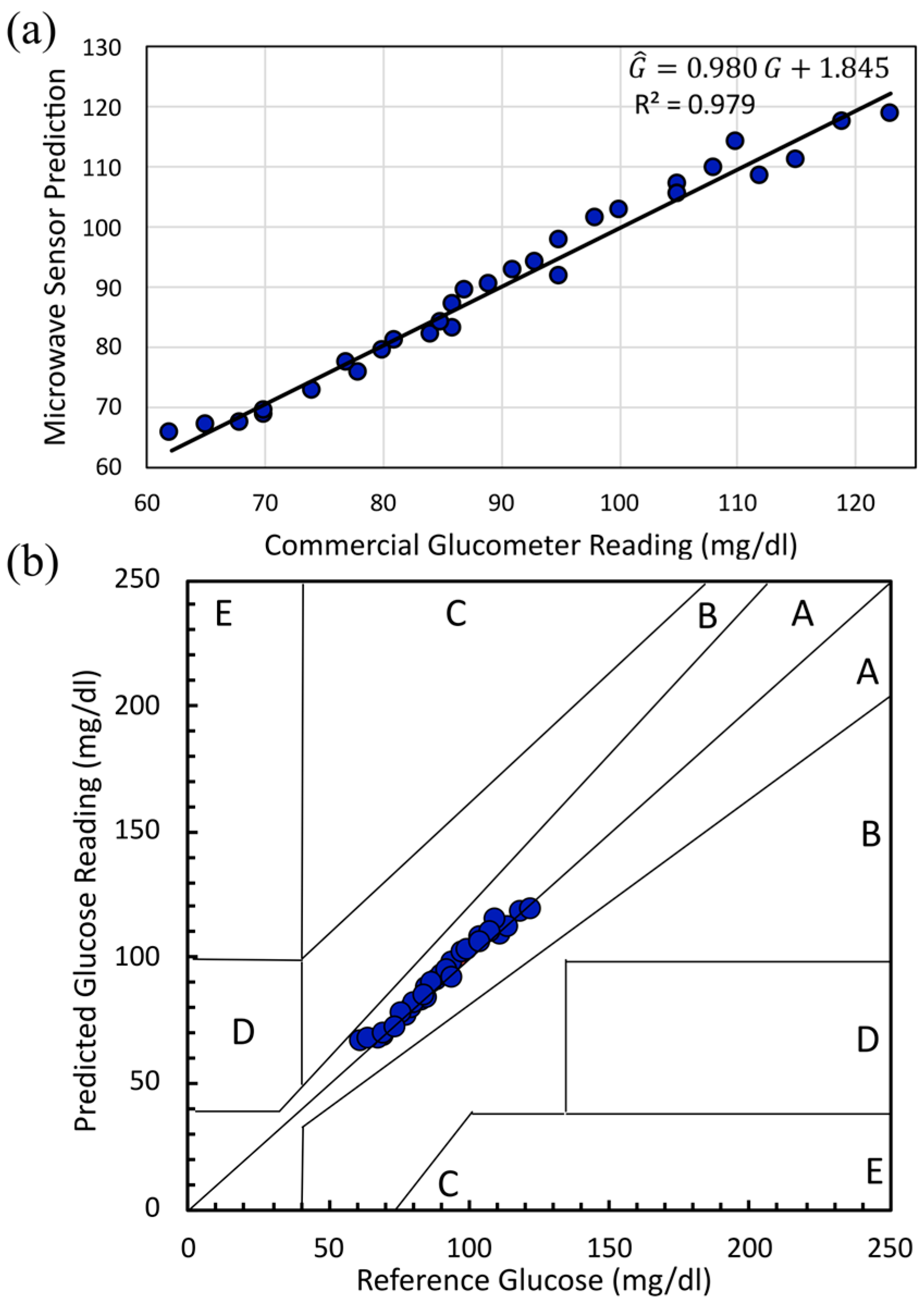
5.2.3. In Vivo Human Validation (N = 31) of a Dual-Finger DHC-CSRR Sensor on Rogers Using an AD8302 Evaluation Board
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Latin symbols | |
| Coefficients of the quadratic voltage–to–glucose calibration | |
| Intercept of the regression between predicted and reference glucose (mg/dL) | |
| Confidence interval of the mean | |
| Diagonal length of outer hexagonal CSRR cell (mm) | |
| Diagonal length of inner hexagonal CSRR cell (mm) | |
| Residual error for sample , (mg/dL) | |
| Fundamental resonant frequency (Hz) | |
| Second-mode resonant frequency (Hz) | |
| Blood glucose concentration (mg/dL) | |
| Predicted glucose concentration from regression model (mg/dL) | |
| Substrate height/thickness (mm) | |
| Number of groups (participants) in pooled–variance analysis | |
| Slope of the regression between predicted and reference glucose (dimensionless) | |
| Number of repeated measurements per participant | |
| Total number of samples in the dataset | |
| Resonator quality factor, (dimensionless) | |
| Amplitude resolution with respect to change in real permittivity, (dB) | |
| Amplitude resolution with respect to change in loss tangent, (dB) | |
| Sample standard deviation of repeated measurements (dB) | |
| Pooled standard deviation across participants (dB) | |
| |S21| | Magnitude of the transmission coefficient (dB) |
| |S21| | Change in |S21| with glucose or dielectric properties (dB) |
| 3 dB bandwidth around the resonance (Hz) | |
| Standard error of the mean, (dB) | |
| Expanded uncertainty of the mean at 95% confidence (dB) | |
| Output voltage of the AD8302 board/sensor chain (V) | |
| Mean voltage across all participants (V or mV) | |
| Mean |S21| amplitude for repeated measurements at a given glucose level (dB) | |
