Smart Healthcare at Home: A Review of AI-Enabled Wearables and Diagnostics Through the Lens of the Pi-CON Methodology
Abstract
1. Introduction
1.1. The Shift Toward Smart Healthcare at Home
1.2. Growth of AI-Enabled Wearables and Diagnostics
1.3. Usability and Adherence: A Growing Concern
1.4. The Role of AI in Passive, Continuous, Non-Contact Monitoring
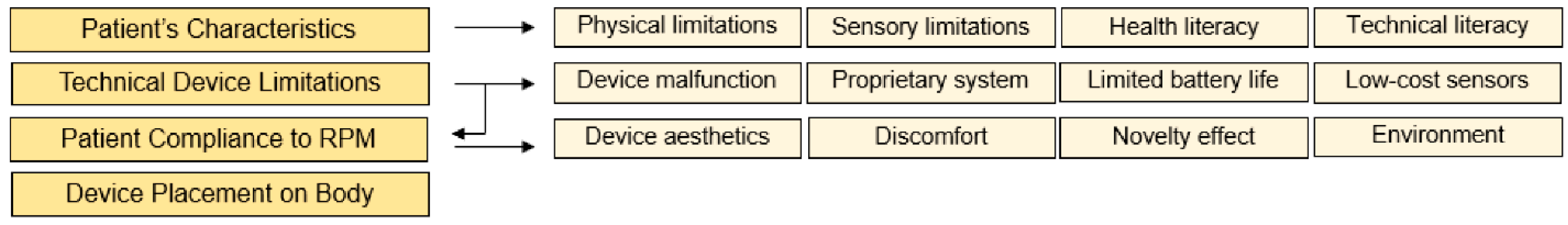
1.5. Introducing the Pi-CON Methodology
2. Review Methodology
3. Overview of Home-Based Health Technologies
3.1. Vital Sign Monitoring Wearables
3.2. Digital Diagnostics and At-Home Testing Tools
3.3. Body Composition Assessment Technologies
4. Previous Work
4.1. Usability, Engagement, and Barriers to Adoption
- (1)
- user interface design and onboarding;
- (2)
- the novelty effect and sustained engagement;
- (3)
- usability across populations;
- (4)
- AI applications in home-based monitoring;
- (5)
- trust, privacy, and data security.
4.2. User Interface Design and Onboarding
4.3. Overcoming the Novelty Effect in AI-IoT Health Systems
4.4. Ensuring Inclusive Usability in AI Driven IoT Devices
4.5. AI Applications in IoT-Based Home Health Monitoring
4.6. Trust, Privacy, and Data Security in AI-IoT Ecosystems
4.7. Toward Passive, Ubiquitous and User-Centered AI-IoT Health Monitoring
5. Discussion
5.1. Key Findings
5.2. Thematic Insights and Integration of the Pi-CON Framework
5.3. Practical Implications
5.4. Future Research Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Census Bureau. The Graying of America: More Older Adults Than Kids by 2035. Available online: https://www.census.gov/library/stories/2018/03/graying-america.html (accessed on 28 June 2025).
- Chen, C.; Ding, S.; Wang, J. Digital health for aging populations. Nat. Med. 2023, 29, 1623–1630. [Google Scholar] [CrossRef]
- Patel, S.Y.; Mehrotra, A.; Huskamp, H.A.; Uscher-Pines, L.; Ganguli, I.; Barnett, M.L. Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US. JAMA Intern. Med. 2021, 181, 388–391. [Google Scholar] [CrossRef]
- Verma, S. Early impact of CMS expansion of Medicare telehealth during COVID-19. Health Aff. Forefr. 2020. [Google Scholar]
- Baumann, S.; Stone, R.; Kim, J.Y.-M. Introducing the Pi-CON Methodology to Overcome Usability Deficits during Remote Patient Monitoring. Sensors 2024, 24, 2260. [Google Scholar] [CrossRef]
- Research and Markets. $38.9 Billion Wearable Medical Devices Industry—Global Market Trajectory & Analytics to 2026. Available online: https://www.businesswire.com/news/home/20220311005277/en/%2438.9-Billion-Wearable-Medical-Devices-Industry---Global-Market-Trajectory-Analytics-to-2026---ResearchAndMarkets.com (accessed on 6 July 2025).
