Cross-Scanner Harmonization of AI/DL Accelerated Quantitative Bi-Parametric Prostate MRI †
Abstract
1. Introduction
2. Materials and Methods
2.1. AI/DL-Accelerated Quantitative T2 and ADC Mapping Protocols
2.2. Patient Studies
2.3. Phantom Measurements
2.4. Quantitative Bi-Parametric (bp) MRI Analysis
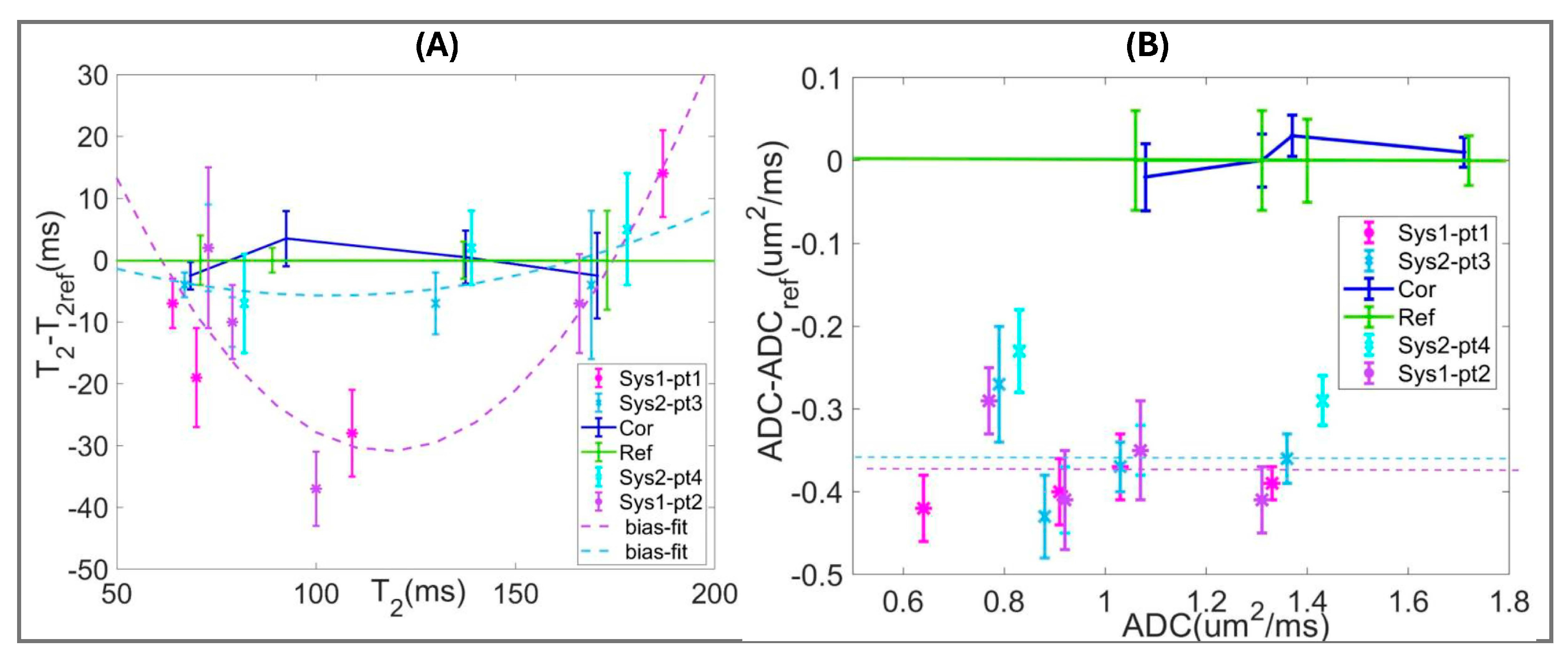
2.5. Phantom Metrics and Protocol Bias Measurement
2.6. Quantitative Lesion Metrics Harmonization
3. Results
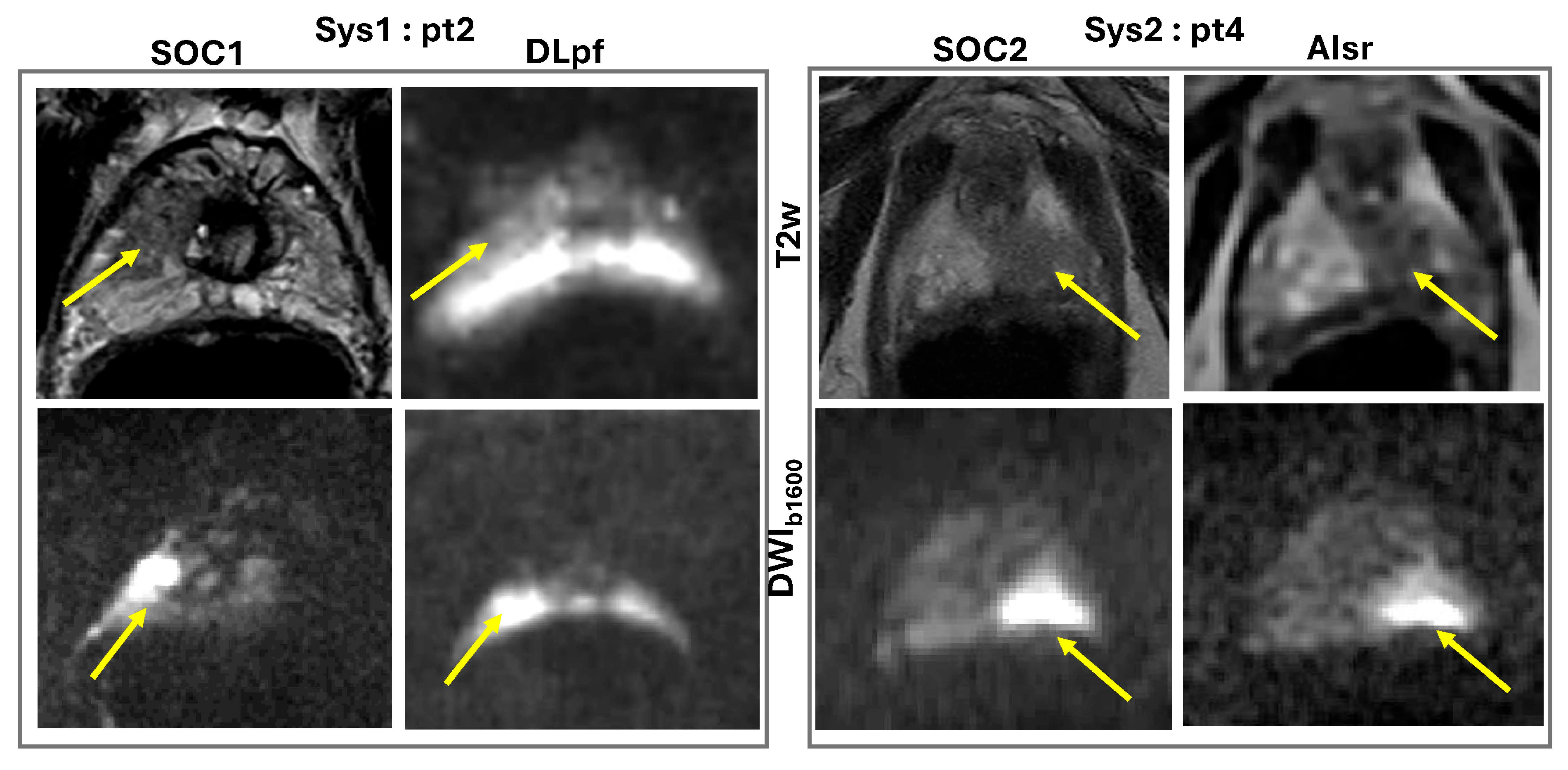
3.1. Qualitative Assessment
3.2. Quantitative Assessment
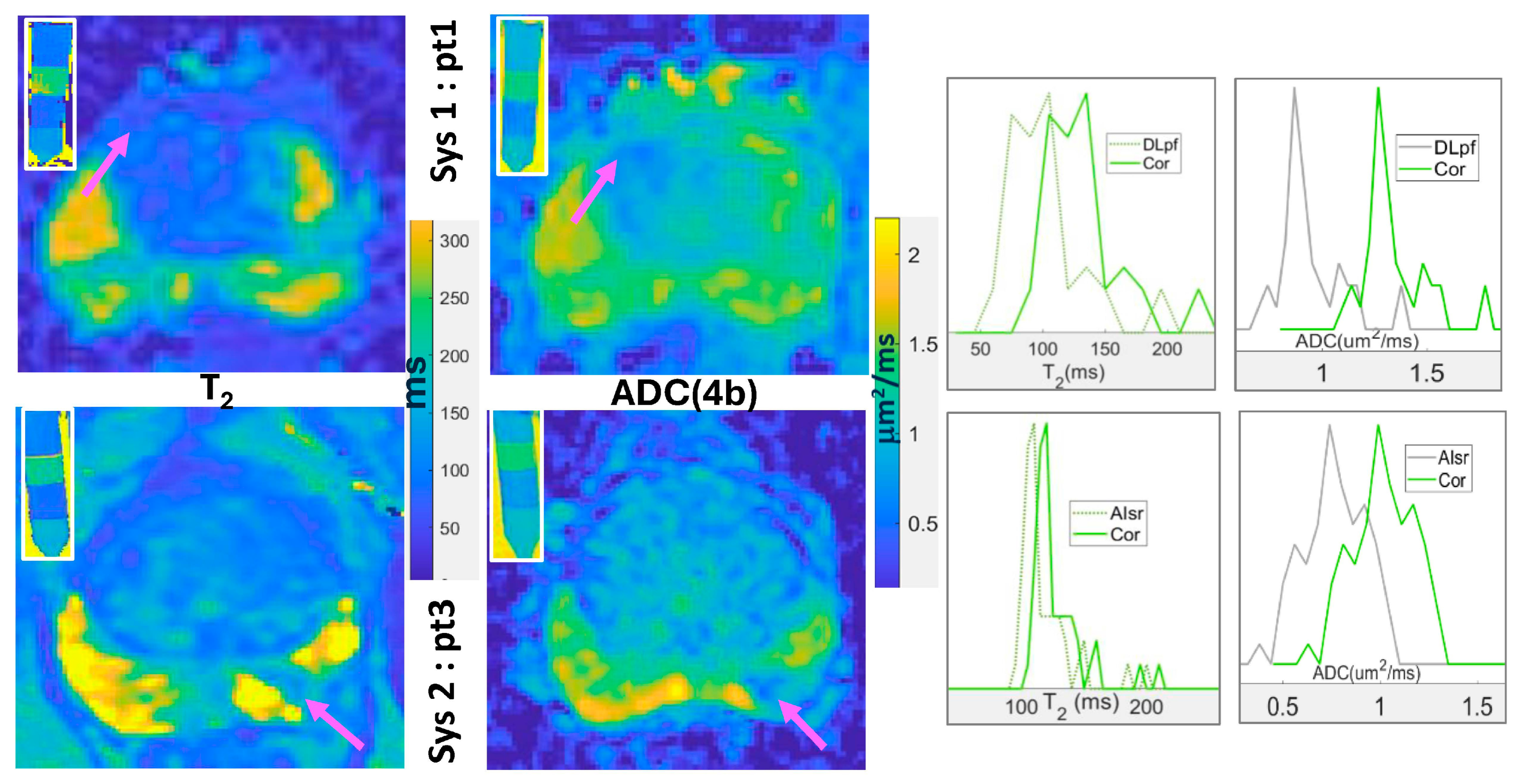
3.3. Quantitative bpMRI Harmonization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient |
| QIBA | Quantitative Imaging Biomarker Alliance |
| NIST | National Institute of Standards |
| DWI | Diffusion weighted imaging |
| T2w | T2 weighted |
| DLpf | Deep-learning partial-Fourier |
| AIsr | Artificial-intelligence super-resolution |
| q-bpMRI | Quantitative bi-parametric MRI |
| SOC | Standard-of-care |
| MESE | Multi-Echo Spin-Echo |
| MEEPI | Multi-Echo echo-planar imaging |
| TE | Echo-time |
| DK | Diffusion kurtosis |
| HW | Half-width |
| Sys | system |
| pt | patient |
| PVP | polyvinylpyrrolidone |
References
- Ferro, M.; Crocetto, F.; Bruzzese, D.; Imbriaco, M.; Fusco, F.; Longo, N.; Napolitano, L.; La Civita, E.; Cennamo, M.; Liotti, A.; et al. Prostate Health Index and Multiparametric MRI: Partners in Crime Fighting Overdiagnosis and Overtreatment in Prostate Cancer. Cancers 2021, 13, 4723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoots, I.G.; Barentsz, J.O.; Bittencourt, L.K.; Haider, M.A.; Macura, K.J.; Margolis, D.J.A.; Moore, C.M.; Oto, A.; Panebianco, V.; Siddiqui, M.M.; et al. PI-RADS Committee Position on MRI Without Contrast Medium in Biopsy-Naive Men With Suspected Prostate Cancer: Narrative Review. AJR Am. J. Roentgenol. 