Wearable Devices for the Quantitative Assessment of Knee Joint Function After Anterior Cruciate Ligament Injury or Reconstruction: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Device Details
3.1.1. Inertial Measurement Units (IMUs)
3.1.2. Accelerometers
3.1.3. Electromagnetic Sensors
3.1.4. Force Sensing Insoles
3.1.5. Other Devices
3.2. Participants’ Demographics
3.2.1. Participants’ Groups
- ACL/ACLR-only,
- Mixed, that is ACLR/ACL-deficient (ACLD) with healthy controls,
- Healthy-only.
3.2.2. Demographics
3.2.3. Activity Level and Athletic Background
3.2.4. Exclusion Criteria
3.3. Task Protocols
3.3.1. Joint Laxity Assessments
3.3.2. Dynamic Functional Tasks
3.3.3. Rehabilitation, Joint Position Sense (JPS) and Loading Protocols
3.3.4. Device Calibration Procedures
3.4. Outcome Measures and Validation
3.4.1. Laxity Tests
3.4.2. Joint Kinematics
3.4.3. Loading Proxies from Insoles and Model-Estimated
3.4.4. Validated Temporospatial Proxies
3.4.5. Patient-Reported Outcome Measures (PROMs)
3.5. Descriptive TRL Mapping
4. Discussion
4.1. Synthesis by Device Type
4.2. Sampling Frequency
4.3. Measurement Protocols and Outcome Metrics
4.4. Methodological Gaps
- Systematic validation protocols against gold-standard systems.
- Transparent reporting of accuracy metrics and statistical agreement.
- Use outcome-specific metrics tailored to the data type (e.g., RMSE for continuous variables, ICC for reliability, F1 score for classification tasks).
- Clear differentiation between validation accuracy, test–retest reliability, and predictive model performance.
- Inclusion of PROMs.
4.5. Technology Readiness and Implementation Roadmap
4.6. Recommendations for Future Research
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Axial Compressive Force |
| ACI | Anterior Cruciate Insufficiency |
| ACL | Anterior Cruciate Ligament |
| ACL-RSI | ACL-Return to Sport Index |
| ACLD | ACL-Deficient |
| ACLI | ACL Intact |
| ACLR | Anterior Cruciate Ligament Reconstruction/Anterior Cruciate Ligament Reconstructed |
| ADL | Activities of Daily Living |
| ADLS | Activities of Daily Living Score |
| ALR | Average Loading Rate |
| AP/ML | Anteroposterior/Mediolateral |
| APT | Acceleration of Posterior Translation |
| ATP | Anterior Tibial Position |
| ATT | Anterior Tibial Translation |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| BPTB | Bone–Patellar Tendon–Bone |
| BTB | Bone–Tendon–Bone |
| CAD | Computer-Aided Design |
| CHT | Crossover Hop Test |
| CKRS | Cincinnati Knee Rating System |
| DMD | Digital Medical Device |
| DOF | Degrees of Freedom |
| DVJ | Drop Vertical Jump |
| EMG | Electromyography |
| EPSRC | Engineering and Physical Sciences Research Council |
| GPT | Generative Pre-trained Transformer |
| GRF | Ground Reaction Force |
| HES | Hospital Episode Statistics |
| IC | Initial Contact |
| ICC | Intraclass Correlation Coefficient |
| ICRS | International Cartilage Repair Society |
| IDEAL | Idea, Development, Exploration, Assessment, Long-term Follow-up |
| IKDC | International Knee Documentation Committee |
| IKDC-SKF | International Knee Documentation Committee Subjective Knee Form |
| ILR | Instantaneous Loading Rate |
| IMP | Impulse |
| IMU | Inertial Measurement Unit |
| IPF | Impact Peak Force |
| IQR | Interquartile range |
| JPS | Joint Position Sense |
| KAM | Knee Abduction Moment |
| KEM | Knee Extension Moment |
| KFEXC | Knee-Flexion Excursion |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| LAP | Load Analysis Program |
| LR | Loading rate |
| LSI | Limb Symmetry Index |
| LSTM | Long Short-Term Memory |
| MCID | Minimal Clinically Important Difference |
| MDC | Minimal Detectable Change |
| MEMS | Micro-Electromechanical Systems |
| MIMU | Magnetic Inertial Measurement Unit |
| MMAT | Mixed Methods Appraisal Tool |
| MRC | Medical Research Council |
| MRI | Magnetic Resonance Imaging |
| MVIC | Maximal Voluntary Isometric Contraction |
| N-pose | Neutral Pose |
| N/A | Not Applicable |
| NIRS | Near-Infrared Spectroscopy |
| NMES | Neuromuscular Electrical Stimulation |
| NR | Not Reported |
| OSF | Open Science Framework |
| PCA | Principal Component Analysis |
| PCC | Population, Concept, Context |
| PCL | Posterior Cruciate Ligament |
| PIF | Peak Impact Force |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA-2020 | PRISMA 2020 Flow Diagram |
| PSM | Pivot-Shift Meter |
| QALY | Quality-Adjusted Life Year |
| QPS | Quantitative Pivot Shift |
| RCT | Randomised Controlled Trial |
| RMS | Root Mean Square |
| RMSE | Root Mean Square Error |
| ROM | Range of Motion |
| rRMSE | Relative Root Mean Square Error |
| RSI | Return to Sport Index |
| RTS | Return to Sport |
| SEM | Standard Error of Measurement |
| sEMG | Surface Electromyography |
| SLS | Single Leg Squat |
| SPIRIT-AI | Standard Protocol Items: Recommendations for Interventional Trials—Artificial Intelligence |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| SVM | Support Vector Machine |
| TAS | Tegner Activity Scale |
| TO | Toe-Off |
| TPR | True Positive Rate |
| TRL | Technology Readiness Level |
| TSLH | Triple Single-Leg Hop |
| UKRI | UK Research and Innovation |
| vGRF | Vertical Ground Reaction Force |
| VKLD | Vermont Knee Laxity Device |
References
- Giuliani, J.R.; Kilcoyne, K.G.; Rue, J.P.H. Anterior Cruciate Ligament Anatomy: A Review of the Anteromedial and Posterolateral Bundles. J. Knee Surg. 2009, 22, 148–154. [Google Scholar] [CrossRef]
- Abeyewardene, S.; Ganss, A.; Menge, T.J.; Hamilton, K.D. Anterior Cruciate Ligament Reconstruction with Quadriceps Tendon Autograft: A Comprehensive Review. J. Surg. 2020. [Google Scholar] [CrossRef]
- Minamoto, Y.; Akagi, R.; Maki, S.; Shiko, Y.; Tozawa, R.; Kimura, S.; Yamaguchi, S.; Kawasaki, Y.; Ohtori, S.; Sasho, T. Automated Detection of Anterior Cruciate Ligament Tears Using a Deep Convolutional Neural Network. BMC Musculoskelet. Disord. 2022, 23, 577. [Google Scholar] [CrossRef]
- Barrack, R.L.; Skinner, H.B.; Buckley, S.L. Proprioception in the Anterior Cruciate Deficient Knee. Am. J. Sports Med. 1989, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Benjaminse, A.; Gokeler, A.; Van Der Schans, C.P. Clinical Diagnosis of an Anterior Cruciate Ligament Rupture: A Meta-Analysis. J. Orthop. Sports Phys. Ther. 2006, 36, 267–288. [Google Scholar] [CrossRef]
- Xergia, S.A.; Pappas, E.; Zampeli, F.; Georgiou, S.; Georgoulis, A.D. Asymmetries in Functional Hop Tests, Lower Extremity Kinematics, and Isokinetic Strength Persist 6 to 9 Months Following Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2013, 43, 154–162. [Google Scholar] [CrossRef]
