Long-Term Evaluation of Bone Healing Monitoring Using an Instrumented Plate with Measurement Sensors (Smart Implant) over 10 Years
Abstract
Highlights
- With first- and second-generation telemetric bone implants, force transmission over the fracture region is possible.
- By this, the status of bone union over time can be monitored.
- Healing process of bone union can successfully be monitored by smart implants.
- Long-term monitoring is possible when using external power sources.
Abstract
1. Introduction
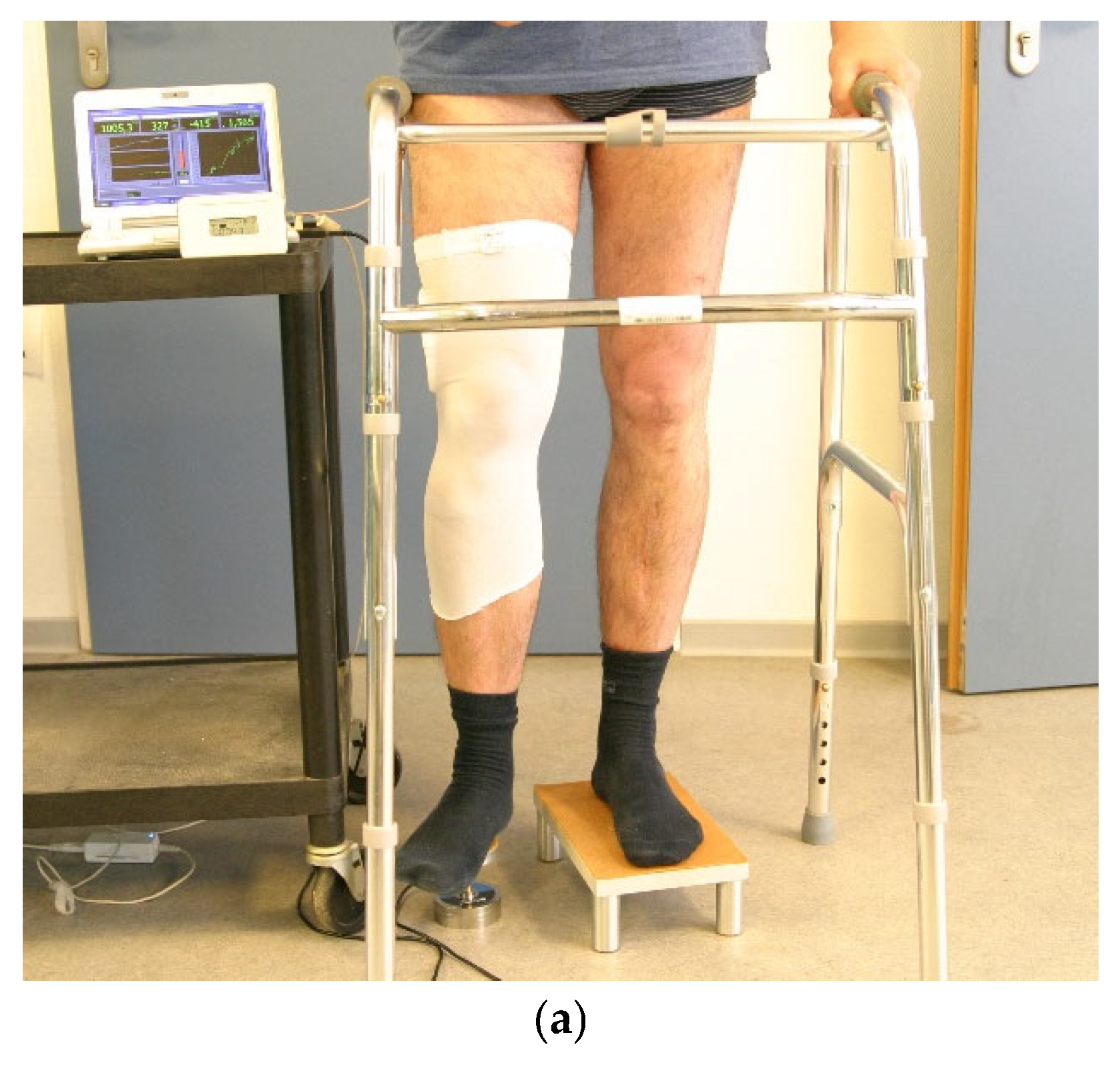
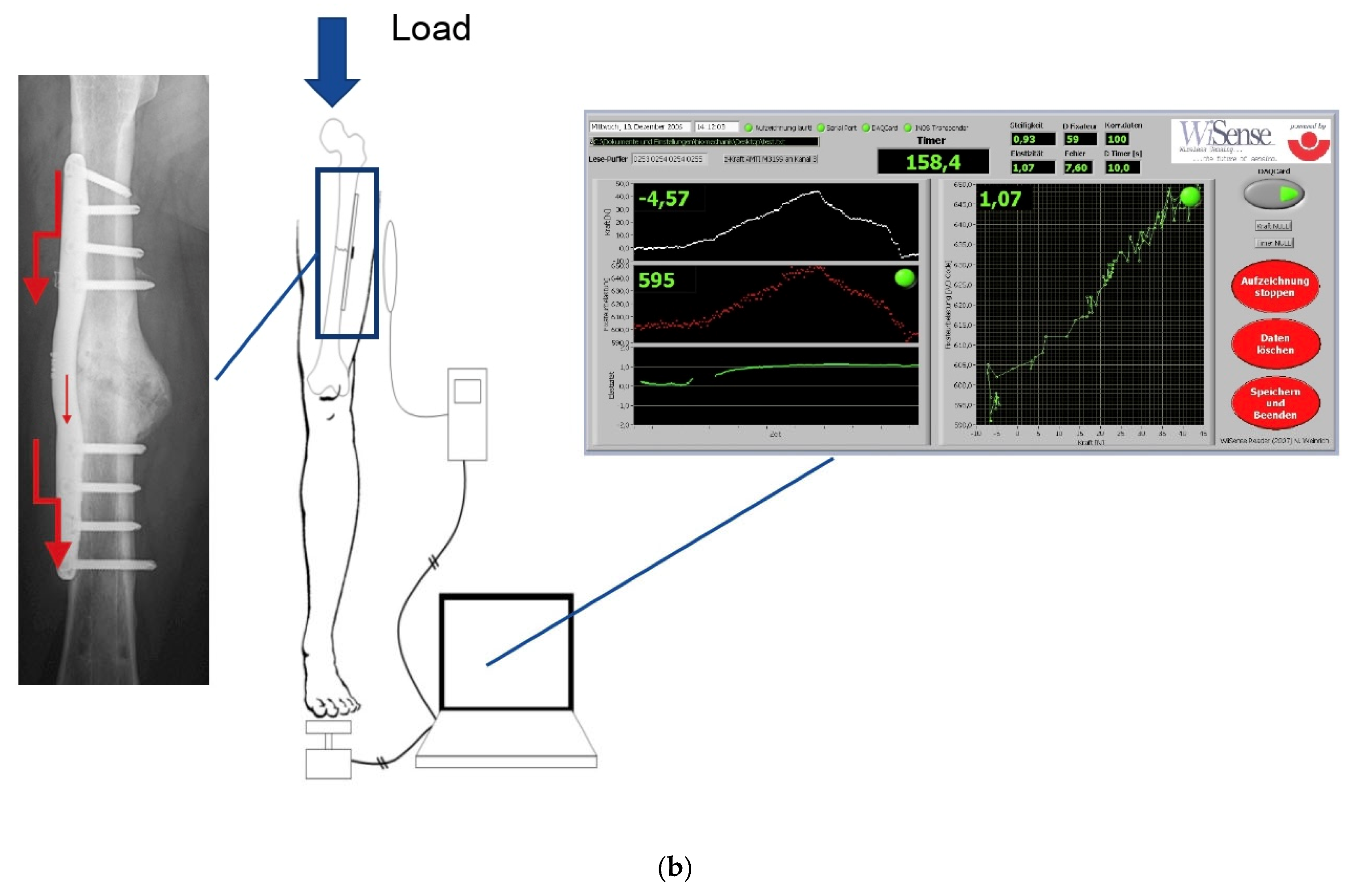
2. Materials and Methods
2.1. Study Subjects
2.2. Material
2.3. Methods