| Predicted glucose for the sample (mg/dL) | |
| Reference the glucometer glucose for the sample (mg/dL) | |
| Greek symbols | |
| Complex relative permittivity, (dimensionless) | |
| Real part of relative permittivity (energy storage) (dimensionless) | |
| Imaginary part of relative permittivity (loss) (dimensionless) | |
| Static (low-frequency) permittivity in Debye model (dimensionless) | |
| High-frequency permittivity limit in Debye model (dimensionless) | |
| Glucose concentration variable in Debye model (mg/dL) | |
| Dielectric loss angle | |
| Loss tangent, (dimensionless) | |
| Angular frequency, (rad/s) | |
| Debye relaxation time (ps) | |
| Degrees of freedom for Student’s –distribution () | |
| Statistical and error metrics | |
| Mean absolute error of glucose prediction (mg/dL) | |
| Mean absolute percentage error of glucose prediction (%) | |
| Mean absolute relative difference between predicted and reference glucose (%) | |
| Root–mean–square error of glucose prediction (mg/dL) | |
| Standard deviation of absolute errors (mg/dL) | |
| Abbreviations and acronyms | |
| AD8302 | Analog Devices gain/phase detector IC |
| BGL | Blood glucose level |
| BMI | Body mass index |
| CGM | Continuous glucose monitoring |
| CSRR | Complementary split ring resonator |
| DHC–CSRR | Double-honeycomb complementary split ring resonator (16–cell array) |
| DMM | Digital multimeter |
| GC | Glucose concentration |
| ISF | Interstitial fluid |
| MI | Minimally invasive |
| NI | Non-invasive |
| QF | Quality factor |
| RF | Radio frequency |
| RO3210 | Rogers RO3210 substrate |
| VNA | Vector network analyzer |
References
- Hossain, J.; Mamun, A.; Islam, R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas 2021 IDF Diabetes Atlas. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581934/ (accessed on 18 October 2025).