- Nagappan, A.; Krasniansky, A.; Knowles, M. Patterns of ownership and usage of wearable devices in the United States, 2020–2022: Survey study. J. Med. Internet Res. 2024, 26, e56504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Ye, Z.; Yang, S.; Liu, M.; Wu, Q.; Zhou, C.; He, P.; Gan, X.; Qin, X. Mobile phone use and risks of overall and 25 site-specific cancers: A prospective study from the UK Biobank Study. Cancer Epidemiol. Biomark. Prev. 2024, 33, 88–95. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Chandra, S.; Yakkala, K.; Kennedy, S.; Agrawal, A.; Sippel, M.; Ramu, P.; Chaudhri, A.; Smith, B.; Criminisi, A.; et al. Smartphone camera based assessment of adiposity: A validation study. npj Digit. Med. 2022, 5, 79. [Google Scholar] [CrossRef]
- Al-Khlaiwi, T.; Habib, S.S.; Alshalan, M.; Al-Qhatani, M.; Alsowiegh, S.; Queid, S.; Alyabis, O.; Al-Khliwi, H. Comparison of mobile phone usage and physical activity on glycemic status, body composition & lifestyle in male Saudi mobile phone users. Heliyon 2022, 8, e10646. [Google Scholar] [CrossRef] [PubMed]
- El-Gayar, O.; Elnoshokaty, A. Factors and design features influencing the continued use of wearable devices. J. Healthc. Inform. Res. 2023, 7, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Guu, T.-W.; Muurling, M.; Khan, Z.; Kalafatis, C.; Aarsland, D.; Brem, A.-K. Wearable devices: Underrepresentation in the ageing society. Lancet Digit. Health 2023, 5, e336–e337. [Google Scholar] [CrossRef]
- Ding, H.; Ho, K.; Searls, E.; Low, S.; Li, Z.; Rahman, S.; Madan, S.; Igwe, A.; Popp, Z.; Burk, A.; et al. Assessment of Wearable Device Adherence for Monitoring Physical Activity in Older Adults: Pilot Cohort Study. JMIR Aging 2024, 7, e60209. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial intelligence enhanced sensors-enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- Veldhuis, L.I.; Woittiez, N.J.C.; Nanayakkara, P.W.B.; Ludikhuize, J. Artificial intelligence for the prediction of in-hospital clinical deterioration: A systematic review. Crit. Care Explor. 2022, 4, e0744. [Google Scholar] [CrossRef]
- Baumann, S.; Stone, R.; Abdelall, E. Introducing a Remote Patient Monitoring Usability Impact Model to Overcome Challenges. Sensors 2024, 24, 3977. [Google Scholar] [CrossRef]
- Baumann, S.; Stone, R.T.; Genschel, U.; Mgaedeh, F. The pi-con methodology applied: Operator errors and preference tracking of a novel ubiquitous vital signs sensor and its user interface. Int. J. Hum. Comput. Interact. 2024, 40, 3782–3804. [Google Scholar] [CrossRef]
- Selvaraju, V.; Spicher, N.; Wang, J.; Ganapathy, N.; Warnecke, J.M.; Leonhardt, S.; Swaminathan, R.; Deserno, T.M. Continuous monitoring of vital signs using cameras: A systematic review. Sensors 2022, 22, 4097. [Google Scholar] [CrossRef]
- Almarshad, M.A.; Islam, M.S.; Al-Ahmadi, S.; BaHammam, A.S. Diagnostic features and potential applications of PPG signal in healthcare: A systematic review. Healthcare 2022, 10, 547. [Google Scholar] [CrossRef]
- Doherty, C.; Baldwin, M.; Keogh, A.; Caulfield, B.; Argent, R. Keeping Pace with Wearables: A Living Umbrella Review of Systematic Reviews Evaluating the Accuracy of Consumer Wearable Technologies in Health Measurement. Sports Med. 2024, 54, 2907–2926. [Google Scholar] [CrossRef]
- Raju, K.V.; Sree, M.A.R.; Brahmaiah, B.; Nori, L.P. Wearable Medical Devices: Standards, Approval Pro-Cess And Regulatory Issues. Pharma Times 2024, 56, 24. [Google Scholar]
