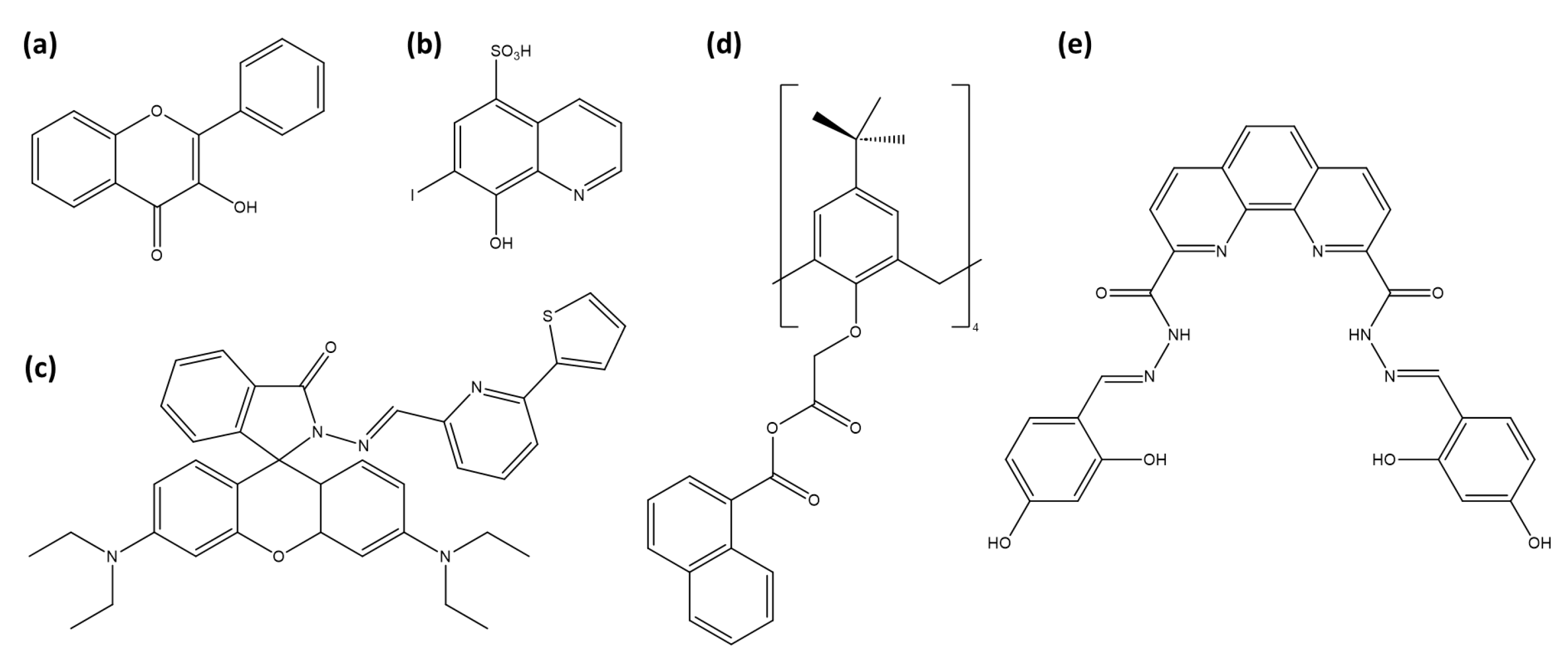
Abstract
With nuclear power playing an increasing role in efforts to reduce carbon emissions, the development of effective and sensitive monitoring tools for (radio)toxic elements in the environment has become essential. This review highlights recent advances in fluorescent probes developed for the detection of key elements associated with the nuclear industry, including uranium, cesium, strontium, technetium, zirconium, and beryllium. Various sensor platforms, ranging from organic ligands and DNAzymes to metal–organic frameworks and quantum dots, offer promising features, such as high sensitivity, selectivity, and suitability for environmental matrices. Several recent designs now achieve detection limits in the nanomolar to picomolar range, revealing new perspectives for environmental and biological applications.
1. Introduction
As the global community confronts the challenges of climate change and the urgent need to reduce greenhouse gas emissions, nuclear energy is increasingly recognized as a low-carbon, large-scale power source capable of complementing renewable energies [1,2,3]. Consequently, many countries have committed to expanding their nuclear energy capacity, resulting in the construction of new reactors and the extended operation of aging facilities. While nuclear power offers clear environmental and energy security advantages, it also raises critical concerns regarding the safe management of radioactive materials and their potential release into the environment [4,5]. Regulatory frameworks at national and international levels, such as IAEA or national nuclear safety agencies, demand the rigorous monitoring of radioactive contaminants across the nuclear fuel cycle, from mining to waste disposal [6]. As nuclear infrastructure ages and scales up, the development of efficient, rapid, and field-deployable detection methods becomes increasingly vital to ensure safety, regulatory compliance, and environmental protection.
Radionuclides originating from the nuclear industry are diverse and include fuel elements, fission products, and activation products [7,8]. Conventional pressurized water reactors use fuel containing uranium-238 (238U), enriched to approximately 3.5% with uranium-235 (235U). After the fuel is spent, it still contains about 95% uranium, with 1% of this being 235U. The spent fuel also contains about 1% plutonium, primarily 239Pu and 240Pu, and less than 0.1% minor actinides. Of these minor actinides, approximately half are neptunium-237, followed by americium-241, americium-243, and curium-244. Table 1 presents the main actinides found in the spent fuel, along with their characteristics.

Table 1.
Actinides’ characteristics.
The remaining 4% of the spent fuel consists of fission products, which are divided into two categories [9]. The first category includes medium-lived isotopes, with half-lives under 50 years, where cesium-137 and strontium-90 are the most prevalent. The second category comprises long-lived isotopes, with half-lives exceeding years and the major contributors being cesium-135, technetium-99, and zirconium-93. Table 2 presents the characteristics of the main fission products.

Table 2.
Fission products’ characteristics. a Noble gas, no impact. b Decay product energy: 3.67 MeV.
Activation products are materials that become radioactive after exposure to neutron radiation in a nuclear reactor. They originate from both the structural materials of the reactor (e.g., cobalt-60, iron-55, nickel-63, manganese-54, and beryllium-10) and the reactor coolant (e.g., nitrogen-16, tritium, carbon-14, and sodium-24) [10]. The nature and quantities of these activation products depend on factors such as the reactor design, the materials used in construction, and the reactor’s operating conditions, including neutron flux and exposure duration.
These elements present significant risks to human health and the environment due to their radiotoxicity and chemical behavior. Chemically, many of these substances tend to bioaccumulate in specific tissues, such as bones or organs. Their radioactivity, which deposits energy in tissues, can cause severe biological damage, such as cancer. This combination of radiological and chemical dangers necessitates safety measures to limit exposure and environmental contamination.
Monitoring environmental contamination using these elements is crucial for assessing and mitigating risks to ecosystems and human health. Various analytical techniques are employed for this purpose, including the development of sensors, compounds, or systems designed to interact with specific target ions and generate measurable responses. These sensors may be chemical, electrochemical, bacterial-cell, or protein-based, but they all rely on selective interactions with their target ions to produce a detectable signal [11]. This signal can manifest as a change in spectroscopic properties (such as absorption or fluorescence), electrochemical behavior (voltammetry, potentiometry), or even biological responses (such as gene expression). This review focuses on a specific class of optical probes: fluorescent sensors.
Fluorescent sensors offer significant advantages for environmental detection, including high sensitivity, rapid response times, and the capability for real-time monitoring [12,13]. These sensors can detect metal ions at low concentrations, often below the ppm level. Furthermore, sensitive and selective sensors reduce the need for complex sample preparation and expensive instrumentation, such as laboratory spectrometers. This makes them cost-effective tools for environmental monitoring, especially in portable devices, enabling direct on-site detection [14].
This review will focus on the fluorescent detection of key elements in the nuclear industry. Concerning transuranium elements, to the best of our knowledge, no fluorescent sensors have been developed for neptunium, plutonium, americium, or curium. The detection and quantification of these radioisotopes primarily rely on spectroscopic techniques such as alpha spectroscopy, liquid scintillation counting, UV-Vis-NIR absorption, Raman scattering, and mass spectrometry methods, including ICP-MS [15,16,17,18]. Thorium has limited use in the nuclear industry and has already been the subject of numerous reviews concerning fluorescent probes [19,20,21]. Fluorescent probes for uranium will be examined in detail, as it is the primary element used in nuclear applications.
As discussed previously, the main fission products are cesium with its two isotopes, followed by strontium, technetium, and zirconium. Therefore, their fluorescent probes will be detailed here. Activation products primarily include cobalt-60, iron-55, nickel-63, manganese-54, and beryllium-10. Except for beryllium, all of these are transition metals. Numerous reviews have already explored recent advancements in the development of fluorescent chemosensors specifically designed for detecting these transition elements [22,23,24,25]. To our knowledge, no review has specifically focused on fluorescent probes for beryllium, making its inclusion here necessary. This review focuses on the diversity of fluorescent probes, highlighting different sensor types, properties, matrices, and applications, and thus complements recent reviews on sensors for radioactive elements [26,27].
2. Fluorescent Sensors
Fluorescent sensors are categorized into three families based on their response to ion complexation: turn-on (fluorescence enhancement), turn-off (fluorescence quenching), and ratiometric (shift in emission wavelength) [28,29,30]. These responses are governed by different fluorescence mechanisms described in the following sections.
2.1. Photoinduced Electron Transfer
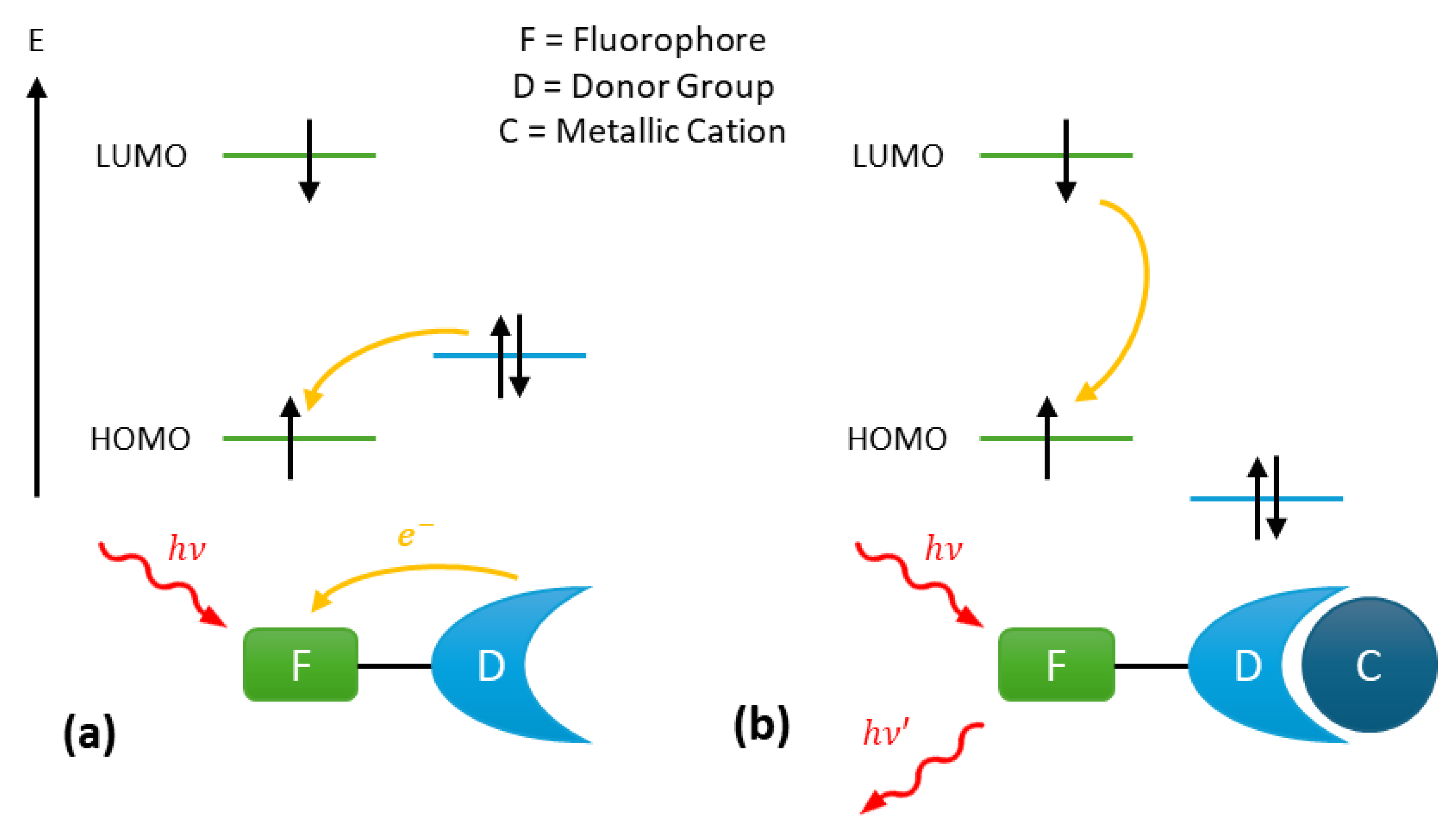
The photoinduced electron transfer (PeT) mechanism is commonly employed in the design of turn-on fluorescent probes. Such probes typically consist of a fluorophore linked via a spacer to a donor group able to complex the target ion with a certain level of selectivity (Figure 1). Upon absorption of a photoelectron, the electron occupying the highest occupied molecular orbital (HOMO) of the fluorophore transitions to the lowest unoccupied molecular orbital (LUMO) of the fluorophore. Subsequently, an electron transfer occurs from the HOMO of the donor group to the HOMO of the fluorophore, resulting in fluorescence quenching (Figure 1a). This process effectively blocks the emission transition of the excited fluorophore from the LUMO to the HOMO.
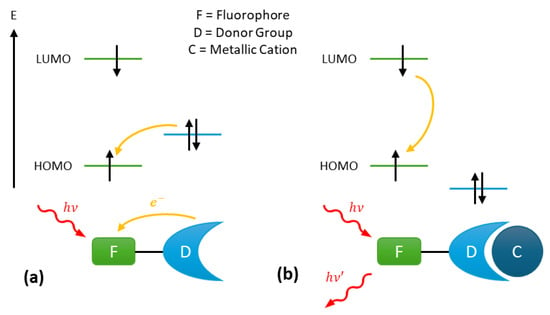
Figure 1.
Fluorescence mechanism of photoinduced electron Transfer (PeT) probes: (a) fluorescence quenching by the donor group and (b) fluorescence enabling via cation complexation on the donor group.
However, when the metal cation is complexed to the donor group, the energy level of the donor group’s HOMO becomes lower than that of the fluorophore’s HOMO (Figure 1b). As a result, the PeT in no longer possible, and the fluorescence from the probe is restored, enabled through the de-excitation of the fluorophore [31].
2.2. Photo-Induced Charge Transfer
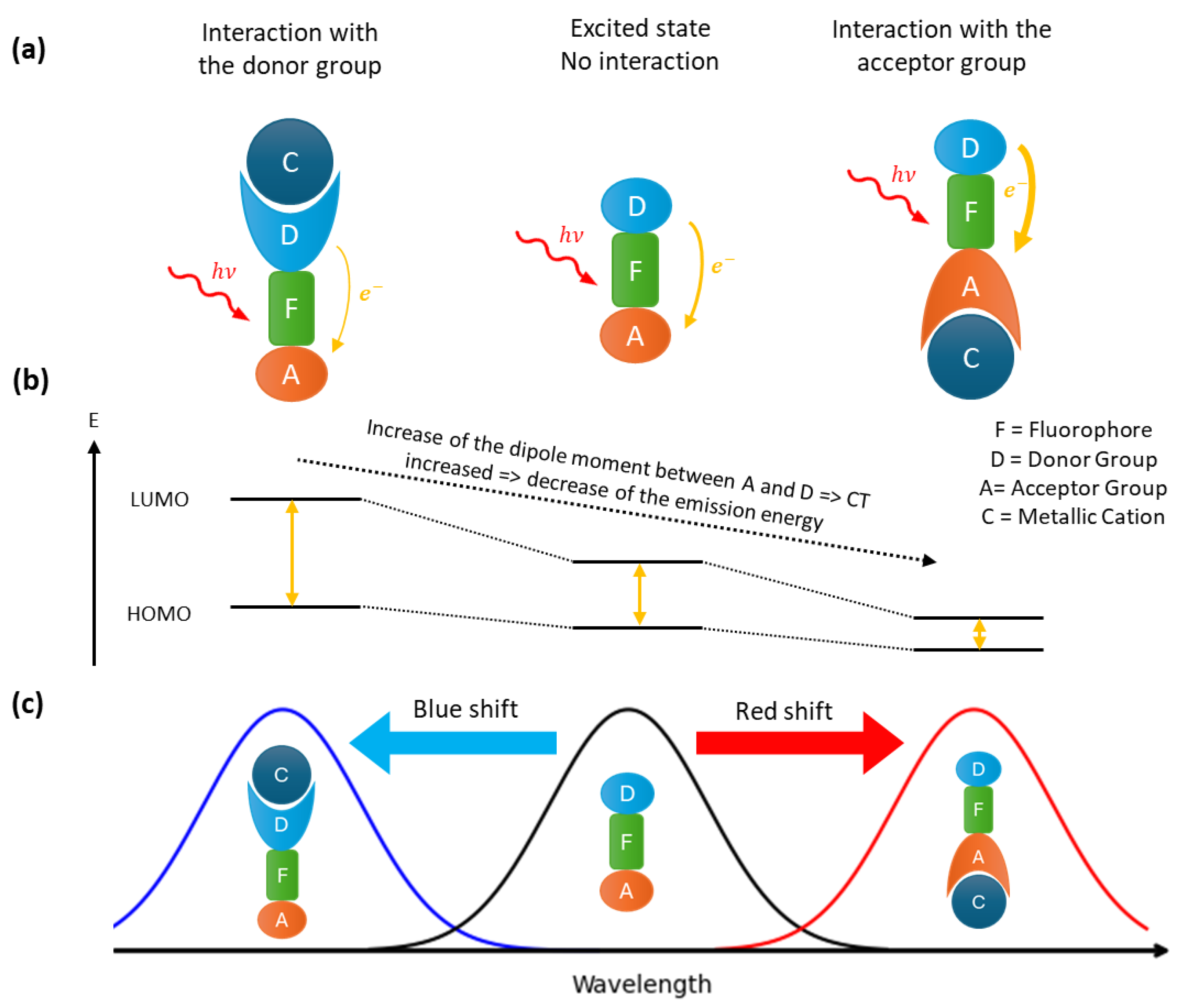
Fluorophores that contain both an acceptor and a donor group facilitate the construction of ratiometric probes. Upon excitation, this kind of fluorophore undergoes an intramolecular charge transfer, known as a photo-induced charge transfer (PCT). The latter having an energy cost, the emitted photon will be of lower energy than the excitation photon, resulting in a red shift in the fluorescence spectrum compared to the absorbance spectrum. PCT depends of the dipole moment between the donor and acceptor groups in the fluorophore (Figure 2). Therefore, the interaction of a cation with one of these groups will change the dipole moment. Depending of the complexation site of the cation, the gap energy between LUMO and HOMO is changed, leading to a shift of the emission wavelength. If the donor site is involved, a blue shift (an increase in the energy gap) will result, whereas complexation via the acceptor site will lead to a red shift (a decrease in the energy gap). If the charge transferred is a proton, the effect is known as an excited state intramolecular proton transfer (ESIPT) [32].
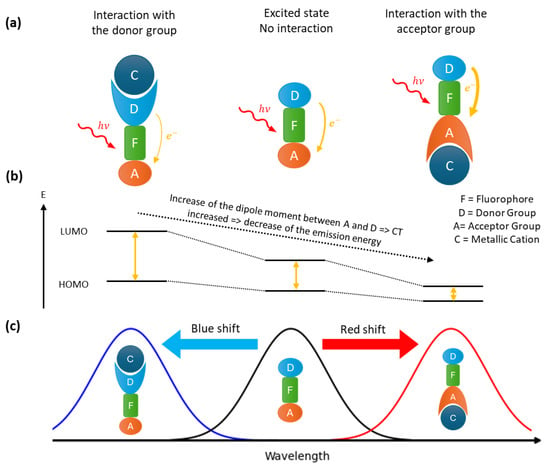
Figure 2.