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Calibration of Minimally Invasive Continuous Glucose Monitoring Sensors: State-of-The-Art and Current Perspectives. Biosensors 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Joshi, A.M.; Mohanty, S.P.; Cenkeramaddi, L.R. Non-Invasive Glucose Measurement Technologies: Recent Advancements and Future Challenges. IEEE Access 2024, 12, 61907–61936. [Google Scholar] [CrossRef]
- Ameen, S.S.M.; Omer, K.M.; Mansour, F.R.; Bedair, A.; Hamed, M. Non-invasive wearable electrochemical sensors for continuous glucose monitoring. Electrochem. Commun. 2025, 173, 107894. [Google Scholar] [CrossRef]
- Rashid, H.O.; Aktar, M.N.; Preda, V.; Nasiri, N. Advances in electrochemical sensors for real-time glucose monitoring. Sens. Diagn. 2024, 3, 893–913. [Google Scholar] [CrossRef]
- Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors 2021, 21, 6820. [Google Scholar] [CrossRef] [PubMed]
- Zilgarayeva, A.; Smailov, N.; Pavlov, S.; Mirzakulova, S.; Alimova, M.; Kulambayev, B.; Nurpeissova, D. Optical sensor to improve the accuracy of non-invasive blood sugar monitoring. Indones. J. Electr. Eng. Comput. Sci. 2024, 34, 1489. [Google Scholar] [CrossRef]
- Kandwal, A.; Sharma, Y.D.; Jasrotia, R.; Kit, C.C.; Lakshmaiya, N.; Sillanpää, M.; Liu, L.W.; Igbe, T.; Kumari, A.; Sharma, R.; et al. A comprehensive review on electromagnetic wave based non-invasive glucose monitoring in microwave frequencies. Heliyon 2024, 10, e37825. [Google Scholar] [CrossRef]
- Kandwal, A.; Liu, L.W.; Deen, M.J.; Jasrotia, R.; Kanaujia, B.K.; Nie, Z. Electromagnetic Wave Sensors for Noninvasive Blood Glucose Monitoring: Review and Recent Developments. IEEE Trans. Instrum. Meas. 2023, 72, 1–15. [Google Scholar] [CrossRef]
- Masini, J.; Shahbaz, R.; Deshours, F.; Alquie, G.; El Bastami, C.; Kokabi, H. Penetration Depth in Multilayered Biological Tissues using a Compact Microwave Biosensor. In Proceedings of the 2022 52nd European Microwave Conference (EuMC), Milan, Italy, 27–29 September 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 408–411. [Google Scholar] [CrossRef]
- Vrba, J.; Karch, J.; Vrba, D. Phantoms for Development of Microwave Sensors for Noninvasive Blood Glucose Monitoring. Int. J. Antennas Propag. 2015, 2015, 570870. [Google Scholar] [CrossRef]
- Odabashyan, L.; Babajanyan, A.; Baghdasaryan, Z.; Kim, S.; Kim, J.; Friedman, B.; Lee, J.-H.; Lee, K. Real-Time Noninvasive Measurement of Glucose Concentration Using a Modified Hilbert Shaped Microwave Sensor. Sensors 2019, 19, 5525. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Ahmed, M.I.; Allam, A.; Pokharel, R.K.; Abdel-Rahman, A.B. A Simulation Study on Triple-Square Complementary Split-Ring Resonator for Non-Invasive Blood and Aqueous Solutions Measurements. In Proceedings of the 2023 11th International Japan-Africa Conference on Electronics, Communications, and Computations (JAC-ECC), Alexandria, Egypt, 18–20 December 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 13–16. [Google Scholar] [CrossRef]
- Afshari, N.; Hashemi, S.M.; Geran, F. Sensitivity evaluation of miniature microstrip line-based sensors using multiple sensing parameters for non-invasive blood glucose monitoring. IET Microw. Antennas Propag. 2023, 17, 872–886. [Google Scholar] [CrossRef]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Quendo, C.; Avila-Navarro, E.; Sabater-Navarro, J.M. Concentration Measurement of Microliter-Volume Water–Glucose Solutions Using Q Factor of Microwave Sensors. IEEE Trans. Instrum. Meas. 2019, 68, 2621–2634. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, X.; Hu, M.; Wang, Z. Non-invasive microwave blood glucose monitoring based on the diffusion limited aggregation earlobe model. Int. J. RF Microw. Comput. Eng. 2022, 32, 12. [Google Scholar] [CrossRef]
