A Fenclorim Molecularly Imprinted Electrochemical Sensor Based on a Polycatechol/Ti3C2Tx Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Synthesis of Layered Titanium Carbide (Ti3C2Tx)
2.4. Electrochemical Preparation of Ti3C2Tx-Modified Electrode (Ti3C2Tx/GCE)
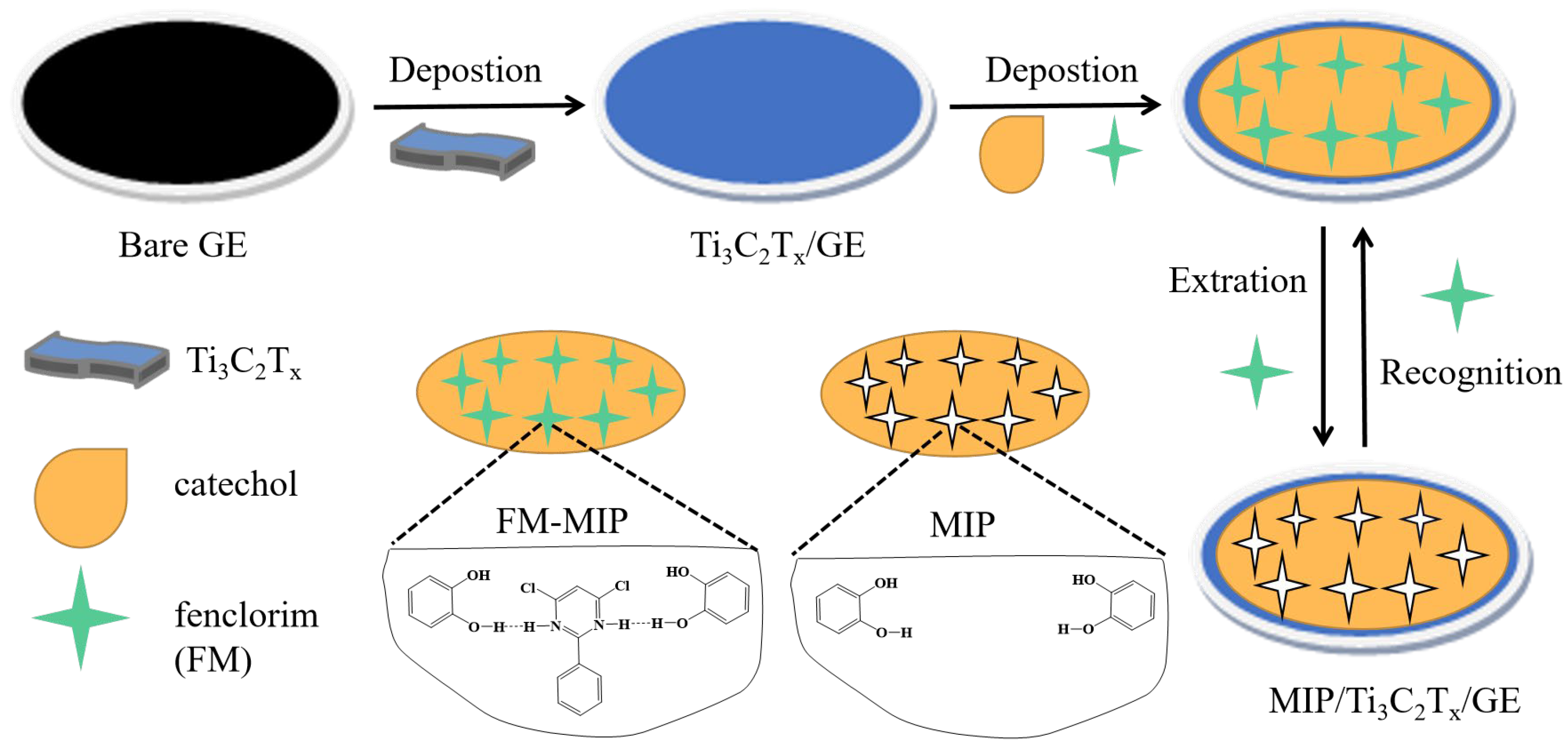
2.5. Fabrication Processes of the MIP and NIP Electrodes
2.6. Optimization of Parameters for the Developed MIP/Ti3C2Tx/GCE Sensor
2.7. Analytical-Performance Measurements
2.8. Detection of FM in the Actual Samples
3. Results and Discussion
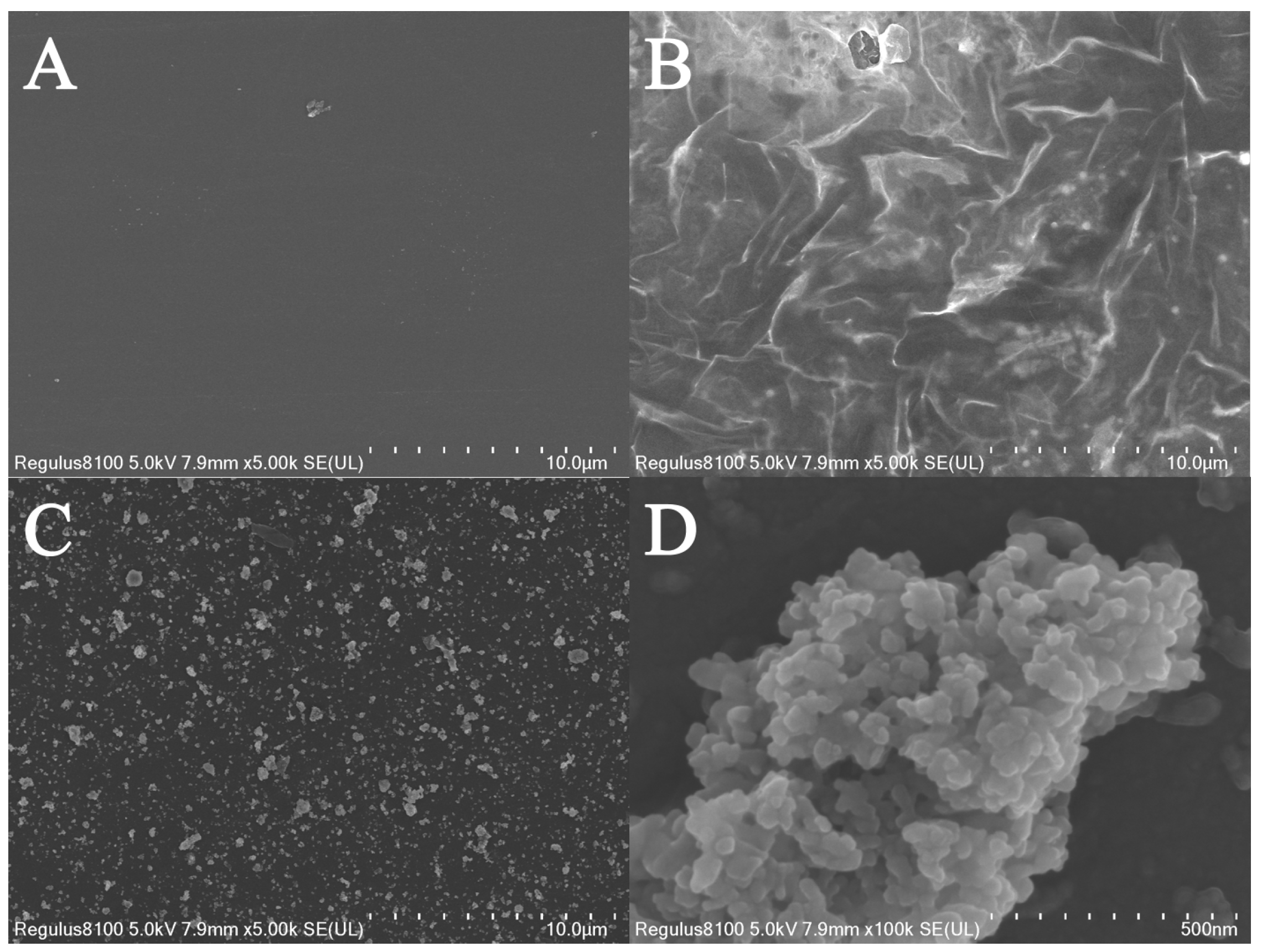
3.1. Characterization of Ti3C2Tx/GCE and MIP/Ti3C2Tx/GCE
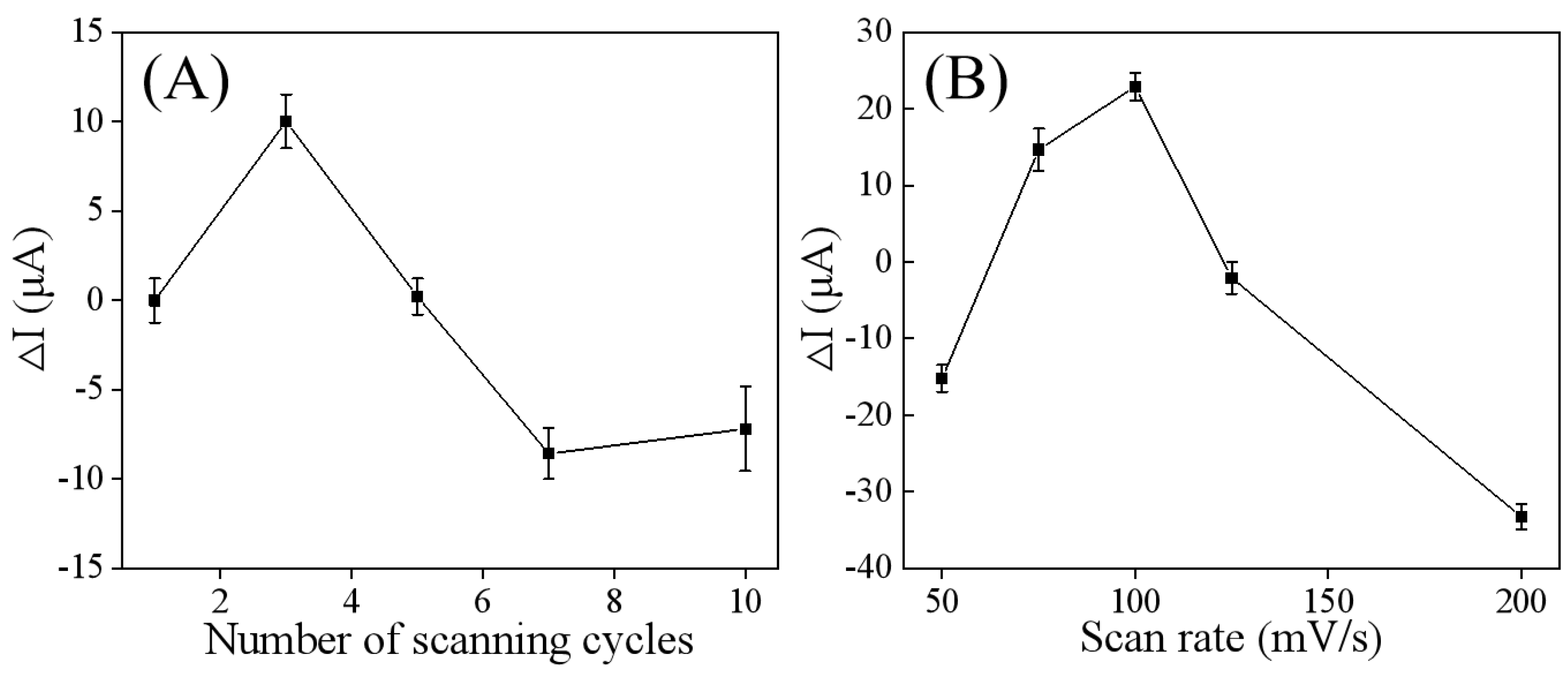
3.2. Ti3C2Tx/GCE Preparation Optimization
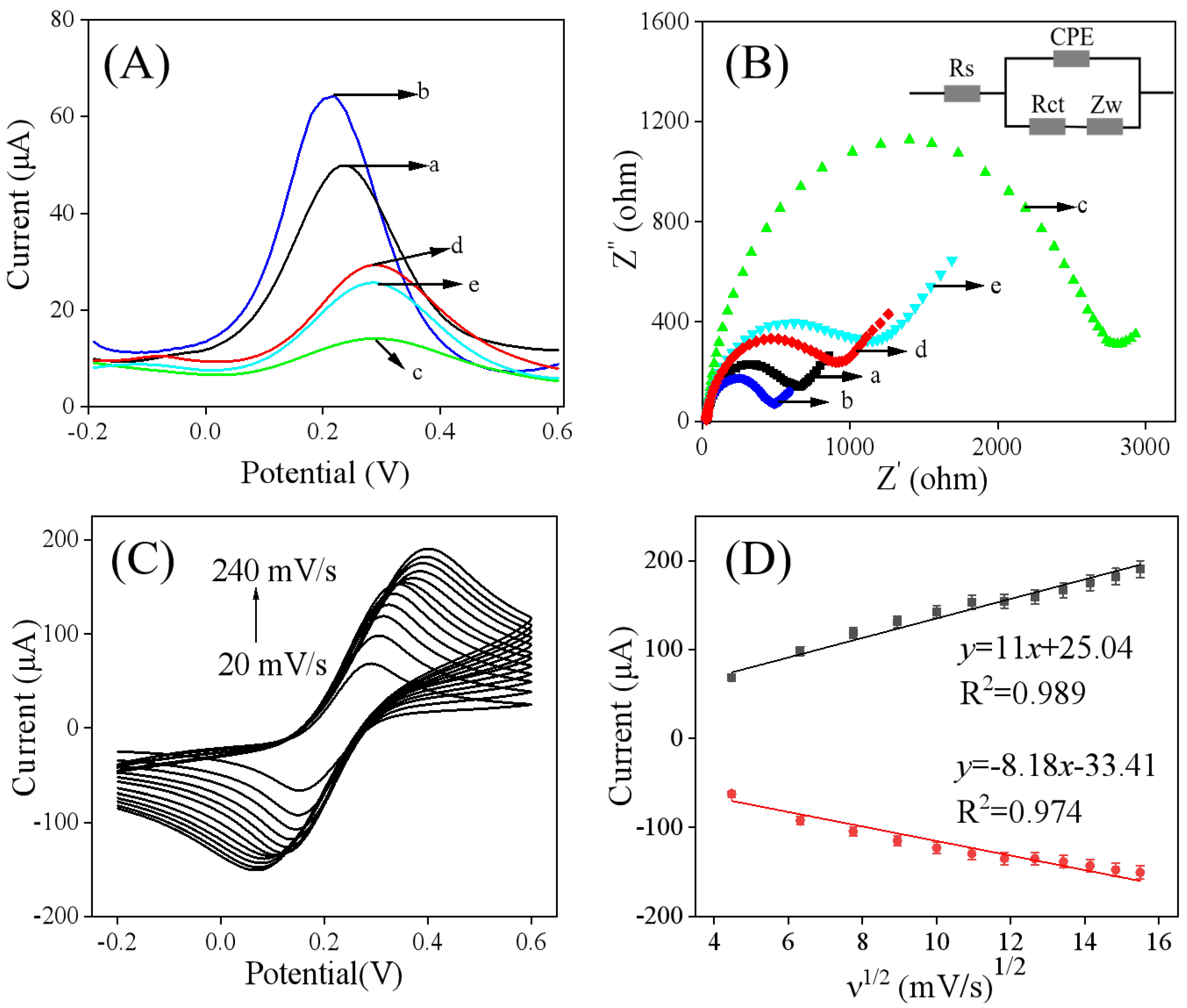
3.3. Electrochemical Investigations
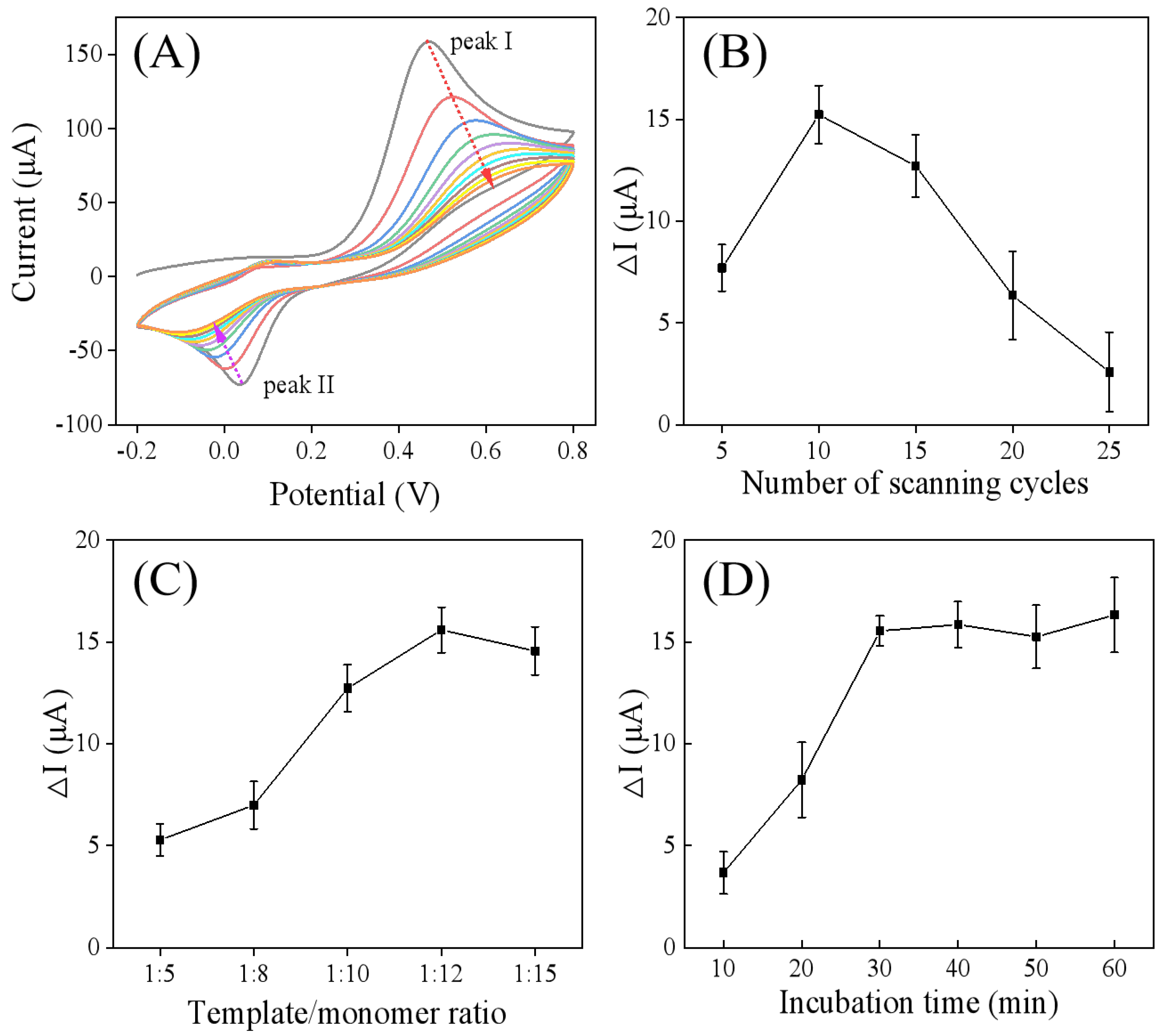
3.4. Preparation and Optimization of Parameters for the Developed MIP/Ti3C2Tx/GCE Sensor
3.5. Analytical Performance of MIP/Ti3C2Tx/GCE for Quantification of Fenclorim
3.6. Selectivity and Stability
3.7. Real-Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, J. Herbicide safeners-commercial products and tools for agrochemical research. Pestic. Outlook 2001, 12, 10–15. [Google Scholar] [CrossRef]