2021, 216, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.R.; Maturen, K.E.; George, A.K.; Borza, T.; Ellimoottil, C.; Montgomery, J.S.; Wei, J.T.; Denton, B.T.; Davenport, M.S. Temporary Health Impact of Prostate MRI and Transrectal Prostate Biopsy in Active Surveillance Prostate Cancer Patients. J. Am. Coll. Radiol. 2019, 16, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Purysko, A.S.; Rosenkrantz, A.B.; Barentsz, J.O.; Weinreb, J.C.; Macura, K.J. PI-RADS Version 2: A Pictorial Update. Radiographics 2016, 36, 1354–1372. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, E.J.; Shankar, P.R.; Westphalen, A.C. A Systematic Review of the Existing Prostate Imaging Reporting and Data System Version 2 (PI-RADSv2) Literature and Subset Meta-Analysis of PI-RADSv2 Categories Stratified by Gleason Scores. AJR Am. J. Roentgenol. 2019, 212, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.A.; Hielscher, T.; Badura, P.; Gortz, M.; Kuder, T.A.; Gnirs, R.; Schwab, C.; Hohenfellner, M.; Schlemmer, H.P.; Bonekamp, D. Contribution of Dynamic Contrast-enhanced and Diffusion MRI to PI-RADS for Detecting Clinically Significant Prostate Cancer. Radiology 2023, 306, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Hobbs, B.P.; Wei, W.; Kundra, V. Dynamic contrast-enhanced MRI for the detection of prostate cancer: Meta-analysis. AJR Am. J. Roentgenol. 2015, 204, W439–W448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, H.J.; Wienke, A.; Surov, A. Discrimination between clinical significant and insignificant prostate cancer with apparent diffusion coefficient—A systematic review and meta analysis. BMC Cancer 2020, 20, 482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moraes, M.O.; Roman, D.H.H.; Copetti, J.; de S Santos, F.; Agra, A.; Noronha, J.A.P.; Carvalhal, G.; Neto, E.J.D.; Zanon, M.; Baldisserotto, M.; et al. Effects