- Hanna, J.; Tawk, Y.; Azar, S.; Ramadan, A.H.; Dia, B.; Shamieh, E.; Zoghbi, S.; Kanj, R.; Costantine, J.; Eid, A.A. Wearable flexible body matched electromagnetic sensors for personalized non-invasive glucose monitoring. Sci. Rep. 2022, 12, 14885. [Google Scholar] [CrossRef]
- Saha, S.; Cano-Garcia, H.; Sotiriou, I.; Lipscombe, O.; Gouzouasis, I.; Koutsoupidou, M.; Palikaras, G.; Mackenzie, R.; Reeve, T.; Kosmas, P.; et al. A Glucose Sensing System Based on Transmission Measurements at Millimetre Waves using Micro strip Patch Antennas. Sci. Rep. 2017, 7, 6855. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, S.; Sun, H. Metamaterials Application in Sensing. Sensors 2012, 12, 2742–2765. [Google Scholar] [CrossRef]
- Baghelani, M.; Abbasi, Z.; Daneshmand, M.; Light, P.E. Non-invasive continuous-time glucose monitoring system using a chipless printable sensor based on split ring microwave resonators. Sci. Rep. 2020, 10, 12980. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Microwave reflective biosensor for glucose level detection in aqueous solutions. Sens. Actuators A Phys. 2020, 301, 111662. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Wang, Z.; Sun, J.; Zhang, X. Highly sensitive blood glucose monitoring sensor with adjustable resonant frequency based on MP-CSRR. Sens. Actuators A Phys. 2024, 366, 115004. [Google Scholar] [CrossRef]
- Debye, P.J.W. Polar Molecules; Dover Publications Inc.: New York, NY, USA, 1960. [Google Scholar]
- Eleiwa, M.A.; Elsherbeni, A.Z. Debye Constants for Biological Tissues from 30 Hz to 20 GHz. Appl. Comput. Electromagn. Soc. Newsl. 2001, 16, 202–213. [Google Scholar]
- Hofmann, M.; Trenz, F.; Weigel, R.; Fischer, G.; Kissinger, D. A microwave sensing system for aqueous concentration measurements based on a microwave reflectometer. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest, Montreal, QC, Canada, 17–22 June 2012; pp. 4–6. [Google Scholar] [CrossRef]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-Based Noninvasive Concentration Measurements for Biomedical Applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Jarvis, J.; Janezic, M.; Riddle, B.; Holloway, C.; Paulter, N.G.; Blendell, J.E. Dielectric and Conductor-Loss Characterization and Measurements on Electronic Packaging Materials; U.S. Department of Commerce: Washington, DC, USA, 2001.
- Baker-Jarvis, J. Dielectric and Conductor-Loss Characterization and Measurements on Electronic Packaging Materials; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2001. [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: Novel design utilizing a four-cell CSRR hexagonal configuration. Sci. Rep. 2020, 10, 15200. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Ahmed, M.I.; Allam, A.; Pokharel, R.K.; Abdel-Rahman, A.B. A Novel Biosensor for Non-Invasive Blood Glucose Measurement Based on Double Square Complimentary Split Ring Resonator. In Proceedings of the 2023 17th European Conference on Antennas and Propagation (EuCAP), Florence, Italy, 26–31 March 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Hasan, N.; Tamanna, S.; Singh, P.; Nadeem, D.; Rudramuni, M. Cylindrical Dielectric Resonator Antenna Sensor for Non-Invasive Glucose Sensing Application. In Proceedings of the 2019 6th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 7–8 March 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 961–964. [Google Scholar] [CrossRef]
- Mansour, E.; Allam, A.; Abdel-Rahman, A.B. A novel approach to non-invasive blood glucose sensing based on a single-slot defected ground structure. Int. J. Microw. Wirel. Technol. 2022, 15, 32–40. [Google Scholar] [CrossRef]
- Tlili, B.; Alhomsi, M.; Benchoubane, Y.; Keshkar, M.; Rishani, N.R. Non Invasive Glucose Detection Using Multiple Cell Triangular CSRR Sensor. In Proceedings of the 2024 IEEE Conference on Antenna Measurements and Applications (CAMA), Da Nang, Vietnam, 9–11 October 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Islam, R.; Islam, M.T.; M., M.S.; Bais, B.; Almalki, S.H.A.; Alsaif, H.; Islam, S. Metamaterial sensor based on rectangular enclosed adjacent triple circle split ring resonator with good quality factor for microwave sensing application. Sci. Rep. 2022, 12, 6792. [Google Scholar] [CrossRef] [PubMed]