- Georgoulis, A.D.; Pappa, L.; Moebius, U.; Malamou-Mitsi, V.; Pappa, S.; Papageorgiou, C.O.; Agnantis, N.J.; Soucacos, P.N. The Presence of Proprioceptive Mechanoreceptors in the Remnants of the Ruptured ACL as a Possible Source of Re-Innervation of the ACL Autograft. Knee Surg. Sports Traumatol. Arthrosc. 2001, 9, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Frobell, R.B.; Roos, E.M.; Roos, H.P.; Ranstam, J.; Lohmander, L.S. A Randomized Trial of Treatment for Acute Anterior Cruciate Ligament Tears. N. Engl. J. Med. 2010, 363, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Granan, L.P.; Forssblad, M.; Lind, M.; Engebretsen, L. The Scandinavian ACL Registries 2004-2007: Baseline Epidemiology. Acta Orthop. 2009, 80, 563–567. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Granan, L.P.; Dunn, W.R.; Amendola, A.; Andrish, J.T.; Brophy, R.; Carey, J.L.; Flanigan, D.; Huston, L.J.; Jones, M.; et al. Cross-Cultural Comparison of Patients Undergoing ACL Reconstruction in the United States and Norway. Knee Surg. Sports Traumatol. Arthrosc. 2009, 18, 98–105. [Google Scholar] [CrossRef]
- Mall, N.A.; Chalmers, P.N.; Moric, M.; Tanaka, M.J.; Cole, B.J.; Bach, B.R.; Paletta, G.A. Incidence and Trends of Anterior Cruciate Ligament Reconstruction in the United States. Am. J. Sports Med. 2014, 42, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Matar, H.E.; Platt, S.R.; Bloch, B.V.; Board, T.N.; Porter, M.L.; Cameron, H.U.; James, P.J. Three Orthopaedic Operations, over 1,000 Randomized Controlled Trials, in over 100,000 Patients. Bone Jt. Res. 2022, 11, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S.S.; Dowen, D.; James, P.; Serrano-Pedraza, I.; Reed, M.R.; Deehan, D. Complications Following Anterior Cruciate Ligament Reconstruction in the English NHS. Knee 2012, 19, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McCammon, J.; Martin, R.K.; Prior, H.J.; Leiter, J.; MacDonald, P.B. Epidemiological Trends of Anterior Cruciate Ligament Reconstruction in a Canadian Province. Clin. J. Sport. Med. 2020, 30, e207–e213. [Google Scholar] [CrossRef]
- Prentice, H.A.; Lind, M.; Mouton, C.; Persson, A.; Magnusson, H.; Gabr, A.; Seil, R.; Engebretsen, L.; Samuelsson, K.; Karlsson, J.; et al. Patient Demographic and Surgical Characteristics in Anterior Cruciate Ligament Reconstruction: A Description of Registries from Six Countries. Br. J. Sports Med. 2018, 52, 716–722. [Google Scholar] [CrossRef]
- Mancino, F.; Kayani, B.; Gabr, A.; Fontalis, A.; Plastow, R.; Haddad, F.S. Anterior Cruciate Ligament Injuries in Female Athletes: Risk Factors and Strategies for Prevention. Bone Jt. Open 2024, 5, 94–100. [Google Scholar] [CrossRef]
- Matar, H.E.; Platt, S.R.; Bloch, B.V.; James, P.J.; Cameron, H.U. A Systematic Review of Randomized Controlled Trials in Anterior Cruciate Ligament Reconstruction: Standard Techniques Are Comparable (299 Trials With 25,816 Patients). Arthrosc. Sports Med. Rehabil. 2021, 3, e1211–e1226. [Google Scholar] [CrossRef]
- Musahl, V.; Engler, I.D.; Nazzal, E.M.; Dalton, J.F.; Lucidi, G.A.; Hughes, J.D.; Zaffagnini, S.; Della Villa, F.; Irrgang, J.J.; Fu, F.H.; et al. Current Trends in the Anterior Cruciate Ligament Part II: Evaluation, Surgical Technique, Prevention, and Rehabilitation. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 34–51. [Google Scholar] [CrossRef]
- British Association for Surgery of the Knee; British Orthopaedic Sports Trauma and Arthroscopy Association Best Practice for Management of Anterior Cruciate Ligament (ACL) Injuries. 2023. Available online: https://www.boa.ac.uk/static/88a4c3e3-df3e-4e51-a92e7d2f86d7d82a/Best-Practice-Book-for-management-of-Anterior-Cruciate-Ligament-injuries.pdf (accessed on 3 July 2025).
- Mather, R.C.; Hettrich, C.M.; Dunn, W.R.; Cole, B.J.; Bach, B.R.; Huston, L.J.; Reinke, E.K.; Spindler, K.P. Cost-Effectiveness Analysis of Early Reconstruction versus Rehabilitation and Delayed Reconstruction for Anterior Cruciate Ligament Tears. Am. J. Sports Med. 2014, 42, 1583–1591. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Appleby, D. Cost-Effectiveness Analysis of the Most Common Orthopaedic Surgery Procedures: Knee Arthroscopy and Knee Anterior Cruciate Ligament Reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1317–1322. [Google Scholar] [CrossRef]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. Fifty-Five per Cent Return to Competitive Sport Following Anterior Cruciate Ligament Reconstruction Surgery: An Updated Systematic Review and Meta-Analysis Including Aspects of Physical Functioning and Contextual Factors. Br. J. Sports Med. 2014, 48, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- De Fontenay, B.P.; Argaud, S.; Blache, Y.; Monteil, K. Motion Alterations after Anterior Cruciate Ligament Reconstruction: Comparison of the Injured and Uninjured Lower Limbs during a Single-Legged Jump. J. Athl. Train. 2014, 49, 311–316. [Google Scholar] [CrossRef]
- Paterno, M.V.; Rauh, M.J.; Schmitt, L.C.; Ford, K.R.; Hewett, T.E. Incidence of Second ACL Injuries 2 Years after Primary ACL Reconstruction and Return to Sport. Am. J. Sports Med. 2014, 42, 1567–1573. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in Healthcare Wearable Devices. NPJ Flex. Electron. 2021, 5, 9. [Google Scholar] [CrossRef]
- Bellitti, P.; Borghetti, M.; Lopomo, N.F.; Sardini, E.; Serpelloni, M. Smart Brace for Static and Dynamic Knee Laxity Measurement. Sensors 2022, 22, 5815. [Google Scholar] [CrossRef]
- Prill, R.; Walter, M.; Królikowska, A.; Becker, R. A Systematic Review of Diagnostic Accuracy and Clinical Applications of Wearable Movement Sensors for Knee Joint Rehabilitation. Sensors 2021, 21, 8221. [Google Scholar] [CrossRef]
- Tan, T.; Gatti, A.A.; Fan, B.; Shea, K.G.; Sherman, S.L.; Uhlrich, S.D.; Hicks, J.L.; Delp, S.L.; Shull, P.B.; Chaudhari, A.S. A Scoping Review of Portable Sensing for Out-of-Lab Anterior Cruciate Ligament Injury Prevention and Rehabilitation. NPJ Digit. Med. 2023, 6, 46. [Google Scholar] [CrossRef]
- Golberg, E.; Pinkoski, A.; Beaupre, L.; Rouhani, H. Monitoring External Workload With Wearable Technology After Anterior Cruciate Ligament Reconstruction: A Scoping Review. Orthop. J. Sports Med. 2023, 11, 23259671231191134. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Social Res. Methodol. Theory Pract. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; Mclnerney, P.; Munn, Z.; Tricco, A.; Khalil, H. Chapter 11: Scoping Reviews (2020). In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar]
- UK Research and Innovation Activities Associated with Different Technology Readiness Levels. Available online: https://www.ukri.org/wp-content/uploads/2022/01/EPSRC-11012022-Technologyreadinesslevelsfrombasicresearchtoadoptionanddiffusion.pdf (accessed on 31 August 2025).