of the addition of quantitative apparent diffusion coefficient data on the diagnostic performance of the PI-RADS v2 scoring system to detect clinically significant prostate cancer. World J. Urol. 2020, 38, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Ushinsky, A.; Yang, A.; Nguyentat, M.; Fardin, S.; Uchio, E.; Lall, C.; Lee, T.; Houshyar, R. Utility of quantitative apparent diffusion coefficient measurements and normalized apparent diffusion coefficient ratios in the diagnosis of clinically significant peripheral zone prostate cancer. Br. J. Radiol. 2018, 91, 20180091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoang Dinh, A.; Souchon, R.; Melodelima, C.; Bratan, F.; Mege-Lechevallier, F.; Colombel, M.; Rouviere, O. Characterization of prostate cancer using T2 mapping at 3T: A multi-scanner study. Diagn. Interv. Imaging 2015, 96, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.; Giganti, F.; Johnston, E.W.; Sidhu, H.S.; Panagiotaki, E.; Punwani, S.; Alexander, D.C.; Atkinson, D. Simplified Luminal Water Imaging for the Detection of Prostate Cancer From Multiecho T(2) MR Images. J. Magn. Reson. Imaging 2019, 50, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; O’Connor, G.; Lo, W.C.; Jiang, Y.; Margevicius, S.; Schluchter, M.; Ponsky, L.E.; Gulani, V. Targeted Biopsy Validation of Peripheral Zone Prostate Cancer Characterization With Magnetic Resonance Fingerprinting and Diffusion Mapping. Investig. Radiol. 2019, 54, 485–493. [Google Scholar] [CrossRef]
- Margolis, D.J.A.; Chatterjee, A.; deSouza, N.M.; Fedorov, A.; Fennessy, F.M.; Maier, S.E.; Obuchowski, N.; Punwani, S.; Purysko, A.; Rakow-Penner, R.; et al. Quantitative Prostate MRI, From the AJR Special Series on Quantitative Imaging. AJR Am. J. Roentgenol. 2024, 225, e2431715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, A.; Mercado, C.; Bourne, R.M.; Yousuf, A.; Hess, B.; Antic, T.; Eggener, S.; Oto, A.; Karczmar, G.S. Validation of Prostate Tissue Composition by Using Hybrid Multidimensional MRI: Correlation with Histologic Findings. Radiology 2022, 302, 368–377. [Google Scholar] [CrossRef]