- Liu, S.; Deng, X.; Zhou, X.; Bai, L. Assessing the toxicity of three “inert” herbicide safeners toward Danio rerio: Effects on embryos development. Ecotoxicol. Environ. Saf. 2021, 207, 111576. [Google Scholar] [CrossRef]
- Su, L.; Caywood, L.M.; Sivey, J.D.; Dai, N. Sunlight photolysis of safener benoxacor and herbicide metolachlor as mixtures on simulated soil surfaces. Environ. Sci. Technol. 2019, 53, 6784–6793. [Google Scholar] [CrossRef]
- McFadden, M.E.; Reber, K.P.; Sivey, J.D.; Cwiertny, D.M.; LeFevre, G.H. Microbial biotransformation products and pathways of dichloroacetamide herbicide safeners. Environ. Sci. Technol. Lett. 2022, 10, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kral, A.E.; Pflug, N.C.; McFadden, M.E.; LeFevre, G.H.; Sivey, J.D.; Cwiertny, D.M. Photochemical transformations of dichloroacetamide safeners. Environ. Sci. Technol. 2019, 53, 6738–6746. [Google Scholar] [CrossRef] [PubMed]
- McFadden, M.E.; Hladik, M.L. Cyprosulfamide: Analysis of the herbicide safener and two of its degradates in surface water and groundwater from the Midwestern United States. ACS Agric. Sci. Technol. 2021, 1, 355–361. [Google Scholar] [CrossRef]
- Simonsen, D.; Livania, V.; Cwiertny, D.M.; Samuelson, R.J.; Sivey, J.D.; Lehmler, H.-J. A systematic review of herbicide safener toxicity. Crit. Rev. Toxicol. 2024, 54, 805–855. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, F.; Fu, Y. Research progress on the action mechanism of herbicide safeners: A review. J. Agric. Food Chem. 2023, 71, 3639–3650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, F.; Fu, Y. Herbicide safeners: From molecular structure design to safener activity. J. Agric. Food Chem. 2024, 72, 2451–2466. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, J.; Huang, Y.; Wang, Z.; Cheng, B.; Ma, J.; Wei, Y.; Meng, Y.; Lu, H. Benoxacor caused developmental and cardiac toxicity in zebrafish larvae. Ecotoxicol. Environ. Saf. 2021, 224, 112696. [Google Scholar] [CrossRef]
- Hu, L.; Yao, Y.; Cai, R.; Pan, L.; Liu, K.; Bai, L. Effects of fenclorim on rice physiology, gene transcription and pretilachlor detoxification ability. BMC Plant Biol. 2020, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.I.; Norsworthy, J.K.; Kumar, N.; Kim, T.H. Environmental release of a safener fenclorim from rice (Oryza sativa L.) following seed treatment. Agric. Environ. Lett. 2025, 10, e70011. [Google Scholar] [CrossRef]
- Woodward, E.E.; Hladik, M.L.; Kolpin, D.W. Occurrence of dichloroacetamide herbicide safeners and co-applied herbicides in midwestern US streams. Environ. Sci. Technol. Lett. 2018, 5, 3–8. [Google Scholar] [CrossRef]