- Yona, T.; Peskin, B.; Fischer, A.G. Lower Limb Kinematic Changes during Stair Navigation 3 and 5 Months after Anterior Cruciate Ligament Reconstruction: A Longitudinal Analysis in Real-World Settings. PM & R 2025, 17, 663–672. [Google Scholar] [CrossRef]
- Di Paolo, S.; Bragonzoni, L.; Della Villa, F.; Grassi, A.; Zaffagnini, S. Do Healthy Athletes Exhibit At-Risk Biomechanics for Anterior Cruciate Ligament Injury during Pivoting Movements? Sports Biomech. 2024, 23, 2995–3008. [Google Scholar] [CrossRef] [PubMed]
- Benjaminse, A.; Nijmeijer, E.M.; Gokeler, A.; Di Paolo, S. Application of Machine Learning Methods to Investigate Joint Load in Agility on the Football Field: Creating the Model, Part I. Sensors 2024, 24, 3652. [Google Scholar] [CrossRef] [PubMed]
- Button, K.; Felemban, M.; Davies, J.; Nicholas, K.; Parry-Williams, J.; Muaidi, Q.; Al-Amri, M. A Standardised Template for Reporting Lower Limb Kinematic Waveform Movement Compensations from a Sensor-Based Portable Clinical Movement Analysis Toolkit. IPEM-Transl. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Baldazzi, A.; Molinaro, L.; Taborri, J.; Margheritini, F.; Rossi, S.; Bergamini, E. Reliability of Wearable Sensors-Based Parameters for the Assessment of Knee Stability. PLoS ONE 2022, 17, e0274817. [Google Scholar] [CrossRef]
- Fan, B.; Xia, H.; Xu, J.; Li, Q.; Shull, P.B. IMU-Based Knee Flexion, Abduction and Internal Rotation Estimation during Drop Landing and Cutting Tasks. J. Biomech. 2021, 124, 110549. [Google Scholar] [CrossRef]
- Albano, D.; Lambiase, G.; Romano, B.; Vastola, R. Nonlinear Analysis of Knee Kinematic Variability after ACL Reconstruction for the Return to Sport. J. Phys. Educ. Sport 2021, 21, 922–926. [Google Scholar] [CrossRef]
- Labbé, D.R.; Li, D.; Grimard, G.; de Guise, J.A.; Hagemeister, N. Quantitative Pivot Shift Assessment Using Combined Inertial and Magnetic Sensing. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2330–2338. [Google Scholar] [CrossRef]
- Portillo-Ortíz, N.K.; Sigala-González, L.R.; Ramos-Moctezuma, I.R.; Bermúdez Bencomo, B.L.; Gomez Salgado, B.A.; Ovalle Arias, F.C.; Leal-Berumen, I.; Berumen-Nafarrate, E. Standardizing and Classifying Anterior Cruciate Ligament Injuries: An International Multicenter Study Using a Mobile Application. Diagnostics 2025, 15, 19. [Google Scholar] [CrossRef]
- Niederer, D.; Behringer, M.; Stein, T. Functional Outcomes after Anterior Cruciate Ligament Reconstruction: Unravelling the Role of Time between Injury and Surgery, Time since Reconstruction, Age, Gender, Pain, Graft Type, and Concomitant Injuries. BMC Sports Sci. Med. Rehabil. 2023, 15, 49. [Google Scholar] [CrossRef]
- Mengis, N.; Schmidt, S.; Ellermann, A.; Sobau, C.; Egloff, C.; Kreher, M.M.; Ksoll, K.; Schmidt-Lucke, C.; Rippke, J.N. A Novel Sensor-Based Application for Home-Based Rehabilitation Can Objectively Measure Postoperative Outcomes Following Anterior Cruciate Ligament Reconstruction. J. Pers. Med. 2023, 13, 1398. [Google Scholar] [CrossRef]
- Sun, T.; Li, D.; Fan, B.; Tan, T.; Shull, P.B. Real-Time Ground Reaction Force and Knee Extension Moment Estimation During Drop Landings Via Modular LSTM Modeling and Wearable IMUs. IEEE J. Biomed. Health Inform. 2023, 27, 3222–3233. [Google Scholar] [CrossRef]
- Ahmadian, N.; Nazarahari, M.; Whittaker, J.L.; Rouhani, H. Quantification of Triple Single-Leg Hop Test Temporospatial Parameters: A Validated Method Using Body-Worn Sensors for Functional Evaluation after Knee Injury. Sensors 2020, 20, 3464. [Google Scholar] [CrossRef]
- Kawanishi, Y.; Nozaki, M.; Kobayashi, M.; Yasuma, S.; Fukushima, H.; Murase, A.; Takenaga, T.; Yoshida, M.; Kuroyanagi, G.; Kawaguchi, Y.; et al. Preoperative Knee Instability Affects Residual Instability as Evaluated by Quantitative Pivot-Shift Measurements During Double-Bundle ACL Reconstruction. Orthop. J. Sports Med. 2020, 8, 2325967120959020. [Google Scholar] [CrossRef] [PubMed]
- Dowling, A.V.; Favre, J.; Andriacchi, T.P. Inertial Sensor-Based Feedback Can Reduce Key Risk Metrics for Anterior Cruciate Ligament Injury during Jump Landings. Am. J. Sports Med. 2012, 40, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Diermeier, T.; Meredith, S.J.; Irrgang, J.J.; Zaffagnini, S.; Kuroda, R.; Hochino, Y.; Samuelsson, K.; Smith, C.N.; Popchak, A.; Musahl, V.; et al. Patient-Reported and Quantitative Outcomes of Anatomic Anterior Cruciate Ligament Reconstruction With Hamstring Tendon Autografts. Orthop. J. Sports Med. 2020, 8, 2325967120926159. [Google Scholar] [CrossRef] [PubMed]
- Musahl, V.; Burnham, J.; Lian, J.; Popchak, A.; Svantesson, E.; Kuroda, R.; Zaffagnini, S.; Samuelsson, K.; Sheean, A.; Burnham, J.M.; et al. High-Grade Rotatory Knee Laxity May Be Predictable in ACL Injuries. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3762–3769. [Google Scholar] [CrossRef]
- Lopomo, N.; Signorelli, C.; Bonanzinga, T.; Muccioli, G.M.M.; Visani, A.; Zaffagnini, S. Quantitative Assessment of Pivot-Shift Using Inertial Sensors. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 713–717. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Kim, H.; Shultz, S.J. Neuromuscular Fatigue and Tibiofemoral Joint Biomechanics When Transitioning from Non-Weight Bearing to Weight Bearing. J. Athl. Train. 2015, 50, 23–29. [Google Scholar] [CrossRef]
- Labbe, D.R.; de Guise, J.A.; Mezghani, N.; Godbout, V.; Grimard, G.; Baillargeon, D.; Lavigne, P.; Fernandes, J.; Ranger, P.; Hagemeister, N. Feature Selection Using a Principal Component Analysis of the Kinematics of the Pivot Shift Phenomenon. J. Biomech. 2010, 43, 3080–3084. [Google Scholar] [CrossRef]
- Kuroda, R.; Hoshino, Y.; Nagamune, K.; Kubo, S.; Nishimoto, K.; Araki, D.; Yamaguchi, M.; Yoshiya, S.; Kurosaka, M. Intraoperative Measurement of Pivot Shift by Electromagnetic Sensors. Oper. Tech. Orthop. 2008, 18, 190–195. [Google Scholar] [CrossRef]