- Hectors, S.J.; Semaan, S.; Song, C.; Lewis, S.; Haines, G.K.; Tewari, A.; Rastinehad, A.R.; Taouli, B. Advanced Diffusion-weighted Imaging Modeling for Prostate Cancer Characterization: Correlation with Quantitative Histopathologic Tumor Tissue Composition-A Hypothesis-generating Study. Radiology 2018, 286, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Panagiotaki, E.; Chan, R.W.; Dikaios, N.; Ahmed, H.U.; O’Callaghan, J.; Freeman, A.; Atkinson, D.; Punwani, S.; Hawkes, D.J.; Alexander, D.C. Microstructural characterization of normal and malignant human prostate tissue with vascular, extracellular, and restricted diffusion for cytometry in tumours magnetic resonance imaging. Investig. Radiol. 2015, 50, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, S.L.; McGarry, S.D.; Kaczmarowski, A.; Iczkowski, K.A.; Jacobsohn, K.; Hohenwalter, M.D.; Hall, W.A.; See, W.A.; Banerjee, A.; Charles, D.K.; et al. Optimized b-value selection for the discrimination of prostate cancer grades, including the cribriform pattern, using diffusion weighted imaging. J. Med. Imaging 2018, 5, 011004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simpkin, C.J.; Morgan, V.A.; Giles, S.L.; Riches, S.F.; Parker, C.; deSouza, N.M. Relationship between T2 relaxation and apparent diffusion coefficient in malignant and non-malignant prostate regions and the effect of peripheral zone fractional volume. Br. J. Radiol. 2013, 86, 20120469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gassenmaier, S.; Afat, S.; Nickel, D.; Mostapha, M.; Herrmann, J.; Othman, A.E. Deep learning-accelerated T2-weighted imaging of the prostate: Reduction of acquisition time and improvement of image quality. Eur. J. Radiol. 