Fluorescence mechanism of PCT probes: (a) different interaction of the cation with the fluorophore and the corresponding spectral displacement (b) and fluorescence spectra (c).
2.3. Fluorescence Resonance Energy Transfer
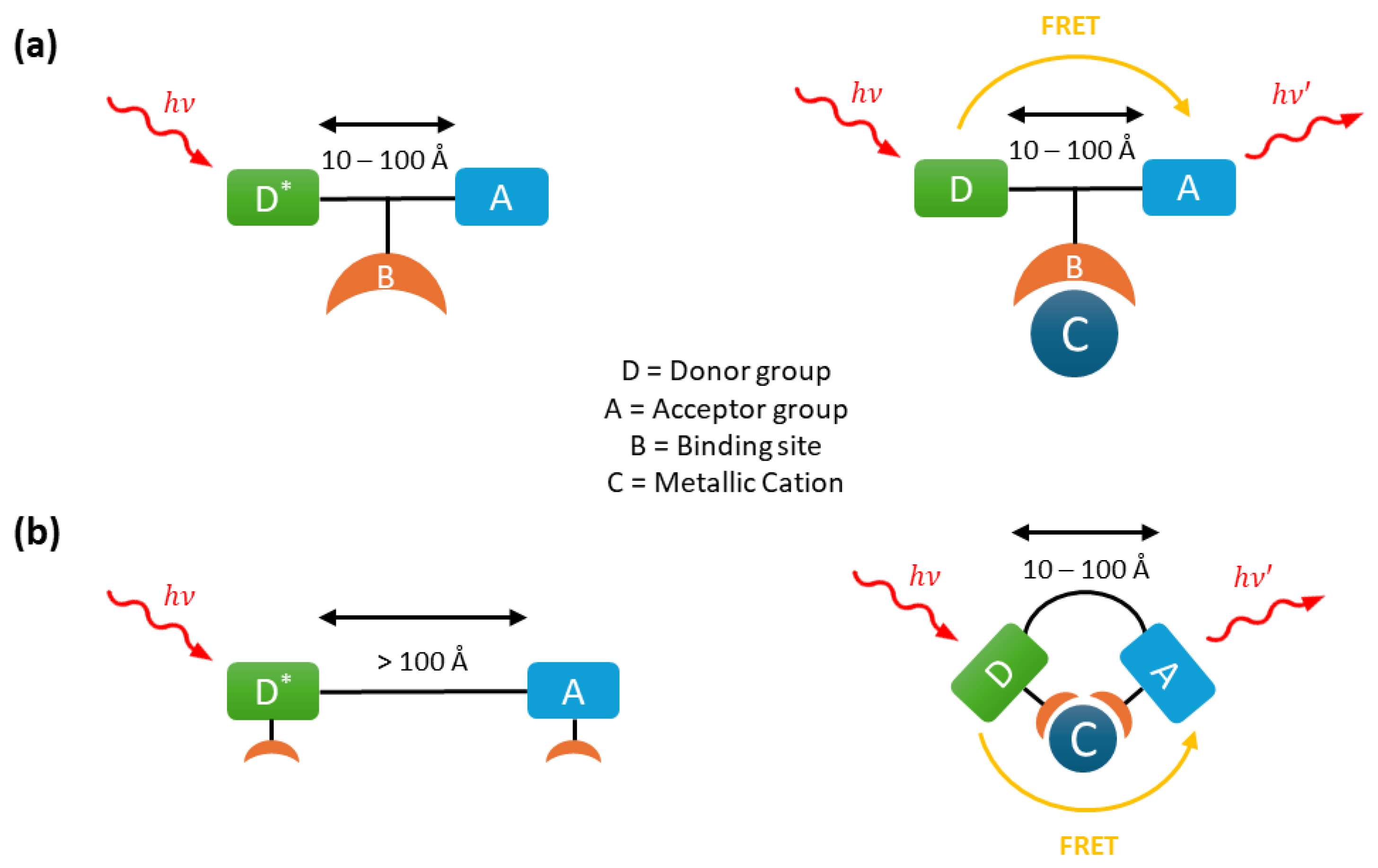
Fluorescence emission can occur after resonance energy transfer (FRET) from a donor group to an acceptor group separated by a distance typically ranging from 10 to 100 Å. This non-radiative energy transfer occurs when the emission spectrum of the donor overlaps with the absorption spectrum of the acceptor. The excited donor transfers its energy via a long-range dipole–dipole interaction to the acceptor, which subsequently emits this energy as fluorescence.
This mechanism is widely employed in ratiometric fluorescent probes through two main designs. In the first, a metal ion can activate FRET by enabling or enhancing the fluorescence emission of either the donor or acceptor group (Figure 3a). This is achieved by increasing the spectral overlap between the emission spectrum of the donor and the excitation spectrum of the acceptor. In the second design, FRET is initially inhibited because the donor and acceptor groups are separated by a distance greater than 100 Å (Figure 3b). However, the binding of a metal ion can alter the molecular conformation, bringing the donor and acceptor closer together and thereby activating FRET.
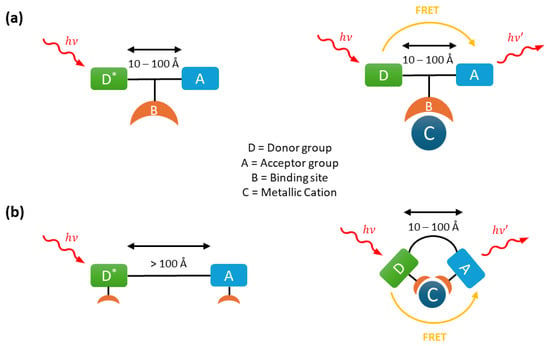
Figure 3.
Fluorescence mechanism of Förster Resonance Energy Transfer (FRET) probes: (a) activation of FRET with cation binding and (b) with metal cation complexation bringing the two groups together.
2.4. Fluorescence Aggregation Mechanisms
Aggregation-induced emission (AIE) and aggregation-caused quenching (ACQ) are two contrasting photophysical phenomena that describe the behavior of fluorescent molecules in their aggregated states. In AIE, molecules are non-emissive in dilute solutions due to non-radiative decay pathways such as intramolecular rotations or vibrations but become fluorescent upon aggregation as these motions are restricted. Due to this property, AIE compounds have significant applications in bioimaging, sensors, and optoelectronics [33]. In the presence of specific ions, AIE sensors undergo aggregation, driven by ion coordination or interaction, which activates fluorescence.
Conversely, ACQ occurs when luminescent molecules lose their fluorescence upon aggregation due to strong intermolecular interactions like - stacking, which create non-radiative pathways such as excimer or exciplex formation. Although commonly observed in planar aromatic systems, ACQ is used to design turn-off fluorescent probes, which are often referred to as ’AIE turn-off’ probes in the literature.
2.5. Absorbance-Caused Enhancement
Absorbance-caused enhancement (ACE) is a fluorescence phenomenon through which the presence of an absorbing species near a fluorophore increases its light absorption, resulting in enhanced fluorescence emission. In ion sensors, ion complexation increases the absorbance of the complex, which in turn amplifies fluorescence and enables ion detection.
3. Fluorescent Probes for Nuclear Industry Elements
3.1. Uranium
Due to its natural abundance and significant role in the nuclear industry, uranium is the primary actinide studied for detection using sensors. A recent review by Wu et al. highlights that fluorescent sensors are the most popular choice, owing to their superior sensitivity and selectivity for uranium over other metals [34]. In nature, uranium is predominantly found in the +VI oxidation state, corresponding to the trans-dioxo form [UO2]2+, commonly known as uranyl. Uranyl ions accept ligands in their equatorial plane, typically with a coordination number ranging from 4 to 6 [35,36,37].
[UO2]2+ ions emit intrinsic fluorescence in the range of 450–600 nm under UV excitation. This property was widely exploited during the latter half of the 20th century for detecting uranium in geological samples [38]. The detection of trace levels of uranium requires laser excitation of the sample, typically using laser fluorimetry or, for enhanced sensitivity and speciation capability, time-resolved laser-induced fluorimetry [39]. The intrinsic fluorescence of uranium can be significantly enhanced by stabilizing it with an appropriate ligand. Dipicolinic acid (DPA), which forms a 1:2 complex with uranyl ions in aqueous medium ([UO2]2+:DPA), serves this purpose effectively [40,41]. DPA enhances the fluorescence emission of uranyl by an approximate factor of six compared to the emission in the absence of the ligand [42]. Trimesic acid and phosphoric acid have also been shown to enhance uranyl fluorescence in an aqueous solution [43,44]. Maji and Viswanathan further demonstrated a co-fluorescence effect with the addition of yttrium ions, enabling uranium detection at the ppm level [44].
However, the detection limits of these techniques, especially for field measurements, was proven to be limited. In response, the past two decades have seen a growing interest in the development of fluorescence sensors for [UO2]2+, significantly enhancing both sensitivity and selectivity.
3.1.1. Organic Ligands
A wide variety of organic fluorescent sensors have been specifically developed for uranyl detection (Table 3). In most cases, a fluorescent moiety is linked to a coordination site for the [UO2]2+ cation. Uranyl complexation enables detection through one of three mechanisms: fluorescence enhancement (turn-on), fluorescence quenching (turn-off), or a shift in fluorescence emission.
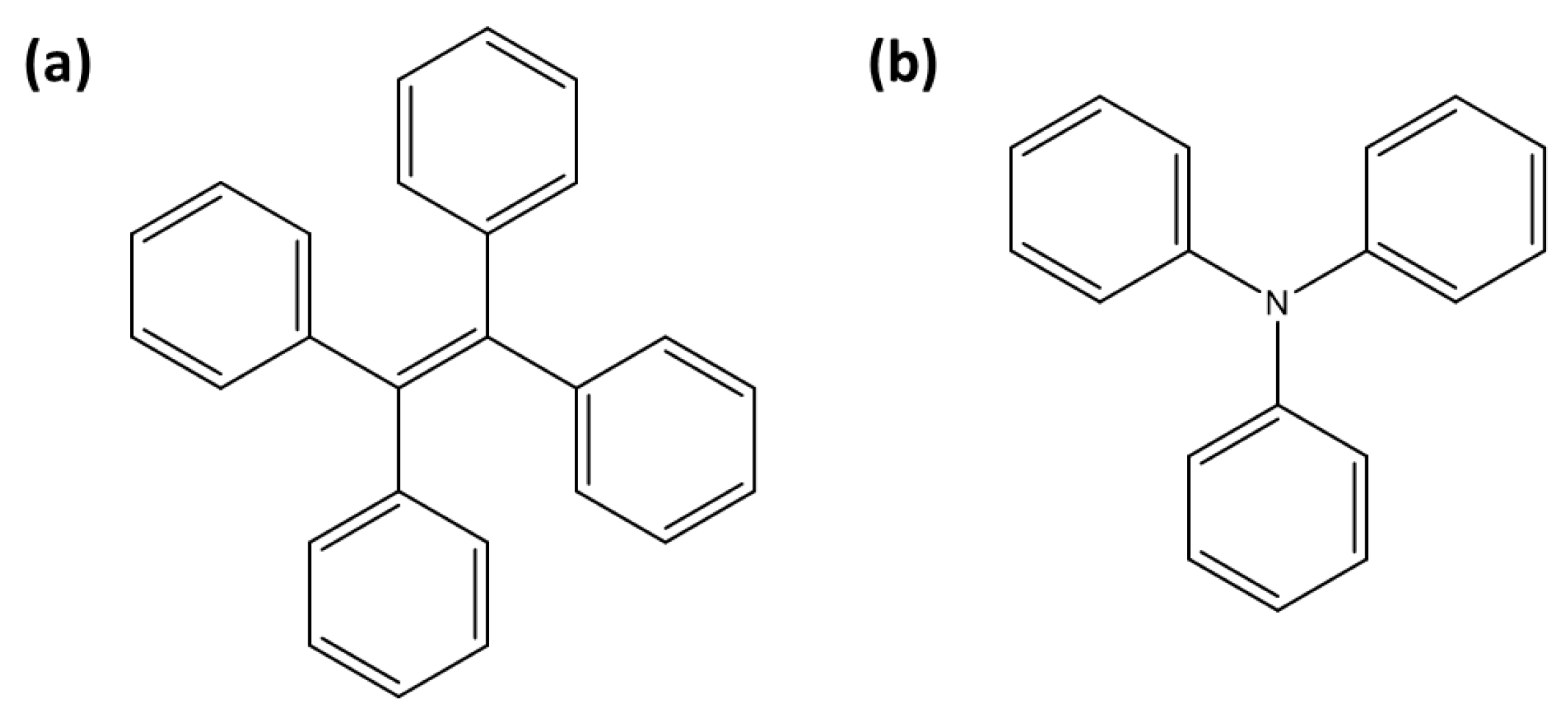
A tetraphenylethylene (TPE) moiety (Figure 4a) linked to a uranyl-specific coordination unit has been widely utilized over the past decade for detecting uranyl ions (Table 3). TPE-based sensors are generally fluorescent, but their fluorescence is quenched upon uranyl complexation through the ACQ effect. Wen et al. reported the design of TPE-T, where the uranyl coordination unit is 2-(4,5-dihydrothiazol-2-yl)phenol [45]. In this system, uranyl ions are stabilized coordination with two TPE-T moieties, leading to ACQ. TPE-T exhibits 96% fluorescence quenching in the presence of uranyl ions, enabling clear differentiation from other metal ions. Additionally, TPE-T operates effectively across a broad pH range and demonstrates strong anti-interference capabilities, making it highly suitable for environmental applications, such as determining uranyl concentrations in river water.
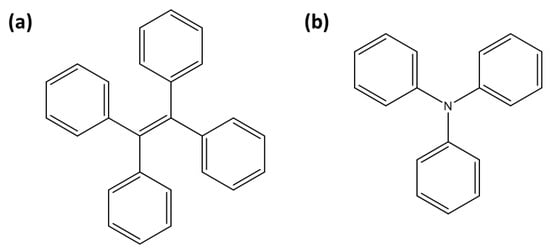
Figure 4.
Representation of fluorescent moieties for [UO2]2+ detection: (a) tetraphenylethylene (TPE) and (b) triphenylamine (TPA).

Table 3.
[UO2]2+ sensors. NMM: N-methyl-mesoporphyrin IX.
Table 3.
[UO2]2+ sensors. NMM: N-methyl-mesoporphyrin IX.
| Type | Compound | Mechanism | / (nm) | LOD (nM) | Matrix | Refs. | |
|---|---|---|---|---|---|---|---|
| TPE- | 2-(4,5-dihydrothiazol-2-yl)phenol | ACQ | 280/494 | nd | River water (pH 2–10) | [45] | |
| based | Salophen | ACQ | 345/548 | 39 | Drinking water (pH 3–9) | [46] | |
| Carbamoylphosphine oxide | ACQ | 330/470 | 32 | Tap water (pH 4) | [47] | ||
| Amidoxime | ACQ | nd/444 | 4.7 | River water (pH 4–7) | [48] | ||
| Amidoxime | ACQ | 340/485 | 7.9 | Drinking water (pH 3–13) | [49] | ||
| Carbodiimide | ACQ/PCT | 346/512 | 0.07 | Nuclear waste and seawater (pH 5–9) | [50] | ||
| 2-hydroxy-benzalaniline | AIE | 350/500 | 11 | Natural water (pH 4–9) | [51] | ||
| TPA- | 2-(4,5-dihydrothiazol-2-yl)phenol | ACQ | 350/510 | 50 | Water (pH 4.5–7)/living cells | [52] | |
| based | 2-(Aminophenyl)iminomethylphenol | ACQ | 380/550 | 39 | Water (pH 2–10)/living cells | [53] | |
| TPA-benzoyl hydrazine | ACQ | 384/510 | 0.2 | Water (pH 2–10)/living cells | [54] | ||
| 4-aldehyde-4,4-bis(4-pyridyl)TPA | ACQ | 430/525 | 0.01 | Groundwater (pH 7.4) | [54] | ||
| Salicyl | 4-pethoxycarboxyl SA | AIE | 370/540 | 0.8 | Nuclear wastewaters (pH 7–10) | [55] | |
| aldehyde | 3-hydroxy-flavone SA | AIE | 370/457 | 2.1 | H2O (pH 5–8.5)/living cells | [56] | |
| azine (SA) | SA and 5-nitro SA | AIE | 365/550 | 23 | H2O/CH3CN mixture | [57] | |
| Other | Calcein | Turn-off | 492/520 | 60 | H2O (pH 4) | [58] | |
| organic | Quinoxalinol salen | Turn-off | 450/540 | nd | H2O/DMF mixture | [59] | |
| ligands | Curcumin | Turn-off | 424/507 | nd | Tap water (pH 4) | [60] | |
| Esculin | Turn-off | 390–455 | 6 | H2O (pH 5–7) | [61] | ||
| Naphthalimide | Turn-off | 403/523 | 4100 | Acetonitrile | [62] | ||
| Furosemide | Turn-on | 320/522 | 500 | H2O (pH 5.5) | [63] | ||
| Fluorophore | Quencher | ||||||
| DNAzymes | Fluorescein | Black Hole | FRET turn-on | nd/520 | 0.05 | Soil samples (pH 5.5) | [64] |
| amidite | Quencher 1 | FRET turn-on | 487/520 | 0.02 | Tap and river waters (pH 5.5) | [65] | |
| (FAM) | Dabcyl | FRET turn-on | nd/520 | 0.6 | Living cells | [66] | |
| Graphene oxide | FRET turn-on | 490/nd | 0.03 | Tap and river waters (pH 5.5) | [67] | ||
| AuNPs | FRET turn-on | nd | 0.01 | Natural waters (pH 5.5) | [68] | ||
| AuNPs | FRET turn-on | nd/520 | 0.0001 | River water (pH 5.5) | [69] | ||
| MoS2 nanosheets | FRET turn-off | 495/525 | 0.002 | River water (pH 5.5) | [70] | ||
| Rhodamine | Guanine bases | FRET turn-on | 399/609 | 0.09 | Natural waters (pH 5.5) | [71] | |
| SYBR Green I | None | FRET turn-off | nd/525 | 0.2 | Natural waters (pH 3.5) | [72] | |
| Eu compound | Salophen-Eu-phosphate | PeT turn-on | no/413 | 8 | Water (pH 4–9) | [73] | |
| Eu compound | Eu-phosphate | PeT turn-on | 400/597 | 10 | Water (pH 7.2–8.8)/cells | [74] | |
Lin et al. introduced a second TPE-based fluorophore utilizing salophen as the uranyl coordination unit [46]. Salophen, N,N′-Bis(salicylidene)-1,2-phenylenediamine, is well known for its strong affinity for [UO2]2+, forming a stable complex with a 1:1 stoechiometry [75]. Similar to TPE-T, uranyl complexation with salophen results in fluorescence quenching, enabling a limit of detection as low as 39 nM. Lin et al. also developed a self-assembled monolayer incorporating TPE in conjunction with carbamoylphosphine oxide as the uranyl coordination unit [47]. These compounds are randomly distributed on a quartz glass substrate, enabling fluorescence quenching upon uranyl complexation. This configuration enhances detection capabilities, offering a robust platform for sensitive uranyl ion sensing.
Ding et al. and Zhan et al. developed uranyl sensors, TPE-A and TPE-ADX, respectively, based on TPE combined with amidoxime as the coordination unit [48,49]. Both sensors utilize fluorescence quenching upon uranyl complexation, facilitated via the interaction of two amidoxime moieties with the uranyl ion. TPE-ADX demonstrates a broader pH range (pH 3–13) compared to TPE-A (pH 4–7). Both sensors achieve low detection limits, with 4.7 nM for TPE-A and 7.9 nM for TPE-ADX. Feng et al. developed another TPE-based fluorescent sensor incorporating carbodiimide to create uranyl-specific coordination sites. This sensor achieves an ultra-low detection limit of 69 pM and demonstrates high selectivity by leveraging ACQ and PCT mechanisms. It operates effectively within a pH range of 5–9.