- Yagi, M.; Kuroda, R.; Nagamune, K.; Yoshiya, S.; Kurosaka, M. Double-Bundle ACL Reconstruction Can Improve Rotational Stability. In Clinical Orthopaedics and Related Research; Lippincott Williams and Wilkins: Ambler, PA, USA, 2007; Volume 454, pp. 100–107. [Google Scholar]
- Grood, E.S.; Suntay, W.J.; Noyes, F.R.; Butler, D.L. Biomechanics of the Knee-Extension Exercise. Effect of Cutting the Anterior Cruciate Ligament. J. Bone Jt. Surg. Ser. A 1984, 66, 725–734. [Google Scholar] [CrossRef]
- Kubo, S.; Muratsu, H.; Yoshiya, S.; Mizuno, K.; Kurosaka, M. Reliability and Usefulness of a New in Vivo Measurement System of the Pivot Shift. In Clinical Orthopaedics and Related Research; Lippincott Williams and Wilkins: Ambler, PA, USA, 2007; Volume 454, pp. 54–58. [Google Scholar]
- Cherelstein, R.E.; Kuenze, C.; Harkey, M.S.; Walaszek, M.C.; Grozier, C.; Brumfield, E.R.; Lewis, J.N.; Hughes, G.A.; Chang, E.S. Evaluating Gait with Force Sensing Insoles 6 Months after Anterior Cruciate Ligament Reconstruction: An Autograft Comparison. Med. Sci. Sports Exerc. 2024, 57, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cherelstein, R.E.; Kuenze, C.M.; Walaszek, M.C.; Brumfield, E.R.; Lewis, J.N.; Hughes, G.A.; Chang, E.S. Patients With a Quadriceps Tendon Autograft Demonstrate Greater Asymmetry in Landing Kinetics Than Patients With a Bone–Patellar Tendon–Bone Autograft 6 Months After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2025, 53, 623–631. [Google Scholar] [CrossRef]
- Luftglass, A.R.; Peebles, A.T.; Miller, T.K.; Queen, R.M. The Impact of Standardized Footwear on Load and Load Symmetry. Clin. Biomech. 2021, 88, 105421. [Google Scholar] [CrossRef]
- Peebles, A.T.; Renner, K.E.; Miller, T.K.; Moskal, J.T.; Queen, R.M. Associations between Distance and Loading Symmetry during Return to Sport Hop Testing. Med. Sci. Sports Exerc. 2019, 51, 624–629. [Google Scholar] [CrossRef]
- Peebles, A.T.; Maguire, L.A.; Renner, K.E.; Queen, R.M. Validity and Repeatability of Single-Sensor Loadsol Insoles during Landing. Sensors 2018, 18, 4082. [Google Scholar] [CrossRef]
- Nyffenegger, D.; Baur, H.; Henle, P.; Busch, A. Cortical Activity during the First 4 Months after Anterior Cruciate Ligament Reconstruction While Performing an Active Knee Joint Position Sense Test: A Pilot Study. Knee 2025, 55, 168–178. [Google Scholar] [CrossRef]
- Busch, A.; Gianotti, L.R.R.; Mayer, F.; Baur, H. Monitoring Cortical and Neuromuscular Activity: Six-Month Insights into Knee Joint Position Sense Following ACL Reconstruction. Int. J. Sports Phys. Ther. 2024, 19, 1209–1303. [Google Scholar] [CrossRef]
- Deiss, V.; Bähler, P.; Kolly, P.; Schärer, A.; Henle, P.; Eichelberger, P.; Lutz, N.; Baur, H. Test-Retest Reliability and Concurrent Validity Assessment of a Novel High-Frequency Sensor Device for Anterior Tibial Translation Measurement in Loaded and Unloaded Condition: An Exploratory Cross-Sectional Study. BMC Musculoskelet. Disord. 2024, 25, 218. [Google Scholar] [CrossRef]
- Fiorentino, N.M.; Atkins, P.R.; Kutschke, M.J.; Goebel, J.M.; Foreman, K.B.; Anderson, A.E. Soft Tissue Artifact Causes Significant Errors in the Calculation of Joint Angles and Range of Motion at the Hip. Gait Posture 2017, 55, 184–190. [Google Scholar] [CrossRef]
- Zhao, J. A Review of Wearable IMU (Inertial-Measurement-Unit)-Based Pose Estimation and Drift Reduction Technologies. In Journal of Physics: Conference Series; IOP Publishing: Philadelphia, PA, USA, 2018; Volume 1087. [Google Scholar]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.S. Validity and Reliability Ofwearable Sensors for Joint Angle Estimation: A Systematic Review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.M.; Haidegger, T.; Birkfellner, W.; Cleary, K.; Peters, T.M.; Maier-Hein, L. Electromagnetic Tracking in Medicine -A Review of Technology, Validation, and Applications. IEEE Trans. Med. Imaging 2014, 33, 1702–1725. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.T.; Deneweth Zendler, J.; Zernicke, R.F. Validation of a Wireless Shoe Insole for Ground Reaction Force Measurement. J. Sports Sci. 2019, 37, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Seiberl, W.; Jensen, E.; Merker, J.; Leitel, M.; Schwirtz, A. Accuracy and Precision of Loadsol® Insole Force-Sensors for the Quantification of Ground Reaction Force-Based Biomechanical Running Parameters. Eur. J. Sport. Sci. 2018, 18, 1100–1109. [Google Scholar] [CrossRef]
- Renner, K.E.; Blaise Williams, D.S.; Queen, R.M. The Reliability and Validity of the Loadsol® under Various Walking and Running Conditions. Sensors 2019, 19, 265. [Google Scholar] [CrossRef]
- Piriyaprasarth, P.; Morris, M.E.; Winter, A.; Bialocerkowski, A.E. The Reliability of Knee Joint Position Testing Using Electrogoniometry. BMC Musculoskelet. Disord. 2008, 9, 6. [Google Scholar] [CrossRef]
- Panisset, J.C.; Ntagiopoulos, P.G.; Saggin, P.R.; Dejour, D. A Comparison of TelosTM Stress Radiography versus RolimeterTM in the Diagnosis of Different Patterns of Anterior Cruciate Ligament Tears. Orthop. Traumatol. Surg. Res. 2012, 98, 751–758. [Google Scholar] [CrossRef]
- Ericsson, D.; Östenberg, A.H.; Andersson, E.; Alricsson, M. Test-Retest Reliability of Repeated Knee Laxity Measurements in the Acute Phase Following a Knee Trauma Using a Rolimeter. J. Exerc. Rehabil. 2017, 13, 550. [Google Scholar] [CrossRef]
- Sigward, S.M.; Cesar, G.M.; Havens, K.L. Predictors of Frontal Plane Knee Moments during Side-Step Cutting to 45° and 110° Men and Women: Implications for ACL Injury HHS Public Access. Clin. J. Sport. Med. 2015, 25, 529–534. [Google Scholar] [CrossRef]
- Weinhandl, J.T.; Irmischer, B.S.; Sievert, Z.A. Sex Differences in Unilateral Landing Mechanics from Absolute and Relative Heights. Knee 2015, 22, 298–303. [Google Scholar] [CrossRef]