2021, 137, 109600. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ohno, Y.; Yamamoto, K.; Murayama, K.; Ikedo, M.; Yui, M.; Hanamatsu, S.; Tanaka, Y.; Obama, Y.; Ikeda, H.; et al. Deep Learning Reconstruction of Diffusion-weighted MRI Improves Image Quality for Prostatic Imaging. Radiology 2022, 303, 373–381. [Google Scholar] [CrossRef] [PubMed]
- van Lohuizen, Q.; Roest, C.; Simonis, F.F.J.; Fransen, S.J.; Kwee, T.C.; Yakar, D.; Huisman, H. Assessing deep learning reconstruction for faster prostate MRI: Visual vs. diagnostic performance metrics. Eur. Radiol. 2024, 34, 7364–7372. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Johnson, P.M.; Knoll, F.; Lui, Y.W. Artificial Intelligence for MR Image Reconstruction: An Overview for Clinicians. J. Magn. Reson. Imaging 2021, 53, 1015–1028. [Google Scholar] [CrossRef]
- Vergara, D.; Armato, S.G.; Hadjiiski, L., 3rd; Drukker, K. Best Practices for Artificial Intelligence and Machine Learning for Computer-Aided Diagnosis in Medical Imaging. J. Am. Coll. Radiol. 2024, 21, 341–343. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Huang, E.; deSouza, N.M.; Raunig, D.; Delfino, J.; Buckler, A.; Hatt, C.; Wang, X.; Moskowitz, C.; Guimaraes, A.; et al. A Framework for Evaluating the Technical Performance of Multiparameter Quantitative Imaging Biomarkers (mp-QIBs). Acad. Radiol. 2023, 30, 147–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- US FDA Center for Devices and Radiological Health. Technical Performance Assessment of Quantitative Imaging in Radiological Device Premarket Submissions; FDA: Silver Spring, MA, USA, 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-performance-assessment-quantitative-imaging-radiological-device-premarket-submissions (accessed on 3 August 2025).