Lin et al. developed a distinct TPE-based sensor, in which TPE is linked to 2-hydroxy-benzalaniline, which operates through its hydrolysis induced via uranyl ions [51]. The hydrolysis product leads to enhanced and blue-shifted fluorescence upon interaction with [UO2]2+, contrasting with the fluorescence quenching observed in previous TPE-based sensors.
Triphenylamine (TPA) (Figure 4b) is widely used in the design of fluorescent sensors due to its strong electron-donating properties [76]. TPA-based sensors generally exhibit fluorescence, which is quenched upon interaction with uranyl ions, making them effective turn-off probes (Table 3). Zheng et al. designed a probe in which TPA is linked to a 2-(4,5-dihydrothiazol-2-yl)phenol coordination unit [52]. It exhibits high selectivity for uranyl ions, even in the presence of other metal ions, with interference from Cu2+ and Ni2+ being manageable through the use of masking agents like EDTA. Subsequently, Wu et al. developed a TPA-based probe, which incorporates a modified benzalaniline as the coordination unit for [UO2]2+ [53]. This sensor demonstrated a low detection limit of 39 nM, and it showed excellent anti-interference capabilities across a pH range of 2–10. Another TPA-based sensor, 4-aldehyde-4,4-bis(4-pyridyl)TPA, achieved an ultralow detection limit of 0.01 nM for uranyl in groundwater, relying on uranyl-triggered protein cleavage that ensures high selectivity by eliminating interference from other cations [54].
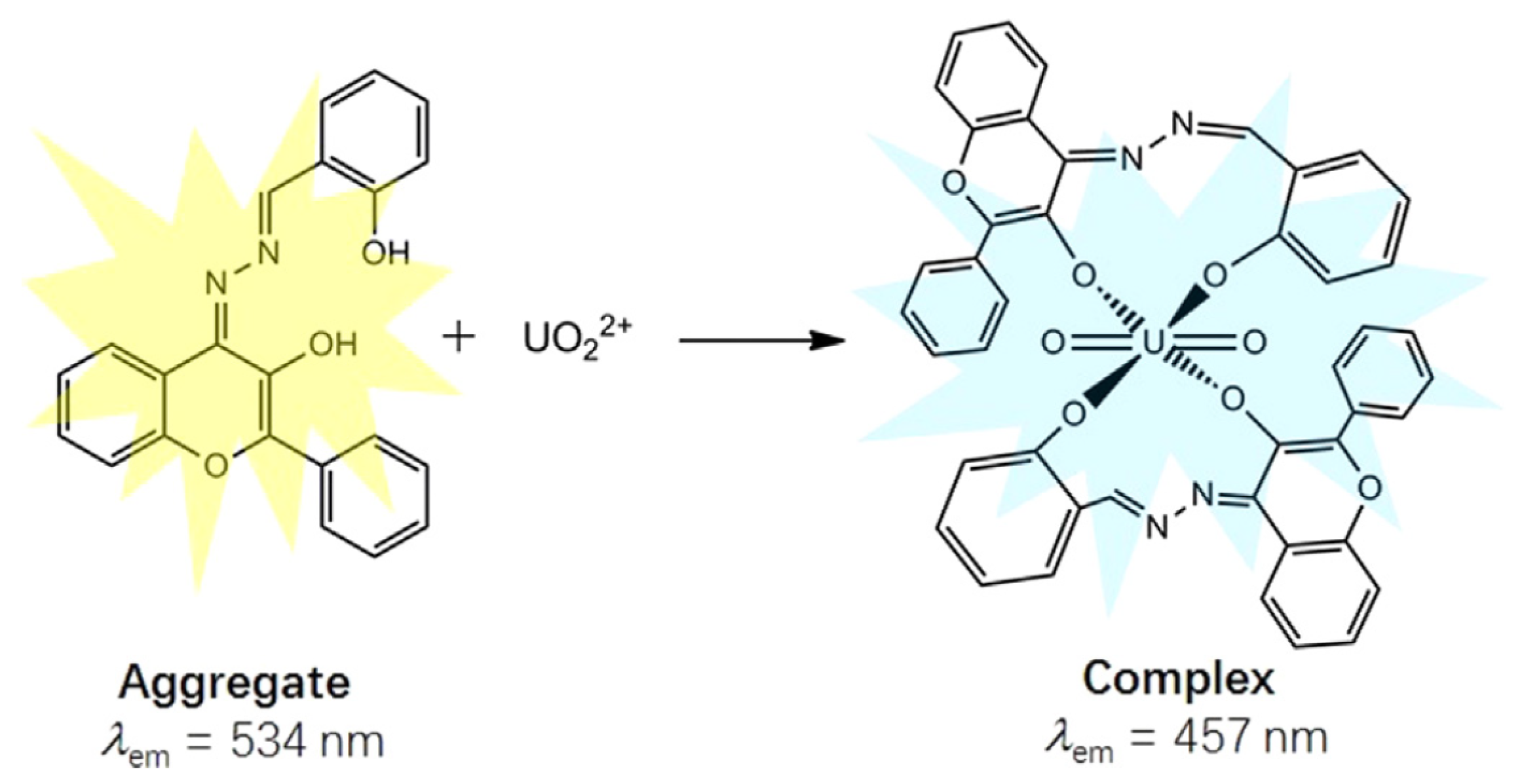
The salicylaldehyde azine (SA) moiety can also function as an AIE fluorophore (Table 3). Chen et al. demonstrated that 4-pethoxycarboxyl SA [55] and 3-hydroxy-flavone SA [56] function as turn-on AIE sensors. These compounds aggregate upon interaction with [UO2]2+, leading to fluorescence emission (Figure 5). Interestingly, SA and 5-nitro SA were shown to exhibit a contrasting behavior: uranyl ions quench the AIE effect in an organoaqueous solvent by disrupting aggregation, as reported by Pham et al. [57].
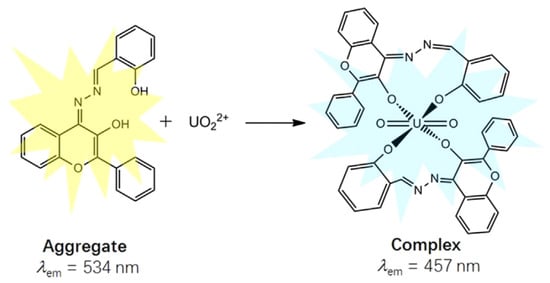
Figure 5.
Schematic presentation for the ratiometric fluorescence change in 3-hydroxy-flavone salicylaldehyde azine upon binding with [UO2]2+ [56].
Nivens et al. demonstrated that the fluorescence of calcein, a dye molecule, is quenched upon complexation with uranyl ions [58]. Following the excitation of the complex at 425 nm, a photochemical reaction occurs, involving the decarboxylation of calcein and the dissociation of the complex. The photochemical oxidation reverses the quenching and enhances the fluorescence signal, making it a useful method for detecting uranyl ions selectively.
Various other organic ligands serve as turn-off probes, in which complexation with [UO2]2+ quenches fluorescence emission (Table 3). Among these are quinoxalinol salen ligands, for which the quenching is accompanied by a blue shift in emission, whereas Cu2+ complexation presents a red shift [59]. Curcumin forms a 1:2 complex with uranyl ions ([UO2]2+:curcumin), leading to a slight red shift in fluorescence and a distinct color change from bright yellow to orange [60]. The fluorescence quenching induced via uranyl complexation with esculin is further enhanced through the adsorption of esculin and uranyl ions onto SBA-15 resin [61]. Additionally, Kim and Tsukahara developed a sensor that combines diaza-18-crown-6 ether as the uranyl coordination site with a naphthalimide moiety as fluorophore [62].
On the other hand, the furosemide compound 4-chloro-2(furan-2-ylmethylamino)-5-sulfamoylbenzoic acid leads to an enhancement in the fluorescence emission by forming a 1:1 complex with uranyl ion [63].
3.1.2. DNA
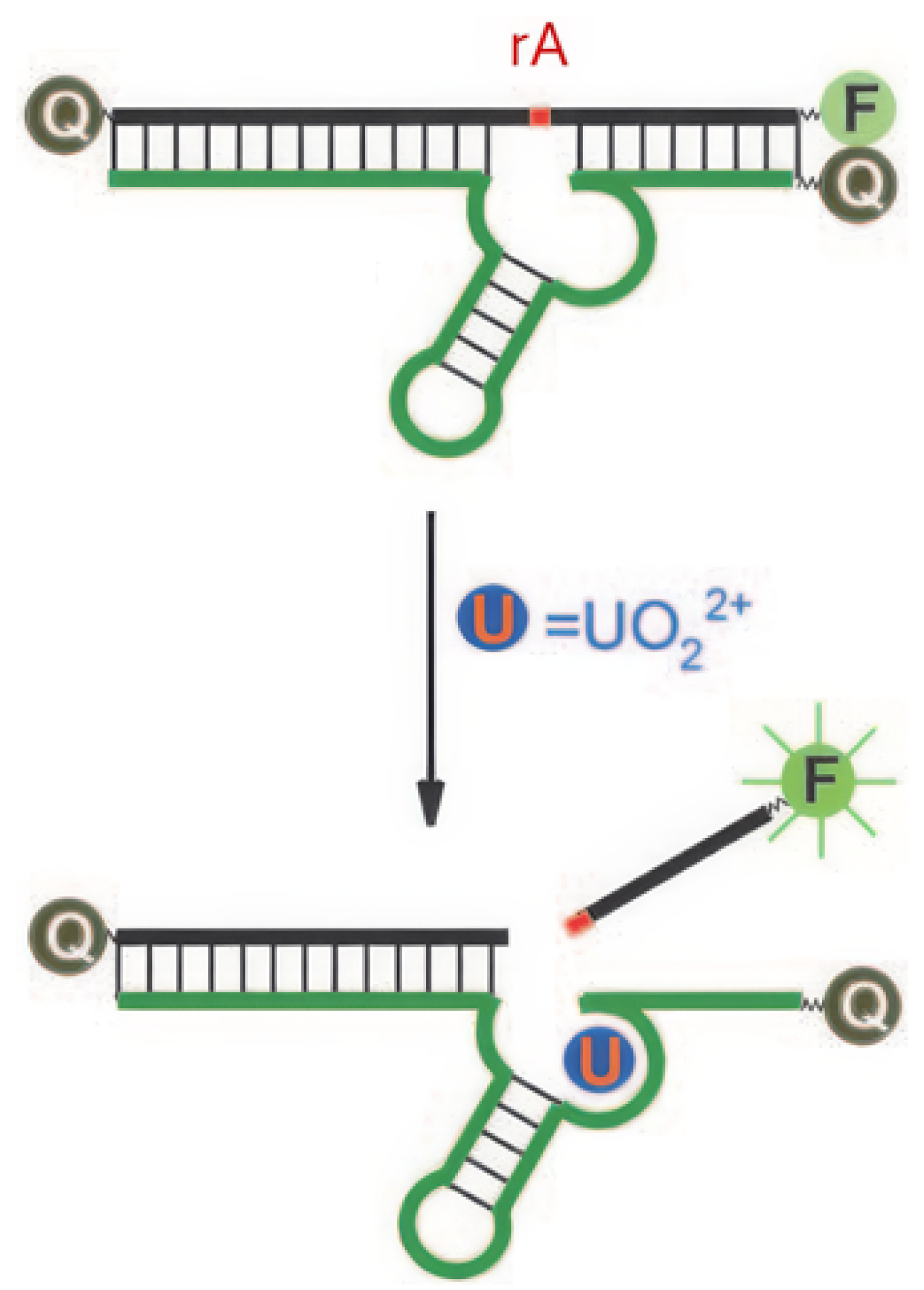
DNAzymes are DNA molecules with catalytic activity, often dependent on metal ion cofactors for their function. They are widely used in fluorescent sensors due to their ability to undergo conformational changes or catalyze specific reactions upon binding with target metal ions [77]. Liu et al. developed a DNAzyme with a selective complexation site for uranyl ions and a fluorophore positioned at the strand’s extremity [64]. The fluorescence emission of the fluorophore is suppressed by a quencher located opposite to it on the complementary strand, an effect attributed to FRET (Figure 6). A ribonucleotide adenosine (rA) site is incorporated near the uranyl complexation site; upon binding to uranyl ions, the DNAzyme catalyzes the cleavage of the rA site (optimal at pH 5.5 [70]), resulting in the release of the strand comprising the fluorophore. Once free from the quencher, fluorescence emission is recovered, enabling the detection of uranyl ions with a detection limit of 45 pM. Gold nanoparticles are integrated into this system to create biocompatible probes for detecting [UO2]2+ in living cells [78]. This model, based on the rA site and the release of a fluorophore, has been taken up by several other studies (Table 3).
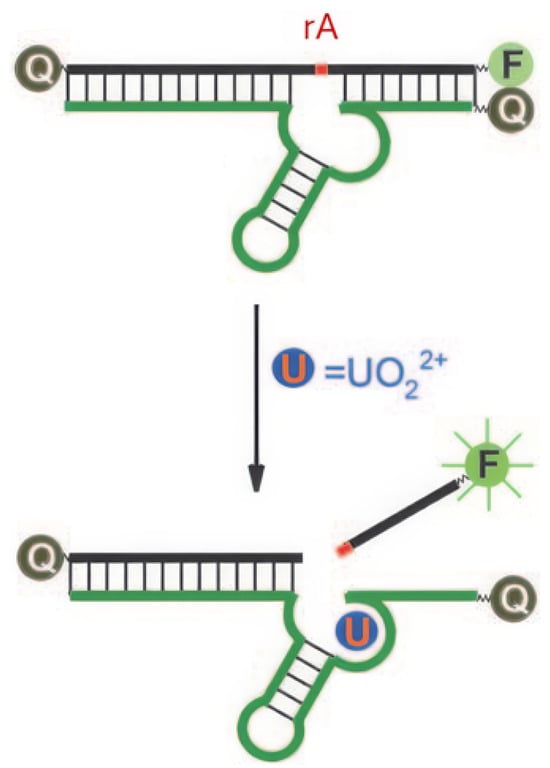
Figure 6.
Principle of fluorescence detection of [UO2]2+ using a DNAzyme [64]. F represents the fluorophore, Q the quencher, and rA the ribonucleotide adenosine.
Fluorescein amidite (FAM) remains one of the most commonly used fluorophores for designing turn-on probes, paired with various quenchers as detailed in Table 3 [64,65,66,67,68,69]. Notable examples include a dual probe for intracellular detection of Pb2+ and [UO2]2+ ions [66], the use of graphene oxide as a free quencher to avoid quencher labeling [67], and DNA amplification strategies to enhance sensitivity [68,69]. Turn-off probes can also be created, through which the quencher is not integrated into the DNAzyme. Instead, once released from the DNAzyme, FAM interacts with MoS2 nanosheets that quench its fluorescence [70].
Xiao et al. used tetramethyl-6-carboxyrhodamine as the fluorophore and four guanine bases as quenchers positioned at the strand’s extremity [79]. A PeT effect occurs between the fluorophore and the quenchers until the fluorophore is released, restoring fluorescence and functioning as a turn-on probe. N-methyl-mesoporphyrin IX is used as the fluorophore, while graphene oxide serves as a free quencher, to design a turn-on probe [71]. Zhu et al. developed a DNAzyme that, upon rA cleavage, undergoes a conformational change to form a G-quadruplex [72]. SYBR Green I, a fluorophore initially bound to the DNAzyme, loses its fluorescence when released, functioning as a turn-off probe.
The use of DNAzymes for [UO2]2+ detection via fluorescence demonstrates excellent limits of detection, with nearly all probes achieving sensitivity at the picomolar level (Table 3). Additionally, these probes exhibit good selectivity for uranyl and are often biocompatible, enabling their use for detection in living cells.
Other oligonucleotide-based sensors have been developed, utilizing fluorophores such as tryptophan [80] or dansyl groups [81] incorporated into a peptidic chain. Additionally, a salophen moiety has been immobilized onto silica gel particles as a solid-phase receptor for uranyl [82]. An oligonucleotide chain labeled with FAM subsequently interacts with the apical oxygen atom of the uranyl ion, forming a sandwich supramolecule. Following HCl elution, the fluorescence intensity is measured, allowing for uranyl detection with a LOD of 0.84 nM.
3.1.3. Others
Jiang et al. developed a surface fluorescence sensor based on a Salophen-europium(III) complex for detecting uranium without external excitation [73]. The fluorescence mechanism is based on the cation–cation interaction (PeT process) between U(VI) and Eu(III) through a phosphate bridge, on the surface of a glass slide, leading to intense fluorescence with a LOD of 8 nM. This europium phosphate uranyl strategy has also been applied to the fluorescence imaging of [UO2]2+ in cells [74].
Several studies report the development of polymer-based sensors for the detection of uranyl ions via fluorescence quenching. The amidoxime group is frequently employed as a coordination site for uranyl within the polymer, enabling detection through the PeT effect [83,84]. In these systems, fluorescence is achieved by incorporating the amidoxime moiety into the polymer chain. Amidoxime groups can also be linked to 1,3,5-triethynylbenzene to create fluorescent microporous polymers, which allow the simultaneous adsorption and detection of uranium [85]. Additionally, other compounds such as porphyrin [86], trimetazidine [87], clopidogrel [88], and quinoline [89] have been incorporated into polymers to detect uranium via fluorescence quenching upon uranyl complexation.
Metal–organic frameworks (MOFs) exhibit high adsorption capacity for uranium. The incorporation of emissive central metal atoms enables luminescent properties, facilitating the development of MOF-based fluorescent sensors. A wide variety of MOF sensors has been developed, which includes different metal atoms such as Tb(III) [90,91,92,93,94,95], Co(II) [96], Zr(IV) [97], Ni [98], and Zn(II) [99,100]. These examples present limits of detection below 100 nM.
Covalent organic frameworks (COFs) have emerged as versatile fluorescent platforms for uranium detection and extraction, combining stability, tunable functionality, and rapid response [101,102,103,104,105,106]. Functionalized architectures such as sp2 carbon-conjugated amidoxime COFs [101], hydroxyl-functionalized 3D COFs [102], and β-keto-enamine COFs [106] enable selective sensing with low detection limits (typically 4–100 nM) in aqueous and environmental matrices. Further refinements include olefin-linked COFs bearing amidoxime, pyridine, and hydroxyl groups with improved recovery [103], exfoliated nanosheet COFs that mitigate aggregation quenching to achieve 10 nM sensitivity [104], and porphyrin-based COFs with sub-nanomolar limits of detection [105]. Collectively, these studies highlight the importance of rational functionalization and structural engineering in advancing COFs as regenerable probes for uranium monitoring in complex matrices.
Quantum dots (QDs) are extensively used as fluorescent probes, primarily in the form of carbon dots [107] and cadmium dots [108]. Carbon dots stand out due to their lower toxicity, which makes them particularly suitable for biological and environmental applications [109].
3.1.4. Conclusions
The development of fluorescent sensors for uranium has made significant strides over recent decades. Researchers have designed various types of selective and sensitive probes, including organic ligands, DNAzymes, MOFs, and QDs. Advances in organic ligand design, especially in the last decade with TPE-based systems, have lowered detection limits into the nanomolar range, addressing environmental and industrial needs. Additionally, DNAzyme sensors containing the rA cleavage site have demonstrated detection limits in the picomolar range, with high sensitivity and biocompatibility, enabling applications in complex biological and environmental matrices. However, despite these promising results, key performance factors such as selectivity in real-world matrices, stability, and probe reusability are not often explored. These criteria must be addressed to ensure that fluorescent sensors can be reliably applied for the field monitoring of uranium contamination.
3.2. Cesium
Cesium-137 and Cesium-135 are medium-lived and long-lived fission products, respectively. 137Cs is more problematic due to its higher specific activity and greater decay energy. Among fission products, cesium poses significant problems after a release because it deposits in the soil and is readily absorbed by living organisms [110]. Its radioactive nature can cause severe health risks, including an increased risk of cancer, making its detection and monitoring crucial for environmental and public health safety [111].