- Wenio, I.; Derewiaka, D.; Majewska, E.; Bartosiewicz, I.; Ryszkowska, E. Development of a rapid method for simultaneous determination of pesticides in plant oil using GC-MS/MS. Appl. Sci. 2024, 14, 4923. [Google Scholar] [CrossRef]
- Hung, M.-W.; Lee, C.-J.; Lin, Y.-H.; Chao, L.-C.; Huang, K.-C.; Tsai, H.-Y.; Thanachayanont, C. Custom-designed portable potentiostat and indirect cyclic voltammetry index analysis for rapid pesticide detection using molecularly imprinted polymer sensors. Sensors 2025, 25, 2999. [Google Scholar] [CrossRef] [PubMed]
- Latif, U.; Yaqub, S.; Dickert, F.L. Sensitive coatings based on molecular-imprinted polymers for triazine pesticides’ detection. Sensors 2024, 24, 5934. [Google Scholar] [CrossRef]
- Nagabooshanam, S.; Roy, S.; Deshmukh, S.; Wadhwa, S.; Sulania, I.; Mathur, A.; Krishnamurthy, S.; Bharadwaj, L.M.; Roy, S.S. Microfluidic affinity sensor based on a molecularly imprinted polymer for ultrasensitive detection of chlorpyrifos. ACS Omega 2020, 5, 31765–31773. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef]
- Hafeez, S.; Khanam, A.; Cao, H.; Chaplin, B.P.; Xu, W. Novel conductive and redox-active molecularly imprinted polymer for direct quantification of perfluorooctanoic acid. Environ. Sci. Technol. Lett. 2024, 11, 871–877. [Google Scholar] [CrossRef]
