Dual-Electrode Glass Ribbons Intended for Use in Microplasma-Based Sensors
Highlights
- Long multimaterial ribbons composed of soda–lime silicate glass and AA 2024 electrodes were successfully developed via the S&D technique for the first time.
- The in situ formation of an oxide layer on the tip of each electrode drastically increased the duration of the plasma emission without the need for additional post-functionalization of the electrode surfaces but required adapted interspacing values.
- The control of the inter-electrode spacing value is critical to improving the microplasma emission time when the ribbon is used as miniaturized gas detector in hydrocarbon emission detection.
- Using an aluminum alloy to make the electrodes can greatly increase the distance between the analyzed region and the location of the measuring and electricity generation devices.
Abstract
1. Introduction
2. Materials and Methods
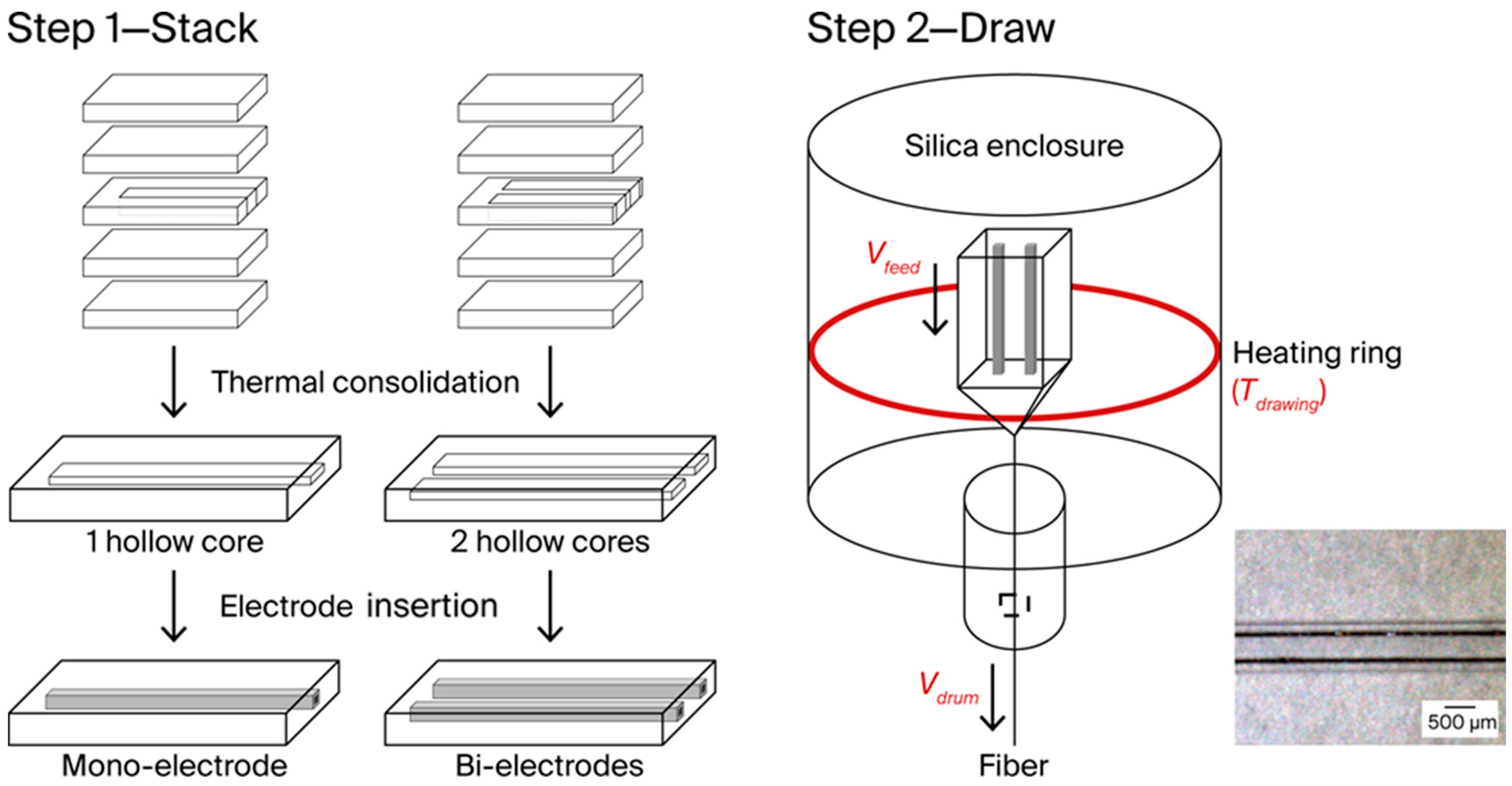
2.1. Materials and S&D Process
- Good electrical properties. At equal weight, aluminum has twice the electrical conductivity of copper, hence its applications in long-distance, high-voltage electricity transmission. Even though the electrical resistivity of aluminum alloys is higher than for pure aluminum (around +40%), an aluminum wire conducts 2.1 times more electricity than a copper wire and 18 times more than a steel wire of the same weight.
- Light weight and better mechanical properties than pure aluminum even at high temperature [28].
- Low cost. For the same electrical conduction, the price of an aluminum alloy conductor is equal to 40% of the price of a copper conductor (data Euralliage®, Gonesse, France). The AA 2024 alloy price is similar to that of 99.9% purity aluminum (2.98 USD/kg for square bars compared to around 2.6 USD/kg for pure aluminum plates).