- Queen, R.M.; Vap, A.; Moorman, C.T.; Garrett, W.E.; Butler, R.J. Gender Differences in Plantar Loading during an Unanticipated Side Cut on Field Turf. Clin. J. Sport. Med. 2016, 26, 157–161. [Google Scholar] [CrossRef]
- Longo, U.G.; Risi Ambrogioni, L.; Berton, A.; Candela, V.; Migliorini, F.; Carnevale, A.; Schena, E.; Nazarian, A.; DeAngelis, J.; Denaro, V. Conservative versus Accelerated Rehabilitation after Rotator Cuff Repair: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2021, 22, 637. [Google Scholar] [CrossRef] [PubMed]
- Bohm, S.; Mersmann, F.; Arampatzis, A. Human Tendon Adaptation in Response to Mechanical Loading: A Systematic Review and Meta-Analysis of Exercise Intervention Studies on Healthy Adults. Sports Med. Open 2015, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Hollman, J.H.; McDade, E.M.; Petersen, R.C. Normative Spatiotemporal Gait Parameters in Older Adults. Gait Posture 2011, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Eur. Urol. 2015, 67, 1142–1151. [Google Scholar] [CrossRef]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K.; Chan, A.W.; Darzi, A.; Holmes, C.; Yau, C.; Ashrafian, H.; et al. Reporting Guidelines for Clinical Trial Reports for Interventions Involving Artificial Intelligence: The CONSORT-AI Extension. Nat. Med. 2020, 26, 1364–1374. [Google Scholar] [CrossRef]
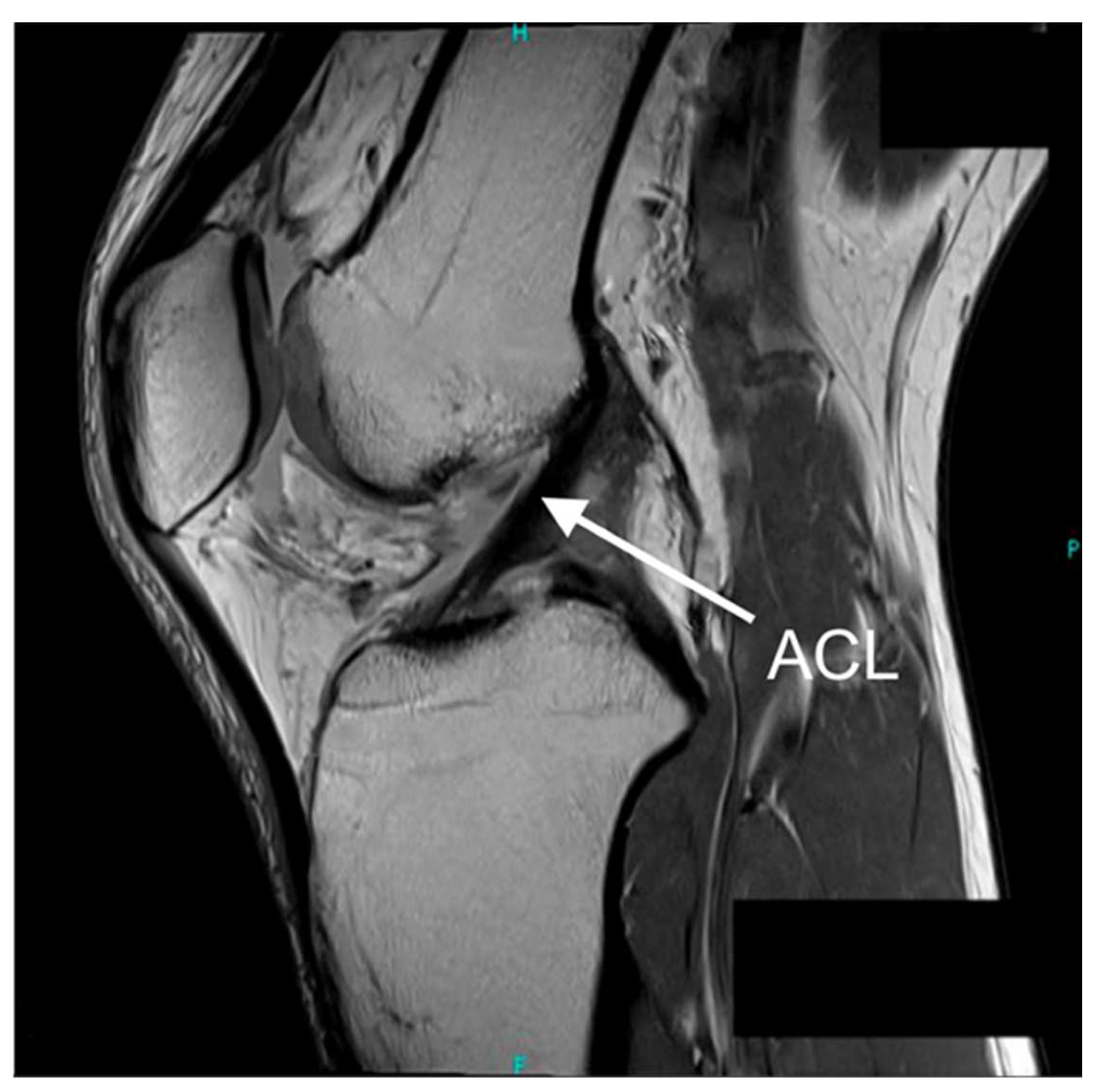
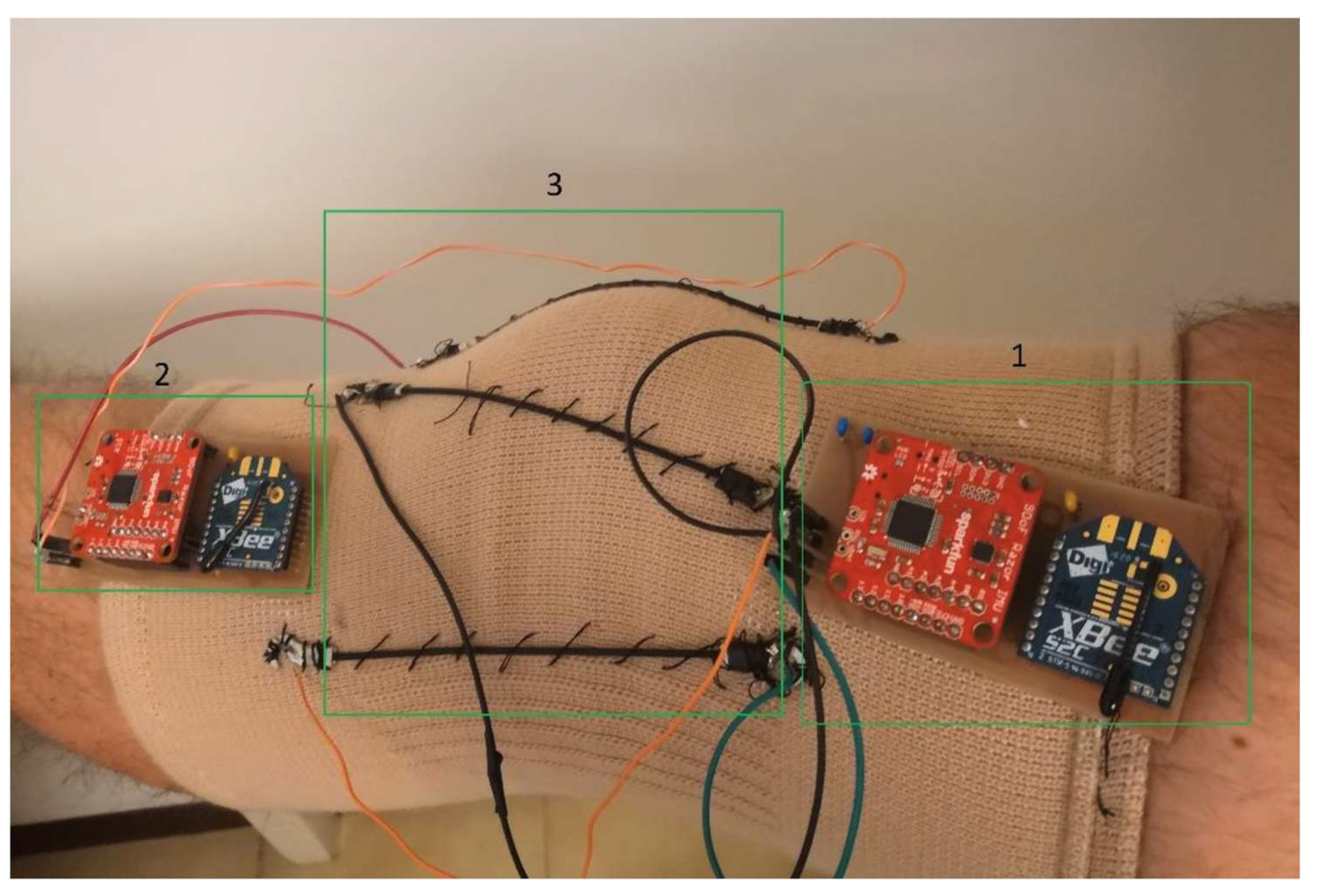


| TRL Band * | Category | Criteria |
|---|---|---|
| 3–4 | Prototype | Researcher-run; laboratory setting; healthy volunteers or small early patient pilots; no clinical workflow; custom pipeline; no regulatory status. |
| 5–6 | Research deployment/ initial clinical studies | Patients in clinical settings; clinician-performed/supervised; standardised protocol; research deployment; outputs not used to guide care; no regulatory claim. |
| 7 | Workflow trials/ multisite pilots | Operational use embedded in clinical workflows; multi-site/registry with standard operating procedure; routine rehab/clinic sessions; trained staff; evidence that outputs are used within the care pathway. |
| 8–9 | Regulatory approval and integration | Regulatory clearance and limited/routine integration into standard care; post-market/real-world evidence. |
| Year | Study | Device Type | Placement | Number of Devices | Sampling Rate | Data Processing Software |
|---|---|---|---|---|---|---|
| 2025 | Yona et al. [34] | IMU (9 axis) | - Pelvis, - Thighs, - Shanks, - Feet | 7 | NR | IBM SPSS (Version 29) |
| 2024 | Di Paolo et al. [35] | IMU (9 axis) | - Upper limbs, - trunk, - Lower limbs | 15 | 60 Hz | Dedicated Xsens software and a custom MATLAB script (The MathWorks, Natick, MA, USA) |
| 2024 | Benjaminse et al. [36] | IMU (9 axis) | - Head (right side), - Sternum, - Hands (posterior side), - Wrists (dorsal side), - Above elbows (lateral side), - Scapula spines (middle), - Posterior superior iliac spines (middle), - Thighs (lateral side), - Tibias (medial surface), - Forefoot (dorsal side) | 17 | 240 Hz | Custom MATLAB script vR2022a (The MathWorks, Natick, MA, USA) |
| 2022 | Button et al. [37] | IMU (9 axis) | - Upper thighs (centrally and halfway between the greater trochanter and lateral epicondyle), - Lower legs (proximal medial surface of each tibia), - Feet (dorsum of each foot), - Sacrum | 7 | NR | MVN BIOMECH studio software (version 4.4), custom written MATLAB code (MATLAB version 2015a; The MathWorks Inc., Natick, MA, USA) |
| 2022 | Bellitti et al. [26] | IMU (9 axis) + stretchable strain sensors | IMUs: - Proximal anterolateral part of the tibia, - Distal lateral part of the femur Stretch sensors: - Lateral side of the knee, - Femur epicondyle, - Anterior aspect of the knee | IMUs: 2 stretch sensors: 3, | 40 Hz | Custom software (written in LabVIEW2017, National Instruments, Austin, TX, USA), MATLAB (MathWorks) |
| 2022 | Baldazzi et al. [38] | IMU (9 axis) | - Medial upper portion of the tibial crest, - Foot dorsum | 2 | 500 Hz | Custom MATLAB scripts and functions |
| 2021 | Fan et al. [39] | IMU (9 axis) | 2 per leg: - Thigh, - Shank | 4 | 100 Hz | Custom |
| 2021 | Albano et al. [40] | IMU (9 axis) | - Thighs (one on each leg), - Shank (one on each leg) | 4 | 60 Hz | Custom MATLAB script, UNO Biomechanics Nonlinear Analysis Toolbox |
| 2015 | Labbé et al. [41] | IMU (9 axis) | - Tibia, - Femur | 2 | 150 Hz | Custom |
| 2024 | Portillo-Ortiz et al. [42] | Smartphone (accelerometer + gyroscope) | - Tibial tuberosity (2 fingers below patella, incline towards medial aspect of tibia) | 1 | NR | Neural-network classification via the app; features processed in MATLAB; CSV assembled with a Python script |
| 2023 | Niederer et al. [43] | IMU (6 axis) | - Tibia (highest circumference of the lower leg) | 1 | 4.5 kHz–9.0 kHz | NR |
| 2023 | Mengis et al. [44] | IMU (6 axis) | - Tibial tuberosity | 1 | NR | Orthelligent HOME app |
| 2023 | Sun et al. [45] | IMU (6 axis) | - Chest (trunk-fifth thoracic vertebrae), - Waist (pelvis-mid-point between left and right anterior superior iliac spine), - Right and left thigh (thigh-midpoint between the left anterior superior iliac spine and left femur medial epicondyle), - Right and left shank (shank-one-third point between left femur medial epicondyle and left tibia apex of medial malleolus near proximal end of tibia), - Right and left foot (second metatarsal) | 8 | 100 Hz | Keras (v2.5.0), TensorFlow (v2.5.0) |
| 2020 | Ahmadian et al. [46] | IMU (6 axis) | Physilog BFSr-3: - Forefoot, - Upper shank (criterion-related validity), Physilog 5: Bilaterally: - Feet, - Shanks (construct validity) | Physilog BFSr-3: 2 Physilog 5: 4 | 500 Hz (criterion-related validity), 256 Hz (construct validity) | Custom |
| 2020 | Kawanishi et al. [47] | IMU (6 axis) | - Tibia (between the lateral aspect of the anterior tibial tuberosity and Gerdy tubercle) | 1 | NR | NR |
| 2012 | Dowling et al. [48] | IMU (6 axis) | - Chest, - Thigh, - Shank | 3 | 240 Hz | Custom real-time feedback |