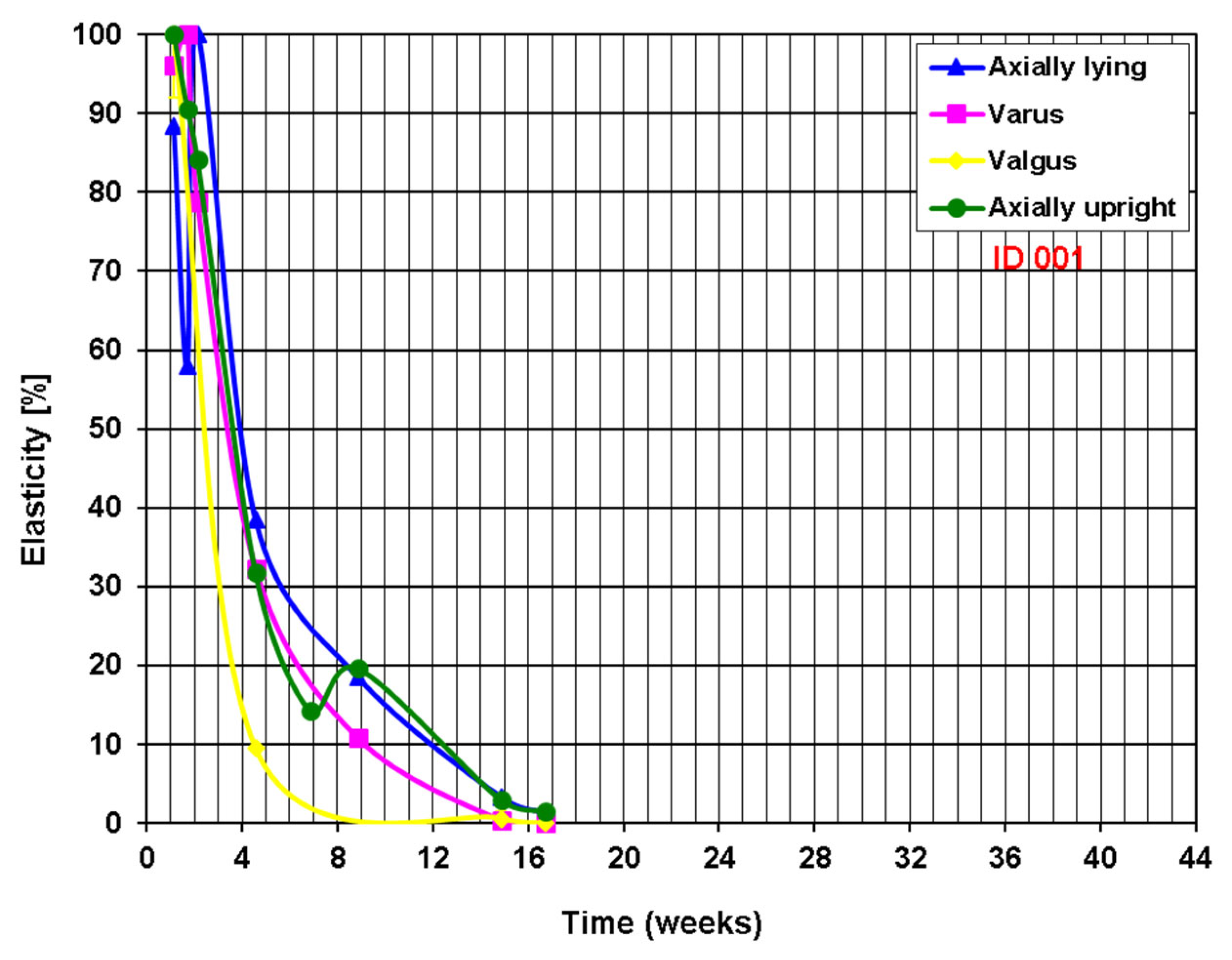
Definition of Healing Status
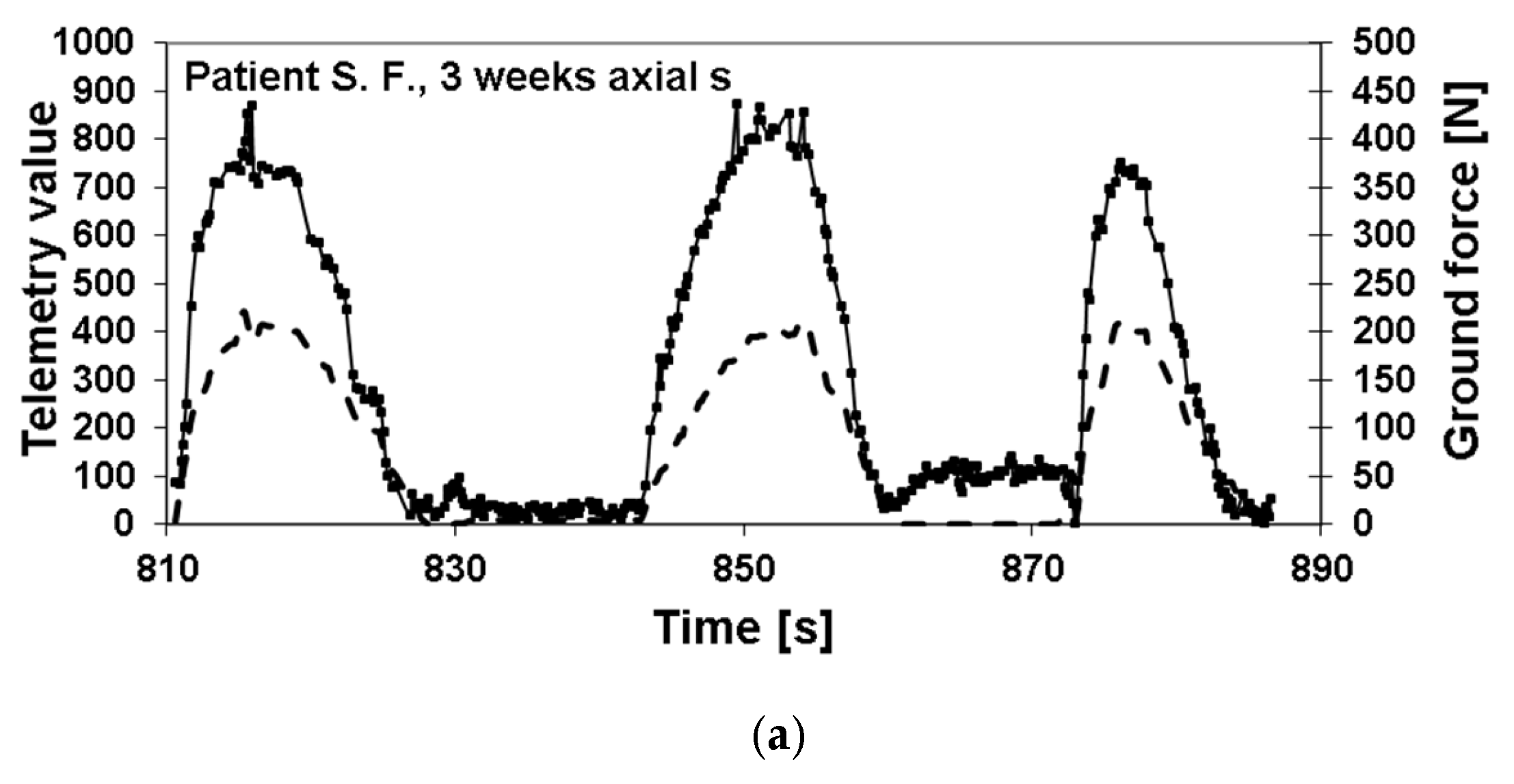
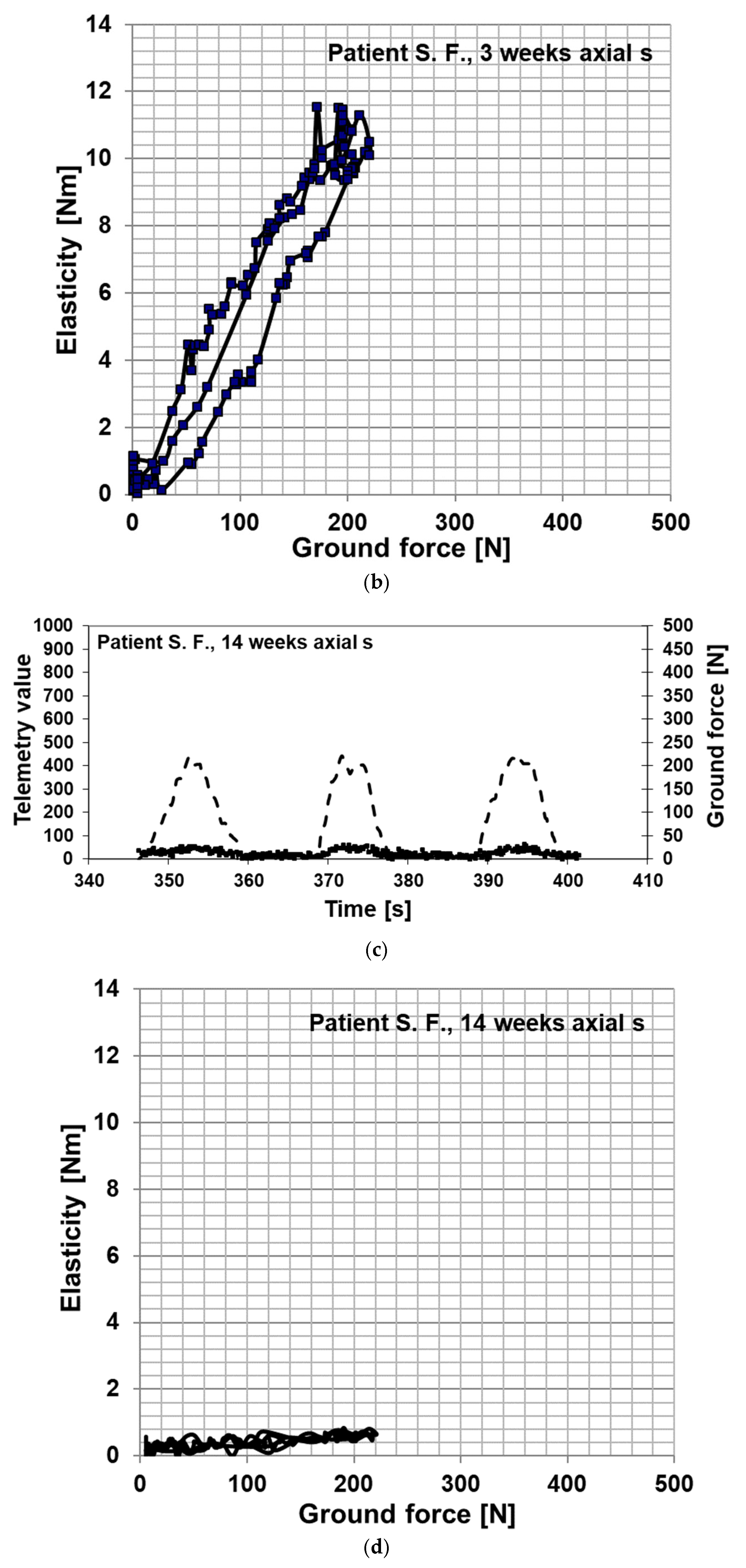
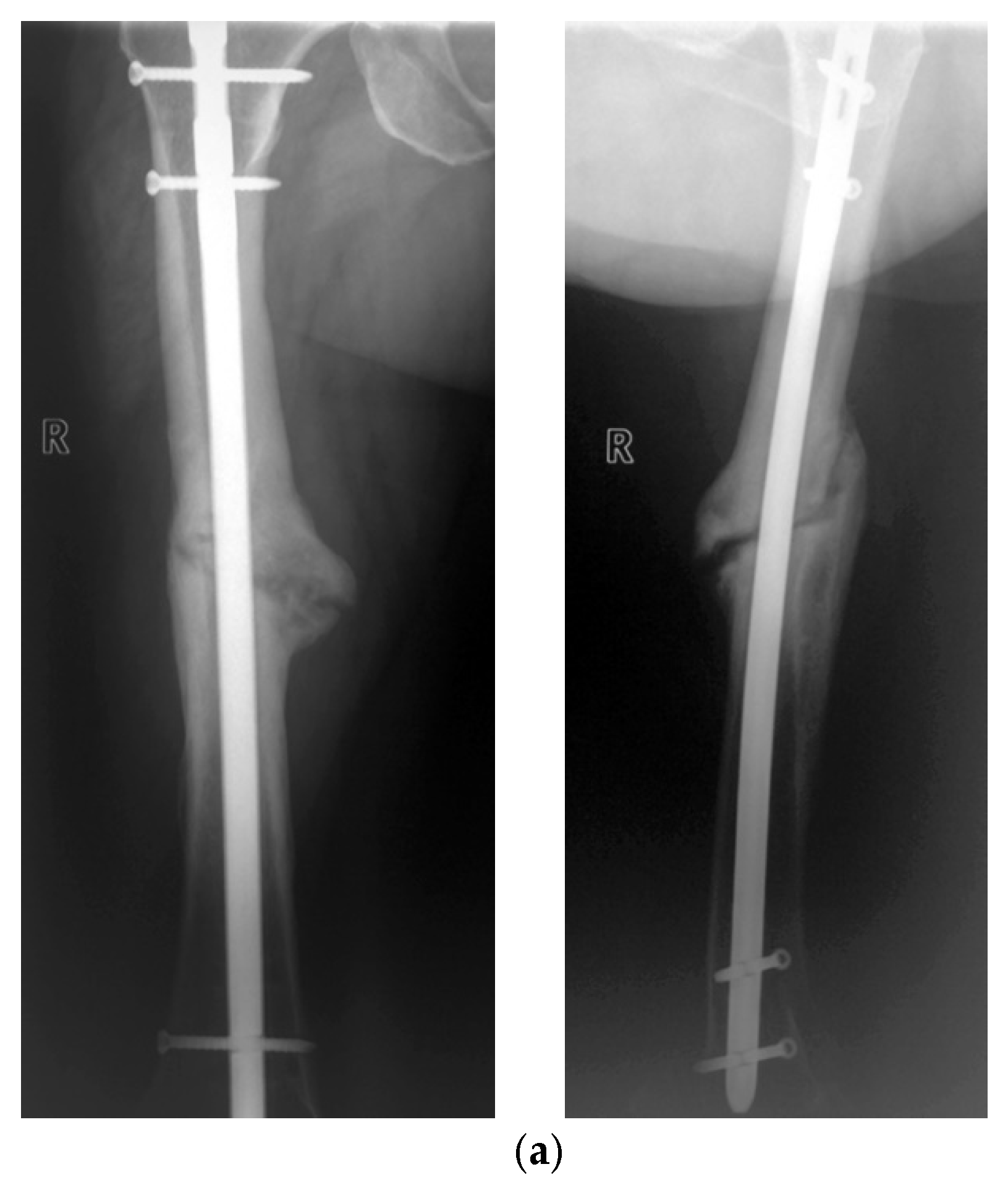
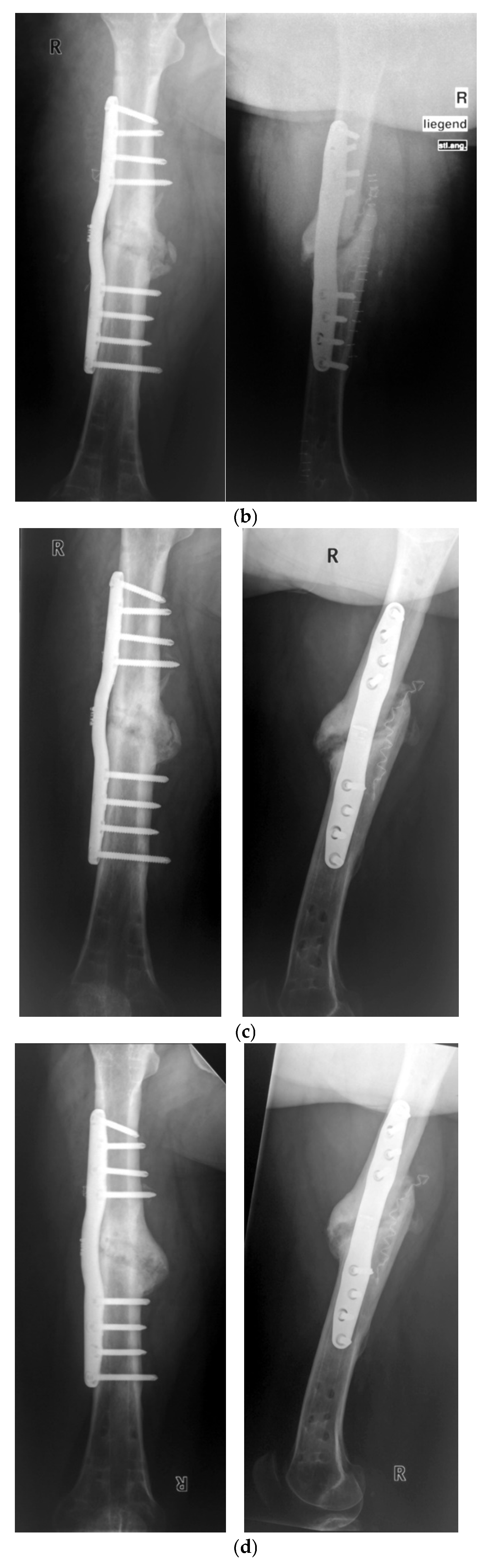