- Shin, D. Embodying algorithms, enactive artificial intelligence and the extended cognition: You can see as much as you know about algorithm. J. Inf. Sci. 2023, 49, 18–31. [Google Scholar] [CrossRef]
- Gill, S.; Bunting, K.V.; Sartini, C.; Cardoso, V.R.; Ghoreishi, N.; Uh, H.-W.; Williams, A.J.; Suzart-Woischnik, K.; Banerjee, A.; Asselbergs, F.W.; et al. Smartphone detection of atrial fibrillation using photoplethysmography: A systematic review and meta-analysis. Heart 2022, 108, 1600–1607. [Google Scholar] [CrossRef]
- Liutkus, J.; Kriukas, A.; Stragyte, D.; Mazeika, E.; Raudonis, V.; Galetzka, W.; Stang, A.; Valiukeviciene, S. Accuracy of a smartphone-based artificial intelligence application for classification of melanomas, melanocytic nevi, and seborrheic keratoses. Diagnostics 2023, 13, 2139. [Google Scholar] [CrossRef]
- Nguyen, N.; Lane, B.; Lee, S.; Gorman, S.L.; Wu, Y.; Li, A.; Lu, H.; Elhadad, N.; Yin, M.; Meyers, K. A mixed methods study evaluating acceptability of a daily COVID-19 testing regimen with a mobile-app connected, at-home, rapid antigen test: Implications for current and future pandemics. PLoS ONE 2022, 17, e0267766. [Google Scholar] [CrossRef]
- Takkalapally, P.; Kasula, B.Y.; Patil, S.C.; Logeshwaran, J. Examining the Benefits of AI in Wearable Sensor-based Healthcare Solutions. In Proceedings of the 2024 15th International Conference on Computing Communication and Networking Technologies (ICCCNT), Kamand, India, 24–28 June 2024; pp. 1–6. [Google Scholar]
- Tewari, N.; Awad, S.; Macdonald, I.A.; Lobo, D.N. A comparison of three methods to assess body composition. Nutrition 2018, 47, 1–5. [Google Scholar] [CrossRef]
- Siedler, M.R.; Rodriguez, C.; Stratton, M.T.; Harty, P.S.; Keith, D.S.; Green, J.J.; Boykin, J.R.; White, S.J.; Williams, A.D.; DeHaven, B.; et al. Assessing the reliability and cross-sectional and longitudinal validity of fifteen bioelectrical impedance analysis devices. Br. J. Nutr. 2023, 130, 827–840. [Google Scholar] [CrossRef]
- Nana, A.; Staynor, J.M.D.; Arlai, S.; El-Sallam, A.; Dhungel, N.; Smith, M.K. Agreement of anthropometric and body composition measures predicted from 2D smartphone images and body impedance scales with criterion methods. Obes. Res. Clin. Pract. 2022, 16, 37–43. [Google Scholar] [CrossRef]
- Kamiar Kordari, K.S. Validating Accuracy: A Deep Dive into Spren VisionTM for Body Composition Analysis. Available online: https://www.spren.com/blog/validating-accuracy-a-deep-dive-into-spren-vision-tm-for-body-composition-analysis (accessed on 28 June 2025).
- Starkoff, B.E.; Nickerson, B.S. Emergence of imaging technology beyond the clinical setting: Utilization of mobile health tools for at-home testing. Nutr. Clin. Pract. 2024, 39, 518–529. [Google Scholar] [CrossRef]
- Lee, T.; Puyol-Antón, E.; Ruijsink, B.; Roujol, S.; Barfoot, T.; Ogbomo-Harmitt, S.; Shi, M.; King, A. An investigation into the causes of race bias in artificial intelligence–based cine cardiac magnetic resonance segmentation. Eur. Heart J.-Digit. Health 2025, 6, 350–358. [Google Scholar] [CrossRef]
- Hassan, M.; Kushniruk, A.; Borycki, E. Barriers to and facilitators of artificial intelligence adoption in health care: Scoping review. JMIR Hum. Factors 2024, 11, e48633. [Google Scholar] [CrossRef]