- Boss, M.A.; Malyarenko, D.; Partridge, S.; Obuchowski, N.; Shukla-Dave, A.; Winfield, J.M.; Fuller, C.D.; Miller, K.; Mishra, V.; Ohliger, M.; et al. The QIBA Profile for Diffusion-Weighted MRI: Apparent Diffusion Coefficient as a Quantitative Imaging Biomarker. Radiology 2024, 313, e233055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malyarenko, D.; Ono, S.; Lynch, T.J.E.; Swanson, S.D. Technical note: Hydrogel-based mimics of prostate cancer with matched relaxation, diffusion and kurtosis for validating multi-parametric MRI. Med. Phys. 2024, 51, 3590–3596. [Google Scholar]
- Dieckmeyer, M.; Ruschke, S.; Eggers, H.; Kooijman, H.; Rummeny, E.J.; Kirschke, J.S.; Baum, T.; Karampinos, D.C. ADC Quantification of the Vertebral Bone Marrow Water Component: Removing the Confounding Effect of Residual Fat. Magn. Reson. Med. 2017, 78, 1432–1441. [Google Scholar]
- Malyarenko, D.; Swanson, S.D.; Richardson, J.; Lowe, S.; O’Connor, J.; Fajardo, J.E.; Jiang, Y.; Wells, S.; Chenevert, T.L. Harmonization of AI/DL accelerated quantitative bi-parametric prostate MRI: Demonstration in multi-parametric phantom and patients. In Proceedings of the 2025 ISMRM, Honolulu, HI, USA, 10–15 May 2025; p. 103. [Google Scholar]
- Lawrence, E.M.; Zhang, Y.; Starekova, J.; Wang, Z.; Pirasteh, A.; Wells, S.A.; Hernando, D. Reduced field-of-view and multi-shot DWI acquisition techniques: Prospective evaluation of image quality and distortion reduction in prostate cancer imaging. Magn. Reson. Imaging 2022, 93, 108–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bischoff, L.M.; Peeters, J.M.; Weinhold, L.; Krausewitz, P.; Ellinger, J.; Katemann, C.; Isaak, A.; Weber, O.M.; Kuetting, D.; Attenberger, U.; et al. Deep Learning Super-Resolution Reconstruction for Fast and Motion-Robust T2-weighted Prostate MRI. Radiology 2023, 308, e230427. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.V.; Daube-Witherspoon, M.E.; Karp, J.S. Validation of phantom-based harmonization for patient harmonization. Med. Phys. 2017, 44, 3534–3544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.; Thomas, J.V.; Nix, J.W.; Gordetsky, J.B.; Li, Y.; Rais-Bahrami, S. Portable Perfusion Phantom Offers Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging for Accurate Prostate Cancer Grade Stratification: A Pilot Study. Acad. Radiol. 2021, 28, 405–413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saito, Y.; Kamagata, K.; Andica, C.; Maikusa, N.; Uchida, W.; Takabayashi, K.; Yoshida, S.; Hagiwara, A.; Fujita, S.; Akashi, T.; et al. Traveling Subject-Informed Harmonization Increases Reliability of Brain Diffusion Tensor and Neurite Mapping. Aging. Dis. 2023, 15, 2770–2785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, P.M.; Tong, A.; Donthireddy, A.; Melamud, K.; Petrocelli, R.; Smereka, P.; Qian, K.; Keerthivasan, M.B.; Chandarana, H.; Knoll, F. Deep Learning Reconstruction Enables Highly Accelerated Biparametric MR Imaging of the Prostate. J. Magn. Reson. Imaging 2022, 56, 184–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saha, A.; Bosma, J.S.; Twilt, J.J.; van Ginneken, B.; Bjartell, A.; Padhani, A.R.; Bonekamp, D.; Villeirs, G.; Salomon, G.; Giannarini, G.; et al. PI-CAI consortium. Artificial intelligence and radiologists in prostate cancer detection on MRI (PI-CAI): An international, paired, non-inferiority, confirmatory study. Lancet Oncol. 2024, 25, 879–887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giganti, F.; Moreira da Silva, N.; Yeung, M.; Davies, L.; Frary, A.; Ferrer Rodriguez, M.; Sushentsev, N.; Ashley, N.; Andreou, A.; Bradley, A.; et al. AI-powered prostate cancer detection: A multi-centre, multi-scanner validation study. Eur. Radiol. 