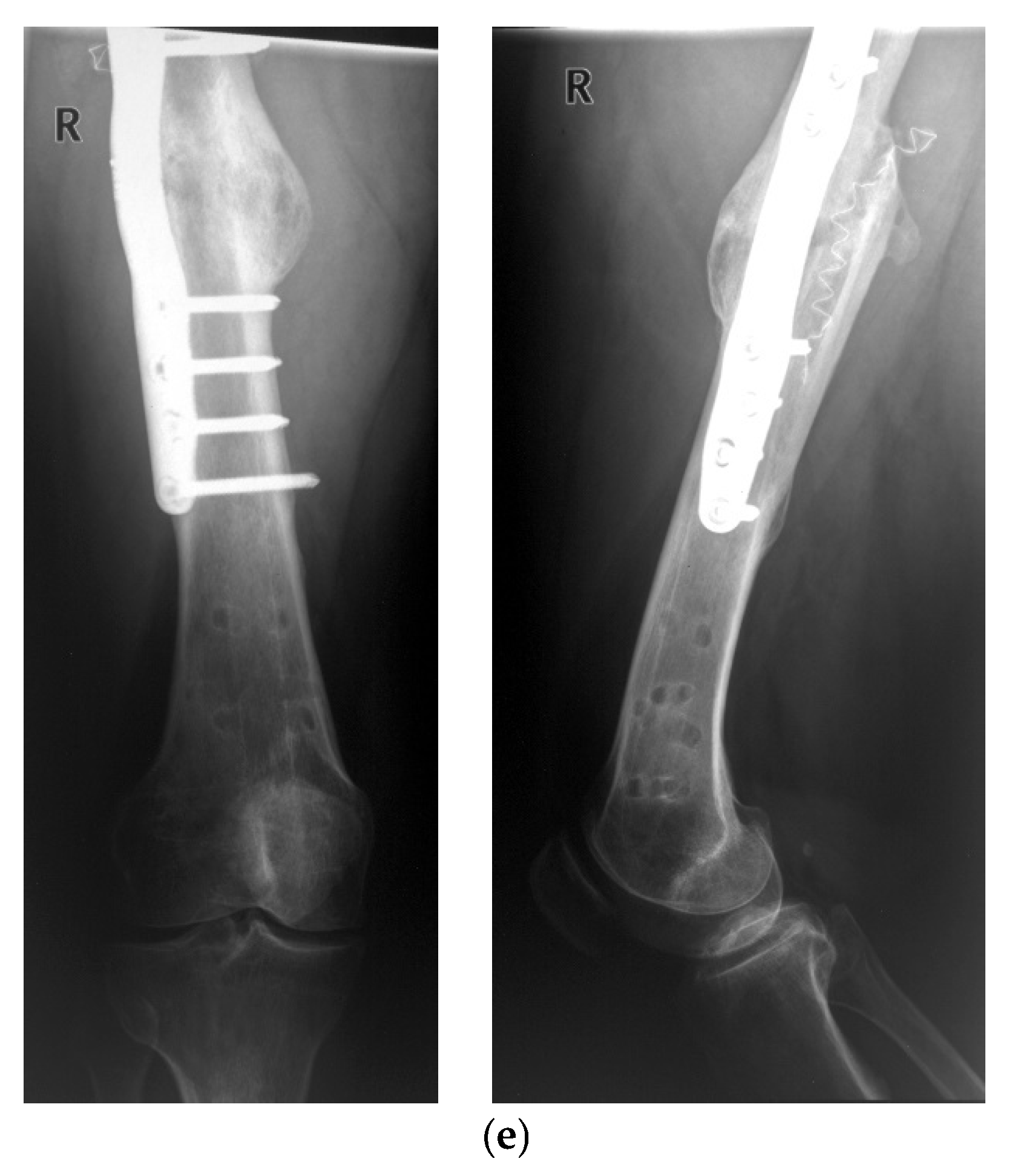
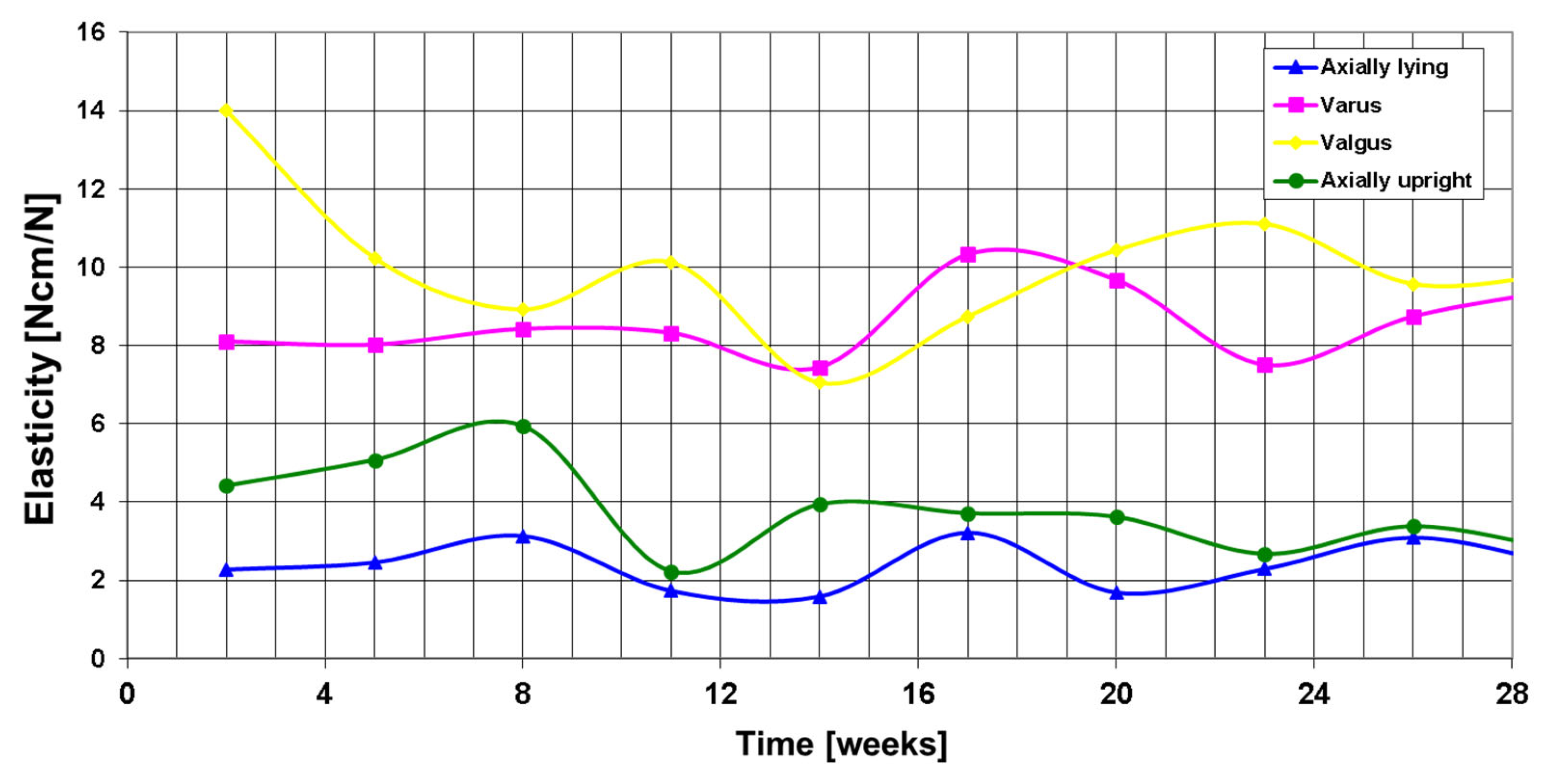
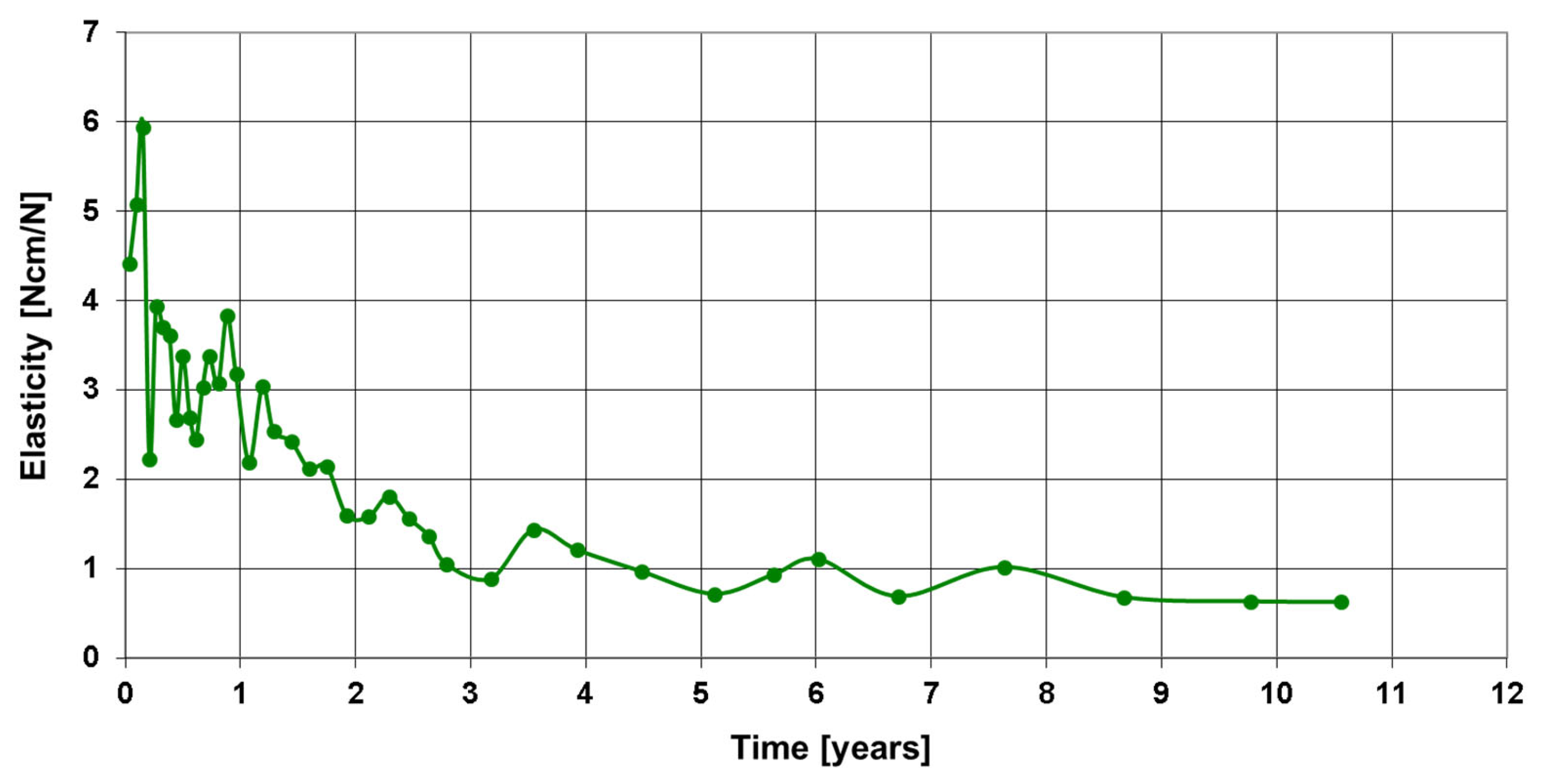
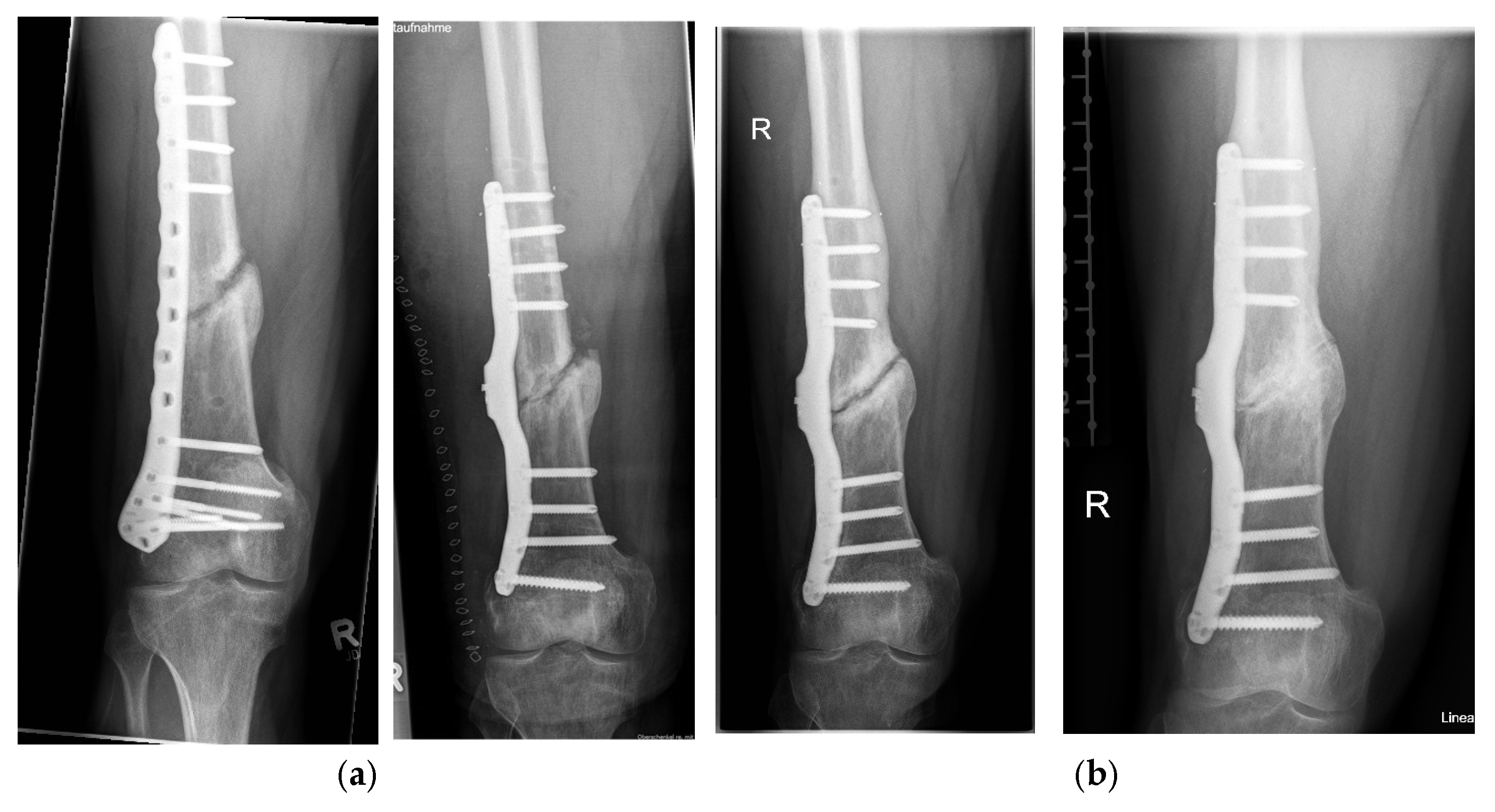
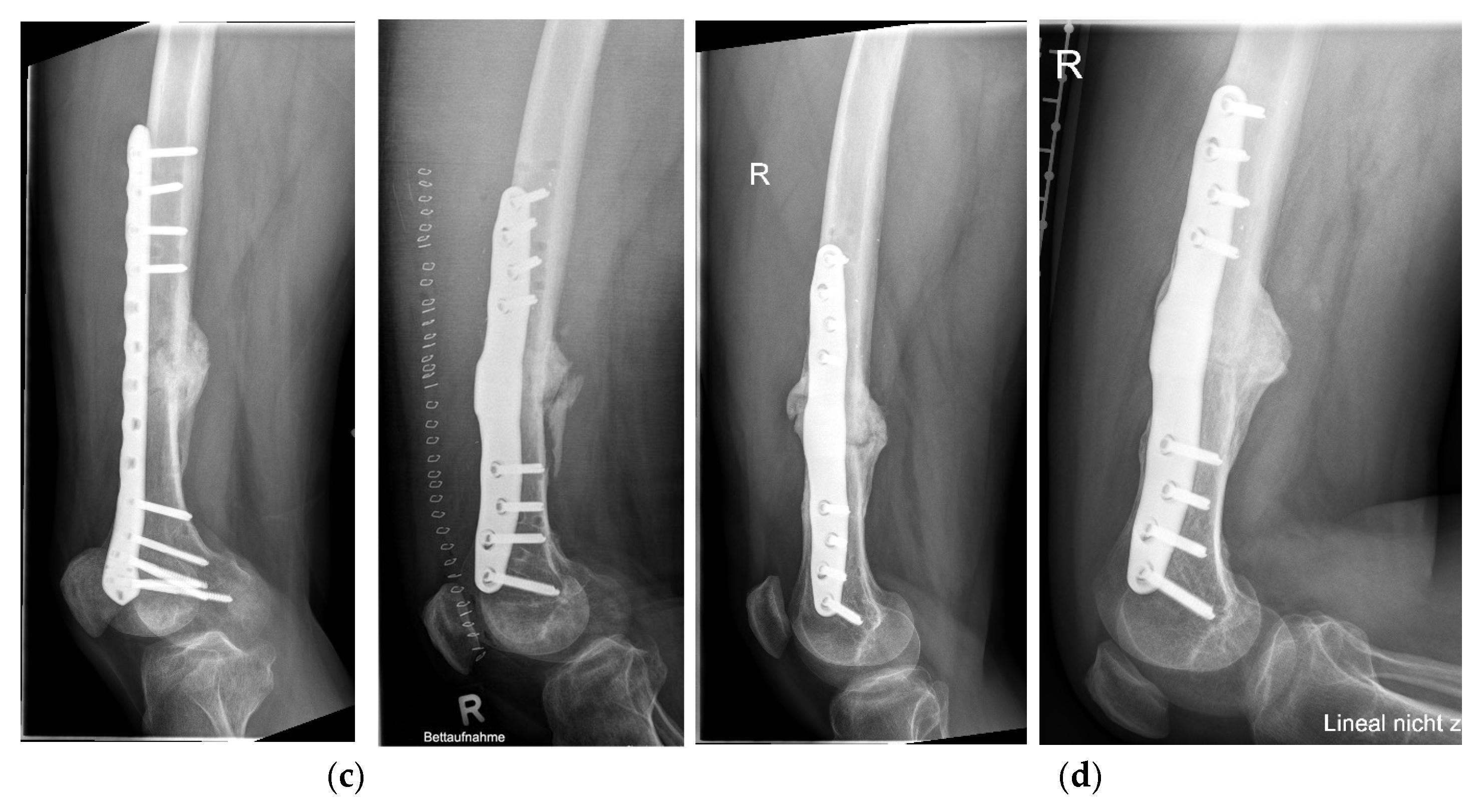