- Bevan, N.; Carter, J.; Earthy, J.; Geis, T.; Harker, S. New ISO standards for usability, usability reports and usability measures. In Human-Computer Interaction. Proceedings of the Theory, Design, Development and Practice: 18th International Conference, HCI International 2016, Toronto, ON, Canada, 17–22 July 2016; Proceedings, Part I 18; Springer: Berlin/Heidelberg, Germany, 2016; pp. 268–278. [Google Scholar]
- Kamnerddee, C.; Putjorn, P.; Intarasirisawat, J. AI-Driven Design Thinking: A Comparative Study of Human-Created and AI-Generated UI Prototypes for Mobile Applications. In Proceedings of the 2024 8th International Conference on Information Technology (InCIT), Chonburi, Thailand, 14–15 November 2024; pp. 237–242. [Google Scholar]
- Li, J.; Washington, P. A comparison of personalized and generalized approaches to emotion recognition using consumer wearable devices: Machine learning study. JMIR AI 2024, 3, e52171. [Google Scholar] [CrossRef]
- Wang, Y. Fatigue-Aware Adaptive Interfaces for Wearable Devices Using Deep Learning. arXiv 2025, arXiv:2506.13203. [Google Scholar] [CrossRef]
- Wang, C.; Cassidy, L.; He, W.; Pierson, T.J.; Kotz, D. Challenges and opportunities in onboarding smart-home devices. In Proceedings of the 25th International Workshop on Mobile Computing Systems and Applications, San Diego, CA, USA, 28–29 February 2024; pp. 60–65. [Google Scholar]
- Massa, C.; De La Vega, M.; Zelaya, M.I. Bridging the Digital Health Literacy Gap in Older Adult Populations Through a Service Design Approach. In Service Design, Creativity, and Innovation in Healthcare: Challenges, Insights, Solutions; Springer: Berlin/Heidelberg, Germany, 2024; pp. 191–208. [Google Scholar]
- Hou, J. Interactive Technologies of Wearable Devices for Elderly: A Literature Review. Open Access Libr. J. 2023, 10, 1–17. [Google Scholar] [CrossRef]
- Denecke, K.; Kaufmann, B.; Reichenpfader, D.; Petersen, C. How to Consider Health Literacy in Digital Health Interventions? In International Conference on Human-Computer Interaction; Springer: Berlin/Heidelberg, Germany, 2024; pp. 259–267. [Google Scholar]
- Jan, M.; Coppin-Renz, A.; West, R.; Le Gallo, C.; Cochran, J.M.; van Heumen, E.; Fahmy, M.; Reuteman-Fowler, J.C. Safety Evaluation in Iterative Development of Wearable Patches for Aripiprazole Tablets With Sensor: Pooled Analysis of Clinical Trials. JMIR Form. Res. 2023, 7, e44768. [Google Scholar] [CrossRef]
- Khatsenko, K.; Khin, Y.; Maibach, H. Allergic Contact Dermatitis to Components of Wearable Adhesive Health Devices. Dermatitis 2020, 31, 283–286. [Google Scholar] [CrossRef]
- Chen, C.; Lifset, E.T.; Han, Y.; Roy, A.; Hogarth, M.; Moore, A.A.; Farcas, E.; Weibel, N. How do Older Adults Set Up Voice Assistants? Lessons Learned from a Deployment Experience for Older Adults to Set Up Standalone Voice Assistants. In Proceedings of the Companion Publication of the 2023 ACM Designing Interactive Systems Conference, Pittsburgh, PA, USA, 10–14 July 2023; Association for Computing Machinery: New York, NY, USA, 2023; pp. 164–168. [Google Scholar]
- Shandilya, E.; Fan, M. Understanding older adults’ perceptions and challenges in using AI-enabled everyday technologies. In Proceedings of the Tenth International Symposium of Chinese CHI, Guangzhou, China, 22–23 October 2022; pp. 105–116. [Google Scholar]
- Evans, H.; Agoro, H. Integrating Generative AI with IoT Devices for Enhanced User Experience. 2023. Available online: https://www.researchgate.net/publication/390232065_Integrating_Generative_AI_with_IoT_Devices_for_Enhanced_User_Experience (accessed on 3 July 2025).