- Kandwal, A.; Igbe, T.; Li, J.; Liu, Y.; Li, S.; Liu, L.W.Y.; Nie, Z. Highly Sensitive Closed Loop Enclosed Split Ring Biosensor With High Field Confinement for Aqueous and Blood-Glucose Measurements. Sci. Rep. 2020, 10, 4081. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Buranrat, B. Advances in a Microwave Sensor-Type Interdigital Capacitor with a Hexagonal Complementary Split-Ring Resonator for Glucose Level Measurement. Chemosensors 2023, 11, 257. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Alquié, G.; Deshours, F.; Kokabi, H. Triple-Poles Complementary Split Ring Resonator for Sensing Diabetics Glucose Levels at cm-Band. arXiv 2019, arXiv:1908.07407. [Google Scholar] [CrossRef]
- Jang, C.; Park, J.-K.; Lee, H.-J.; Yun, G.-H.; Yook, J.-G. Non-Invasive Fluidic Glucose Detection Based on Dual Microwave Complementary Split Ring Resonators with a Switching Circuit for Environmental Effect Elimination. IEEE Sens. J. 2020, 20, 8520–8527. [Google Scholar] [CrossRef]
- Morrison, F.A. Uncertainty Analysis for Engineers and Scientists; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Alquie, G.; Deshours, F.; Kokabi, H.; Shubair, R.M. Non-Invasive Real-Time Monitoring of Glucose Level Using Novel Microwave Biosensor Based on Triple-Pole CSRR. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1407–1420. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Babajanyan, A.; Lee, K.; Friedman, B. Noncontact characterization of glucose by a waveguide microwave probe. Curr. Appl. Phys. 2009, 9, 856–860. [Google Scholar] [CrossRef]
- Huang, S.Y.; Omkar; Yoshida, Y.; Inda, A.J.G.; Xavier, C.X.; Mu, W.C.; Meng, Y.S.; Yu, W. Microstrip Line-Based Glucose Sensor for Noninvasive Continuous Monitoring Using the Main Field for Sensing and Multivariable Crosschecking. IEEE Sens. J. 2019, 19, 535–547. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, J.; Li, G. An Antenna Loaded with Complementary Split Ring Resonator for Non-Invasive Blood Glucose Measurement. In Proceedings of the 2024 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Glasgow, UK, 20–23 May 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Srisai, S.; Kongkaew, P.; Harnsoongnoen, S. Noncontact and Noninvasive Detection of Glucose Concentration Using a Single-Port Microwave Sensor Coupled with Artificial Neural Networks. IEEE Trans. Instrum. Meas. 2025, 74, 9510910. [Google Scholar] [CrossRef]
- Farouk, M.; El-Hameed, A.S.A.; Eldamak, A.R.; Elsheakh, D.N. Noninvasive blood glucose monitoring using a dual band microwave sensor with machine learning. Sci. Rep. 2025, 15, 16271. [Google Scholar] [CrossRef] [PubMed]
- Dexcom, Inc. Dexcom G7 Continuous Glucose Monitoring System; Dexcom, Inc.: San Diego, CA, USA, 2025; Available online: https://www.dexcom.com/en-GB/dexcom-shop/g7/stp-gt-001 (accessed on 6 November 2025).
- Dexcom, Inc. Dexcom CGM Accuracy. Available online: https://www.dexcom.com/accuracy (accessed on 6 November 2025).
- Abbott Diabetes Care. FreeStyle Continuous Glucose Monitoring Systems, Abbott Laboratories. Available online: https://www.freestyle.abbott/eg-en/home.html (accessed on 6 November 2025).
- Alva, S.; Brazg, R.; Castorino, K.; Kipnes, M.; Liljenquist, D.R.; Liu, H. Accuracy of the Third Generation of a 14-Day Continuous Glucose Monitoring System. Diabetes Ther. 2023, 14, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Abbott. How Good Is FreeStyle Libre 3? They’re Here to Testify. Available online: https://www.abbott.com/corpnewsroom/diabetes-care/how-good-is-FreeStyle-Libre-3-they-are-here-to-testify.html (accessed on 6 November 2025).