2025, 35, 4915–4924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sunoqrot, M.R.S.; Saha, A.; Hosseinzadeh, M.; Elschot, M.; Huisman, H. Artificial intelligence for prostate MRI: Open datasets, available applications, and grand challenges. Eur. Radiol. Exp. 2022, 6, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Telecan, T.; Chiorean, A.; Sipos-Lascu, R.; Caraiani, C.; Boca, B.; Hendea, R.M.; Buliga, T.; Andras, I.; Crisan, N.; Lupsor-Platon, M. ISUP Grade Prediction of Prostate Nodules on T2WI Acquisitions Using Clinical Features, Textural Parameters and Machine Learning-Based Algorithms. Cancers 2025, 17, 2035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castaldo, R.; Brancato, V.; Cavaliere, C.; Trama, F.; Illiano, E.; Costantini, E.; Ragozzino, A.; Salvatore, M.; Nicolai, E.; Franzese, M. A Framework of Analysis to Facilitate the Harmonization of Multicenter Radiomic Features in Prostate Cancer. J. Clin. Med. 2022, 12, 140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, F.; Chen, A.A.; Horng, H.; Bashyam, V.; Davatzikos, C.; Alexander-Bloch, A.; Li, M.; Shou, H.; Satterthwaite, T.D.; Yu, M.; et al. Image harmonization: A review of statistical and deep learning methods for removing batch effects and evaluation metrics for effective harmonization. Neuroimage 2023, 274, 120125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boss, M.A.; Snyder, B.S.; Kim, E.; Flamini, D.; Englander, S.; Sundaram, K.M.; Gumpeni, N.; Palmer, S.L.; Choi, H.; Froemming, A.T.; et al. Repeatability and Reproducibility Assessment of the Apparent Diffusion Coefficient in the Prostate: A Trial of the ECOG-ACRIN Research Group (ACRIN 6701). J. Magn. Reson. Imaging 2022, 56, 668–679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lemainque, T.; Yoneyama, M.; Morsch, C.; Iordanishvili, E.; Barabasch, A.; Schulze-Hagen, M.; Peeters, J.M.; Kuhl, C.; Zhang, S. Reduction of ADC bias in diffusion MRI with deep learning-based acceleration: A phantom validation study at 3.0 T. Magn. Reson. Imaging 2024, 110, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.E.; Peskin, A.P.; Wilmes, L.J.; Aliu, S.O.; Jones, E.F.; Li, W.; Kornak, J.; Newitt, D.C.; Hylton, N.M. Variability and bias assessment in breast ADC measurement across multiple systems. J. Magn. Reson. Imaging 2016, 44, 846–855. [Google Scholar] [CrossRef]
- Amouzandeh, G.; Chenevert, T.L.; Swanson, S.D.; Ross, B.D.; Malyarenko, D.I. Technical note: Temperature and concentration dependence of water diffusion in polyvinylpyrrolidone solutions. Med. Phys. 2022, 49, 3325–3332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holz, M.; Heil, S.R.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular 16 liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]


| Protocol | TE (ms) | TR (s) | b[nav] (s/mm2) | Acquired Voxel (mm3) | Scan Duration (min) |
|---|---|---|---|---|---|
| Sys1: | |||||
| SOC1-T2w | 107 | * 9.2/4.8 | NA | 0.5 × 0.6 × 3 | 6:00 |
| SOC1-DWI | 91 | 4.4/4.8 | 0[1], 100[1], 800[3], 1600[20] | 1.75 × 1.75 × 4 | 8:50 |
| DLpf-T2 (MEEPI) | 40, 70, 100, 130, 160 | 5.5/6.8 | NA | 2 × 2 × 3 (off-line map) | 2:10 |
| DLpf-ADC | 80 | 5.5 | 0[1], 100[1], 800[2], 1600[8] | 2 × 2 × 4/3 | 3:30 |
| Sys2: | |||||
| SOC2-T2w | 110 | 4.4 | NA | 0.4 × 0.7 × 3 | 5:00 |
| SOC2-DWI | 77 | 7.2 | 0[2], 100[2], 800[4], 1600[16] | 2.2 × 2.3 × 4 | 8:20 |