| 2020 | Diermeier et al. [49] | Accelerometer + image-analysis system (for lateral compartment translation) | - Tibia (Gerdy tubercle) | 1 | 120 Hz | Specifically developed application |
| 2018 | Musahl et al. [50] | Accelerometer | - Proximal tibia | 1 | NR | Proprietary iPad software (KiRA acceleration); custom iPad Image Analysis (video-based translation) |
| 2012 | Lopomo et al. [51] | Accelerometer | - Tibia | 1 | NR | Klee—dedicated software used for kinematic analysis for the BLU-IGS navigation system |
| 2015 | Schmitz et al. [52] | Electromagnetic sensor | - Lateral thigh (midpoint), - Centre of patella, - Tibial shaft (midpoint) | 3 | 100 Hz | NR |
| 2010 | Labbe et al. [53] | Electromagnetic sensor | - Thigh - Shank | 2 | NR | Custom software developed in MATLAB (Mathworks, Natick, MA) |
| 2008 | Kuroda et al. [54] | Electromagnetic sensor | - Thigh (10 cm above the patella), - Tibia (7 cm below the tibial tubercle) (- attached to a stylus for digitising anatomical landmarks) | 3 receivers and 1 transmitter | 60 Hz | 6 Degrees of Freedom (DOF) tibiofemoral kinematics derived from the joint coordinate system [56] |
| 2007 | Yagi et al. [55] | Electromagnetic sensor | - Tibia (10 cm below tibial tubercle), - Femur (13 cm above patella) | 2 | 60 Hz | 6 Degrees of Freedom (DOF) tibiofemoral kinematics derived from the joint coordinate system [56] |
| 2007 | Kubo et al. [57] | Electromagnetic sensor | - Thigh (1), - Tibia (1—distal to tibial tubercle, 1- proximal to the ankle) | 3 (+ 1 additional sensor to register anatomical reference points) | 40 Hz | NR |
| 2025 | Cherelstein et al. [58] | Force-sensing insole | - Under foot | 2 (1 for each foot) | 100 Hz | Loadsol mobile application and custom processing program [62] |
| 2025 | Cherelstein et al. [59] | Force-sensing insole | - Under foot | 2 (1 for each foot) | 100 Hz | Loadsol mobile application and custom processing program [62] |
| 2021 | Luftglass et al. [60] | Force-sensing insole | - Under foot | 2 (1 for each foot) | 200 Hz (for 33 participants), 100 Hz (for 7 participants) | Load analysis program (LAP): a custom MATLAB user-interface for loadsol® data. |
| 2019 | Peebles et al. [61] | Force-sensing insole | - Under foot | 2 (1 for each foot) | 100 Hz | NR |
| 2018 | Peebles et al. [62] | Force-sensing insole | - Under foot | 2 (1 for each foot) | 2 types: 100 Hz, 200 Hz | MATLAB (Version 9, The Mathworks, Inc, Natick, MA, USA) |
| 2025 | Nyffenegger et al. [63] | Electrogoniometer | - Knee (centre aligned with knee joint; proximal arm to greater trochanter, distal arm to lateral malleolus) | 1 | 4000 Hz | IMAGO Process Master (Pfitec®, Endingen, Germany), Microsoft® Excel spreadsheet (Windows 10, Microsoft Corporation, Redmond, WA, USA). |
| 2024 | Busch et al. [64] | Electrogoniometer | - Knee (centre aligned with knee joint; in the midline between the lateral femoral and tibial epicondyle of the leg) | 1 | 4000 Hz | IMAGO Process Master (Pfitec®, Endingen, Germany), Microsoft® Excel spreadsheet (Windows 10, Microsoft Corporation, Redmond, WA, USA). |
| 2024 | Deiss et al. [65] | Inductive displacement sensor | - Patella, - Tibial tuberosity | 2 | NR | NR |
| Category | Study | Validation Reference | Device Type | Outcome Measure |
|---|---|---|---|---|
| Laxity tests | Lopomo et al. [51] | I | Accelerometer | Three-dimensional acceleration of tibia |
| Kubo et al. [57] | I | Electromagnetic sensor | Tibial posterior translation, tibial lateral translation, max posterolaterally directed velocity | |
| Yagi et al. [55] | I | Electromagnetic sensor | Tibial linear acceleration | |
| Labbé et al. [41] | I | IMU (9 axis) | Tibial velocity spike, femoral velocity spike, tibial acceleration drop, femoral acceleration drop | |
| Bellitti et al. [26] | I | IMU (9 axis) + stretchable strain sensors | Anterior–posterior translation, medial–lateral translation, internal–external rotation, flexion–extension rotation, adduction–abduction rotation | |
| Deiss et al. [65] | I | Inductive displacement sensor | Anterior tibial translation | |
| Musahl et al. [50] | P | Accelerometer | Lateral-compartment tibial translation, lateral-compartment tibial acceleration | |
| Diermeier et al. [49] | P | Accelerometer + image-analysis system | Anterior tibial translation (video analysis), tibial acceleration | |
| Kuroda et al. [54] | P | Electromagnetic sensor | Coupled anterior tibial translation (c-ATT), acceleration of posterior translation (APT) | |
| Portillo-Ortiz et al. [42] | P | Smartphone (accelerometer + gyroscope) | Angular velocity (rotational laxity measurement) 3 axis | |
| Labbe et al. 2010 [53] | N | Electromagnetic sensor | AP/ML/total tibial translation magnitude, tibial internal–external rotation magnitude, tibial adduction–abduction magnitude, AP/ML/total translation velocity, tibial internal–external rotation angular velocity, tibial adduction–abduction angular velocity, AP/ML/total translation accelerations, tibial internal–external rotation angular acceleration, tibial adduction–abduction angular acceleration | |
| Kawanishi et al. [47] | N | IMU (6 axis) | Tibial external rotational angular velocity, tibial acceleration | |
| Joint kinematics | Fan et al. [39] | I | IMU (9 axis) | Knee internal rotation, knee abduction, knee flexion |
| Busch et al. [64] | P | Electrogoniometer | Knee joint angle (absolute error, constant error, variable error) | |
| Nyffenegger et al. [63] | P | Electrogoniometer | Knee joint angle (absolute angular error) (JPS) | |
| Mengis et al. [44] | P | IMU (6 axis) | Knee displacement (mm), extension/flexion angles, knee displacement (degrees), angle reproduction angle (JPS) | |
| Niederer et al. [43] | P | IMU (6 axis) | Knee displacement, angle reproduction error (JPS) | |
| Yona et al. [34] | P | IMU (9 axis) | Knee flexion angle | |