- Medtronic Diabetes. CGM Sensors, Medtronic Diabetes. Available online: https://www.medtronic.com/en-ca/diabetes/support/product-support/cgm-sensors.html (accessed on 6 November 2025).
- Medtronic MiniMed. MiniMed 780G System User Guide with Guardian 4 Sensor. April 2025. Available online: https://www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/MiniMed-780G-system-user-guide-with-Guardian-4-sensor.pdf (accessed on 6 November 2025).
- Ascensia Diabetes Care. Eversense E3 Continuous Glucose Monitoring System. Ascensia Diabetes Care. Available online: https://global.eversensediabetes.com/ (accessed on 6 November 2025).
- Senseonics Holdings, Inc. Senseonics Announces FDA Approval of the Eversense E3 Continuous Glucose Monitoring System for Use for Up to 6 Months. Senseonics Holdings. Available online: https://www.senseonics.com/investor-relations/news-releases/2022/02-11-2022-120033959 (accessed on 6 November 2025).
- Garg, S.K.; Liljenquist, D.; Bode, B.; Christiansen, M.P.; Bailey, T.S.; Brazg, R.L.; Denham, D.S.; Chang, A.R.; Akturk, H.K.; Dehennis, A.; et al. Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: The PROMISE Study. Diabetes Technol. Ther. 2022, 24, 84–92. [Google Scholar] [CrossRef]
- Ascensia Diabetes Care. Ascensia Diabetes Care Successfully Completes Initial European Launch of Next-Generation Eversense E3 CGM System. Ascensia Diabetes Care. Available online: https://www.ascensia.com/press/press-releases/press-release/?id=56abb695-1b13-45b2-a4e2-64eac2507166 (accessed on 6 November 2025).
- Abbott Diabetes Care. FreeStyle Blood Glucose Monitoring Systems. Abbott Laboratories. Available online: https://www.diabetescare.abbott/products.html (accessed on 6 November 2025).
- Tack, C.; Pohlmeier, H.; Behnke, T.; Schmid, V.; Grenningloh, M.; Forst, T.; Pfützner, A. Accuracy Evaluation of Five Blood Glucose Monitoring Systems Obtained from the Pharmacy: A European Multicenter Study with 453 Subjects. Diabetes Technol. Ther. 2012, 14, 330–337. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, R.; Zhao, X.; Zhang, L.; Wang, T.; Yang, S.; Han, N.; Zhu, D. Accuracy evaluation of Roche Accu-Chek Performa blood glucose meters at low glucose concentrations: A nine-year retrospective study. iLABMED 2024, 2, 141–148. [Google Scholar] [CrossRef]
- Shi, J.; Fernández-García, R.; Gil, I. Sensor Technologies for Non-Invasive Blood Glucose Monitoring. Sensors 2025, 25, 3591. [Google Scholar] [CrossRef] [PubMed]


| Debye Parameter | Model Equation |
|---|---|
| 5.38 + 30 × 10−3·χ | |
| 80.68 − 0.207 × 10−3·χ | |
| 9.68 + 0.23 × 10−3·χ |
| Glucose Samples | ||||||
|---|---|---|---|---|---|---|
| 78.275 | 78.295 | 78.315 | 78.395 | 78.602 | 78.949 | |
| tan(δ) | 0.133 | 0.131 | 0.129 | 0.124 | 0.108 | 0.082 |
| Substrate | DOuter | DInner | Thickness (T) | Height (H) |
|---|---|---|---|---|
| Rogers | 6.08 mm | 4.61 mm | 0.37 mm | 1.27 mm |
| FR-4 | 7.60 mm | 5.75 mm | 0.46 mm | 0.88 mm |
| Configuration | Substrate | ∣S21∣ (dB) | Sensitivity dB/(mg/dL) | R2 |
|---|---|---|---|---|
| One Finger | FR-4 Rogers | 0.29 | 7.54 × 10−3 | 0.947 |
| 1.16 | 1.22 × 10−2 | 0.993 | ||
| Two Fingers | FR-4 Rogers | 1.62 | 2.78 × 10−2 | 0.985 |
| 6.82 | 9.35 × 10−2 | 0.973 |