- Mahmodnezhad, S.; Roushani, M.; Karazan, Z.M. An electrochemical sensor based on the molecularly imprinted polymer/ single walled carbon nanotube-modified glassy carbon electrode for detection of zineb fungicide in food samples. Food Control 2025, 168, 110919. [Google Scholar] [CrossRef]
- Rocha, P.; Rebelo, P.; Pacheco, J.G.; Geraldo, D.; Bento, F.; Leão-Martins, J.M.; Delerue-Matos, C.; Nouws, H.P.A. Electrochemical molecularly imprinted polymer sensor for simple and fast analysis of tetrodotoxin in seafood. Talanta 2025, 282, 127002. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Gund, G.S.; Jung, M.G.; Roh, S.H.; Park, J.; Kim, J.K.; Park, H.S. Core–shell structured mxene@carbon nanodots as bifunctional catalysts for solar-assisted water splitting. ACS Nano 2020, 14, 17615–17625. [Google Scholar] [CrossRef]
- Collini, P.; Kota, S.; Dillon, A.D.; Barsoum, M.W.; Fafarman, A.T. Electrophoretic deposition of two-dimensional titanium carbide (MXene) thick films. J. Electrochem. Soc. 2017, 164, D573–D580. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Jorge, P.A.S. Applications of electrochemical impedance spectroscopy in disease diagnosis—A review. Sens. Actuators Rep. 2024, 8, 100205. [Google Scholar] [CrossRef]