- Costa, C.J.; Aparicio, J.T.; Aparicio, M.; Aparicio, S. Gamification and AI: Enhancing User Engagement through Intelligent Systems. arXiv 2024, arXiv:2411.10462. [Google Scholar]
- Lu, T.; Lin, Q.; Yu, B.; Hu, J. A systematic review of strategies in digital technologies for motivating adherence to chronic illness self-care. npj Health Syst. 2025, 2, 13. [Google Scholar] [CrossRef]
- Sandhaus, H.; Choksi, M.Z.; Ju, W. Regaining Trust: Impact of Transparent User Interface Design on Acceptance of Camera-Based In-Car Health Monitoring Systems. In Adjunct Proceedings of the 16th International Conference on Automotive User Interfaces and Interactive Vehicular Applications, Stanford, CA, USA, 22–25 September 2024; Association for Computing Machinery: New York, NY, USA, 2024; pp. 203–208. [Google Scholar]
- Rodrigues, L.; Pereira, F.D.; Toda, A.M.; Palomino, P.T.; Pessoa, M.; Carvalho, L.S.G.; Fernandes, D.; Oliveira, E.H.T.; Cristea, A.I.; Isotani, S. Gamification suffers from the novelty effect but benefits from the familiarization effect: Findings from a longitudinal study. Int. J. Educ. Technol. High. Educ. 2022, 19, 13. [Google Scholar] [CrossRef]
- Shin, G.; Feng, Y.; Jarrahi, M.H.; Gafinowitz, N. Beyond novelty effect: A mixed-methods exploration into the motivation for long-term activity tracker use. JAMIA Open 2019, 2, 62–72. [Google Scholar] [CrossRef]
- Windasari, N.A.; Lin, F. Why do people continue using fitness wearables? The effect of interactivity and gamification. Sage Open 2021, 11, 21582440211056610. [Google Scholar] [CrossRef]
- Hydari, M.Z.; Adjerid, I.; Striegel, A.D. Health wearables, gamification, and healthful activity. Manag. Sci. 2023, 69, 3920–3938. [Google Scholar] [CrossRef]
- Dhiman, N. Enhancing User Engagement in Wearable Apps through Gamification. Available online: https://insights.daffodilsw.com/blog/enhancing-user-engagement-in-wearable-apps-through-gamification (accessed on 28 June 2025).
- Greysen, S.R.; Changolkar, S.; Small, D.S.; Reale, C.; Rareshide, C.A.; Mercede, A.; Snider, C.K.; Greysen, H.M.; Trotta, R.; Halpern, S.D. Effect of behaviorally designed gamification with a social support partner to increase mobility after hospital discharge: A randomized clinical trial. JAMA Netw. Open 2021, 4, e210952. [Google Scholar] [CrossRef]
- AlSaad, R.; Abd-Alrazaq, A.; Boughorbel, S.; Ahmed, A.; Renault, M.-A.; Damseh, R.; Sheikh, J. Multimodal large language models in health care: Applications, challenges, and future outlook. J. Med. Internet Res. 2024, 26, e59505. [Google Scholar] [CrossRef]
- Kargarandehkordi, A.; Slade, C.; Washington, P. Personalized AI-driven real-time models to predict stress-induced blood pressure spikes using wearable devices: Proposal for a prospective cohort study. JMIR Res. Protoc. 2024, 13, e55615. [Google Scholar] [CrossRef]
- Moore, K.; O’SHea, E.; Kenny, L.; Barton, J.; Tedesco, S.; Sica, M.; Crowe, C.; Alamäki, A.; Condell, J.; Nordström, A.; et al. Older Adults’ Experiences With Using Wearable Devices: Qualitative Systematic Review and Meta-synthesis. JMIR Mhealth Uhealth 2021, 9, e23832. [Google Scholar] [CrossRef]
- Muñoz Esquivel, K.; Gillespie, J.; Kelly, D.; Condell, J.; Davies, R.; McHugh, C.; Duffy, W.; Nevala, E.; Alamäki, A.; Jalovaara, J.; et al. Factors Influencing Continued Wearable Device Use in Older Adult Populations: Quantitative Study. JMIR Aging 2023, 6, e36807. [Google Scholar] [CrossRef]
- Oh, S.S.; Kim, K.-A.; Kim, M.; Oh, J.; Chu, S.H.; Choi, J. Measurement of Digital Literacy Among Older Adults: Systematic Review. J. Med. Internet Res. 2021, 23, e26145. [Google Scholar] [CrossRef]
- Fakhimi, M.M.; Hughes, A.; Gustavson, A.M. Evaluating Smart Home Usability and Accessibility in Early Detection and Intervention of Mental Health Challenges Among Older Adults: A Narrative Review and Framework. J. Ageing Longev. 2025, 5, 3. [Google Scholar] [CrossRef]