| Glucose Concentration (mg/dL) | Mean S21 (dB) | Standard Deviation (dB) | (dB) |
|---|---|---|---|
| 60 | −5.898 | 0.284 | 0.706 |
| 70 | −6.638 | 0.239 | 0.595 |
| 90 | −9.456 | 0.181 | 0.448 |
| 150 | −12.777 | 0.162 | 0.402 |
| Sensing Technique | Frequency (GHz) | Test Method | GC (mg/dL) | Sensing Parameter | Sensitivity dB/(mg/mL) |
|---|---|---|---|---|---|
| Six CSRR Cells [26] | 2.5–3.5 | Aqueous Solution | 0–160 | S21 | 7.47 × 10−3 |
| Triangular CSRR [37] | 2.1 | Aqueous Solution | 50–200 | S21 | 7.10 × 10−2 |
| Triple Pole CSRR [44] | 2.3 | Synthesis Blood Samples | 70–120 | S21 | 6.20 × 10−2 |
| Dielectric Waveguide Probe [45] | 2.0–2.5 | Aqueous Solution | 0–30,000 | S11 | 3.00 × 10−5 |
| Microstrip line [46] | 1.4–1.9 | Aqueous Solution | 78–5000 | S11 | 1.80 × 10−3 |
| T-patch CSRR [47] | 1.02–2.24 | Aqueous Solution | 0–594 | S11 | 1.60 × 10−3 |
| Single Port Sensor [48] | 2.0–3.0 | Aqueous Solution | 0–20,000 | S11 | 6.40 × 10−2 |
| Three-Ring SRR [49] | 2.4–5.2 | Single-Finger | 0–400 | S11 | 1.10 × 10−2 |
| This Work | 1.5 | Dual-Finger | 60–150 | S21 | 9.36 × 10−2 |
| Characteristic | Value |
|---|---|
| Age (years) | 22.3 ± 3.3 |
| Height (cm) | 173.9 ± 9.8 |
| Weight (kg) | 76.6 ± 11.5 |
| BMI (kg/m2) | 25.3 ± 2.9 |
| Glucose (mg/dL) | 90.8 ± 16.5 |
| Male, n (%) | 19 (61.3%) |
| Female, n (%) | 12 (38.7%) |
| System | Accuracy (MARD or Equivalent) | Sensitivity/Dynamics | Invasiveness | Approximate Cost/Burden |
|---|---|---|---|---|
| Dexcom G7 (iCGM) [50] | MARD ≈ 8.2% [51] | 5 min continuous readings; hypo/hyper alarms; non-adjunctive dosing | Minimally invasive 10-day subcutaneous filament | High recurring sensor cost; typically, several hundred USD/month |
| FreeStyle Libre 3 [52] | >90% of readings within ±20%/20 mg/dL [53] | 1 min readings to smartphone; optional alarms | Minimally invasive, worn up to 14 days [54] | Lower-cost CGM; ≈ USD 70–80 per sensor |
| Medtronic Guardian 4 [55] | MARD ≈ 8.2–8.5% [56] | 5 min readings; predictive low-glucose management in hybrid closed-loop pumps | Minimally invasive 7-day subcutaneous sensor + transmitter [56] | Recurring sensor costs comparable to other CGMs |
| Eversense E3 [57] | MARD ≈ 8.5–8.8% over 180 days [58,59] | Continuous long-term CGM; on-body vibrating alerts and app trends | Implantable optical sensor in the upper arm (6 months) + external transmitter [60] | Higher annual cost, including insertion/removal procedures; fewer insertions per year |
| Capillary finger-stick glucometer [61] | 98.8% of readings within ±15 mg/dL or ±15% [62] | High single-point accuracy; no continuous trend; depends on test frequency [63] | Fully invasive repeated finger-prick sampling | Low device cost: ongoing strip cost dominates, highly payer-dependent |
| Proposed dual-finger DHC-CSRR sensor (this work) | 97.83%; 100% within ±15 mg/dL (ISO 15197) and in Clarke zone A (60–125 mg/dL) | Non-invasive amplitude-based readout; local sensitivity ≈1.099 mg/dL/mV and ≈0.0935 dB/(mg/dL) | Fully non-invasive dual-finger contact on a planar microwave structure; no skin penetration | Research prototype; planar RF hardware with no per-sensor consumables, only periodic calibration |
| Type | Substrate Material | (1–10) GHz | Typical Thickness Range (mm) | Main Advantages | Main Limitations |