2.2. Characterization Techniques
3. Results
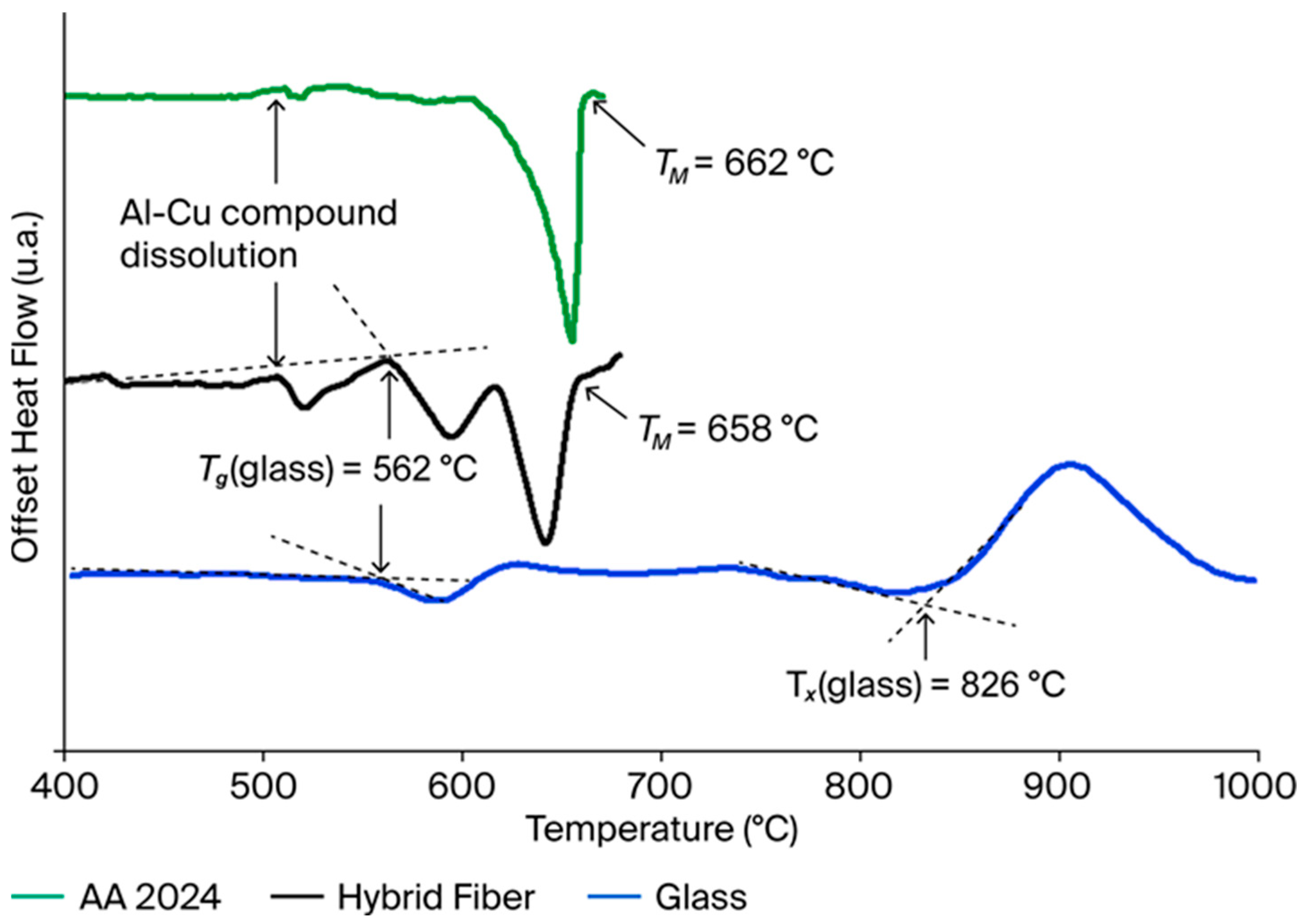
3.1. Thermal Analysis Results
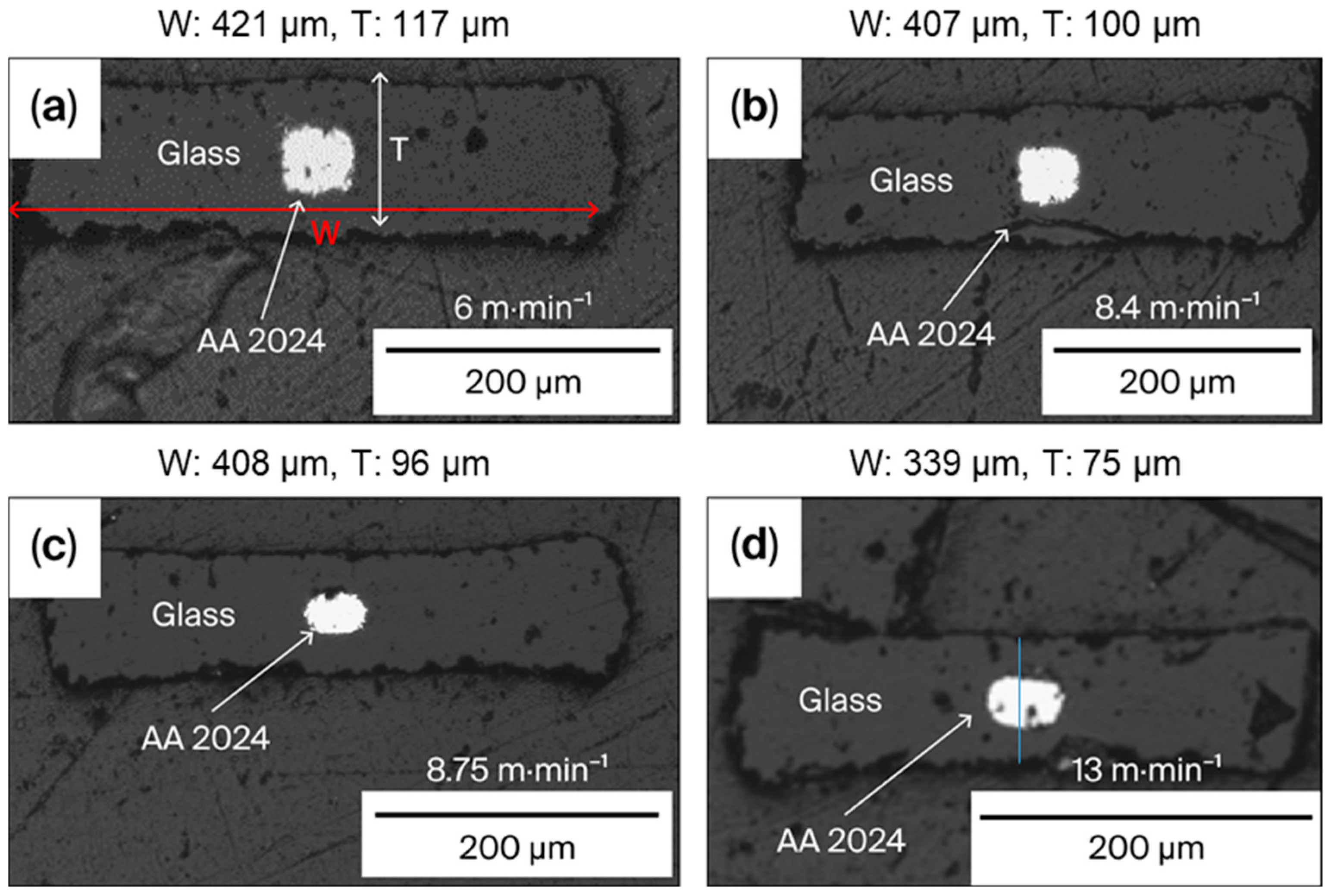
3.2. Control of the Electrode Shapes and Microstructures
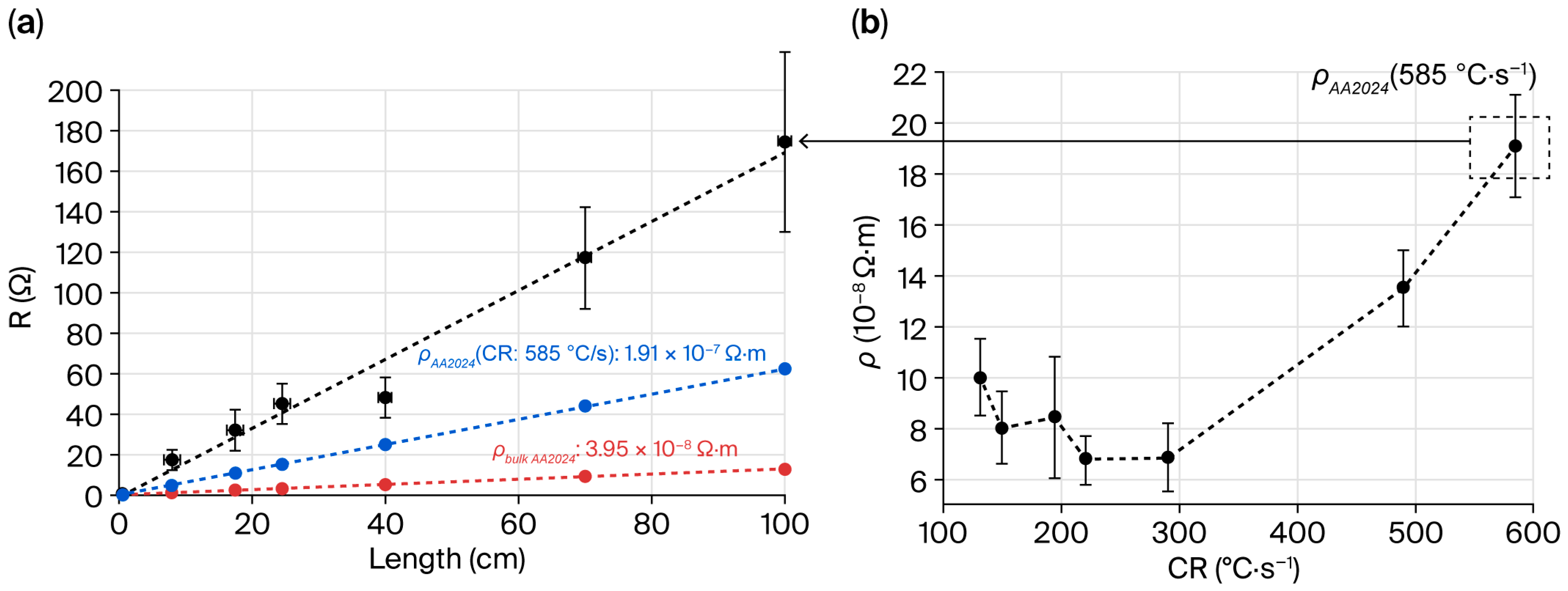
3.3. Electrical Properties of the Drawn Fiber
3.4. Bi-Electrode Fibers and Plasma Generation
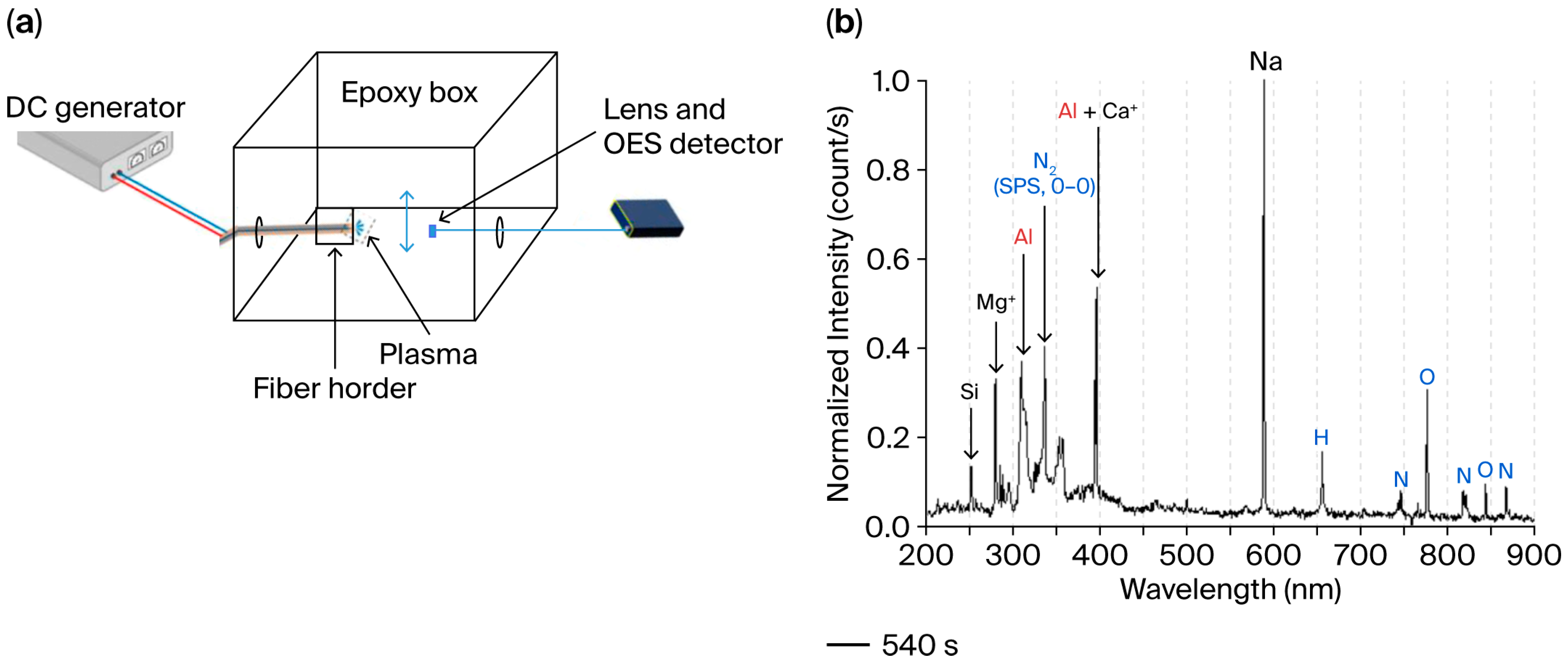
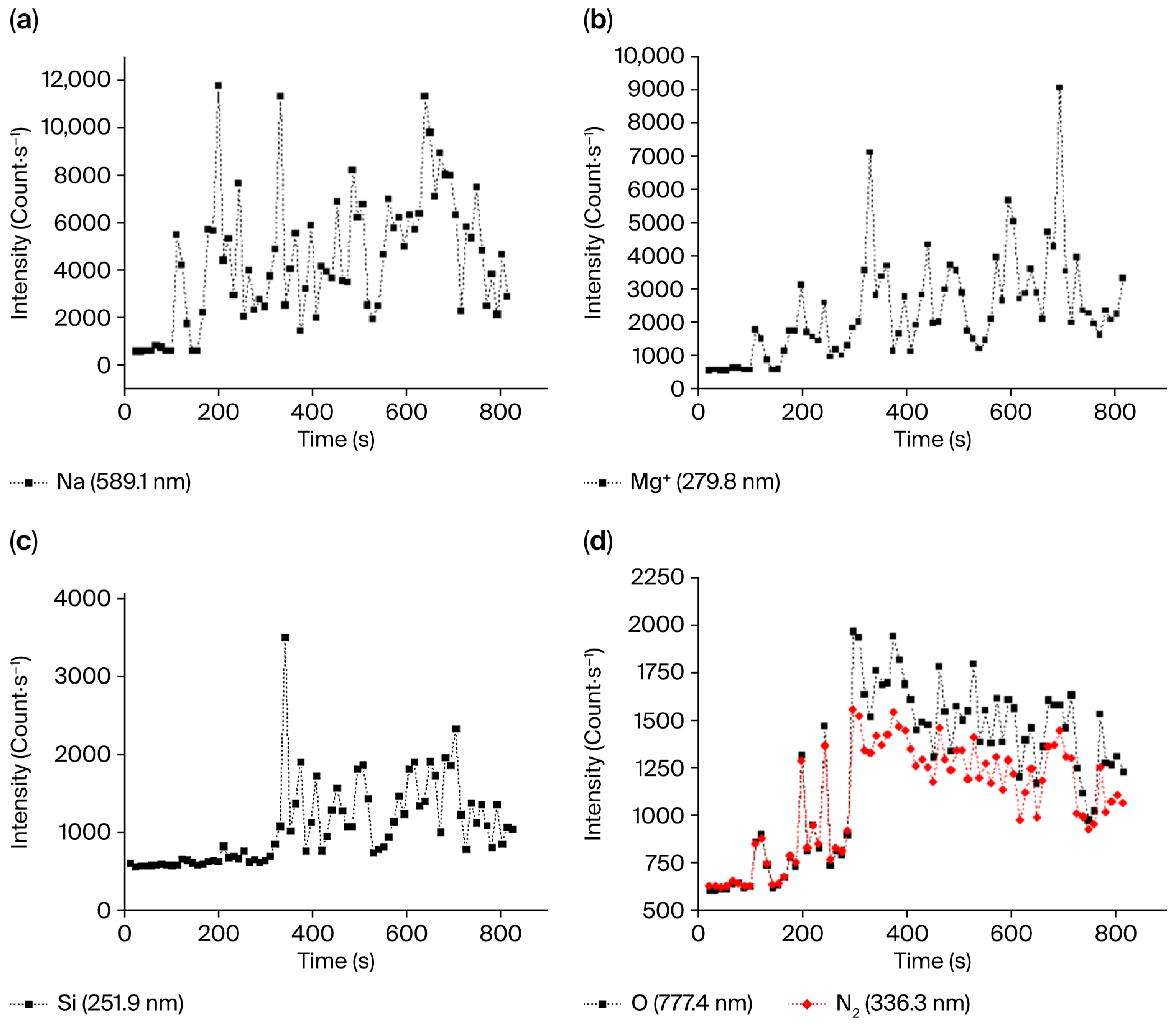
3.5. Plasma Analysis
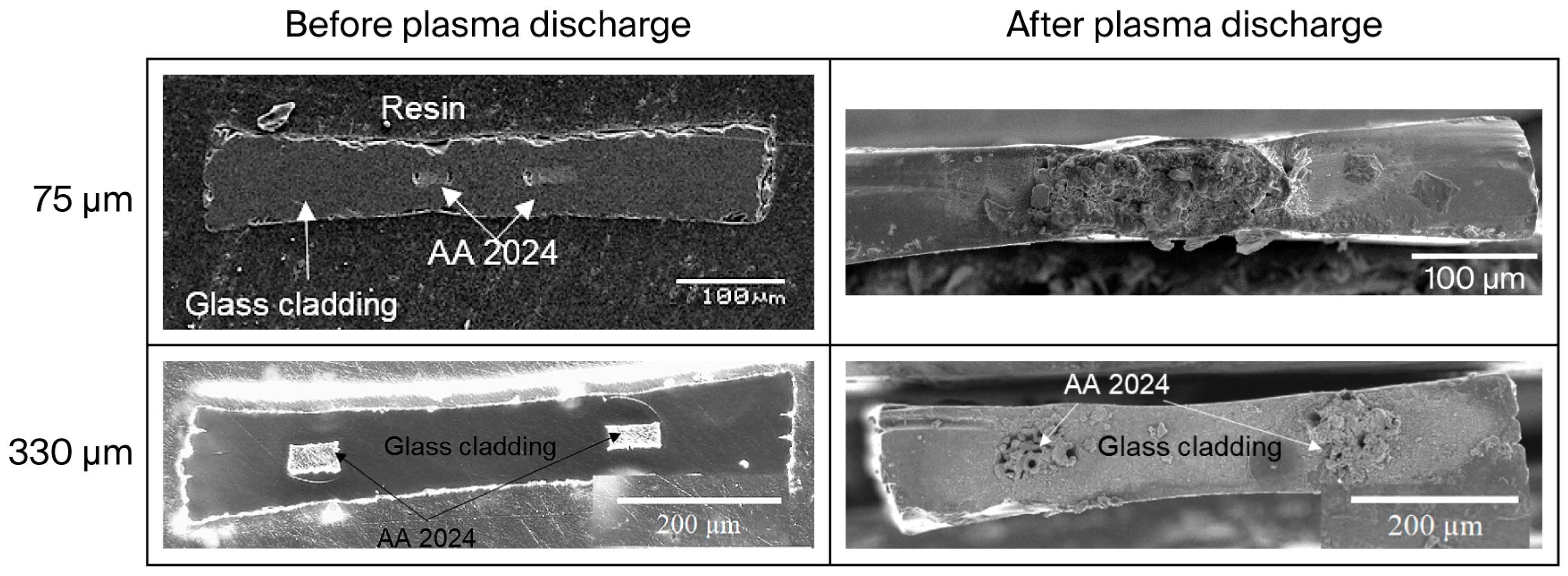
3.6. Bi-Electrode Fiber Behavior During Plasma Discharge in Air
3.7. Bi-Electrode Fibers Used as Gas Sensor
4. Discussion
4.1. S&D Parameters and Optimization of Electrical Properties of Multimaterial Fibers