- Kim, J.C.; Saguna, S.; Åhlund, C. Acceptability of a health care app with 3 user interfaces for older adults and their caregivers: Design and evaluation study. JMIR Hum. Factors 2023, 10, e42145. [Google Scholar] [CrossRef]
- Vigouroux, N.; Vella, F.; Lepage, G.; Campo, E. Usability study of tactile and voice interaction modes by people with disabilities for home automation controls. In Proceedings of the International Conference on Computers Helping People with Special Needs, Lecco, Italy, 11–15 July 2022; Springer: Berlin/Heidelberg, Germany, 2022; pp. 139–147. [Google Scholar]
- Pedroso, A.F.; Khera, R. Leveraging AI-enhanced digital health with consumer devices for scalable cardiovascular screening, prediction, and monitoring. npj Cardiovasc. Health 2025, 2, 34. [Google Scholar] [CrossRef]
- Švihrová, R.; Rossi, A.D.; Marzorati, D.; Tzovara, A.; Faraci, F.D. Designing digital health interventions with causal inference and multi-armed bandits: A review. Front. Digit. Health 2025, 7, 1435917. [Google Scholar] [CrossRef]
- Torres-Soto, J.; Ashley, E.A. Multi-task deep learning for cardiac rhythm detection in wearable devices. npj Digit. Med. 2020, 3, 116. [Google Scholar] [CrossRef]
- Shen, Y.; Voisin, M.; Aliamiri, A.; Avati, A.; Hannun, A.; Ng, A. Ambulatory atrial fibrillation monitoring using wearable photoplethysmography with deep learning. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019; pp. 1909–1916. [Google Scholar]
- Vo, K.; El-Khamy, M.; Choi, Y. PPG-to-ECG Signal Translation for Continuous Atrial Fibrillation Detection via Attention-based Deep State-Space Modeling. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FA, USA, 15–19 July 2024; pp. 1–7. [Google Scholar]
- Graybeal, A.J.; Brandner, C.F.; Tinsley, G.M. Visual body composition assessment methods: A 4-compartment model comparison of smartphone-based artificial intelligence for body composition estimation in healthy adults. Clin. Nutr. 2022, 41, 2464–2472. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Rodriguez, C.; Siedler, M.R.; Tinoco, E.; White, S.J.; LaValle, C.; Brojanac, A.; DeHaven, B.; Rasco, J.; Florez, C.M.; et al. Mobile phone applications for 3-dimensional scanning and digital anthropometry: A precision comparison with traditional scanners. Eur. J. Clin. Nutr. 2024, 78, 509–514. [Google Scholar] [CrossRef]
- Davis Iii, K.M.; Ruotsalo, T. Physiological Data: Challenges for Privacy and Ethics. Computer 2025, 58, 33–44. [Google Scholar] [CrossRef]
- Abdelall, E.; Almasarwah, N.; Stone, R.; Mgaedeh, F.; Yared, T.; Baumann, S. An Investigation of Activity Trackers’ Potential Harmful Impacts on Mental Health and Factors Controlling User Intention for Adoptability. In Proceedings of the International Conference on Computers and Industrial Engineering, Sharjah-Dubai, United Arab Emirates, 30 October–2 November 2023; Elsevier: Amsterdam, The Netherlands, 2023. Available online: https://www.researchgate.net/publication/380515396_AN_INVESTIGATION_OF_ACTIVITY_TRACKERS’_POTENTIAL_HARMFUL_IMPACTS_ON_MENTAL_HEALTH_AND_FACTORS_CONTROLLING_USER_INTENTION_FOR_ADOPTABILITY (accessed on 12 July 2025).
- Yang, Q.; Liu, Y.; Chen, T.; Tong, Y. Federated machine learning: Concept and applications. ACM Trans. Intell. Syst. Technol. (TIST) 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Abbas, S.R.; Abbas, Z.; Zahir, A.; Lee, S.W. Federated Learning in Smart Healthcare: A Comprehensive Review on Privacy, Security, and Predictive Analytics with IoT Integration. Healthcare 2024, 12, 2587. [Google Scholar] [CrossRef]
- Calderon, N. HIPAA vs. GDPR Compliance: What’s the Difference. Medstack. Available online: https://medstack.co/blog/hipaa-vs-gdpr/ (accessed on 5 July 2025).