|---|---|---|---|---|---|
| Rigid | FR-4 epoxy–glass laminate | 3.8–4.5 | 0.8–2.0 (1.6 mm standard) | Very low cost; compatible with standard PCB fabrication; suitable for proof-of-concept resonators and antennas | high dielectric loss, reducing Q-factor and sensitivity; ε and loss vary with batch and moisture; limited suitability for precision metrology. |
| Rigid | Rogers RO4350B (hydrocarbon/ceramic) | 3.3–3.7 | 0.10–1.52 | Low-loss supports high-Q resonators and stable S-parameter measurements. | Higher cost than FR-4; rigid and non-conformal, limiting direct on-skin or curved-body placement |
| Rigid | Rogers RO3210 (ceramic-filled PTFE) | 10.2 | 0.25–1.27 | Very high εr enabling compact resonators and strong near-field confinement; enhances sensitivity to small permittivity changes. | Narrower impedance bandwidth, more sensitive to fabrication tolerances; rigid and higher material cost. |
| Flexible | Rogers R/flex 3600 (LCP foil) | 2.6–2.7 | 0.025–0.050 | Flexible, low-loss, and moisture-stable, suitable for conformal on skin for CGM. | Requires specialized flexible-circuit processing; higher cost and limited availability than standard rigid laminates. |
| Flexible | Kapton® polyimide film | 3.2–3.5 | 0.025–0.125 | Thermally stable low-loss flexible film; widely used in printed circuits and lightweight wearable sensor prototypes. | Bendable but not stretchable; repeated bending can induce micro-cracks and resonance detuning over time. |
| Flexible | PDMS (polydimethylsiloxane) | 2.6–3.0 | 0.5–2.0 (cast layers) | Soft, skin-conformal and biocompatible; excellent for on-skin coupling and integration of microfluidic channels with glucose solutions or sweat. | Higher dielectric loss than LCP or polyimide; thickness uniformity depends on the casting process; long-term creep can gradually shift resonance. |
| Flexible | Textile substrate (e.g., denim) | 1.6–1.8 | 1.0–2.5 | Intrinsically wearable and breathable, enables garment-level integration of antennas. | εr and loss are strongly affected by sweat, humidity, and pressure; poor repeatability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, E.; Ahmed, M.I.; Allam, A.; Pokharel, R.K.; Abdel-Rahman, A.B. Sensitivity Evaluation of a Dual-Finger Metamaterial Biosensor for Non-Invasive Glycemia Tracking on Multiple Substrates. Sensors 2025, 25, 7034. https://doi.org/10.3390/s25227034
Mansour E, Ahmed MI, Allam A, Pokharel RK, Abdel-Rahman AB. Sensitivity Evaluation of a Dual-Finger Metamaterial Biosensor for Non-Invasive Glycemia Tracking on Multiple Substrates. Sensors. 2025; 25(22):7034. https://doi.org/10.3390/s25227034
Chicago/Turabian StyleMansour, Esraa, Mohamed I. Ahmed, Ahmed Allam, Ramesh K. Pokharel, and Adel B. Abdel-Rahman. 2025. "Sensitivity Evaluation of a Dual-Finger Metamaterial Biosensor for Non-Invasive Glycemia Tracking on Multiple Substrates" Sensors 25, no. 22: 7034. https://doi.org/10.3390/s25227034
APA StyleMansour, E., Ahmed, M. I., Allam, A., Pokharel, R. K., & Abdel-Rahman, A. B. (2025). Sensitivity Evaluation of a Dual-Finger Metamaterial Biosensor for Non-Invasive Glycemia Tracking on Multiple Substrates. Sensors, 25(22), 7034. https://doi.org/10.3390/s25227034