- Fu, D.; Chen, T.; Cheng, Y.; Li, A.; Liu, H.; Cheng, Z.; Li, P.; Liu, J. A molecularly imprinted electrochemical sensing platform based on the signal amplification system fabricated with the theoretically optimized monomer for specific determination of formaldehyde. Sens. Actuators B 2021, 344, 130260. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Liu, X.; Zhao, A.; Hu, C.; Gan, T.; Liu, Y. Rational design of a mesoporous silica@ZIF-8 based molecularly imprinted electrochemical sensor with high sensitivity and selectivity for atropine monitoring. J. Electroanal. Chem. 2021, 903, 115843. [Google Scholar] [CrossRef]
- Parnianchi, F.; Kashanian, S.; Nazari, M.; Santoro, C.; Bollella, P.; Varmira, K. Highly selective and sensitive molecularly imprinting electrochemical sensing platform for bilirubin detection in saliva. Microchem. J. 2021, 168, 106367. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Wang, L. Porous carbon derived from ZIF-8 modified molecularly imprinted electrochemical sensor for the detection of tert-butyl hydroquinone (TBHQ) in edible oil. Food Chem. 2021, 365, 130462. [Google Scholar] [CrossRef]
- Pourghobadi, R.; Nematollahi, D.; Baezzat, M.R.; Alizadeh, S.; Goljani, H. Electropolymerization of catechol on wireless graphite electrode. Unusual cathodic polycatechol formation. J. Electroanal. Chem. 2020, 866, 114180. [Google Scholar] [CrossRef]
- Jozef, U. Strategy for determination of LOD and LOQ values–some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar]