- Aminifar, A.; Shokri, M.; Aminifar, A. Privacy-preserving edge federated learning for intelligent mobile-health systems. Future Gener. Comput. Syst. 2024, 161, 625–637. [Google Scholar] [CrossRef]
- Khan, R.; Taj, S.; Ma, X.; Noor, A.; Zhu, H.; Khan, J.; Khan, Z.U.; Khan, S.U. Advanced federated ensemble internet of learning approach for cloud based medical healthcare monitoring system. Sci. Rep. 2024, 14, 26068. [Google Scholar] [CrossRef]
- Baumann, S.; Stone, R.; Abdelall, E.; Srikrishnan, V.; Schnieders, T.; Fales, C.; Mumani, A. Implementing Blockchain to Enhance Usability of Patient-Generated Data. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Seattle, WA, USA, 28 October–1 November 2019; Volume 63, pp. 1344–1348. [Google Scholar] [CrossRef]
- Rosenbacke, R.; Melhus, Å.; McKee, M.; Stuckler, D. How Explainable Artificial Intelligence Can Increase or Decrease Clinicians’ Trust in AI Applications in Health Care: Systematic Review. JMIR AI 2024, 3, e53207. [Google Scholar] [CrossRef]
- Chen, Y.; Esmaeilzadeh, P. Generative AI in medical practice: In-depth exploration of privacy and security challenges. J. Med. Internet Res. 2024, 26, e53008. [Google Scholar] [CrossRef]
- Lee, A.R.; Koo, D.; Kim, I.K.; Lee, E.; Yoo, S.; Lee, H.-Y. Opportunities and challenges of a dynamic consent-based application: Personalized options for personal health data sharing and utilization. BMC Med. Ethics 2024, 25, 92. [Google Scholar] [CrossRef]
- Pourpanah, F.; Etemad, A. Exploring the landscape of ubiquitous in-home health monitoring: A comprehensive survey. ACM Trans. Comput. Healthc. 2024, 5, 1–43. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, Z. Ambient Sound in Healthcare Settings and Its Effects on Patients and Staff: A Systematic Review. Alam Cipta: Int. J. Sustain. Trop. Des. Res. Pract. 2022, 15, 44–54. [Google Scholar] [CrossRef]
- Abedi, H.; Ansariyan, A.; Morita, P.; Wong, A.; Boger, J.; Shaker, G. AI-Powered Non-Contact In-Home Gait Monitoring and Activity Recognition System Based on mm-Wave FMCW Radar and Cloud Computing. IEEE Internet Things J. 2022, 10, 9465–9481. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Zhou, M.; Ren, A.; Tian, Z. Remote monitoring of human vital signs based on 77-GHz mm-wave FMCW radar. Sensors 2020, 20, 2999. [Google Scholar] [CrossRef]
- Xu, H.; Ebrahim, M.P.; Hasan, K.; Heydari, F.; Howley, P.; Yuce, M.R. Accurate heart rate and respiration rate detection based on a higher-order harmonics peak selection method using radar non-contact sensors. Sensors 2021, 22, 83. [Google Scholar] [CrossRef]
- Allado, E.; Poussel, M.; Renno, J.; Moussu, A.; Hily, O.; Temperelli, M.; Albuisson, E.; Chenuel, B. Remote photoplethysmography is an accurate method to remotely measure respiratory rate: A hospital-based trial. J. Clin. Med. 2022, 11, 3647. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Sivakumar, S.; Lim, K.H. Review on remote heart rate measurements using photoplethysmography. Multimed. Tools Appl. 2024, 83, 44699–44728. [Google Scholar] [CrossRef]
- El-Hajj, C.; Kyriacou, P.A. Cuffless blood pressure estimation from PPG signals and its derivatives using deep learning models. Biomed. Signal Process. Control 2021, 70, 102984. [Google Scholar] [CrossRef]
- Saman, B.; Eid, M.M.; Eid, M.M. Recently employed engineering techniques to reduce the spread of COVID-19 (corona virus disease 2019): A review study. Indones. J. Electr. Eng. Comput. Sci. 2021, 22, 277–286. [Google Scholar] [CrossRef]