| Di Paolo et al. [35] | P | IMU (9 axis) | Knee flexion angle, knee valgus angle | |
| Baldazzi et al. [38]. | P | IMU (9 axis) | Tibial angular displacement—root mean square (RMS) of angular velocity (foot and leg), peak angular velocity (foot and leg), RMS of acceleration (foot and leg), sway path (tibia), sway area (tibia), sway area eccentricity (tibia) | |
| Schmitz et al. [52] | N | Electromagnetic sensor | Anterior tibial translation, knee-flexion excursion, peak knee-flexion angular acceleration (secondary outcome) | |
| Dowling et al. [48] | N | IMU (6 axis) | Max knee flexion angle, first peak of thigh coronal angular velocity | |
| Albano et al. [40] | N | IMU (9 axis) | Maximal Lyapunov exponent (LyE) of knee flexion–extension angle | |
| Button et al. [37] | N | IMU (9 axis) | Knee joint angle waveforms in sagittal and frontal planes | |
| Loading proxies/kinetics | Peebles et al. [62] | I | Force-sensing insole | Peak impact force (PIF), loading rate (LR), impulse (IMP) (total force applied over time), limb symmetry index (LSI) for IP/LR/IMP |
| Sun et al. [45] | I | IMU (6 axis) | Vertical ground reaction force (vGRF), external knee extension moment (KEM) | |
| Benjaminse et al. [36] | I | IMU (9 axis) | Knee abduction moment (KAM) class (classification models), peak KAM (regression models) | |
| Peebles et al. [61] | P | Force-sensing insole | Peak impact force (PIF), loading rate (LR), impulse (IMP) (total force applied over time), LSI for IP/LR/IMP | |
| Luftglass et al. [60] | P | Force-sensing insole | Peak impact force, impulse (total force applied over time), LSI derived from the above metrics | |
| Cherelstein et al. [58] | P | Force-sensing insole | Peak impact force (PIF), instantaneous loading rate (ILR), average loading rate (ALR), impulse (area under the force–time curve from heel strike to toe-off, LSI derived from the above metrics | |
| Cherelstein et al. [59] | P | Force-sensing insole | Peak impact force (PIF), average loading rate, impulse, LSI derived from the above metrics | |
| Validated temporospatial proxies | Ahmadian et al. [46] | I | IMU (6 axis) | Foot-ground initial contact (IC) instants, foot-ground terminal contact (TC) instances, flying and landing times, individual distances, foot forwards progression distances, time and distance-based LSI |
| Study | TRL Band | Justification |
|---|---|---|
| Niederer et al. [43] | 7 | Nationwide rehabilitation registry using Orthelligent Pro tibial IMU with standard operating procedure-based, clinician-run testing (hop/jump, Y-Balance, JPS) across multiple centres; operational use in practice, but no explicit regulatory status. |
| Yagi et al. [55] | 5–6 | Pivot-shift under general anaesthesia at 1-year follow-up using thigh/shank Polhemus Fastrak sensors; validated pipeline (r = 0.995; ≤0.85 mm error); research deployment in a clinical setting, not routine care. |
| Kuroda et al. [54] | 5–6 | Intraoperative pivot-shift under GA using strapped FASTRAK sensors and stylus digitisation; 6-DoF kinematics with c-ATT/APT outcomes; patient cohorts in theatre; research workflow, no routine integration. |
| Lopomo et al. [51] | 5–6 | Intra-operative pivot-shift under general anaesthesia using strapped KiRA accelerometer validated against navigation; good repeatability/correlation; single-site research setup, not routine care. |
| Musahl et al. [50] | 5–6 | Multi-centre, surgeon-performed preoperative pivot-shift under general anaesthesia using tibial IMU (KiRA) and iPad image-analysis; trained users; research setting; no routine workflow/regulatory use. |
| Kawanishi et al. [47] | 5–6 | Intraoperative pivot-shift under general anaesthesia with a strapped tibial IMU quantifying acceleration and external rotation angular velocity; 91 ACLR cases, surgeon-run protocol, receiver operating characteristic cut-offs for predicting residual instability; research workflow, no routine integration/regulatory status. |
| Diermeier et al. [49] | 5–6 | Multi-centre (4 sites) pivot-shift quantified preoperatively, at time zero after ACLR (under general anaesthesia) and after 24 months using a strapped KiRA accelerometer and a tablet image-analysis; surgeon-standardised protocol; research deployment (no routine workflow/regulatory status). |
| Labbe et al. [53] | 5–6 | Clinician-performed pivot shift in ACL-deficient patients using thigh/shank electromagnetic sensors; multi-surgeon cohort; custom MATLAB feature extraction/PCA; research workflow, no routine clinical integration. |
| Labbé et al. [41] | 5–6 | Clinical pivot-shift in ACL-deficient patients (no GA) using two strapped IMUs (tibia and femur); “femoral acceleration drop” strongly matches clinical grade (r = 0.84); research use, not routine. |
| Kubo et al. [57] | 5–6 | Clinic pivot-shift in ACL-deficient patients using Polhemus Fastrak; correlated with IKDC; bone–pin validation (r = 0.995, ≤0.85 mm error); researcher-run, single-site |
| Portillo-Ortiz et al. [42] | 5–6 | Multi-centre outpatient clinics; trained surgeons; immediate feedback; framed as trial version; not yet routine/regulated integration. |
| Button et al. [37] | 5–6 | Used in ACLR patients within a physiotherapy department; seven IMUs with a custom MATLAB reporting tool (gait/squat/stair ascent); clinician-run agreement study; complete research workflow but not embedded as routine care and no regulatory status stated |
| Yona et al. [34] | 5–6 | ACLR patients tested on full staircase during clinic visits; complete workflow but not embedded as routine care; no regulatory claims. |
| Mengis et al. [44] | 5–6 | Single-centre clinical validation of a commercial tibial IMU during supervised rehabilitation visits at 3 and 6 months post-ACLR; standardised test battery (ROM, Y-Balance, vertical/side hops) with significant correlations to IKDC; conducted within an RCT; regulatory status not stated. |