Cesium ions present an oxidation state of +1, forming highly electropositive Cs+ ions. Due to its large ionic size and low charge density, Cs+ prefers forming complexes with bulky and non-polarizing ligands. The detection of cesium ions using fluorescent sensors has been extensively explored, leveraging various molecular designs and photophysical mechanisms. Kumar et al., in their review, pointed out two types of fluorescent sensors for Cs+: crown ether-based and calixarene-based crowns [112].
3.2.1. Crown Ether-Based
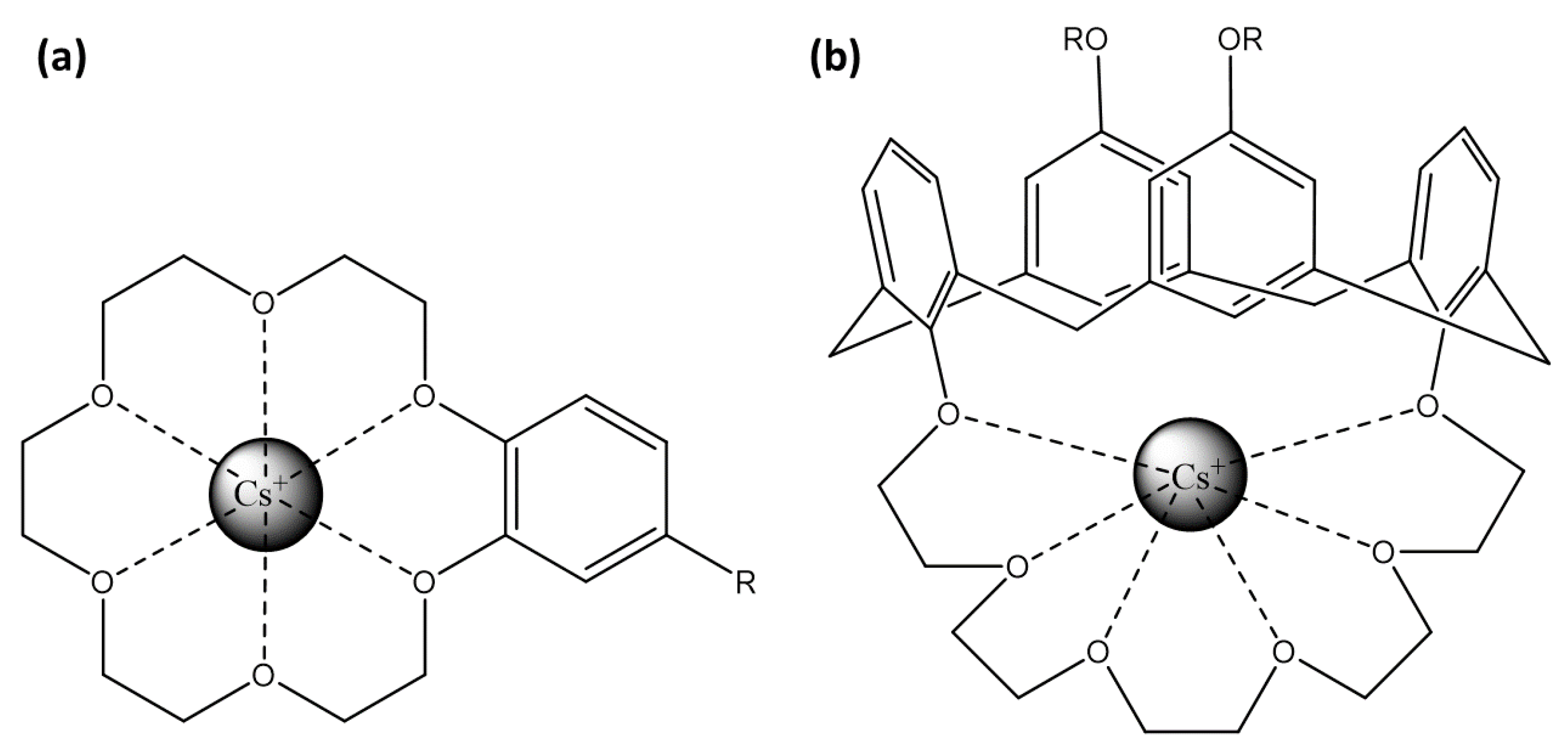
Crown-ether-based fluorescent probes achieve ion selectivity through the size of their cyclic cavity and the nature of donor atoms [113]. Their strong, size-specific affinity for alkali and alkaline–earth metal ions allows integration into sensors to enhance both selectivity and binding efficiency (Figure 7a). When cesium ions interact with these crown ethers, the resulting complex induces a change in the photophysical properties of the attached fluorophore, often through the PeT mechanism.
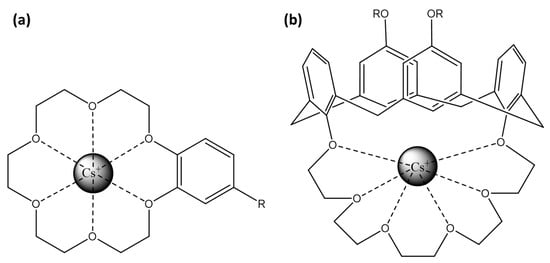
Figure 7.
Representation of Cs+ binding site: (a) crown-6-ether and (b) calix[4]arene with crown-6 ring.
Xia et al. demonstrated that a dicyano-substituted distyrylbenzene between 2 crown-6-ethers exhibits 1:1 coordination with Cs+, enhancing fluorescence with a selectivity for Cs+ greater than for K+ and Na+ [114] (Table 4). Otsuki et al. introduced two phthalimide units between the two crown-6-ethers, showing that 1:1 face-to-face coordination with Cs+ results in fluorescence quenching, whereas 1:2 coordination enhances fluorescence [115]. However, the selectivity of this sensor is higher for K+ and Na+ than for Cs+. Seo et al. combined an anthracene unit with a crown-5-ether, disabling the PeT effect for the Cs+ fluorescence detection, with a high selectivity for Cs+ than for other alkali metal ions [116]. Li et al. present a method for the adsorption and enrichment of Cs+ specifically using mesoporous silica containing dibenzo-24-crown-8-ether as the binding site for the alkali cation [117]. Here, the binding site does not rely on a fluorophore; instead, the addition of PbBr2 leads to the formation and growth of CsPbBr3 nanocrystals, which become fluorescent upon light excitation.

Table 4.
Cesium sensors.
3.2.2. Calixarene-Based Crowns
Calixarene-based receptors interact with a wide range of cations, with selectivity largely determined via cavity size, geometry, and donor atom type [135,136]. Alkali and alkaline earth metals typically bind through ion–dipole interactions with phenolic or ether oxygens, while transition and heavy metals form stronger coordination bonds with nitrogen, sulfur, or oxygen substituents. Lanthanides and actinides engage in multidentate interactions, through which their size and charge density play a crucial role in binding strength. Overall, subtle structural modifications of calixarenes enable fine-tuning of cation recognition, making them versatile platforms for selective sensing.
Concerning cesium ion, calix[4]arenes allow its coordination through electrostatic interactions. Particularly, 1,3-alternate calix[4]arenes with crown-6 rings (Figure 7b) are highly selective for cesium over sodium and moderately selective over potassium [137]. The crown-6 rings within these structures provide a robust and selective binding site for cesium, while the calix[4]arenes offer a versatile platform for attaching various fluorophores.
The fluorescence mechanism of these chemosensors depends on the fluorophore group attached to the calix[4]arene (Table 4). For example, cyanoanthracene [118,119,120,121,122], pyrene [123], and dansyl groups [125] rely on the PeT mechanism, while coumarin [124] operates through FRET. Dioxycoumarin [126,127,128,129] and BODIPY [130] fluorophores work through the PCT mechanism. To improve the solubility in water of these sensors, calix[4]arenes can be functionalized with sulfonate, carboxylate, or phosphate groups [127,129]. Additionally, modifying coumarin with cyano and benzothiazole groups can significantly increase the Stokes shift [128], enhancing the sensor’s performance and detection capabilities.
3.2.3. Others
Other examples of chemosensors for Cs+ include a squaraine-based sensor that detects Cs+ ions through fluorescence quenching [132], a polyethylene glycol chain linking hydroxyphenyl and nitrophenyl moieties that utilizes the PCT effect [131], and a naphthalene-based macrocycle that becomes soluble and fluorescent upon binding to Cs+ [134]. Additionally, a sensor bearing imine and amide linkages, which provide binding sites for Cs+, can aggregate into fluorescent organic nanoparticles in aqueous media and emit fluorescence through the AIE effect [133].
3.2.4. Conclusion
Two prominent types of sensors, crown-6-ether and calix[4]arenes, have been widely utilized for the selective detection of Cs+ ions. Both feature crown-6 rings that provide a highly selective binding site for cesium among alkali cations. Calix[4]arenes have become the primary scaffold for constructing these sensors. The diversity of fluorophores that can be attached to these binding sites influences key sensor characteristics, such as solubility and emission wavelength, allowing for adaptable detection strategies across a variety of environments. Indeed, the cesium detection described here is primarily performed in organic solvents due to fluorophore solubility constraints. However, a few studies have developed water-soluble fluorophores, such as calix[4]arenes bearing dioxycoumarin groups, allowing for the possibility of detecting cesium directly in natural water [127,129].
In addition, dual-function applications are possible, where fluorescent probes could aid both in the selective binding and in the preconcentration of radioactive cesium isotopes, thereby facilitating radiometric detection. This is particularly promising when such sensors are integrated into solid supports. By concentrating trace levels of cesium, these systems could enhance the ability to track radioactive isotopes of cesium in the environment [138].
3.3. Strontium
90Sr, the main radioactive isotope of strontium with a half-life of 28.8 years, is a product of nuclear fission in reactors and nuclear explosions. Due to its chemical similarity to calcium, it can replace calcium in bones, posing serious health risks [139]. Managing strontium-90 contamination involves limiting exposure and using chelating agents to reduce its presence in the body. Fluorescent probes for strontium detection are presented in Table 5.
3.3.1. Fura-2
Fura-2, a widely used fluorophore in biology that contains a benzofuran group, was used to measure Ca2+ levels in tissue cells [140]. This fluorophore forms complexes with Ca2+, as well as other divalent cations such as Sr2+ and Ba2+, with nearly the same selectivity [141,142,143]. However, Fura-2, the first fluorophore studied for strontium detection, has not been further investigated for this purpose since the early 20th century.

Table 5.
Strontium sensors.
Table 5.
Strontium sensors.
| Binding Site | Fluorophore | Mechanism | / (nm) | LOD (nM) | Matrix | Refs. |
|---|---|---|---|---|---|---|
| PEG chain | Pyrene | Excimer Formation | 341/400 | nd | Acetonitrile | [144] |
| Crown-ether | 1,8-dioxyxanthone | PeT turn-on | 308–314/424–437 | nd | Methanol | [145] |
| Merocyanine | PCT | 399/612 | nd | Acetonitrile | [146] | |
| BIC | ESIPT | 369/414 | 40 | H2O (5% DMSO) | [147] | |
| 369/384 | 2000 | H2O (NaLa micelle) | ||||
| Calixarene | Pyrene | Excimer Formation | 325/397 | 100 | Acetonitrile | [148] |
| Quinoline | PeT turn-on | 330/470 | 0.001 | Waste water (pH 7) in acetonitrile | [149] | |
| 284/575 | 1.4 | Natural water (pH 7) in acetonitrile | [150] | |||
| Vinylpyridinium | Turn-off | 405/520 | 91 | DMF/H2O (9:1) | [151] | |
| G-quadruplex | Thiazole orange | Turn-on | 480/535 | 10 | H2O (pH 7) | [152] |
| Thioflavin T | Turn-off | 446/490 | 2.1 | Natural water (pH 8.3) | [153] | |
| FONs | Urea derivate | PeT turn-on | /380 | 190,000 | Water (pH 2–7) | [154] |
| Pyrene | Excimer Formation | 345/480 | 9 | Natural water (pH 2.7–11.2) | [155] |
3.3.2. PEG Chain and Crown Ether
Suzuki et al. demonstrated a probe consisting of two pyrene units connected via a PEG chain, where Sr2+ binding induces a conformational change [144]. The fluorescence, initially due to excimer formation of pyrene units, is shifted through monomer emission upon Sr2+ binding. A PEG chain with four or five ether units showed the strongest response.
As for cesium detection, crown ether-based fluorophores are also utilized for strontium detection. Prodi et al. utilized a 1,8-dioxyxanthone chromophore incorporated into crown ether structures of varying lengths, revealing that complexation with Sr2+, Ca2+, and Ba2+ significantly enhances fluorescence [145]. PEG chain length affects both binding affinity and fluorescence quantum yield. Plaza et al. employed a crown-ether-linked merocyanine fluorophore, observing a blue shift in emission upon Sr2+ binding due to the PCT effect, with notable selectivity over other ions such as Li+ and Ca2+ [146]. Akutsu-Suyama et al. reported another crown ether-based fluorophore, N-(2-hydroxy-3-(1H-benzimidazol-2-yl)-phenyl)-1-aza-18-crown-6-ether (BIC), which exhibits enhanced fluorescence in the presence of Sr2+ and shows selectivity over Na+, K+, Mg2+, Ca2+, and Ba2+ ions [147]. The detection of Sr2+ with this hydrophobic fluorophore was enabled in aqueous solutions by adding DMSO or using a sodium laurate micellar system.
3.3.3. Calixarene
PyCalix is a fluorescent probe based on a calixarene scaffold featuring two pyrene moieties that exhibit intramolecular excimer emission [148]. Upon binding with Sr2+ cations, the separation of the pyrene units by Sr2+ leads to the suppression of intramolecular excimer emission and an increase in monomer fluorescence emissions accompanied by a blue shift. PyCalix shows high selectivity for Sr2+ and Ca2+ over other alkali and alkaline earth metals. However, its similar affinity for Ca2+ may interfere with the selective detection of Sr2+.
Sutariya et al. designed two calixarene-based compounds containing quinoline groups as the fluorescent units [149,150]. Both probes demonstrate high selectivity for Sr2+ over a wide range of other cations, with detection limits of 1 nM.
Fang et al. developed a sensor based on a calix[4]arene scaffold, which is modified to function as molecular tweezers through the incorporation of two vinylpyridinium iodide and two acetoxymethyl groups [151]. The sensor exhibits fluorescence quenching in the presence of Ca2+, Sr2+, and Ba2+, accompanied by red shifts in their UV-vis spectra, depending on the cation (570 nm for Sr2+). With its good cell permeability and water solubility, this sensor is a promising candidate for environmental monitoring and biological imaging applications.
3.3.4. G-Quadruplex
G-quadruplex DNA structures, which are guanine-rich sequences stabilized by cations, are often used to detect strontium by luminescence due to their high selectivity for Sr2+ over other metal ions such as Ca2+, Na+, and Mg2+ [156,157,158]. Qu et al. developed a method for detecting strontium ions by fluorescence, utilizing G-quadruplex structures in combination with thiazole orange as the fluorophore [152]. The interaction of single-walled carbon nanotubes with the DNA strand leads to quenching of thiazole orange fluorescence. When Sr2+ ions induce the formation of G-quadruplexes, these structures dissociate from the nanotubes, resulting in fluorescence restoration. More recently, Feng et al. utilized the ability of thioflavin T to induce G-quadruplex formation in DNA to develop a switch-off probe for strontium [153]. Since Sr2+ has a higher affinity for G-quadruplexes than thioflavin T, the fluorescence intensity decreases with the addition of strontium, allowing for a ratiometric probe that achieves a detection limit of 2 nM.
3.3.5. Fluorescent Organic Nanoparticles
Kaur et al. synthesized fluorescent organic nanoparticles (FONs) using the reprecipitation method with 1-(2-hydroxybenzyl)-3-naphthalen-1-yl-1-propyl-urea [154]. The binding of strontium ions to these FONs resulted in an increase in fluorescence due to the inhibition of the PeT process, achieving a detection limit of 2 × µM. In a subsequent study, Kaur et al. introduced new FONs based on a pyrene-derived polymeric compound, which emitted fluorescence through an intramolecular excimer formation process [155]. The binding of strontium ions quenched this excimer formation, resulting in significantly higher sensitivity, with a detection limit of 9 nM for Sr2+. These FONs demonstrated high efficiency and selectivity for Sr2+ detection in aqueous media, showing promising practical applications in tap water, river water, and oral care products.
3.3.6. Conclusion
Strontium detection using fluorescent probes has advanced significantly with the development of various types of sensors, incorporating different mechanisms and Sr2+ complexation sites, such as crown ethers, calixarenes, and G-quadruplexes. Initially, achieving selectivity over other divalent cations was a challenge, but this issue has been addressed over the past two decades, resulting in the development of selective probes. Contrary to cesium radioisotopes (134Cs and 137Cs), which can be readily detected via their -emissions, strontium-90, a -emitter, is more challenging to detect in the field. The development of chemosensors could play a valuable role as screening tools for its rapid and selective detection.
3.4. Technetium
99Tc, an isotope of technetium, the lightest non-stable element, is a byproduct of nuclear reactors. This isotope raises substantial environmental concerns due to its long half-life and high mobility in environmental systems, especially in the pertechnetate form (TcO4−), which can readily migrate through soil and groundwater [159]. The perrhenate ion, ReO4−, is frequently employed as a non-radioactive surrogate of TcO4− to study technetium detection through fluorescence due to its comparable physical and chemical properties. Fluorescent probes for technetium detection are presented in Table 6.

Table 6.
Technetium sensors. a 2-phenylpyridine, b 2,2 ′-bipyridine.
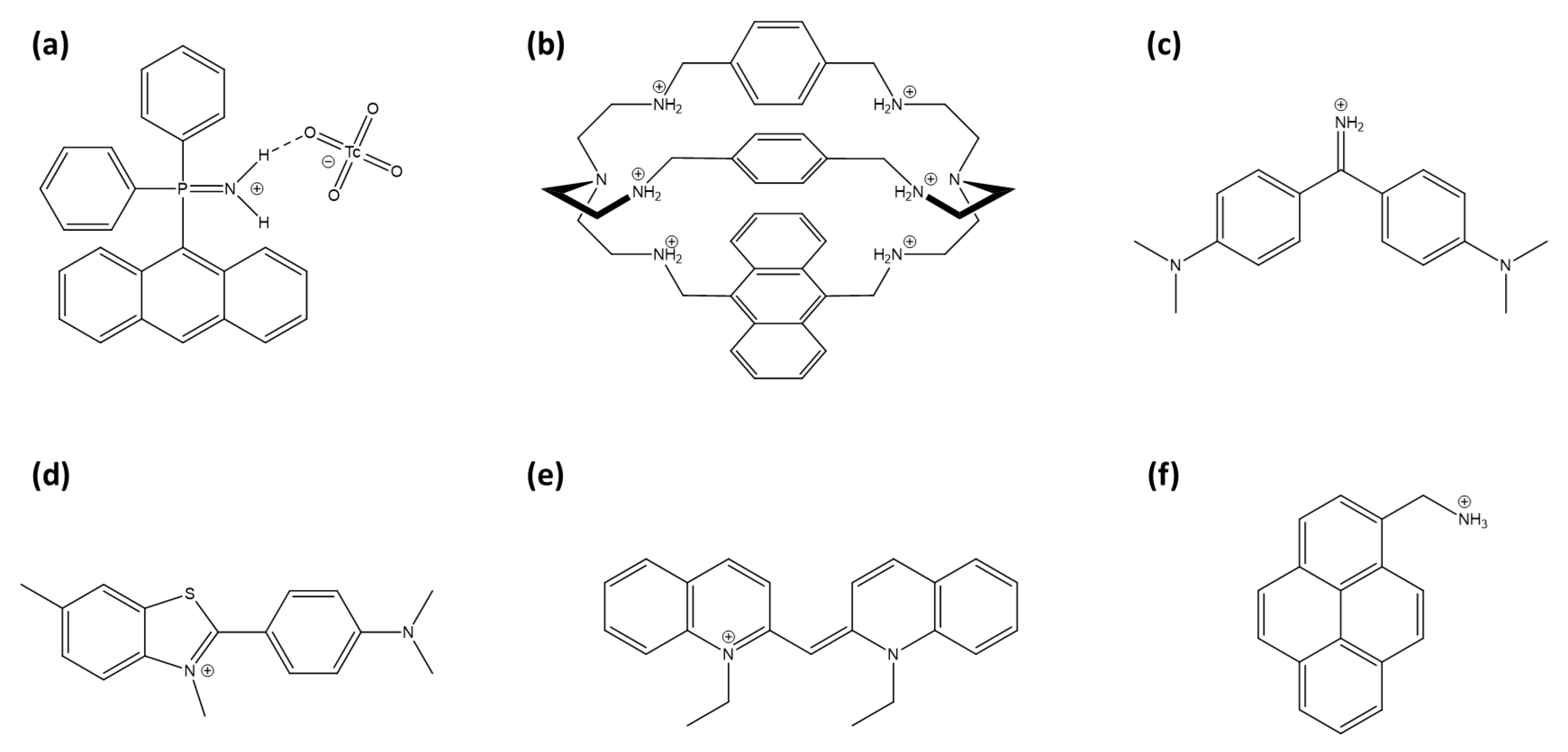
Arrigo et al. developed phosphiniminium cations linked to anthracene moieties (Figure 8a) that exhibit high selectivity for TcO4−, even in the presence of competing anions such as chloride, nitrate, and phosphate [160]. Although no significant spectral shift was detected, which limits its effectiveness as a fluorescent probe, the system demonstrated potential as a scintillation sensor for 99Tc detection due to its responsiveness to beta emissions from technetium’s radioactive decay.