- Huber, W. Basic calculations about the limit of detection and its optimal determination. Accred. Qual. Assur. 2003, 8, 213–217. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Ding, B.; Cai, R.; Bai, L. Selective Action mechanism of fenclorim on rice and echinochloa crusgalli is associated with the inducibility of detoxifying enzyme activities and antioxidative defense. J. Agric. Food. Chem. 2021, 69, 5830–5839. [Google Scholar] [CrossRef] [PubMed]

| Electrode | Rs (ohm·cm2) | Rct (ohm·cm2) | Warburg | CPE | |
|---|---|---|---|---|---|
| Y0 (S·s5·cm−2) | Y0 (S·sn·cm2) | n | |||
| Bare GCE | 2.08 | 39.68 | 0.007 | 1.904 × 10−5 | 0.87 |
| Ti3C2Tx/GCE | 1.46 | 30.54 | 0.015 | 1.772 × 10−5 | 0.80 |
| MIP without elution | 1.64 | 188.20 | 0.015 | 2.074 × 10−5 | 0.89 |
| MIP/Ti3C2Tx/GCE | 1.78 | 56.71 | 0.003 | 2.112 × 10−5 | 0.87 |
| MIP incubated in 50 nM FM | 1.89 | 69.58 | 0.005 | 1.342 × 10−5 | 0.85 |
| Method | Linear Range | LOD | Reference |
|---|---|---|---|
| HPLC-DAD | - | <0.02 μg g−1 (88.89 nM) | [12] |
| Solid-phase extraction tandem GC/MS | - | 2.00 ng/L (8.89 pM) | [13] |
| MIP-Ti3C2Tx/GCE | 5.0–300 nM | 1.56 nM | This paper |
| Sample | Add (nM) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Paddy field water | 5 | 4.93 | 93.07 | 4.5 |