| Cherelstein et al. [59] | 5–6 | Multi-site clinic-based DVJ testing at 6 ± 1 months post-ACLR using force-sensing insoles (100 Hz) with a standardised calibration; outcomes PIF, ALR, impulse (LSI); supervised clinical assessments within a study, no routine workflow/regulatory claims. |
| Cherelstein et al. [58] | 5–6 | Multi-site rehab-clinic/clinical-lab treadmill gait at 6 ± 1 months post-ACLR using loadsol insoles (100 Hz) with standardised calibration; outcomes = PIF, ILR, ALR, impulse (LSI); researcher-supervised, no stated regulatory status or routine workflow integration. |
| Peebles et al. [62] | 3–4 | Laboratory validity/repeatability study in healthy athletes using loadsol insoles during single-leg hop and stop-jump; compared to force plates (100 Hz and newer 200 Hz version: validity improves, but absolute loads are underestimated); researcher-run, no clinical workflow. |
| Luftglass et al. [60] | 3–4 | Laboratory study in healthy adults using loadsol (200 Hz) and the LAP MATLAB interface for landing kinetics/LSI; researcher-run, no clinic deployment or regulatory status. |
| Peebles et al. [61] | 3–4 | Research-run RTS testing with loadsol insoles (100 Hz) during single/triple/crossover hops in ACLR vs healthy; outcomes = LSI of impact peak, loading rate, impulse; no clinical workflow/regulatory use. |
| Fan et al. [39] | 3–4 | Laboratory validation in healthy adults using four Xsens IMUs (100 Hz) on thigh/shank during drop landing and 45° cutting; new two-step complementary filter + single-pose calibration; errors vs Vicon = 1.07° flexion, 2.87° abduction, 2.64° internal rotation; researcher-run, no clinic workflow or regulatory use. |
| Di Paolo et al. [35] | 3–4 | Laboratory screening in healthy athletes with 15-IMU Xsens and custom processing; change of direction/deceleration tasks; Vicon used only for moments; research workflow, no clinical integration/regulatory status. |
| Benjaminse et al. [36] | 3–4 | Lab model-development study in healthy youth female footballers using 17-IMU, motion capture and force plates during unanticipated sidestep cutting; ML classifies high vs low KAM (AUC 0.81–0.85); researcher-run workflow, no field/clinical deployment or regulatory status. |
| Sun et al. [45] | 3–4 | Laboratory model-development in healthy males using 8 IMUs with motion capture and force plates; modular LSTM estimates vertical ground reaction forces / knee-extension moment in real time for single/double-leg drop landings; researcher-run; no clinical workflow/regulatory status. |
| Ahmadian et al. [46] | 3–4 | Laboratory setting, researcher-run pipeline; not embedded in routine care; no regulatory claims. Two foot/shank IMUs; validated IC/TC, times, and distances vs motion capture; exploratory patient–control cohort; KOOS correlations; no clinical workflow use. |
| Dowling et al. [48] | 3–4 | Laboratory drop-jump training in healthy athletes with 3 cabled IMUs and custom real-time feedback; Vicon/force plate used only as reference; researcher-run, no clinical workflow/regulatory status. |
| Baldazzi et al. [38] | 3–4 | Lab protocol in healthy soccer players using two strapped IMUs on tibia and foot during single-leg squat and crossover hop; reports reliability (ICC 0.29–0.84, MDC) for stability metrics; researcher-run, no clinical workflow/regulatory status. |
| Albano et al. [40] | 3–4 | Laboratory treadmill walking with four Xsens Dot sensors on thighs and legs; knee flexion–extension derived from inclinometry; Lyapunov exponent (LyE) variability metric; n = 4 (ACLR and healthy); researcher-run, no clinical workflow/regulatory status. |
| Busch et al. [64] | 3–4 | Laboratory JPS pilot with electrogoniometer, sEMG and dry-EEG (DSI-24); ACLR patients measured at 1.5/3–4/6 months; researcher-run, single-site; no clinical workflow or regulatory status. |
| Nyffenegger et al. [63] | 3–4 | Laboratory pilot, exploratory neurophysiology; small early postoperative cohort; no clinic workflow or deployment. |
| Schmitz et al. [52] | 3–4 | Lab VKLD test in healthy adults with strapped miniBIRD trackers measuring anterior tibial translation (ATT) and knee-flexion under 40% BW; researcher-run; no clinical workflow/regulatory status. |
| Bellitti et al. [26] | 3–4 | Smart knee brace (2 IMUs and 3 stretch sensors); lab characterisation vs Xsens plus small pilot (n = 4) of Lachman/drawer/pivot-shift vs optical; researcher-run; no clinical integration/regulatory. |
| Deiss et al. [65] | 3–4 | Laboratory prototype ATT device (two inductive displacement sensors at patella and tibial tuberosity) in healthy volunteers; good test–retest (Lachman ICC 0.90–0.94) and concurrent validity vs Lachmeter; researcher-run, no clinical integration/regulatory. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptaszyk, O.; Boutefnouchet, T.; Cummins, G.; Kim, J.M.; Ding, Z. Wearable Devices for the Quantitative Assessment of Knee Joint Function After Anterior Cruciate Ligament Injury or Reconstruction: A Scoping Review. Sensors 2025, 25, 5837. https://doi.org/10.3390/s25185837
Ptaszyk O, Boutefnouchet T, Cummins G, Kim JM, Ding Z. Wearable Devices for the Quantitative Assessment of Knee Joint Function After Anterior Cruciate Ligament Injury or Reconstruction: A Scoping Review. Sensors. 2025; 25(18):5837. https://doi.org/10.3390/s25185837
Chicago/Turabian StylePtaszyk, Oliwia, Tarek Boutefnouchet, Gerard Cummins, Jin Min Kim, and Ziyun Ding. 2025. "Wearable Devices for the Quantitative Assessment of Knee Joint Function After Anterior Cruciate Ligament Injury or Reconstruction: A Scoping Review" Sensors 25, no. 18: 5837. https://doi.org/10.3390/s25185837
APA StylePtaszyk, O., Boutefnouchet, T., Cummins, G., Kim, J. M., & Ding, Z. (2025). Wearable Devices for the Quantitative Assessment of Knee Joint Function After Anterior Cruciate Ligament Injury or Reconstruction: A Scoping Review. Sensors, 25(18), 5837. https://doi.org/10.3390/s25185837