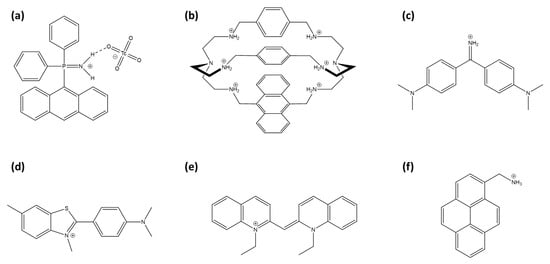
Figure 8.
Representation of TcO4− and ReO4− sensors: (a) (9-anthracenyl)Ph2P=NH2+ cation, (b) modified azacryptand cage with anthracene, (c) auramine O, (d) thioflavin-T, (e) pseudocyanine and (f) 1-pyrenemethylamine.
Amendola et al. developed a fluorescent sensor for TcO4− in water, using an azacryptand cage [161]. An anthracene incorporated into the azacryptand structure (Figure 8b) enhances the detection of TcO4− through fluorescence quenching via the PeT effect in aqueous solutions at pH 2. This receptor is highly selective, showing no interference from environmentally prevalent anions such as chloride, nitrate, and sulfate.
The weak hydration properties of perrhenate and pertechnetate anions facilitate the formation of contact ion pairs with certain cationic fluorescent probes. Auramine O (Figure 8c), a cationic molecular rotor dye, forms fluorescent aggregates with ReO4−, resulting in both an emission enhancement and a red shift (AIE effect) [162]. Thioflavin-T and pseudoisocyanine (respectively, Figure 8d,e), other cationic fluorescent molecules, act as turn-on probes by aggregating with perrhenate anions [163,164]. Notably, pseudoisocyanine exhibits a particularly low limit of detection for perrhenate anions, reported at 0.2 µM, outperforming other sensors.
1-pyrenemethylamine (PMA, Figure 8f) is used as a turn-off probe with perrhenate anion [165]. The selectivity of the sensor is attributed to the strong hydrogen bonding and electrostatic interactions between a ReO4− anion and the amino group of PMA, facilitating a photoinduced electron transfer process.
On the other hand, various macrostructures have been developed for the fluorescence detection of TcO4− and ReO4−. Fluorescent MOFs are one such approach, with Rapti et al., who developed two Zr-based MOFs, MOR-1 and MOR-2, demonstrating effective sorption capacities for TcO4− and ReO4− [166]. Notably, MOR-2 functions as a selective luminescent sensor for ReO4− in highly acidic conditions, where fluorescence quenching is based on a ligand-to-anion electron transfer. Xu et al. presented an example of a cationic photoactive porous aromatic framework material functionalized with an Ir(III) organometallic complex [167]. The detection mechanism for TcO4− involves a strong, selective interaction between the Ir(III) complex and the anion, resulting in a fluorescence turn-on response. Li et al. described the coordination polymer TJNU-302, a cationic Ag(I) coordination polymer that incorporates a fluorescent ligand, 1,2,4,5-tetra(pyridin-4-yl)benzene [168]. TJNU-302 shows high selectivity and efficiency in capturing and sensing perrhenate ions, with fluorescence quenching attributed to hydrogen bonding between ReO4− and the framework.
Choi and Lee developed a carbon quantum-dot-based sensor specifically designed for detecting perrhenate anions in aqueous solutions [169]. This sensor uses cationic carbon quantum dots functionalized with quaternary ammonium groups to enhance selectivity and sensitivity for ReO4−. The fluorescence mechanism relies on PeT process facilitated via electrostatic interactions between the cationic carbon quantum dots’ surface and the anions, resulting in fluorescence intensity reduction. Yi et al. introduced an ionic liquid-modified covalent organic framework incorporating nitrogen groups to bind ReO4− anion [170]. The fluorescence quenching mechanism in this framework involves intramolecular charge transfer, upon ReO4− binding.
Tc(CO)3+, a cationic form of 99Tc, is also present in significant amounts in nuclear waste. With the aim of monitoring Tc(CO)3+ in nuclear waste, Branch et al. developed a method to convert Tc(CO)3+ into a fluorescent complex using a bipyridyl ligand [171]. This complexation induces fluorescence, enabling the detection of Tc(CO)3+ with a limit of detection of 0.2 µM.
The fluorescence-based detection of technetium has progressed through the development of small-molecule probes and macrostructures designed to target the pertechnetate anion or its non-radioactive surrogate, the perrhenate anion in aqueous medium. These include organic ligands utilizing hydrogen bonding, MOFs, and carbon quantum dots achieving detection limits in the micromolar range.
3.5. Zirconium
Zirconium’s corrosion resistance, heat tolerance, and low neutron absorption make it ideal for cladding fuel rods in water-cooled reactors, with nearly 90% of global zirconium production dedicated to nuclear energy. Zirconium is commonly used in medical implants due to its biocompatibility and low chemical toxicity [172]. However, detecting zirconium could serve as a safety marker around nuclear reactors. The long-lived fission product 93Zr has low specific activity and radiation energy, resulting in minimal radiological risk. Zirconium is found in the environment in stable forms such as ZrSiO4 and ZrO2, which are largely insoluble in water, keeping zirconium fixed in soils and sediments [173]. Under certain conditions, zirconium can exist as Zr4+ ions or in various hydroxo and oxo complexes. Fluorescent probes for technetium detection are presented in Table 7.

Table 7.
Zirconium sensors.
The first published work appeared in 1951, when Alford et al. reported a fluorometric method for determining zirconium using 3-hydroxyflavone (Figure 9a) as a fluorescent probe [177]. This procedure involves forming a fluorescent complex between zirconium and 3-hydroxyflavone in a sulfuric acid solution, emitting blue fluorescence under ultraviolet light. The fluorescence intensity is directly proportional to zirconium concentration, enabling a quantitative analysis.
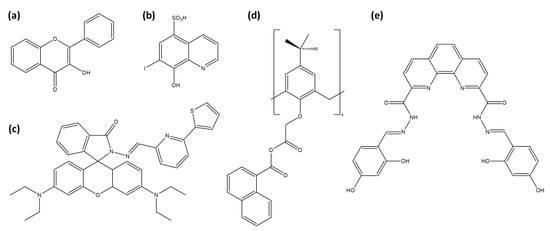
Figure 9.
Representation of Zr4+ sensors: (a) 3-hydroxyflavone, (b) Ferron, (c) RhPT, (d) calix[4]arene with naphthalene units and (e) phenanthroline dicarbohydrazide based chemosensor.
Sánchez et al. explored Ferron (8-hydroxy-7-iodo-quinoline-sulfonic acid, Figure 9b) in -cyclodextrin under acidic conditions to detect zirconium at trace levels [178]. Encapsulation within cyclodextrin enhances fluorescence by shielding the excited species from quenching and nonradiative decay processes [183]. The hydrophobic cavity of cyclodextrin favors cation complexation, with Zr4+ complexation enhancing fluorescence, achieving a detection limit of 0.8 µM.
Mahapatra et al. introduced RhPT (Figure 9c), a rhodamine-based chemosensor, for detecting Zr4+ ions [174]. Upon zirconium binding, RhPT undergoes a structural shift from a spirolactam to a ring-opened form, which activates a delocalized xanthene tautomer within the rhodamine group. This transformation greatly enhances fluorescence and changes the naked-eye color from colorless to pink. The process is reversible, as demonstrated by the disappearance of the fluorescence upon the addition of excess EDTA. RhPT shows promise for live cell imaging, making it suitable for real-time monitoring of zirconium within complex biological systems. Sutariya et al. present a calix[4]arene-based fluorescent sensor (Figure 9d) specifically designed for the selective detection of Zr4+ and Fe2+ ions, thanks to its naphthalene units [175]. This sensor shows a significant fluorescence enhancement upon complexation with Zr4+, while complexation with Fe2+ inhibits fluorescence. In addition, other tested ions (Nd3+, La3+, Fe3+, Pr3+, Ce3+, Zn2+, Cd2+, Mn2+, Ca2+, Ba2+, Co2+, Hg2+, Ni2+, Pb2+, Sr2+, Cu2+, Li+, Ag+, Na+, K+, As3+) have no effect on the fluorescence. Selva-Kumar and Ashok-Kumar introduced a phenanthroline-based fluorescent probe (Figure 9e) designed for detecting Zr4+ in aqueous media [179]. This probe selectively binds zirconium ions with a 1:2 stoichiometry (probe:Zr). It exhibits an increase in absorbance at 412 nm upon interaction with Zr4+, accompanied by significant fluorescence enhancement via the PeT process.
Meng et al. developed a fluorescence turn-on probe for detecting zirconium ions using two complementary DNA strands [176]. Each strand is modified with pyrene on one end (3′ or 5′) and a phosphate group on the other. Upon binding with Zr4+, these oligonucleotides form a hairpin structure, bringing the pyrene molecules into close proximity and generating an excimer fluorescence signal. The introduction of a cyclodextrin further amplifies the fluorescence signal, enhancing the sensitivity of the probe, which achieves a detection limit of 2 × nM. The probe exhibits high selectivity for Zr4+ over other metal ions, making it suitable for environmental and industrial applications.
A cobalt-based metal-organic framework was developed by Kirandeep et al. using a mixed-ligand approach that incorporates both N,N’-donor and polycarboxylate acid ligands to increase the interaction with the cations [180]. This MOF acts as a turn-on fluorescent sensor for Zr4+ via the absorbance-caused enhancement mechanism, demonstrating high selectivity and sensitivity with a detection limit of 67 nM. Moreover, MOF1 shows promise as a selective adsorbent for organic dyes, specifically Reactive Black 5 and Orange G. Chen et al. introduce a reusable Eu-based coordination polymer probe for Zr4+ detection, formulated as [Eu(L)1.5(phen)(H2O)] [181]. This probe is composed of 9,10-anthracenedicarboxylic acid and 1,10-phenanthroline, resulting in a complex with strong blue–violet fluorescence emission. The highly selective interaction with Zr4+ leads to fluorescence quenching, attributed to static weak interactions. Recently, Liao et al. developed fluorescent carbon quantum dots synthesized from o-phenylenediamine and L-cysteine [182]. These quantum dots exhibit high selectivity and sensitivity in detecting zirconium ions, with fluorescence quenching upon Zr4+ interaction. Additionally, they show a reversible pH response, with fluorescence quenching in acidic conditions (pH 1–5) and restoration in neutral or basic environments, making them suitable for pH-sensing applications.
Despite zirconium’s low toxicity, several fluorescent probes have been developed for its detection. Early systems such as 3-hydroxyflavone and Ferron operated in strongly acidic conditions and achieved only micromolar sensitivity, whereas modern probes demonstrate nanomolar detection in more practical matrices. The calixarene–naphthalene sensor offers the best sensitivity (1.4 nM) in river water, while phenanthroline (9 nM) and Co-MOF (67 nM) also perform well in aqueous environments. Carbon quantum dots combine good sensitivity (68 nM) with a broad pH tolerance and live-cell imaging capability. Importantly, the Eu-based coordination polymer achieves 200 nM detection limits while maintaining stability across pH 1–8, making it a strong candidate for reusable sensing platforms. Collectively, these examples show that zirconium probes can combine high sensitivity with adaptability to diverse matrices, supporting their potential in environmental monitoring, industrial safety, and biological applications.
3.6. Beryllium
Recognized for its exceptional physical properties, beryllium has been widely used in electronics and aerospace since the mid-20th century, owing to its high conductivity and thermal stability, and for its low density. Moreover, beryllium finds numerous applications in the nuclear industry. Thanks to its high neutron scattering cross section, it serves as a neutron reflector in nuclear reactors, enhancing the efficiency of reactions. Additionally, beryllium oxide acts as a neutron moderator, playing a crucial role in the control and regulation of nuclear reactions within the reactor core.
It is important to note that beryllium is highly toxic, posing significant health risks [184,185]. Classified as a carcinogenic element, it can lead to the development of chronic beryllium disease. Consequently, stringent measures must be implemented in industries dealing with beryllium to safeguard the health of employees. The development of fluorophores to monitor beryllium started in the 1950s, as scientists suspected an impact of beryllium on human health.
Morin (2′,3,4′,5,7-pentahydroxyflavone) emits a yellow-green fluorescence when it is complexed with beryllium in an alkaline solution [186], with the highest sensitivity at 0.2 M NaOH [187]. But Morin seems not to be selective since zinc, calcium, and lithium interfere with beryllium [188]. Additionally, three different complexes can form between beryllium and morin (M): BeM, Be2M, and Be2M2, depending on the pH, making it a complicated system for fluorescence detection [189].
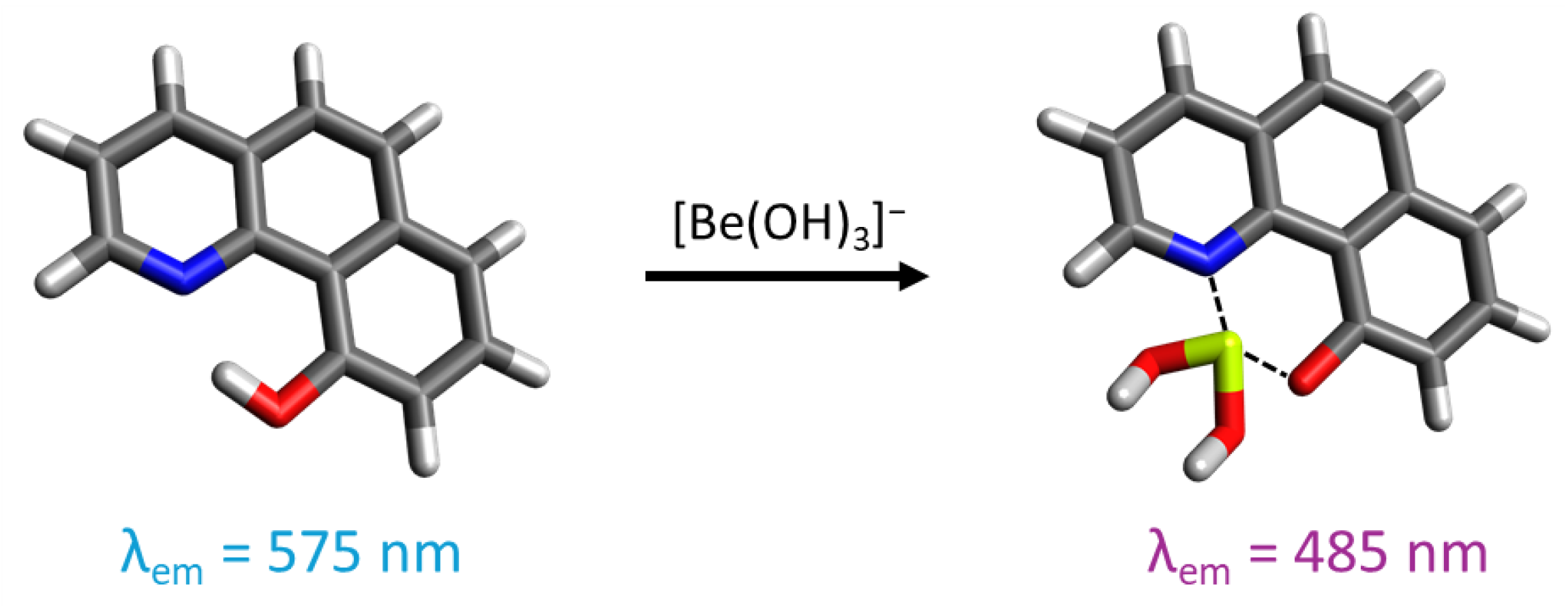
The compound 10-hydroxybenzo[h]quinoline (HBQ) features a strong hydrogen bond between the hydroxy group and benzoquinolinic nitrogen. Owing to its -electron system, it displays significant fluorescence [190,191,192,193]. The excitation of the fluorophore in its enol form induces the keto form through tautomerism with proton transfer (ESIPT process) from the hydroxy oxygen to the benzoquinolinic nitrogen. Subsequently, the excited-state keto form emits fluorescence before returning to the enol form through a reverse proton transfer. HBQ can also form a six-membered chelate ring with Be(II) by replacing the proton, causing a one-hundred-nanometer shift in the fluorescence emission maxima by inhibiting the ESIPT process. As HBQ is not soluble in water, its sulfonate derivative, HBQS (10-hydroxybenzo[h]quinoline-7-sulfonate), demonstrates good water solubility, making it a good probe for beryllium detection in solution (Figure 10).
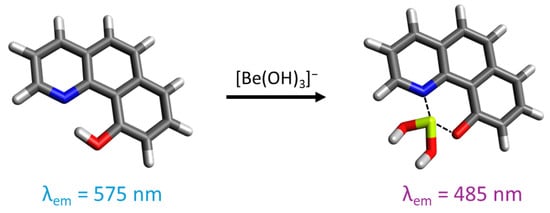
Figure 10.
Representation of 10-hydroxybenzo[h]quinoline-7-sulfonate and its interaction with beryllium in an alkaline medium. For the illustration, the oxygen atoms are colored in red, the nitrogen atoms in blue, the sulfur atoms in yellow, the carbon atoms in gray, the beryllium atom in light green, and the hydrogen atoms in white.
Matsumiya et al. first developed an urban air analysis method using the fluorescence technique with HBQS in 2001 [194]. Following filter dissolution with nitric acid, beryllium is quantified in an alkaline solution (pH 12) through complexation with HBQS at a molar ratio of 1:1. The method achieved an LOD of 5 pg/cm3. Another method was developed to detect beryllium on surfaces using a filter-swiping technique [195]. Beryllium on the swipe is dissolved with ammonium bifluoride, before the addition of a detection solution, containing HBQS, EDTA as a masking agent, and L-lysine as buffer at pH 12.85. Building upon this approach, a portable fluorescence method has been established for the analysis of air and wipe samples with a limit of detection of 13.6 ng/swipe [196].
The close proximity between the hydroxy group and the benzoquinolinic nitrogen suggests that beryllium exhibits a high formation constant compared to larger metal cations. Additionally, alkaline conditions can lead to the hydrolysis of certain metal cations. EDTA is employed as a masking agent, given its weak equilibrium constant with Be(II) (10−3.9) compared to >108 for other metal ions [197]. Consequently, no significant interference was observed from common metal cations, even with a molar excess of 105 [194]. Fe and, to a lesser extent, TiO2 are the most significant interfering species, primarily due to the presence of hydrolyzed particles in suspension. However, this interference can be avoided by filtering the solution, which may exhibit visible color [195,196,198].