4.2. Microplasma Discharge
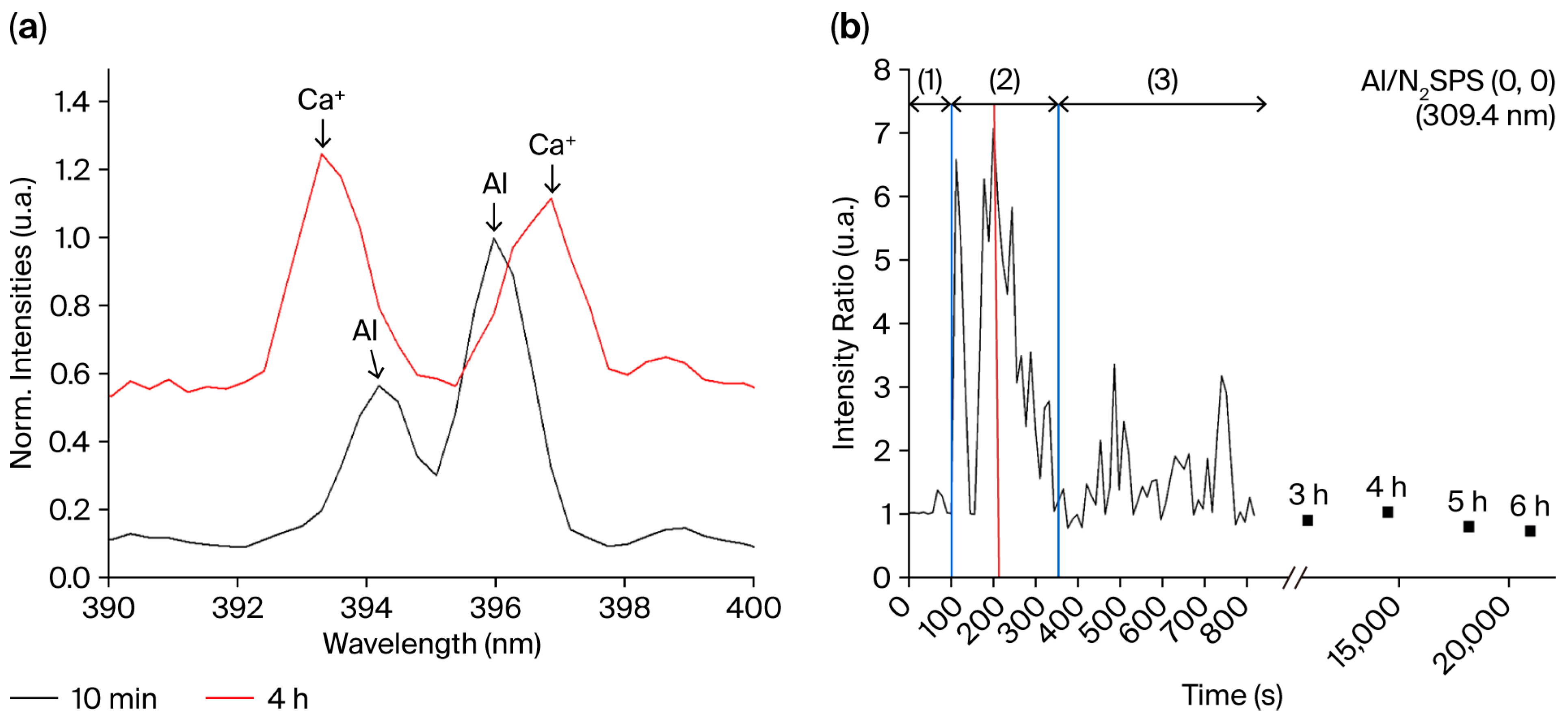
- (1)
- Sputtering of the Al: emission increases (Figure 8b).
- (2)
- Oxidation of the AA 2024 electrode surface due to the presence of oxygen in the plasma (Al emission line decrease, Figure 8b).
- (3)
- Incorporation in the plasma of the glass cladding elements leading to the formation of the oxide layer. The triple junction effect enhances the interaction between the glass surrounding electrodes and the plasma because of the electric field amplification due to the free spaces between the glass and the electrodes [61]. This enhancement makes discharges more probable on the glass close to the electrodes and explains why most of the glass remains almost untouched by the discharge (see Figure 6, where the central part of the glass is not damaged).
4.3. Microplasma Gas Sensor
5. Conclusions
- (1)
- The selection of an aluminum alloy such as AA 2024, instead of a pure metal, maintains the continuity of the electrodes during thermal drawing. The viscosity increases and the fluidity decreases due to the presence of alloying elements (Cu and Mg for the AA 2024) relative to pure aluminum, leading to a significant reduction in the electrode discontinuity. The right choice of Tdrawing is also essential to maintaining a high viscosity for the glass, thus preserving the preform shape (reducing dimensions) and containing the molten alloy.
- (2)
- An understanding of the microstructural changes and porosity formation is essential to controlling the electrical resistivity value. The cooling rate (i.e., vdrum and Tdrawing) is the key parameter. With the chosen bi-electrode geometry and materials, the CR remains between 200 and 300 °C/s.
- (3)
- The inter-electrode spacing value is critical for inducing the formation of a non-bridging layer of oxides between the electrodes during plasma generation. The in situ formation of an oxide layer on the tip of each electrode drastically increases the duration of the plasma emission without the need for additional post-functionalization of the electrode surface.