| 50 | 52.36 | 104.71 | 3.5 | |
| 200 | 198.89 | 99.45 | 2.4 | |
| Pond water | 5 | 5.46 | 109.28 | 4.9 |
| 50 | 50.25 | 100.51 | 2.5 | |
| 200 | 203.76 | 101.88 | 3.8 | |
| River water | 5 | 50.41 | 100.82 | 4.2 |
| 50 | 48.33 | 96.66 | 4.0 | |
| 200 | 205.69 | 102.84 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tang, X.; Chen, H.; Wu, X.; Fu, Z.; Peng, M.; Jin, C.; Guo, J. A Fenclorim Molecularly Imprinted Electrochemical Sensor Based on a Polycatechol/Ti3C2Tx Composite. Sensors 2025, 25, 5838. https://doi.org/10.3390/s25185838
Liu X, Tang X, Chen H, Wu X, Fu Z, Peng M, Jin C, Guo J. A Fenclorim Molecularly Imprinted Electrochemical Sensor Based on a Polycatechol/Ti3C2Tx Composite. Sensors. 2025; 25(18):5838. https://doi.org/10.3390/s25185838
Chicago/Turabian StyleLiu, Xiu, Xing Tang, Hongjun Chen, Xiang Wu, Zitong Fu, Mingyu Peng, Chenzhong Jin, and Jun Guo. 2025. "A Fenclorim Molecularly Imprinted Electrochemical Sensor Based on a Polycatechol/Ti3C2Tx Composite" Sensors 25, no. 18: 5838. https://doi.org/10.3390/s25185838
APA StyleLiu, X., Tang, X., Chen, H., Wu, X., Fu, Z., Peng, M., Jin, C., & Guo, J. (2025). A Fenclorim Molecularly Imprinted Electrochemical Sensor Based on a Polycatechol/Ti3C2Tx Composite. Sensors, 25(18), 5838. https://doi.org/10.3390/s25185838