Matsumiya and Hoshino introduced a derivative of HBQ, (2-(2′-hydroxyphenyl)-10-hydroxybenzo[h]quinoline), used as a precolumn chelating reagent for beryllium in RP-HPLC [199]. The eluate is then detected via fluorometry with a detection limit of 39 fg/cm−3, and it does not present interferences from other metal ions.
HBQ was further functionalized onto a silicon nanopillar surface, onto which a 1 mL aliquot containing beryllium in a basic solution was applied [200]. Fluorescence emission, observed via microscopy, undergoes a shift when Be(II) is complexed with the Si-HBQ substrate (detection limit is 0.6 pg/cm−3). The presence of a thin layer of porous silicon oxide on the surface enhances the contact surface area, thereby amplifying fluorescence. The surface can then be rinsed with nitric acid to remove beryllium, enabling reuse of the substrate.
In addition to HBQ derivatives, a few fluorophores have been studied for beryllium detection. 4-methyl-6-acetyl-7-hydroxycoumarin forms a water-insoluble complex with beryllium, which can be extracted into benzene for measuring its fluorescence emission [201]. The concentration range for Be(II) is 0.5 to 10 ng/mL of benzene. The selectivity of this fluorophore for beryllium is influenced by the presence of citrate, EDTA, Ti, Zr, Hf, Cr, Zn, Sn, Sb, and Bi when their ratio compared to Be exceeds 2. Chromotropic acid and beryllium form a 1:1 complex (pH 4.8–6.0) that emits fluorescence, enabling beryllium detection within the range of 0.1 to 60 ng/cm−3 with no interferences reported among the sixty anions tested [202]. 2,6-diphenyl-4-benzo-9-crown-3-pyrane serves as a turn-on fluorescent probe for Be(II) in a MeOH/H2O (70:30, v/v) solution via the PCT process [203]. It exhibits a low LOD of 2 nM and shows no interference from tested metal ions.
Fluorescent probes for beryllium detection have significantly advanced, starting with Morin in the 1950s, despite its lack of selectivity. More sophisticated fluorophores like HBQ and its derivatives have since emerged, offering high sensitivity, low detection limits, and minimal interference from other metals. These advancements enable reliable beryllium monitoring in diverse environments while addressing its toxicity and environmental persistence.
4. Conclusions
Fluorescent probes have emerged as versatile tools for detecting toxic and radiotoxic elements relevant to the nuclear industry, including uranium, cesium, strontium, technetium, zirconium, and beryllium. Over the past decades, a wide range of sensing platforms, such as organic ligands, DNAzymes, MOFs, and quantum dots, have been developed. Recent breakthroughs, particularly with DNAzyme-based probes, COFs, and AIE systems, have pushed detection limits to the pico- and nanomolar range, enabling highly sensitive monitoring in both environmental and biological matrices.
These advances hold strong promise for real-world applications. Fluorescent probes can support environmental surveillance around nuclear facilities, assist in nuclear waste management, and track contamination from mining activities. In biological systems, several sensors have demonstrated compatibility with complex media, opening opportunities for studying radionuclide bioavailability, toxicity, and biodistribution. Importantly, fluorescence-based approaches provide a clear advantage for detecting alpha- and beta-emitting radionuclides, where radiometric techniques often require extensive sample preparation or lack sufficient sensitivity.
Despite these advances, several challenges remain before fluorescent probes can be reliably deployed in real-world settings. Improving selectivity in complex mixtures, enhancing sensor stability and reusability, and ensuring fluorophore resistance to both photodegradation and radiolysis are critical for long-term performance. Recent studies have highlighted strategies such as protective matrix embedding, the attachment of protective and anti-fading groups, and the optimization of the measurement medium as promising directions [204,205]. Future work should also emphasize the integration of these probes into portable devices, including microfluidic chips or point-of-care diagnostic platforms, while promoting cross-disciplinary research bridging chemistry, materials science, and radiochemistry. Addressing these issues will be essential to translate laboratory progress into robust, field-ready tools for nuclear safety, environmental monitoring, and biomedical applications.
Author Contributions
Investigation, writing and editing, C.P.-P.; review, C.P.-P., D.B., and D.L.; supervision, D.B., and D.L.; funding acquisition and project administration, D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Nuclear Safety Commission (CNSC): RGPIN-2025-03950 and Small Modular Reactors Research Grant ALLRP 580453-2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PeT | Photoinduced electron transfer |
| PCT | Photo-induced charge transfer |
| ESIPT | Excited-state intramolecular proton transfer |
| AIE | Aggregation-induced emission |
| ACQ | Aggregation-caused quenching |
| ACE | Absorbance-caused enhancement |
References
- Buongiorno, J.; Corradini, M.; Parsons, J.; Petti, D. Nuclear Energy in a Carbon-Constrained World: Big Challenges and Big Opportunities. IEEE Power Energ. Mag. 2019, 17, 69–77. [Google Scholar] [CrossRef]
- Mathew, M. Nuclear energy: A pathway towards mitigation of global warming. Prog. Nucl. Energ. 2022, 143, 104080. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Dai, L.; Liu, M.; Xiao, Y.; Cong, T.; Gu, H. The role of nuclear energy in the carbon neutrality goal. Prog. Nucl. Energ. 2023, 162, 104772. [Google Scholar] [CrossRef]
- Allen, T.; Busby, J.; Meyer, M.; Petti, D. Materials challenges for nuclear systems. Mater. Today 2010, 13, 14–23. [Google Scholar] [CrossRef]
- Myasoedov, B.F.; Kalmykov, S.N. Nuclear power industry and the environment. Mendeleev Commun. 2015, 25, 319–328. [Google Scholar] [CrossRef]
- Burns, S. Milestones in Nuclear Law: A Journey in Nuclear Regulation. In Nuclear Law; T.M.C. Asser Press: The Hague, The Netherlands, 2022; pp. 55–73. [Google Scholar] [CrossRef]
- Bruno, J.; Ewing, R.C. Spent Nuclear Fuel. Elements 2006, 2, 343–349. [Google Scholar] [CrossRef]
- Tomar, B.S. Nuclear Fuel Cycle, 1st ed.; Springer: Singapore, 2023. [Google Scholar]
- Nawada, H.P. Status of Minor Actinide Fuel Development; Number 1411 in Publication, Internat; Atomic Energy Agency: Vienna, Austria, 2009. [Google Scholar]
- Nagasaki, S. Radioactive Waste Engineering and Management; Number v.6 in An Advanced Course in Nuclear Engineering Series; Springer: Japan, Tokyo, 2015. [Google Scholar]
- Kim, H.; Jang, G.; Yoon, Y. Specific heavy metal/metalloid sensors: Current state and perspectives. Appl. Microbiol. Biot. 2019, 104, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular fluorescent sensors: An historical overview and update. Coordin. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef]
- Demchenko, A.P. Introduction to Fluorescence Sensing: Volume 2: Target Recognition and Imaging; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Bowyer, A.A.; New, E.J. Fluorescent Platforms for Environmental Sensing. In Fluorescent Chemosensors; The Royal Society of Chemistry: London, UK, 2023; pp. 378–405. [Google Scholar] [CrossRef]
- Qiao, J.; Hou, X.; Miró, M.; Roos, P. Determination of plutonium isotopes in waters and environmental solids: A review. Anal. Chim. Acta 2009, 652, 66–84. [Google Scholar] [CrossRef]
- Mhatre, A.; Agarwal, C.; Parayil, R.T.; Tripathi, R. Surface grafted scintillation sensors for selective detection of low level plutonium alpha activity. Sens. Actuators B 2023, 390, 134021. [Google Scholar] [CrossRef]
- Habibi, A.; Varela, D.D.; Baconet, I.; Haloche, D.; Jaegler, H.; Augeray, C.; Morin, M. Direct determination of 237Np in nuclear effluent by ICP-MS/MS. J. Anal. Atom. Spectrom. 2024, 39, 2058–2065. [Google Scholar] [CrossRef]
- Sharma, P.; Govindaraj, J.; Poulin-Ponnelle, C.; Lariviere, D. Quantification of plutonium in nuclear, environmental, and biological samples: A review. Microchem. J. 2025, 212, 113099. [Google Scholar] [CrossRef]
- Wen, J.; Dong, L.; Hu, S.; Li, W.; Li, S.; Wang, X. Fluorogenic Thorium Sensors Based on 2,6-Pyridinedicarboxylic Acid-Substituted Tetraphenylethenes with Aggregation-Induced Emission Characteristics. Chem. Asian J. 2015, 11, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Dehaen, W. Small-molecule-based fluorescent probes for f-block metal ions: A new frontier in chemosensors. Coordin. Chem. Rev. 2021, 427, 213524. [Google Scholar] [CrossRef]
- Kumar R, S.; Bhaskar, R.; Sharma, H.K.; Ashok Kumar, S.; Sahoo, S.K. Historical overview and recent progress on supramolecular sensors for thorium recognition. Trends Anal. Chem. 2024, 172, 117551. [Google Scholar] [CrossRef]
- Dutta, M.; Das, D. Recent developments in fluorescent sensors for trace-level determination of toxic-metal ions. Trends Anal. Chem. 2012, 32, 113–132. [Google Scholar] [CrossRef]
- Rasheed, T.; Li, C.; Bilal, M.; Yu, C.; Iqbal, H.M. Potentially toxic elements and environmentally-related pollutants recognition using colorimetric and ratiometric fluorescent probes. Sci. Total Environ. 2018, 640–641, 174–193. [Google Scholar] [CrossRef]
- Goshisht, M.K.; Patra, G.K.; Tripathi, N. Fluorescent Schiff base sensors as a versatile tool for metal ion detection: Strategies, mechanistic insights, and applications. Mater. Adv. 2022, 3, 2612–2669. [Google Scholar] [CrossRef]
- Khan, J. Optical Chemosensors Synthesis and Appplication for Trace Level Metal Ions Detection in Aqueous Media: A Review. J. Fluoresc. 2024, 35, 561–582. [Google Scholar] [CrossRef]
- Peng, R.; Wang, G.; Wang, C.; Zhang, T.; Qin, N.; Li, X.; Yan, S.; Liu, X. Detection of radioactive ions: Current status of nanomaterial-based sensors. Microchim. Acta 2025, 192, 465. [Google Scholar] [CrossRef]
- Pei, Y.; Fang, L.; Zhang, J.; Wang, Z.; Zheng, L.; Zhang, Y.; Zhu, J.; Wang, Z.; Zhang, C.; Pan, J.B. Advancing environmental safety and public health: A comprehensive review of luminescent probes for radioactive element detection. Analyst 2025. [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Afrin, A.; Jayaraj, A.; Gayathri, M.S.; Chinna Ayya Swamy, P. An overview of Schiff base-based fluorescent turn-on probes: A potential candidate for tracking live cell imaging of biologically active metal ions. Sens. Diagn. 2023, 2, 988–1076. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Chapter Solvent and Environmental Effects; Springer: Boston, MA, USA, 2006; pp. 205–235. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Chapter Mechanisms and Dynamics of Fluorescence Quenching; Springer: Boston, MA, USA, 2006; pp. 331–351. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Q.; Mao, Y.; Wang, X.; Wang, Y.; Hu, Q.; Wang, H.; Wang, X. Sensors for determination of uranium: A review. Trends Anal. Chem. 2019, 118, 89–111. [Google Scholar] [CrossRef]
- Hay, P.J.; Martin, R.L.; Schreckenbach, G. Theoretical Studies of the Properties and Solution Chemistry of AnO22+ and AnO2+ Aquo Complexes for An = U, Np, and Pu. J. Phys. Chem. A 2000, 104, 6259–6270. [Google Scholar] [CrossRef]
- Ikeda-Ohno, A.; Hennig, C.; Tsushima, S.; Scheinost, A.C.; Bernhard, G.; Yaita, T. Speciation and Structural Study of U(IV) and -(VI) in Perchloric and Nitric Acid Solutions. Inorg. Chem. 2009, 48, 7201–7210. [Google Scholar] [CrossRef]
- de Jong, W.A.; Apra, E.; Windus, T.L.; Nichols, J.A.; Harrison, R.J.; Gutowski, K.E.; Dixon, D.A. Complexation of the Carbonate, Nitrate, and Acetate Anions with the Uranyl Dication: Density Functional Studies with Relativistic Effective Core Potentials. J. Phys. Chem. A 2005, 109, 11568–11577. [Google Scholar] [CrossRef]
- Moulin, C.; Beaucaire, C.; Decambox, P.; Mauchien, P. Determination of uranium in solution at the ng l−1 level by time-resolved laser-induced spectrofluorimetry: Application to geological samples. Anal. Chim. Acta 1990, 238, 291–296. [Google Scholar] [CrossRef]
- Rathore, D. Advances in technologies for the measurement of uranium in diverse matrices. Talanta 2008, 77, 9–20. [Google Scholar] [CrossRef]
- Yusov, A.B.; Mishkevich, V.I.; Fedoseev, A.M.; Grigor’ev, M.S. Complexation of An(VI) (An = U, Np, Pu, Am) with 2,6-pyridinedicarboxylic acid in aqueous solutions. Synthesis and structures of new crystalline compounds of U(VI), Np(VI), and Pu(VI). Radiochemistry 2013, 55, 269–278. [Google Scholar] [CrossRef]
- Autillo, M.; Islam, M.A.; Héron, J.; Guérin, L.; Acher, E.; Tamain, C.; Illy, M.C.; Moisy, P.; Colineau, E.; Griveau, J.C.; et al. Temperature Dependence of 1H Paramagnetic Chemical Shifts in Actinide Complexes, Beyond Bleaney’s Theory: The Dipicolinic Acid Complexes (An=Np, Pu) as an Example. Chem. Eur. J. 2021, 27, 7138–7153. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Viswanathan, K. Sensitization of uranium fluorescence using 2,6-pyridinedicarboxylic acid: Application for the determination of uranium in the presence of lanthanides. J. Lumin. 2009, 129, 1242–1248. [Google Scholar] [CrossRef]
- Kumar, S.A.; Shenoy, N.S.; Pandey, S.; Sounderajan, S.; Venkateswaran, G. Direct determination of uranium in seawater by laser fluorimetry. Talanta 2008, 77, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Viswanathan, K. Enhancement of uranyl fluorescence using trimesic acid: Ligand sensitization and co-fluorescence. J. Lumin. 2011, 131, 1848–1852. [Google Scholar] [CrossRef]
- Wen, J.; Huang, Z.; Hu, S.; Li, S.; Li, W.; Wang, X. Aggregation-induced emission active tetraphenylethene-based sensor for uranyl ion detection. J. Hazard. Mater. 2016, 318, 363–370. [Google Scholar] [CrossRef]
- Lin, N.; Ren, W.; Hu, J.; Gao, B.; Yuan, D.; Wang, X.; Fu, J. A novel tetraphenylethene-based fluorescent sensor for uranyl ion detection with aggregation-induced emission character. Dye. Pigment. 2019, 166, 182–188. [Google Scholar] [CrossRef]
- Lin, N.; Tao, R.; Chen, Z.; Pan, Q.; Zhu, Z.; Gao, B.; Ren, W. Design and fabrication of a new fluorescent film sensor towards uranyl ion via self-assembled monolayer. J. Lumin. 2022, 242, 118562. [Google Scholar] [CrossRef]
- Ding, H.; Li, C.; Zhang, H.; Lin, N.; Ren, W.S.; Li, S.; Liu, W.; Xiong, Z.; Xia, B.; Wang, C.C. A simple fluorescent sensor for highly sensitive detection of . Chin. Chem. Lett. 2023, 34, 107725. [Google Scholar] [CrossRef]
- Zhan, Y.; Lu, X.; Lan, T.; Tian, Q.; Shen, J.; He, W. An AIE fluorescence sensor based on amidoxime for rapid detection of uranyl ions in aqueous solutions. Dye. Pigment. 2023, 216, 111299. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, S.; Cao, M.; Du, X.; Wang, H.; Cao, X.; Feng, L.; Yuan, Y.; Wang, N. Highly sensitive and specific uranyl ion detection by a fluorescent sensor containing uranyl-specific recognition sites. Sci. Bull. 2024, 70, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Hu, J.; Ren, W.; Gao, B.; Fu, J.; Luo, W. A turn-on fluorescent chemodosimeter for uranyl ions based on uranyl-promoted hydrolysis reaction. J. Lumin. 2021, 234, 117947. [Google Scholar] [CrossRef]
- Zheng, M.; Yin, Q.; Wang, D.; Zhao, Z.; Hu, Q.; Wang, H. A fluorescent probe of uranyl for acid and high water system and imaging in living cells. Microchem. J. 2021, 167, 106302. [Google Scholar] [CrossRef]
- Wu, X.Y.; Cui, A.Q.; Ye, J.B.; Song, G.; Wu, Y.N.; Wu, Y.X.; Lai, J.P.; Sun, H. Novel biocompatible and sensitive visual sensor based on aggregation-induced emission for on-site detection of radioactive uranium in water and live cell imaging. Sci. Total Environ. 2023, 858, 159796. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Z.; Zhang, D.; Zhao, X.; Guo, Z.; Huang, X.; Qu, J.; Zhang, J.; Wang, Y.; Jiao, Z. Highly selective and portable fluorescent detection of uranyl in groundwater via AIEgen-labeled protein cleavage. Spectrochim. Acta Part A 2025, 344, 126685. [Google Scholar] [CrossRef]
- Chen, X.; He, L.; Wang, Y.; Liu, B.; Tang, Y. Trace analysis of uranyl ion () in aqueous solution by fluorescence turn-on detection via aggregation induced emission enhancement effect. Anal. Chim. Acta 2014, 847, 55–60. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.; Feng, M.; Xiang, Y.; Tong, A.; He, L.; Liu, B.; Tang, Y. An aggregation induced emission enhancement-based ratiometric fluorescent sensor for detecting trace uranyl ion () and the application in living cells imaging. J. Lumin. 2017, 186, 301–306. [Google Scholar] [CrossRef]
- Pham, X.Q.; Kumar, N.; Ha-Thi, M.H.; Leray, I. Sensitive and selective detection of uranyl ions based on aggregate-breaking mechanism. J. Photochem. Photobiol. A 2019, 373, 139–145. [Google Scholar] [CrossRef]
- Nivens, D.A.; Zhang, Y.; Angel, S. Detection of uranyl ion via fluorescence quenching and photochemical oxidation of calcein. J. Photochem. Photobiol. A 2002, 152, 167–173. [Google Scholar] [CrossRef]