- (4)
- Such a device (the multimaterial fiber developed in this study combined with an OES detector) can be used as a miniaturized gas detector for hydrocarbon emission detection. Proof of concept has only been demonstrated. The sensor properties still need to be finalised. Further work is required to build on the findings regarding the reproducibility of the sensitivity of such a system to different concentrations of harmful substances, or mixtures of them, for example.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, G.; Stolyarov, A.M.; Abouraddy, A.F. Multimaterial Fibers. Int. J. Appl. Glass Sci. 2012, 3, 349–368. [Google Scholar] [CrossRef]
- Abouraddy, A.F.; Bayindir, M.; Benoit, G.; Hart, S.D.; Kuriki, K.; Orf, N.; Shapira, O.; Sorin, F.; Temelkuran, B.; Fink, Y. Towards multimaterial multifunctional fibres that see, hear, sense and communicate. Nat. Mater. 2007, 6, 336–347. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wang, Z.; Wei, L. Advanced Multi-Material Optoelectronic Fibers: A Review. J. Light. Technol. 2021, 39, 3836–3845. [Google Scholar] [CrossRef]
- Park, S.; Loke, G.; Fink, Y.; Anikeev, P. Flexible fiber-based optoelectronics for neutral interfaces. Chem. Soc. Rev. 2019, 48, 1826–1852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; Li, W.; Pu, J.; Wang, Z.; He, B.; Yuan, S.; Xin, J.; Huang, L.; Luo, Z.; et al. Scalable Production of Functional Fibers with Nanoscale Features for Smart Textiles. ACS Nano 2024, 18, 29394–29420. [Google Scholar] [CrossRef]
- Du, M.; Lv, S.; Qiu, J.; Guo, T.; Zhou, S. Multimaterial Fibers for Multifunctional Sensing Applications. Laser Photonics Rev. 2024, 18, 2301125. [Google Scholar] [CrossRef]
- Alhomsi, S.; Teule-Gay, L.; Canioni, L.; Bousquet, B.; Danto, S. Generation of stabilized electrically-induced micro-plasma at the tip of multimaterial optical fibers. Opt. Fiber Technol. 2023, 81, 103508. [Google Scholar] [CrossRef]
- Strutynski, C.; Meza, R.A.; Teulé-Gay, L.; El-Dib, G.; Poulon-Quintin, A.; Salvetat, J.-P.; Vellutini, L.; Dussauze, M.; Cardinal, T.; Danto, S. Stack-and-Draw Applied to the Engineering of Multi-Material Fibers with Non-Cylindrical Profiles. Adv. Funct. Mater. 2021, 31, 2011063. [Google Scholar] [CrossRef]
- Xiao, X.; Le Berre, S.; Fobar, D.G.; Burger, M.; Skrodzki, P.J.; Hartig, K.C.; Motta, A.T.; Jovanovic, I. Measurement of chlorine concentration on steel surfaces via fiber-optic laser-induced breakdown spectroscopy in double-pulse configuration. Spectrochim. Acta Part B At. Spectro. 2018, 141, 44–52. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Mariotti, D.; Sankaran, R.M.; Eden, J.G.; Ostrikov, K. Microplasmas for Advanced Materials and Devices. Adv. Mater. 2020, 32, 1905508. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.; Chiles, I.; Gordon, M. Slit-based supersonic microplasma jets: Scalable sources for nanostructured thin film deposition. Appl. Phys. Lett. 2013, 103, 163115. [Google Scholar] [CrossRef]
- Rahul, R.; Stan, O.; Rahman, A.; Littlefield, E.; Hoshimiya, K.; Yalin, A.P.; Sharma, A.; Pruden, A.; Moore, C.A.; Yu, Z.; et al. Optical and RF electrical characteristics of atmospheric pressure open-air Hollow slot microplasmas and application to bacterial inactivation. J. Phys. D Appl. Phys. 2005, 38, 1750–1759. [Google Scholar] [CrossRef]
- Zuo, X.; Wei, Y.; Chen, L.W.; Meng, Y.D. Non-equilibrium atmospheric pressure microplasma jet: An approach to endoscopic therapies. Phys. Plasmas 2013, 20, 083507. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ballato, J.; Foy, P.; Hawkins, T.; Wei, Y.; Li, J.; Kim, S.-O. Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens. Bioelectron. 2011, 28, 333–338. [Google Scholar] [CrossRef]
- Kim, J.Y.; Wei, Y.; Li, J.; Foy, P.; Hawkins, T.; Ballato, J.; Kim, S.-O. Single-Cell-Level Microplasma Cancer Therapy. Small 2011, 7, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Du, M.; Huang, L.; Zheng, J.; Wei, L.; Qiu, J.; Ren, C.; Zhou, S. Multimaterial Glass Fiber Probe for Deep Neural Stimulation and Detection. Adv. Opt. Mater. 2023, 11, 2202184. [Google Scholar] [CrossRef]
- Alexander Schmidt, M.; Argyros, A.; Sorin, F. Hybrid Optical Fibers: Hybrid Optical Fibers—An Innovative Platform for In-Fiber Photonic Devices. Adv. Opt. Mater. 2016, 4, 13–36. [Google Scholar] [CrossRef]
- Strutynski, C.; Teulé-Gay, L.; Danto, S.; Cardinal, T. Optical Emission Detector Based on Plasma Discharge Generation at the Tip of a Multimaterial Fiber. Sensors 2020, 20, 2353. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Gokcekaya, O.; Md Masum Billah, K.; Ertugrul, O.; Jiang, J.; Sun, J.; Hussain, S. Multi-material additive manufacturing: A systematic review of design, properties, applications, challenges, and 3D printing of materials and cellular metamaterials. Mat. Des. 2023, 226, 111661. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Xiao, K.; He, B.; Zhao, J.; Yang, X.; Liu, Q.; Sharma, A.K.; Leal Junior, A.; Min, R. A comprehensive review of optical fiber technologies in optogenetics and their prospective developments in future clinical therapies. Opt. Las. Technol. 2024, 179, 111332. [Google Scholar] [CrossRef]
- Tao, G.; Abouraddy, A.F. Multimaterial fibers: A new concept in infrared fiber optics. In Fiber Optic Sensors and Application XI; SPIE: Washington, DC, USA, 2014; Volume 9098. [Google Scholar] [CrossRef]