| AIsr-T2 (MESE) | 25, 65, 105, 145, 185 | 8/12.2 | NA | 2 × 2.3 × 3 | 1:45 |
| AIsr-ADC | 77 | 3.9/5.4 | 0[1], 100[1], 800[2], 1600[8] | 2 × 2 × 3/4 | 4:00 |
| Image | Eval. Criteria | Sys1-pt1 (PIRADS 4) | Sys1-pt2 (PIRADS 5) | Sys2-pt3 (PIRADS 4) | Sys2-pt4 (PIRADS 5) |
|---|---|---|---|---|---|
| ADC | Dx quality | 3/3 | 2/3 | 3/4 | 3/3 |
| distortion | 3/3 | 2/2 | 4/3 | 4/4 | |
| resolution | 3/2 | 2/2 | 4/4 | 4/4 | |
| SNR | 4/3 | 2/2 | 3/4 | 4/4 | |
| median | 3/3 | 2/2 | 3.5/4 | 4/4 | |
| DWIb1600 | Dx quality | 3/3 | 2/2 | 3/3 | 4/3 |
| distortion | 3/3 | 2/2 | 4/3 | 4/4 | |
| resolution | 2/2 | 2/2 | 4/3 | 3/4 | |
| SNR | 4/4 | 2/2 | 2/3 | 4/4 | |
| median | 3/3 | 2/2 | 3.5/3 | 4/4 | |
| T2w | Dx quality | 2/2 | 1/1 | 3/3 | 4/3 |
| distortion | 2/3 | 1/2 | 3/3 | 3/3 | |
| resolution | 2/2 | 2/2 | 2/2 | 3/2 | |
| SNR | 3/2 | 2/2 | 4/4 | 4/4 | |
| median | 2/2 | 1.5/2 | 3/3 | 3.5/3 |
| Protocol (Ts ± 0.5 °C) | Parameter Mean [HW] | GS7 | nTZ | nPZ | Atr |
|---|---|---|---|---|---|
| DKref (22.0) | ADC ± 0.015 (mm2/ms) | 1.06 [0.06] | 1.33 [0.06] | 1.42 [0.04] | 1.72 [0.04] |
| Sys1-pt1 (21.4) | 0.64 [0.04] | 0.91 [0.04] | 1.03 [0.02] | 1.33 [0.02] | |
| Sys1-pt2 (21.1) | 0.77 [0.05] | 0.92 [0.06] | 1.07 [0.06] | 1.31 [0.04] | |
| Sys2-pt3 (21.0) | 0.79 [0.07] | 0.88 [0.06] | 1.03 [0.04] | 1.36 [0.04] | |
| Sys2-pt4 (23.5) | 0.83 [0.05] | 0.92 [0.04] | 1.07 [0.03] | 1.43 [0.03] | |
| T2ref (22.0) | T2 ± 1.5 (ms) | 71 [4] | 89 [2] | 137 [3] | 173 [8] |
| Sys1-pt1 (21.4) | 64 [4] | 70 [8] | 109 [7] | 187 [7] | |
| Sys1-pt2 (21.1) | 73 [12] | 79 [6] | 100 [6] | 166 [8] | |
| Sys2-pt3 (21.0) | 67 [2] | 79 [4] | 130 [5] | 169 [10] | |
| Sys2-pt4 (23.5) | 73 [4] | 82 [5] | 139 [6] | 178 [9] |
| Parameter Mean [HW] | Sys1-pt1 | Sys1-pt2 | Sys2-pt3 | Sys2-pt4 |
|---|---|---|---|---|
| T2meas ± 3 (ms) | 90 [25] | 76 [20] | 110 [10] | 82 [16] |
| T2cor ± 3 (ms) | 120 [25] | 96 [20] | 120 [10] | 91 [16] |
| ADC(4b)meas ± 0.03 (mm2/ms) | 0.93 [0.09] | 1.01 [0.18] | 0.75 [0.24] | 0.56 [0.12] |
| ADC(4b)cor ± 0.03 (mm2/ms) | 1.31 [0.09] | 1.39 [0.18] | 1.11 [0.24] | 0.92 [0.12] |
| ADC(2b)meas ± 0.03 (mm2/ms) | 1.18 [0.16] | 1.16 [0.31] | 0.98 [0.36] | 0.77 [0.23] |
| ADC(2b)cor ± 0.03 (mm2/ms) | 1.37 [0.16] | 1.35 [0.31] | 1.16 [0.36] | 0.95 [0.23] |
| ADC(DK)meas ± 0.03 (mm2/ms) | 1.45 [0.2] | 1.36 [0.44] | 1.2 [0.38] | 0.91 [0.24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyarenko, D.; Swanson, S.D.; Richardson, J.; Lowe, S.; O’Connor, J.; Jiang, Y.; Chahine, R.; Wells, S.A.; Chenevert, T.L. Cross-Scanner Harmonization of AI/DL Accelerated Quantitative Bi-Parametric Prostate MRI. Sensors 2025, 25, 5858. https://doi.org/10.3390/s25185858
Malyarenko D, Swanson SD, Richardson J, Lowe S, O’Connor J, Jiang Y, Chahine R, Wells SA, Chenevert TL. Cross-Scanner Harmonization of AI/DL Accelerated Quantitative Bi-Parametric Prostate MRI. Sensors. 2025; 25(18):5858. https://doi.org/10.3390/s25185858
Chicago/Turabian StyleMalyarenko, Dariya, Scott D. Swanson, Jacob Richardson, Suzan Lowe, James O’Connor, Yun Jiang, Reve Chahine, Shane A. Wells, and Thomas L. Chenevert. 2025. "Cross-Scanner Harmonization of AI/DL Accelerated Quantitative Bi-Parametric Prostate MRI" Sensors 25, no. 18: 5858. https://doi.org/10.3390/s25185858
APA StyleMalyarenko, D., Swanson, S. D., Richardson, J., Lowe, S., O’Connor, J., Jiang, Y., Chahine, R., Wells, S. A., & Chenevert, T. L. (2025). Cross-Scanner Harmonization of AI/DL Accelerated Quantitative Bi-Parametric Prostate MRI. Sensors, 25(18), 5858. https://doi.org/10.3390/s25185858