- DeVore II, M.A.; Kerns, S.A.; Gorden, A.E.V. Characterization of Quinoxolinol Salen Ligands as Selective Ligands for Chemosensors for Uranium. Eur. J. Inorg. Chem. 2015, 2015, 5708–5714. [Google Scholar] [CrossRef]
- Zhu, J.H.; Zhao, X.; Yang, J.; Tan, Y.T.; Zhang, L.; Liu, S.P.; Liu, Z.F.; Hu, X.L. Selective colorimetric and fluorescent quenching determination of uranyl ion via its complexation with curcumin. Spectrochim. Acta Part A 2016, 159, 146–150. [Google Scholar] [CrossRef]
- He, W.; Ma, J.; Qian, J.; Liu, H.; Hua, D. Adsorption-assistant detection of trace uranyl ion with high sensitivity and selectivity in the presence of SBA-15. J. Radioanal. Nucl. Chem. 2018, 316, 201–207. [Google Scholar] [CrossRef]
- Kim, M.S.; Tsukahara, T. Studies on Coordination and Fluorescence Behaviors of a Novel Uranyl Ion-Selective Chemosensor Bearing Diaza 18-Crown-6 Ether and Naphthalimide Moieties. ACS Earth Space Chem. 2020, 4, 2270–2280. [Google Scholar] [CrossRef]
- Elabd, A.A.; Elhefnawy, O.A. An Efficient and Sensitive Optical Sensor Based on Furosemide as a new Fluoroionophore for Determination of Uranyl ion. J. Fluoresc. 2015, 26, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Brown, A.K.; Meng, X.; Cropek, D.M.; Istok, J.D.; Watson, D.B.; Lu, Y. A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and millionfold selectivity. Proc. Natl. Acad. Sci. USA 2007, 104, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Cheng, Y.; Li, M.; Li, Z.; Dai, J. Detection of uranyl ions by single-hairpin based self-hybridization chain reaction. Talanta 2025, 285, 127374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liang, W.; Li, D.; Yuan, R.; Xiang, Y. Dual-color encoded DNAzyme nanostructures for multiplexed detection of intracellular metal ions in living cells. Biosens. Bioelectron. 2016, 85, 573–579. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, H.; Huang, Y.; Wang, J.; Gu, M.; Liu, Y.; Wang, N.; Wang, J.; Hu, S.; Deng, R. A smart DNAzyme/graphene oxide nanosystem for fluorescent sensing of uranyl ion with high sensitivity and selectivity. Microchem. J. 2022, 180, 107596. [Google Scholar] [CrossRef]
- Yun, W.; Wu, H.; Yang, Z.; Wang, R.; Wang, C.; Yang, L.; Tang, Y. A dynamic, ultra-sensitive and “turn-on” strategy for fluorescent detection of uranyl based on DNAzyme and entropy-driven amplification initiated circular cleavage amplification. Anal. Chim. Acta 2019, 1068, 104–110. [Google Scholar] [CrossRef]
- Yun, W.; Wu, H.; Liu, X.; Zhong, H.; Fu, M.; Yang, L.; Huang, Y. Ultra-sensitive fluorescent and colorimetric detection of UO22+ based on dual enzyme-free amplification strategies. Sens. Actuators B 2018, 255, 1920–1926. [Google Scholar] [CrossRef]
- Zhang, H.; Ruan, Y.; Lin, L.; Lin, M.; Zeng, X.; Xi, Z.; Fu, F. A turn-off fluorescent biosensor for the rapid and sensitive detection of uranyl ion based on molybdenum disulfide nanosheets and specific DNAzyme. Spectrochim. Acta Part A 2015, 146, 1–6. [Google Scholar] [CrossRef]
- Li, M.H.; Wang, Y.S.; Cao, J.X.; Chen, S.H.; Tang, X.; Wang, X.F.; Zhu, Y.F.; Huang, Y.Q. Ultrasensitive detection of uranyl by graphene oxide-based background reduction and RCDzyme-based enzyme strand recycling signal amplification. Biosens. Bioelectron. 2015, 72, 294–299. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Y.; Xu, S.; Zhang, X. G-quadruplex-assisted enzyme strand recycling for amplified label-free fluorescent detection of . Chin. Chem. Lett. 2019, 30, 58–62. [Google Scholar] [CrossRef]
- Jiang, M.; Xiao, X.; He, B.; Liu, Y.; Hu, N.; Su, C.; Li, Z.; Liao, L. A europium (III) complex-based surface fluorescence sensor for the determination of uranium (VI). J. Radioanal. Nucl. Chem. 2019, 321, 161–167. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Xiong, L.; Song, J.; Zhou, H.; Li, L.; Zhen, D. Development of rare earth europium composites for highly sensitive fluorescence enhancement for detection of uranyl ions in water and cells. J. Radioanal. Nucl. Chem. 2024, 334, 1931–1939. [Google Scholar] [CrossRef]
- Sessler, J.; Melfi, P.; Pantos, G. Uranium complexes of multidentate N-donor ligands. Coordin. Chem. Rev. 2006, 250, 816–843. [Google Scholar] [CrossRef]
- Karak, A.; Manna, S.K.; Mahapatra, A.K. Triphenylamine-based small-molecule fluorescent probes. Anal. Methods 2022, 14, 972–1005. [Google Scholar] [CrossRef]
- Zhou, W.; Saran, R.; Liu, J. Metal Sensing by DNA. Chem. Rev. 2017, 117, 8272–8325. [Google Scholar] [CrossRef]
- Wu, P.; Hwang, K.; Lan, T.; Lu, Y. A DNAzyme-Gold Nanoparticle Probe for Uranyl Ion in Living Cells. J. Am. Chem. Soc. 2013, 135, 5254–5257. [Google Scholar] [CrossRef]
- Xiao, S.J.; Zuo, J.; Zhu, Z.Q.; Ouyang, Y.Z.; Zhang, X.L.; Chen, H.W.; Zhang, L. Highly sensitive DNAzyme sensor for selective detection of trace uranium in ore and natural water samples. Sens. Actuators B 2015, 210, 656–660. [Google Scholar] [CrossRef]
- Yang, C.T.; Han, J.; Gu, M.; Liu, J.; Li, Y.; Huang, Z.; Yu, H.Z.; Hu, S.; Wang, X. Fluorescent recognition of uranyl ions by a phosphorylated cyclic peptide. Chem. Commun. 2015, 51, 11769–11772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, M.; Wang, X.; Gao, L.; Zhang, B.; Wang, L.; Kong, J.; Li, L. A novel fluorescence sensor for uranyl ion detection based on a dansyl-modified peptide. Spectrochim. Acta Part A 2023, 292, 122403. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liao, L.; Zhao, M.; Lin, Y.; Xiao, X.; Nie, C. Separation and determination of trace uranium using a double-receptor sandwich supramolecule method based on immobilized salophen and fluorescence labeled oligonucleotide. Anal. Chim. Acta 2012, 729, 80–84. [Google Scholar] [CrossRef]
- Ma, J.; He, W.; Han, X.; Hua, D. Amidoximated fluorescent polymer based sensor for detection of trace uranyl ion in aqueous solution. Talanta 2017, 168, 10–15. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Yin, Q.; Wang, Y.; Wang, H. A conjugated fluorescent polymer sensor with amidoxime and polyfluorene entities for effective detection of uranyl ion in real samples. Spectrochim. Acta Part A 2021, 244, 118864. [Google Scholar] [CrossRef]
- Xu, M.; Wang, T.; Gao, P.; Zhao, L.; Zhou, L.; Hua, D. Highly fluorescent conjugated microporous polymers for concurrent adsorption and detection of uranium. J. Mater. Chem. A 2019, 7, 11214–11222. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Zhang, S.; Huang, L.; Wang, S.; Hua, D. Determination of trace uranyl ion by thermoresponsive porphyrin–terminated polymeric sensor. Talanta 2015, 131, 198–204. [Google Scholar] [CrossRef]
- Elabd, A.; Attia, M. A new thin film optical sensor for assessment of UO22+ based on the fluorescence quenching of Trimetazidine doped in sol gel matrix. J. Lumin. 2015, 165, 179–184. [Google Scholar] [CrossRef]
- Elabd, A.; Attia, M. Spectroflourimetric assessment of UO22+ by the quenching of the fluorescence intensity of Clopidogrel embedded in PMMA matrix. J. Lumin. 2016, 169, 313–318. [Google Scholar] [CrossRef]
- Güney, O.; Atçakan, E. Synthesis and characterization of quinoline-derivated fluorescent sol–gel-imprinted polymer as a chemosensor for sensing of uranyl ion. J. Sol-Gel Sci. Technol. 2016, 81, 534–543. [Google Scholar] [CrossRef]
- Liu, W.; Dai, X.; Bai, Z.; Wang, Y.; Yang, Z.; Zhang, L.; Xu, L.; Chen, L.; Li, Y.; Gui, D.; et al. Highly Sensitive and Selective Uranium Detection in Natural Water Systems Using a Luminescent Mesoporous Metal–Organic Framework Equipped with Abundant Lewis Basic Sites: A Combined Batch, X-ray Absorption Spectroscopy, and First Principles Simulation Investigation. Environ. Sci. Technol. 2017, 51, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Bogale, R.F.; Shi, Y.; Chen, Y.; Liu, X.; Zhang, S.; Yang, Y.; Zhao, J.; Ning, G. A Water-Stable Dual-Channel Luminescence Sensor for UO22+ Ions Based on an Anionic Terbium(III) Metal–Organic Framework. Chem.-Eur. J. 2017, 23, 7657–7662. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.Q.; Wu, X.Y.; Ye, J.B.; Song, G.; Chen, D.Y.; Xu, J.; Liu, Y.; Lai, J.P.; Sun, H. “Two-in-one” dual-function luminescent MOF hydrogel for onsite ultra-sensitive detection and efficient enrichment of radioactive uranium in water. J. Hazard. Mater. 2023, 448, 130864. [Google Scholar] [CrossRef]
- Lei, M.; Jia, Y.; Zhang, W.; Xie, J.; Xu, Z.; Wang, Y.; Du, W.; Liu, W. Ultrasensitive and Selective Detection of Uranium by a Luminescent Terbium–Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 51086–51094. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Z.; Lei, J.; Liang, J.; Wang, W.; Xiao, Y.; Liu, F.; Dai, X.; Hu, B. A Luminescent Sensor for Trace Uranyl in Nature Water Systems Using a Terbium(III)-Organic Framework. Inorg. Chem. 2025, 64, 14741–14746. [Google Scholar] [CrossRef]
- Xie, J.; Liang, J.; Lei, J.; Xiao, Y.; Luo, F.; Hu, B. Highly Sensitive and Selective Detection of Uranyl Ions Based on a Tb3+-Functionalized MOF via Competitive Host–Guest Coordination. Inorg. Chem. 2025, 64, 3616–3625. [Google Scholar] [CrossRef]
- Mondal, S.; Tedy, A.M.; Chand, S.; Sahoo, R.; Manna, A.K.; Das, M.C. Mechanistical Insights into the Ultrasensitive Detection of Radioactive and Chemotoxic Ions by a Porous Anionic Co-Metal–Organic Framework. Inorg. Chem. 2024, 63, 10403–10413. [Google Scholar] [CrossRef]
- Li, S.Q.; Peng, H.B.; Cao, X.H.; Dong, Z.M.; Wang, Y.Q.; Zhang, Z.B.; Liu, Y.H. Defect-engineered UiO-66-NH2 (AO)-3: Achieving enhanced fluorescence sensing of uranyl ions. Inorg. Chim. Acta 2025, 581, 122655. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, L.; Wu, K.; Yin, M.; Lu, Y.; Yuan, Z.; Jiang, W.; Wang, J.; Wang, X.; Wang, S. Non-rare earth doped metal-organic framework for fluorescent detection of uranyl in real seawater. Sens. Actuators B 2025, 436, 137643. [Google Scholar] [CrossRef]
- Hou, J.X.; Gao, J.P.; Liu, J.; Jing, X.; Li, L.J.; Du, J.L. Highly selective and sensitive detection of Pb2+ and UO22+ ions based on a carboxyl-functionalized Zn(II)-MOF platform. Dye. Pigment. 2019, 160, 159–164. [Google Scholar] [CrossRef]
- Qin, X.; Yang, W.; Yang, Y.; Gu, D.; Guo, D.; Pan, Q. A Zinc Metal–Organic Framework for Concurrent Adsorption and Detection of Uranium. Inorg. Chem. 2020, 59, 9857–9865. [Google Scholar] [CrossRef]
- Cui, W.R.; Zhang, C.R.; Jiang, W.; Li, F.F.; Liang, R.P.; Liu, J.; Qiu, J.D. Regenerable and stable sp2 carbon-conjugated covalent organic frameworks for selective detection and extraction of uranium. Nat. Commun. 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.R.; Chen, Y.R.; Xu, W.; Liu, K.; Qiu, W.B.; Li, Y.; Qiu, J.D. A three-dimensional luminescent covalent organic framework for rapid, selective, and reversible uranium detection and extraction. Sep. Purif. Technol. 2023, 306, 122726. [Google Scholar] [CrossRef]
- Zhen, D.; Liu, C.; Deng, Q.; Li, L.; Grimes, C.A.; Yang, S.; Cai, Q.; Liu, Y. Novel Olefin-Linked Covalent Organic Framework with Multifunctional Group Modification for the Fluorescence/Smartphone Detection of Uranyl Ion. ACS Appl. Mater. 2024, 16, 27804–27812. [Google Scholar] [CrossRef]
- Xu, D.; Lu, S.; Hao, X.; Yang, S.; Lu, J.; Huang, Y.; Chen, W.; Hao, H.; Huang, S.; Chen, L.; et al. Highly Sensitive Detection of ppb-Level Uranyl via Exfoliation-Activated Luminescent Covalent–Organic Framework Nanosheets. ACS Mater. Lett. 2025, 7, 2716–2724. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, X.; Mai, Z.; Yang, L.; Zheng, S.; Liao, J.; Gao, F.; Zhang, Y.; Jiao, Z. Development of COF-MF-based fluorescent sensor for on-site, and portable detection of uranium in ore samples. Int. J. Environ. An. Chem. 2025, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Deng, Q.; Yu, Y.; Tang, X.; Li, L.; Grimes, C.A.; Yang, S.; Cai, Q.; Zhen, D. A β-keto-enamine covalent organic framework fluorescent switch for selective and sensitive detection. Sens. Actuators B 2025, 433, 137564. [Google Scholar] [CrossRef]
- Guin, S.K.; Ambolikar, A.S.; Paul Guin, J.; Neogy, S. Exploring the excellent photophysical and electrochemical properties of graphene quantum dots for complementary sensing of uranium. Sens. Actuators B 2018, 272, 559–573. [Google Scholar] [CrossRef]
- Dutta, R.; Kumar, A. Highly Sensitive and Selective Method for Detecting Ultratrace Levels of Aqueous Uranyl Ions by Strongly Photoluminescent-Responsive Amine-Modified Cadmium Sulfide Quantum Dots. Anal. Chem. 2016, 88, 9071–9078. [Google Scholar] [CrossRef]
- Shamsipur, M.; Molaei, K.; Molaabasi, F.; Hosseinkhani, S.; Alizadeh, N.; Alipour, M.; Moassess, S. One-step synthesis and characterization of highly luminescent nitrogen and phosphorus co-doped carbon dots and their application as highly selective and sensitive nanoprobes for low level detection of uranyl ion in hair and water samples and application to cellular imaging. Sens. Actuators B Chem. 2018, 257, 772–782. [Google Scholar] [CrossRef]
- Burger, A.; Lichtscheidl, I. Stable and radioactive cesium: A review about distribution in the environment, uptake and translocation in plants, plant reactions and plants’ potential for bioremediation. Sci. Total Environ. 2018, 618, 1459–1485. [Google Scholar] [CrossRef]
- Venturi, S. Cesium in Biology, Pancreatic Cancer, and Controversy in High and Low Radiation Exposure Damage—Scientific, Environmental, Geopolitical, and Economic Aspects. Int. J. Environ. Res. Public Health 2021, 18, 8934. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Leray, I.; Depauw, A. Chemically derived optical sensors for the detection of cesium ions. Coordin. Chem. Rev. 2016, 310, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.D.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.S.; Schmehl, R.H.; Li, C.J. A novel caesium selective fluorescent chemosensor. Chem. Commun. 2000, 695–696. [Google Scholar] [CrossRef]
- Otsuki, J.; Yamagata, T.; Ohmuro, K.; Araki, K.; Takido, T.; Seno, M. Fluorescent Monitoring of Complexation of Phthalimide-Fused Crown Ethers with Alkali Metal Ions. Bull. Chem. Soc. Jpn. 2001, 74, 333–337. [Google Scholar] [CrossRef]
- Seo, H.S.; Karim, M.M.; Lee, S.H. Selective Fluorimetric Recognition of Cesium Ion by 15-Crown-5-Anthracene. J. Fluoresc. 2008, 18, 853–857. [Google Scholar] [CrossRef]
- Li, Z.; Deng, H.; Jiang, Y.; Ju, J.; Huang, X.; Zhang, L.; Ruan, G.; Chen, Z.; Huang, Y. Fluorescence based visual sensing of cesium ions enabled by space-confined converting enriched cesium ions to CsPbBr3 nanocrystals in crown ether functionalized mesoporous silica. Sens. Actuators B 2024, 419, 136381. [Google Scholar] [CrossRef]
- Ji, H.; Hettich, R.L.; Brown, G.M.; Dabestani, R. Optical Sensing of Cesium Using 1,3-Alternate Calix[4]-mono- and Di(anthrylmethyl)aza-crown-6. Photochem. Photobiol. 1999, 70, 882–886. [Google Scholar] [CrossRef]
- Ji, H.F.; Brown, G.M.; Dabestani, R. Calix[4]arene-based Cs+ selective optical sensor. Chem. Commun. 1999, 609–610. [Google Scholar] [CrossRef]
- Ji, H.F.; Dabestani, R.; Brown, G.M.; Sachleben, R.A. A new highly selective calix[4]crown-6 fluorescent caesium probe. Chem. Commun. 2000, 833–834. [Google Scholar] [CrossRef]
- Ji, H.F.; Dabestani, R.; Brown, G.M. A Supramolecular Fluorescent Probe, Activated by Protons To Detect Cesium and Potassium Ions, Mimics the Function of a Logic Gate. J. Am. Chem. Soc. 2000, 122, 9306–9307. [Google Scholar] [CrossRef]
- Kim, J.S.; Noh, K.H.; Lee, S.H.; Kim, S.K.; Kim, S.K.; Yoon, J. Molecular Taekwondo. 2. A New Calix[4]azacrown Bearing Two Different Binding Sites as a New Fluorescent Ionophore. J. Org. Chem. 2002, 68, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shon, O.J.; Rim, J.A.; Kim, S.K.; Yoon, J. Pyrene-Armed Calix[4]azacrowns as New Fluorescent Ionophores: “Molecular Taekowndo” Process via Fluorescence Change. J. Org. Chem. 2002, 67, 2348–2351. [Google Scholar] [CrossRef]
- Lee, M.H.; Quang, D.T.; Jung, H.S.; Yoon, J.; Lee, C.H.; Kim, J.S. Ion-Induced FRET On-Off in Fluorescent Calix[4]arene. J. Org. Chem. 2007, 72, 4242–4245. [Google Scholar] [CrossRef] [PubMed]
- Roper, E.D.; Talanov, V.S.; Gorbunova, M.G.; Bartsch, R.A.; Talanova, G.G. Optical Determination of Thallium(I) and Cesium(I) with a Fluorogenic Calix[4]arenebis(crown-6 ether) Containing One Pendent Dansyl Group. Anal. Chem. 2007, 79, 1983–1989. [Google Scholar] [CrossRef]
- Leray, I.; Asfari, Z.; Vicens, J.; Valeur, B. Synthesis and binding properties of calix[4]biscrown-based fluorescent molecular sensors for caesium or potassium ions. J. Chem. Soc. Perkin Trans. 2002, 2, 1429. [Google Scholar] [CrossRef]
- Souchon, V.; Leray, I.; Valeur, B. Selective detection of cesium by a water-soluble fluorescent molecular sensor based on a calix[4]arene-bis(crown-6-ether). Chem. Commun. 2006, 4224–4226. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pham-Xuan, Q.; Depauw, A.; Hemadi, M.; Ha-Duong, N.T.; Lefevre, J.P.; Ha-Thi, M.H.; Leray, I. New sensitive and selective calixarene-based fluorescent sensors for the detection of Cs+ in an organoaqueous medium. New J. Chem. 2017, 41, 7162–7170. [Google Scholar] [CrossRef]