- Chen, G.; Xie, J.; Dai, G.; Zheng, P.; Hu, X.; Lu, H.; Xu, L.; Chen, X.; Chen, X. Validity of wrist and forehead temperature in temperature screening in the general population during the outbreak of 2019 novel coronavirus: A prospective real-world study. MedRxiv 2020. MedRxiv:2020-03. [Google Scholar]
- Shaik, T.; Tao, X.; Higgins, N.; Li, L.; Gururajan, R.; Zhou, X.; Acharya, U.R. Remote patient monitoring using artificial intelligence: Current state, applications, and challenges. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2023, 13, e1485. [Google Scholar] [CrossRef]
- Baumann, S.; Stone, R.T. Applying user-centered design and the Pi-CON methodology for vital signs sensor development. J. Med. Eng. Technol. 2023, 47, 277–287. [Google Scholar] [CrossRef]
- Hariri, M.A. Triggered Screen Restriction: A Novel Gamification Framework. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2024. [Google Scholar]
- Paul, M.M.; Khera, N.; Elugunti, P.R.; Ruff, K.C.; Hommos, M.S.; Thomas, L.F.; Nagaraja, V.; Garrett, A.L.; Pantoja-Smith, M.; Delafield, N.L.; et al. The State of Remote Patient Monitoring for Chronic Disease Management in the United States. J. Med. Internet Res. 2025, 27, e70422. [Google Scholar] [CrossRef]
- Alanazi, M.B.; Aldfery, T.R.; Aldfery, M.Z.; Aldhafiri, S.K.; Aldfery, S.Z.; Aldhafeeri, H.A.; Aldhafeeri, N.E.; Aldhafeeri, A.E.; Aldhafeeri, N.R.; Alanazi, E.A.; et al. Telehealth in Nursing Assessing the Effectiveness of Remote Patient Monitoring on Health Outcomes. J. Int. Crisis Risk Commun. Res. 2024, 7, 1219. [Google Scholar]
- Romanchuk, O.; Polianska, O.; Polianskyi, I.; Yasinska, O. Telerehabilitation. Current opportunities and problems of remote patient monitoring. Неoнатoлoгія, хірургія та перинатальна медицина 2024, 14, 183–190. [Google Scholar]
- Ianculescu, M.; Constantin, V.Ș.; Paraschiv, E.A.; Alexandru, A. Design and Development of Comprehensive Health Tracking Devices for Enhanced Health Monitoring. In Proceedings of the 2024 E-Health and Bioengineering Conference (EHB), IASI, Romania, 14–15 November 2024. [Google Scholar]
- Zhao, C.; Ding, H.; Xu, Z.; Chen, S. RFID-Based Respiration Monitoring Under Daily Body Motion Disturbances. Int. J. Hum.–Comput. Interact. 2025, 41, 7798–7814. [Google Scholar] [CrossRef]
- Hassanpour, A.; Yang, B. Contactless Vital Sign Monitoring: A Review Towards Multi-Modal Multi-Task Approaches. Sensors 2025, 25, 4792. [Google Scholar] [CrossRef]
- Susarla, P.; Mukherjee, A.; Cañellas, M.L.; Casado, C.Á.; Wu, X.; Silvén, O.; Jayagopi, D.B.; López, M.B. Non-contact multimodal indoor human monitoring systems: A survey. Inf. Fusion 2024, 110, 102457. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, S.; Stone, R.T.; Abdelall, E. Smart Healthcare at Home: A Review of AI-Enabled Wearables and Diagnostics Through the Lens of the Pi-CON Methodology. Sensors 2025, 25, 6067. https://doi.org/10.3390/s25196067
Baumann S, Stone RT, Abdelall E. Smart Healthcare at Home: A Review of AI-Enabled Wearables and Diagnostics Through the Lens of the Pi-CON Methodology. Sensors. 2025; 25(19):6067. https://doi.org/10.3390/s25196067
Chicago/Turabian StyleBaumann, Steffen, Richard T. Stone, and Esraa Abdelall. 2025. "Smart Healthcare at Home: A Review of AI-Enabled Wearables and Diagnostics Through the Lens of the Pi-CON Methodology" Sensors 25, no. 19: 6067. https://doi.org/10.3390/s25196067
APA StyleBaumann, S., Stone, R. T., & Abdelall, E. (2025). Smart Healthcare at Home: A Review of AI-Enabled Wearables and Diagnostics Through the Lens of the Pi-CON Methodology. Sensors, 25(19), 6067. https://doi.org/10.3390/s25196067