- Tao, G.; Abouraddy, A.F.; Stolyarov, A.M.; Fink, Y. Multimaterial Fibers. In Lab-On-Fiber Technology; Cusano, A., Consales, M., Crescitelli, A., Ricciardi, A., Eds.; Springer Series in Surface Sciences; Springer International Publishing: Cham, Switzerland, 2015; Volume 56, Chapter 1; pp. 1–26. [Google Scholar] [CrossRef]
- Lee, K.; Hu, P.; Blows, J.L.; Thorncraft, D.; Baxter, J. 200-m optical fiber with an integrated electrode and its poling. Opt. Lett. 2004, 29, 2124–2126. [Google Scholar] [CrossRef] [PubMed]
- Strutynski, C.; Desevedavy, F.; Lemiere, A.; Jules, J.-C.; Gadret, G.; Cardinal, T.; Smektala, F.; Danto, S. Tellurite-based core-clad dual electrodes composite fibers. Opt. Mat. Express 2017, 7, 503–1508. [Google Scholar] [CrossRef]
- Martins, A.A.; Pinheiro, M.J. On the propulsive force developed by asymmetric capacitors in a vacuum. Phys. Procedia 2011, 20, 112–119. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2018, 27, 079501. [Google Scholar] [CrossRef]
- Napolitano, A.; Hawkins, E.G. Viscosity of a Standard Soda-Lime-Silica Glass. J. Res. Natl. Bur. Stand. A Phys. Chem. 1964, 68A, 439–448. [Google Scholar] [CrossRef]
- Polmear, I.J. Light Alloys: From Traditional Alloys to Nanocrystals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9780080973920. [Google Scholar] [CrossRef]
- Tan, C.M.; Yu, W.B.; Jun, W. Making Wafer Bonding Viable for Mass Production. MRS Online Proc. Libr. 2005, 869, 28. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Q.; Zuo, Y.; Cheng, L.; Wang, J. The control of as-cast structure of 2024 aluminum alloy with intensive melt shearing and its effect on microstructure and mechanical properties after T4 treatment. J. Mater. Res. Technol. 2023, 24, 3179–3193. [Google Scholar] [CrossRef]
- Matula, R.A. Electrical resistivity of copper, gold, palladium and silver. J. Phys. Chem. Ref. Data 1979, 8, 1147–1298. [Google Scholar] [CrossRef]
- Bakonyi, I. Accounting for the resistivity contribution of grain boundaries in metals: Critical analysis of reported experimental and theoretical data for Ni and Cu. Eur. Phys. J. Plus 2021, 136, 410. [Google Scholar] [CrossRef]
- Czerwinski, F. Aluminum alloys for electrical engineering: A review. J. Mater. Sci. 2024, 59, 14847–14892. [Google Scholar] [CrossRef]
- Sili, E.; Koliatene, F.; Cambronne, J.P. Pressure and temperature effects on the paschen curve. In Proceedings of the 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; pp. 464–467. [Google Scholar] [CrossRef]
- NIST. Atomic Spectra Database Lines (Wavelenght Order). 2009. Available online: http://www.physics.nist.gov (accessed on 2 November 2025).
- Levens, P.J.; Labrosse, N. Modelling of Mg II lines in solar prominences. Astron. Astrophys. 2019, 625, A30. [Google Scholar] [CrossRef]
- Gornushkin, I.; Glaus, R.; Nagli, L. Stimulated emission in aluminum laser-induced plasma: Kinetic model of population inversion. Appl. Opt. 2017, 56, 695–701. [Google Scholar] [CrossRef]
- Goueguel, C.L.; Singh, J.P.; McIntyre, D.L.; Jain, J.; Karamalidis, A.K. Effect of Sodium Chloride Concentration on Elemental Analysis of Brines by Laser-Induced Breakdown Spectroscopy (LIBS). Appl. Spectrosc. 2014, 68, 213–221. [Google Scholar] [CrossRef]
- Sansonetti, J.E. Wavelenghts, transition probabilities, and Energy Levels for the Spectra of Sodium (NaI–NaXI). J. Phys. Chem. Ref. Data 2008, 37, 1659–1763. [Google Scholar] [CrossRef]
- Wiese, W.L.; Fuhr, J.R.; Deters, T.M. Atomic Transition Probabilities of Carbon, Nitrogen, and Oxygen, A critical Data Compilation. J. Phys. Chem. Ref. Data 1981, 7, 21–522. Available online: https://srd.nist.gov/JPCRD/jpcrdM7.pdf (accessed on 2 November 2025).
- Bouglas, L.; Sofikitis, D.; Everest, M.A.; Alexander, A.J.; Rakitzis, T.P. (2 + 1) laser-induced fluorescence of spin-polarized hydrogen atoms. J. Chem. Phys. 2010, 133, 174308. [Google Scholar] [CrossRef] [PubMed]
- Ono, R. Optical diagnostics of reactive species in atmospheric-pressure nonthermal plasma. J. Phys. D Appl. Phys. 2016, 49, 083001. [Google Scholar] [CrossRef]
- Guerin, M.R. Chemical Composition of Cigarette Smoke. Banbury Report No 3: A Safe Cigarette? Gori, G.B., Bock, F.G., Eds.; Cold Spring Harbor Laboratory: New York, NY, USA, 1980; pp. 191–204. ISBN 10-0879692022. [Google Scholar]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. In Chemistry and Toxicology of Cigarette Smoke and Biomarkers of Exposure and Harm; Bookshelf ID: NBK53017; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010; Chapter 3. [Google Scholar]
- Tane, E.G.; Amorós-Pérez, A.; Martínez-Gómez, L.; Román-Martínez, M.C.; Lillo-Ródenas, M.A. Review and comparative analysis of the particulate matter generated in conventional cigarettes and heated tobacco product—Mainstream and environmental emissions. Environ. Adv. 2024, 16, 100552. [Google Scholar] [CrossRef]
- Belov, I.; Vanneste, J.; Aghaee, M.; Paulussen, S.; Bogaerts, A. Synthesis of micro-and nanomaterials in CO2 and CO dielectric Barrier Discharges. Plasma Process. Polym. 2017, 14, 201600065. [Google Scholar] [CrossRef]
- Hawtof, D.W. Induction Heating in Optical Fiber Draw Processing; Rudnev, V., Totten, G.E., Eds.; ASM International: Detroit, MI, USA, 2014; Volume 4C, pp. 795–798. ISBN 978-1-62708-167-2. [Google Scholar] [CrossRef]
- Dinsdale, A.T.; Quested, P.N. The viscosity of aluminum and its alloys, A review of data and models. J. Mater. Sci. 2004, 39, 7221–7228. [Google Scholar] [CrossRef]