- Pham, X.Q.; Jonusauskaite, L.; Depauw, A.; Kumar, N.; Lefevre, J.; Perrier, A.; Ha-Thi, M.H.; Leray, I. New water-soluble fluorescent sensors based on calix[4]arene biscrown-6 for selective detection of cesium. J. Photochem. Photobiol. 2018, 364, 355–362. [Google Scholar] [CrossRef]
- Depauw, A.; Kumar, N.; Ha-Thi, M.H.; Leray, I. Calixarene-Based Fluorescent Sensors for Cesium Cations Containing BODIPY Fluorophore. J. Phys. Chem. A 2015, 119, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Akamatsu, M.; Okamoto, K.; Sumita, M.; Tateyama, Y.; Sakai, H.; Hill, J.P.; Abe, M.; Ariga, K. Micrometer-level naked-eye detection of caesium particulates in the solid state. Sci. Technol. Adv. Mat. 2013, 14, 015002. [Google Scholar] [CrossRef] [PubMed]
- Radaram, B.; Mako, T.; Levine, M. Sensitive and selective detection of cesium via fluorescence quenching. Dalton Trans. 2013, 42, 16276. [Google Scholar] [CrossRef]
- Chopra, S.; Singh, N.; Thangarasu, P.; Bhardwaj, V.K.; Kaur, N. Fluorescent organic nanoparticles as chemosensor for nanomolar detection of Cs+ in aqueous medium. Dye. Pigment. 2014, 106, 45–50. [Google Scholar] [CrossRef]
- Azadbakht, R.; Khanabadi, J. A novel fluorescent nano-chemosensor for cesium ions based on naphthalene macrocyclic derivative. Spectrochim. Acta Part A 2015, 139, 279–285. [Google Scholar] [CrossRef]
- Diamond, D.; McKervey, M.A. Calixarene-based sensing agents. Chem. Soc. Rev. 1996, 25, 15. [Google Scholar] [CrossRef]
- Mokhtari, B.; Pourabdollah, K.; Dallali, N. A review of calixarene applications in nuclear industries. J. Radioanal. Nucl. Chem. 2010, 287, 921–934. [Google Scholar] [CrossRef]
- Casnati, A.; Pochini, A.; Ungaro, R.; Ugozzoli, F.; Arnaud, F.; Fanni, S.; Schwing, M.J.; Egberink, R.J.M.; de Jong, F.; Reinhoudt, D.N. Synthesis, Complexation, and Membrane Transport Studies of 1,3-Alternate Calix[4]arene-crown-6 Conformers: A New Class of Cesium Selective Ionophores. J. Am. Chem. Soc. 1995, 117, 2767–2777. [Google Scholar] [CrossRef]
- Sato, I.; Okada, K.; Sasaki, J.; Chida, H.; Satoh, H.; Miura, K.; Kikuchi, K.; Otani, K.; Sato, S. Distribution of radioactive cesium and stable cesium in cattle kept on a highly contaminated area of Fukushima nuclear accident. Anim. Sci. J. 2014, 86, 716–720. [Google Scholar] [CrossRef]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Schilling, W.P.; Rajan, L.; Strobl-Jager, E. Characterization of the Bradykinin-stimulated Calcium Influx Pathway of Cultured vascular Endothelial Cells. J. Biol. Chem. 1989, 264, 12838–12848. [Google Scholar] [CrossRef] [PubMed]
- Usachev, Y.M.; Mironov, S.L. Effects of strontium and barium ions on calcium bindings and transport in nerve cells. Neurophysiology 1990, 21, 587–591. [Google Scholar] [CrossRef]
- Hatae, J.; Fujishiro, N.; Kawata, H. Spectroscopic Properties of Fluorescence Dye Fura-2 with Various Divalent Cations. Jpn. J. Physiol. 1996, 46, 423–429. [Google Scholar] [CrossRef][Green Version]
- Suzuki, Y.; Morozumi, T.; Nakamura, H.; Shimomura, M.; Hayashita, T.; Bartsh, R.A. New Fluorimetric Alkali and Alkaline Earth Metal Cation Sensors Based on Noncyclic Crown Ethers by Means of Intramolecular Excimer Formation of Pyrene. J. Phys. Chem. B 1998, 102, 7910–7917. [Google Scholar] [CrossRef]
- Prodi, L.; Bolletta, F.; Zaccheroni, N.; Watt, C.I.F.; Mooney, N.J. A New Family of Luminescent Sensors for Alkaline Earth Metal Ions. Chem.-Eur. J. 1998, 4, 1090–1094. [Google Scholar] [CrossRef]
- Plaza, P.; Leray, I.; Changenet-Barret, P.; Martin, M.M.; Valeur, B. Reversible Bulk Photorelease of Strontium Ion from a Crown Ether-Linked Merocyanine. ChemPhysChem 2002, 3, 668. [Google Scholar] [CrossRef]
- Akutsu-Suyama, K.; Mori, S.; Hanashima, T. Design and characterization of a 2-(2’-hydroxyphenyl)benzimidazole-based Sr2+-selective fluorescent probe in organic and micellar solution systems†. Photochem. Photobiol. Sci. 2019, 18, 2531–2538. [Google Scholar] [CrossRef]
- Ji, H.F.; Yang, Y.; Xu, X.; Brown, G. A calixarene based fluorescent Sr2+ and Ca2+ probe. Org. Biomol. Chem. 2006, 4, 770. [Google Scholar] [CrossRef]
- Sutariya, P.G.; Pandya, A.; Modi, N.R.; Menon, S.K. A highly efficient PET switch on–off–on fluorescence receptor based on calix[4]arene for the selective recognition of Cd2+ and Sr2+. Analyst 2013, 138, 2244. [Google Scholar] [CrossRef]
- Sutariya, P.G.; Soni, H.; Gandhi, S.A.; Pandya, A. Turn on fluorescence strip based sensor for recognition of Sr2+ and CN− via lower-rim substituted calix[4]arene and its computational investigation. Spectrochim. Acta Part A 2020, 238, 118456. [Google Scholar] [CrossRef]
- Fang, J.A.; Zhao, J.L.; Liao, X.; Zeng, X.; Chen, K.; Wei, X.Y.; Su, S.B.; Luo, Q.Y.; Redshaw, C.; Jin, Z. Molecular Tweezers-like Calix[4]arene Based Alkaline Earth Metal Cation (Ca2+, Sr2+, and Ba2+) Chemosensor and Its Imaging in Living Cells and Zebrafish. Inorg. Chem. 2019, 58, 14720–14727. [Google Scholar] [CrossRef]
- Qu, K.; Zhao, C.; Ren, J.; Qu, X. Human telomeric G-quadruplex formation and highly selective fluorescence detection of toxic strontium ions. Mol. BioSyst. 2012, 8, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, H.; Liu, T.; Feng, T.; Cao, M.; Zhang, J.; Liu, T.; Guo, Z.; Galiotis, C.; Yuan, Y.; et al. Ultrasensitive and highly selective detection of strontium ions. Nat. Sustain 2023, 6, 789–796. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, A.; Kaur, N.; Singh, N. Development of Chemosensor for Sr2+ Using Organic Nanoparticles: Application of Sensor in Product Analysis for Oral Care. Org. Biomol. Chem. 2014, 12, 8230–8238. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, G.; Singh, A.; Singh, N.; Kaur, N. Polyamine Based Ratiometric Fluorescent Chemosensor for Strontium Metal Ion in Aqueous Medium: Application in Tap Water, River Water, and in Oral Care. ACS Sustain. Chem. Eng. 2015, 4, 94–101. [Google Scholar] [CrossRef]
- Leung, K.H.; Ma, V.P.Y.; He, H.Z.; Chan, D.S.H.; Yang, H.; Leung, C.H.; Ma, D.L. A highly selective G-quadruplex-based luminescent switch-on probe for the detection of nanomolar strontium(ii) ions in sea water. RSC Adv. 2012, 2, 8273. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Ju, X.J.; Zhou, X.L.; Mu, X.T.; Tian, X.Y.; Zhang, L.; Liu, Z.; Wang, W.; Xie, R.; Chu, L.Y. A novel chemosensor for sensitive and facile detection of strontium ions based on ion-imprinted hydrogels modified with guanosine derivatives. J. Hazard. Mater. 2022, 421, 126801. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, C.; Chen, K.; Wang, Z.; He, M.; Wang, P.; Chen, K.; Jiao, Y.; Yang, Y. G-quadruplex DNA-based colorimetric biosensor for the ultrasensitive visual detection of Sr2+ ions using MnO2 nanorods as oxidase mimetics. Microchim. Acta 2024, 191, 213. [Google Scholar] [CrossRef]
- Meena, A.H.; Arai, Y. Environmental geochemistry of technetium. Environ. Chem. Lett. 2017, 15, 241–263. [Google Scholar] [CrossRef]
- Arrigo, L.M.; Galenas, M.; Bassil, D.B.; Tucker, S.A.; Kannan, R.; Katti, K.V.; Barnes, C.L.; Jurisson, S.S. Fluorescent phosphinimine as possible precursor to an anionic and fluorescent sensor for Tc-99. Radiochim. Acta 2008, 96, 835–844. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Alberto, R.; Braband, H. Fluorescent sensing of 99Tc pertechnetate in water. Chem. Sci. 2014, 5, 1820–1826. [Google Scholar] [CrossRef]
- Desai, A.M.; Singh, P.K. Ratiometric fluorescence turn-on sensing of perrhenate anion, a non-radioactive surrogate of hazardous pertechnetate, in aqueous solution. Sens. Actuators B 2018, 277, 205–209. [Google Scholar] [CrossRef]
- Desai, A.M.; Singh, P.K. An Ultrafast Molecular-Rotor-Based Fluorescent Turn-On Sensor for the Perrhenate Anion in Aqueous Solution. Chem.-Eur. J. 2019, 25, 2035–2042. [Google Scholar] [CrossRef]
- Pandey, S.P.; Desai, A.M.; Singh, P.K. A highly sensitive fluorescence “turn on” detection of perrhenate Anion, a non-radioactive surrogate of hazardous pertechnetate anion. Sens. Actuators B 2020, 323, 128675. [Google Scholar] [CrossRef]
- Singh, G.; Pandey, S.P.; Singh, P.K. A dual intensity and lifetime based fluorescence sensor for perrhenate anion. Sens. Actuators B 2021, 330, 129346. [Google Scholar] [CrossRef]
- Rapti, S.; Diamantis, S.A.; Dafnomili, A.; Pournara, A.; Skliri, E.; Armatas, G.S.; Tsipis, A.C.; Spanopoulos, I.; Malliakas, C.D.; Kanatzidis, M.G.; et al. Exceptional TcO4- Sorption Capacity and Highly Efficient ReO4- Luminescence Sensing by Zr4+ MOFs. J. Mater. Chem. A 2018, 6, 20813–20821. [Google Scholar] [CrossRef]
- Xu, D.; Chen, L.; Dai, X.; Li, B.; Wang, Y.; Liu, W.; Li, J.; Tao, Y.; Wang, Y.; Liu, Y.; et al. A Porous Aromatic Framework Functionalized with Luminescent Iridium(III) Organometallic Complexes for Turn-On Sensing of 99TcO4-. ACS Appl. Mater. Interfaces 2020, 12, 15288–15297. [Google Scholar] [CrossRef]
- Li, C.P.; Zhou, H.; Chen, J.; Wang, J.J.; Du, M.; Zhou, W. A Highly Efficient Coordination Polymer for Selective Trapping and Sensing of Perrhenate/Pertechnetate. ACS Appl. Mater. Interfaces 2020, 12, 15246–15254. [Google Scholar] [CrossRef]
- Choi, M.R.; Lee, B. Synthesis of cationic carbon quantum dot-based dual emission fluorescence sensor for detecting perrhenate anions in aqueous solutions. Opt. Mater. 2022, 134, 113190. [Google Scholar] [CrossRef]
- Yi, S.M.; Zhang, C.R.; Jiang, W.; Liu, X.; Niu, C.P.; Qi, J.X.; Chen, X.J.; Liang, R.P.; Qiu, J.D. Ionic Liquid Modified Covalent Organic Frameworks for Efficient Detection and Adsorption of ReO4-/TcO4-. J. Environ. Chem. Eng. 2022, 10, 107666. [Google Scholar] [CrossRef]
- Branch, S.D.; French, A.D.; Lines, A.M.; Soderquist, C.Z.; Rapko, B.M.; Heineman, W.R.; Bryan, S.A. In Situ Spectroscopic Analysis and Quantification of [Tc(CO)3]+ in Hanford Tank Waste. Environ. Sci. Technol. 2018, 52, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef] [PubMed]
- Aja, S.U.; Wood, S.A.; Williams-Jones, A.E. The aqueous geochemistry of Zr and the solubility of some Zr-bearing minerals. Appl. Geochem. 1995, 10, 603–620. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Manna, S.K.; Mukhopadhyay, S.K.; Banik, A. First Rhodamine-Based “Off–On” Chemosensor with High Selectivity and Sensitivity for Zr4+ and Its Imaging in Living Cell. Sens. Actuators B 2013, 183, 350–355. [Google Scholar] [CrossRef]
- Sutariya, P.G.; Soni, H.; Gandhi, S.A.; Soni, S.S.; Prasad, J. A dual-response naphthalene-armed calix[4]arene based fluorescence receptor for Zr[IV] and Fe[II] via Ligand to metal charge transfer. Sens. Actuators B 2021, 331, 129417. [Google Scholar] [CrossRef]
- Meng, H.M.; Fu, T.; Zhang, X.B.; Wang, N.N.; Tan, W.; Shen, G.L.; Yu, R.Q. Efficient Fluorescence Turn-On Probe for Zr via a Target-Triggered DNA Molecular Beacon Strategy. Anal. Chem. 2012, 84, 2124–2128. [Google Scholar] [CrossRef]
- Alford, W.; Shapiro, L.; White, C. Fluorometric Determination of Zirconium in Minerals. Anal. Chem. 1951, 23, 1149–1152. [Google Scholar] [CrossRef]
- Sánchez, F.G.; Gómez, J.C.M.; López, M.H. Spectrofluorimetric Determination of Zirconium Based on the Inclusion Complex of 8-Hydroxy 7 Iodo Quinoline Sulfonic Acid with β-Cyclodextrin As Ligand. Anal. Lett. 1990, 23, 923–933. [Google Scholar] [CrossRef]
- Selva-Kumar, R.; Ashok-Kumar, S. Highly selective phenanthroline based light-up fluorescent probe for monitoring Zr[IV] in aqueous medium. Inorg. Chem. Commun. 2021, 125, 108406. [Google Scholar] [CrossRef]
- Kirandeep; Kumar, A.; Sharma, A.; Sahoo, S.C.; Zangrando, E.; Saini, V.; Kataria, R.; Kumar Mehta, S. Metal organic framework as “turn-on” fluorescent sensor for Zr[IV] ions and selective adsorbent for organic dyes. Microchem. J. 2021, 171, 106824. [Google Scholar] [CrossRef]
- Chen, M.; Wang, M.; Liu, C.; Yang, T.; Zhou, X.; You, Y. A reusable fluorescence Eu-based coordination polymer for the detection of Zr4+ ions in aqueous media. New J. Chem. 2022, 46, 19991–19996. [Google Scholar] [CrossRef]
- Liao, X.; Zhou, L.; Lu, X.; Zou, H.; Cao, J.; Pan, J.; Li, C.; Zheng, Y. Multifunctional fluorescent carbon quantum dots for Zr4+ ion detection, pH response and cell imaging. J. Mater. Sci. Mater. Electron. 2023, 34, 1205. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, Z.; Lu, Z.; Jing, H.; Qi, W. Comparative Study of Fluorescence Enhancement of Some Fluorescence Systems in Different β-Cyclodextrin Derivatives and Cyclodextrin–Surfactant Media. Microchem. J. 1996, 53, 361–370. [Google Scholar] [CrossRef]
- Strupp, C. Beryllium metal II. A review of the available toxicity data. Ann. Occup. Hyg. 2011, 55, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Elguero, J.; Alkorta, I. The dubious origin of beryllium toxicity. Struct. Chem. 2023, 34, 391–398. [Google Scholar] [CrossRef]
- Zermatten, H.L.J. A Reaction for Beryllium in Minerals and Rocks. Publisher unknown, 1933. [Google Scholar]
- Welford, G.; Harley, J. Fluorimetric Determination of trace amounts of beryllium. Am. Ind. Hyg. Assoc. Q. 1952, 13, 232–234. [Google Scholar] [CrossRef]
- Sandell, E.B. Morin Reaction for Beryllium. Ind. Eng. Chem. Anal. Ed. 1940, 12, 762–764. [Google Scholar] [CrossRef]
- Fletcher, M.H. Fluorometric Study of the Beryllium-Morin System. Anal. Chem. 1965, 37, 550–557. [Google Scholar] [CrossRef]
- Martinez, M.L.; Cooper, W.C.; Chou, P.T. A novel excited-state intramolecular proton transfer molecule, 10-hydroxybenzo[h]quinoline. Chem. Phys. Lett. 1992, 193, 151–154. [Google Scholar] [CrossRef]
- Sytnik, A.; Kasha, M. Excited-state intramolecular proton transfer as a fluorescence probe for protein binding-site static polarity. Proc. Natl. Acad. Sci. USA 1994, 91, 8627–8630. [Google Scholar] [CrossRef]
- Chou, P.T.; Wei, C.Y. Photophysics of 10-Hydroxybenzo[h]quinoline in Aqueous Solution. J. Phys. Chem. 1996, 100, 17059–17066. [Google Scholar] [CrossRef]
- Hristova, S.; Dobrikov, G.; Kamounah, F.S.; Kawauchi, S.; Hansen, P.E.; Deneva, V.; Nedeltcheva, D.; Antonov, L. 10-Hydroxybenzo[h]quinoline: Switching between single- and double-well proton transfer through structural modifications. RSC Adv. 2015, 5, 102495–102507. [Google Scholar] [CrossRef]
- Matsumiya, H.; Hoshino, H.; Yotsuyanagi, T. A novel fluorescence reagent, 10-hydroxybenzo[h]quinoline-7-sulfonate, for selective determination of beryllium(II) ion at pg cm23 levels. Analyst 2001, 126, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Minogue, E.; Ehler, D.; Burrell, A.; McCleskey, T.; Taylor, T. Development of a New Fluorescence Method for the Detection of Beryllium on Surfaces. J. ASTM Int. 2005, 2, 13168. [Google Scholar] [CrossRef]
- Agrawal, A.; Cronin, J.; Tonazzi, J.; McCleskey, T.M.; Ehler, D.S.; Minogue, E.M.; Whitney, G.; Brink, C.; Burrell, A.K.; Warner, B.; et al. Validation of a standardized portable fluorescence method for determining trace beryllium in workplace air and wipe samples. J. Environ. Monit. 2006, 8, 619. [Google Scholar] [CrossRef]
- Sillén, L.G.; Martell, A.E.; Bjerrum, J.; Schwarzenbach, G.K. Stability Constants of Metal-Ion Complexes, 2nd ed.; Number 17 in Chemical Society; Special Publications, Chemical Society: London, UK, 1964; p. 754. [Google Scholar]
- Giguère, M.C. Mise au Point D’une Méthode D’analyse en Fluorescence Pour la Détermination du Béryllium Dans l’air en Milieu de Travail. Master’s Thesis, Université du Québec à Montréal, Montréal, QC, Canada, 2010. [Google Scholar]
- Matsumiya, H.; Hoshino, H. Selective Determination of Beryllium(II) Ion at Picomole per Decimeter Cubed Levels by Kinetic Differentiation Mode Reversed-Phase High-Performance Liquid Chromatography with Fluorometric Detection Using 2-(2‘-Hydroxyphenyl)-10-hydroxybenzo[h]quinoline as Precolumn Chelating Reagent. Anal. Chem. 2003, 75, 413–419. [Google Scholar] [CrossRef]
- Charlton, J.J.; Jones, N.C.; Wallace, R.A.; Smithwick, R.W.; Bradshaw, J.A.; Kravchenko, I.I.; Lavrik, N.V.; Sepaniak, M.J. Nanopillar Based Enhanced-Fluorescence Detection of Surface-Immobilized Beryllium. Anal. Chem. 2015, 87, 6814–6821. [Google Scholar] [CrossRef]
- Yoshida, H.; Ito, T.; Murata, A. Fluorimetric determination of beryllium with 4-methyl-6-acetyl-7-hydroxycoumarin. Fresenius’ J. Anal. Chem. 1990, 338, 738–740. [Google Scholar] [CrossRef]
- Pal, B.K.; Baksi, K. Chromotropic acid as fluorogenic reagent. I. Fluorimetric determination of beryllium. Mikrochim. Acta 1992, 108, 275–283. [Google Scholar] [CrossRef]
- Hosseini, M.; Vaezi, Z.; Ganjali, M.R.; Faridbod, F.; Abkenar, S.D. Fluorescence “Turn-On” chemosensor for the selective detection of beryllium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 161–164. [Google Scholar] [CrossRef]
- Goode, J.A.; Rushworth, J.V.H.; Millner, P.A. Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2014, 31, 6267–6276. [Google Scholar] [CrossRef]
- Demchenko, A.P. Photobleaching of organic fluorophores: Quantitative characterization, mechanisms, protection. Methods Appl. Fluoresc. 2020, 8, 022001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).