- Hidnert, P.; Krider, H.S. Thermal expansion of aluminum and some aluminum alloys. J. Res. Natl. Bur. Stan. 1952, 48, 209–220. [Google Scholar] [CrossRef]
- Kaygısız, Y.; Maraşlı, N. Microstructural, mechanical, and electrical characterization of directionally solidified Al–Cu–Mg eutectic alloy. Phys. Met. Metallogr. 2017, 118, 389–398. [Google Scholar] [CrossRef]
- Gunes, I.; Erdogan, M.; Çengelci, B. Mechanical and electrical properties of artificially aged aluminum alloy AA 2024. Metal. Mat. 2016, 54, 379–387. [Google Scholar] [CrossRef]
- Jeon, J.H.; Bae, D.H. Effect of cooling rate on the thermal and electrical conductivities of an A356 sand cast alloy. J. All. compd. 2019, 808, 151756. [Google Scholar] [CrossRef]
- Hakamada, M.; Kuromura, T.; Chen, Y.; Kusuda, H.; Mabuchi, M. Influence of Porosity and Pore Size on Electrical Resistivity of Porous Aluminum Produced by Spacer Method. Mater. Trans. 2007, 48, 32–36. [Google Scholar] [CrossRef]
- Yang, H.; Guo, Z.; Yang, H. Effect of vacuum on porosity and mechanical properties of high-pressure die-cast pure copper. China Foundry 2019, 16, 232–237. [Google Scholar] [CrossRef]
- Hegemann, D. Plasma activation mechanisms governed by specific energy input: Potential and perspectives. Plasma Process. Polym. 2023, 20, 2300010. [Google Scholar] [CrossRef]
- Selivonin, I.V.; Lazukin, A.V.; Moralev, I.A.; Krivov, S.A. Effect of electrode degradation on the electrical characteristics of surface dielectric barrier discharge. Plasma Sources Sci. Technol. 2018, 27, 085003. [Google Scholar] [CrossRef]
- Hart, L.D.; Lense, E. (Eds.) Alumina Chemicals: Science and Technology Handbook; John Wiley & Sons: Hoboken, NJ, USA, 1990; pp. 293–294. ISBN 978-86156-205-0. [Google Scholar]
- Kumar, B.; Kaushik, B.K.; Negi, Y.S. Perspectives and challenges for organic thin film transistors: Materials, devices, processes and applications. J. Mater. Sci. Mater. Electron. 2014, 25, 1–30. [Google Scholar] [CrossRef]
- McLaren, C.; Balabajew, M.; Gellert, M.; Roling, B.; Jain, H. Depletion Layer Formation in Alkali Silicate Glasses by Electro-Thermal Poling. J. Electrochem. Soc. 2016, 163, H809. [Google Scholar] [CrossRef][Green Version]
- Saim, N.; Bitam-Megherbi, F. Experimental Investigation of the Triple-Junction Effect on the Electric Discharge Characteristics and Modeling of the Maximum Discharge Current. ECTI Trans. Electr. Eng. Electron. Commun. 2021, 19, 115–126. [Google Scholar] [CrossRef]
- Johnson, R.C. The structure and origin of the swan band spectrum of carbon. Philos. Trans. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1927, 226, 157–230. [Google Scholar] [CrossRef]
- McConkey, J.W.; Malone, C.P.; Johnson, P.V.; Winstead, C.; McKoy, V.; Kanik, I. Electron impact dissociation of oxygen-containing molecules—A critical review. Phys. Rep. 2008, 466, 1–103. [Google Scholar] [CrossRef]
- Kustova, E.V.; Aliat, A.; Chikhaoui, A. Vibration-electronic and chemical kinetics of non-equilibrium radiative diatomic gas flows. Chem. Phys. Lett. 2001, 344, 638–646. [Google Scholar] [CrossRef]
- Serdyuchenko, J.A.; Mintusov, E.; Frederickson, K.; Lempert, W.R.; Rich, J.W.; Adamovich, I.V. Isotope effect in Boudouard disproportionation reaction in optically pumped CO. Chem. Phys. 2009, 63, 24–32. [Google Scholar] [CrossRef]
- Grigorian, G.; Cenian, A. Formation and Excitation of CN Molecules in He–CO–N2–O2 Discharge Plasmas. Plasma Chem. Plasma Process. 2011, 31, 337–352. [Google Scholar] [CrossRef]
- Brandis, A.M.; Laux, C.O.; Magin, T.; McIntyre, T.J.; Morgan, R.G. Comparison of titan entry radiation shock-tube data with collisional-radiative models. J. Thermophys. Heat Transf. 2014, 28, 32–38. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, C.; Chang, K.-S. Shock-Tube Determination of CN formation rate in a CO-N2 mixture. J. Thermophys. Heat Transfer. 2007, 21, 50–56. [Google Scholar] [CrossRef]
- Global MRV. Hydrocarbon Emission Control: Discover the Power of PEMS/iPEMS; Global MRV: Cheektowaga, NY, USA, 2024; Available online: https://www.globalmrv.com/hydrocarbon-emission-control-discover-the-power-of-pems-ipems/ (accessed on 2 November 2025).



| Material | Composition (%at) | Tg (±2 °C) | Tx (±2 °C) | TM (±2 °C) | n@589 nm (±0.003) | ρ (10−8 Ω.m) | Density |
|---|---|---|---|---|---|---|---|
| Al-alloy AA 2024 | 96.0% Al; 2.0% Cu; 1.6% Mg; Traces (Si, Fe…) | - | - | 662 | - | 3.95 | 2.71 |
| Glass | 72.7% SiO2; 12.0% Na2O; 6.9% CaO; 8.0% MgO; 0.4% Al2O3 | 562 | 826 | - | 1.517 | - | 2.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnardel, M.; Poulon-Quintin, A.; Danto, S.; Bousquet, B.; Teulé-Gay, L.; Cardinal, T. Dual-Electrode Glass Ribbons Intended for Use in Microplasma-Based Sensors. Sensors 2025, 25, 6814. https://doi.org/10.3390/s25226814
Bonnardel M, Poulon-Quintin A, Danto S, Bousquet B, Teulé-Gay L, Cardinal T. Dual-Electrode Glass Ribbons Intended for Use in Microplasma-Based Sensors. Sensors. 2025; 25(22):6814. https://doi.org/10.3390/s25226814
Chicago/Turabian StyleBonnardel, Mathieu, Angeline Poulon-Quintin, Sylvain Danto, Bruno Bousquet, Lionel Teulé-Gay, and Thierry Cardinal. 2025. "Dual-Electrode Glass Ribbons Intended for Use in Microplasma-Based Sensors" Sensors 25, no. 22: 6814. https://doi.org/10.3390/s25226814
APA StyleBonnardel, M., Poulon-Quintin, A., Danto, S., Bousquet, B., Teulé-Gay, L., & Cardinal, T. (2025). Dual-Electrode Glass Ribbons Intended for Use in Microplasma-Based Sensors. Sensors, 25(22), 6814. https://doi.org/10.3390/s25226814