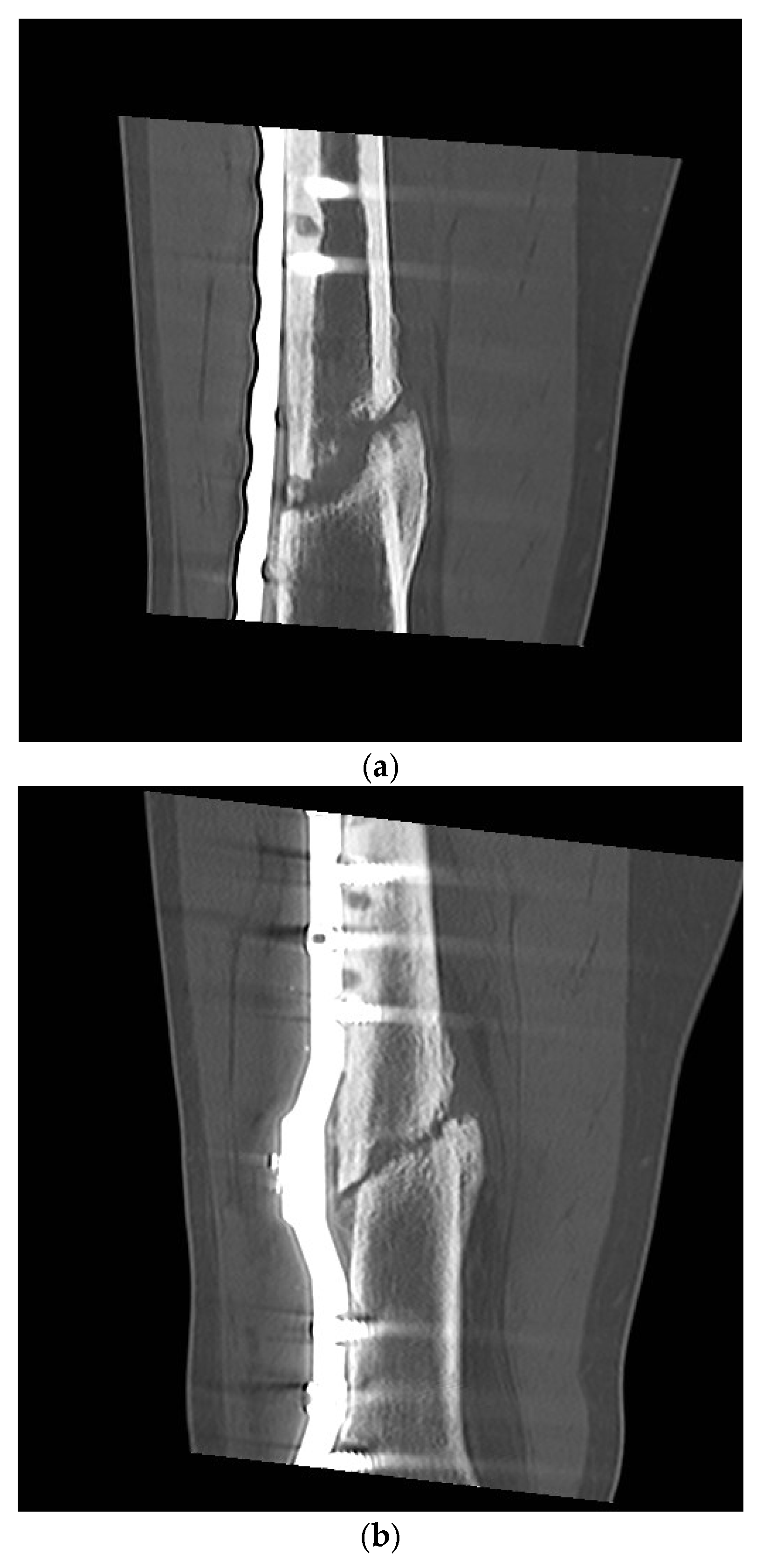
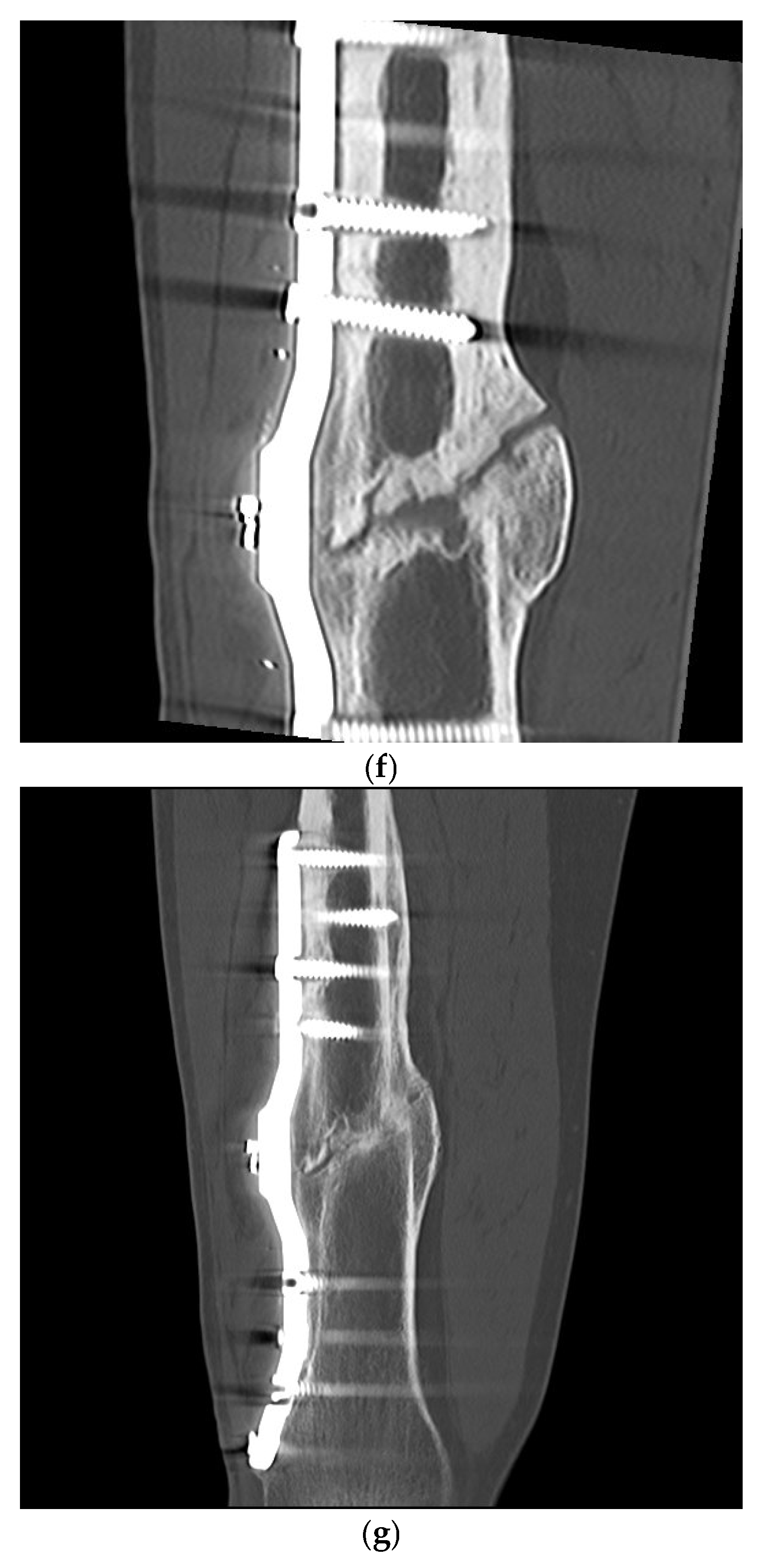
3. Results
Postoperative Procedure and Further Course
4. Discussion
5. Conclusions
5.1. Major Findings
5.2. Clinical and Health-System Implications
5.3. Technological Vision and Platform Development
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torrance, G.W. Utility approach to measuring health-related quality of life. J. Chronic Dis. 1987, 40, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Schottel, P.C.; O’Connor, D.P.; Brinker, M.R. Time trade-off as a measure of health-related quality of life: Long bone non-unions have a devastating impact. J. Bone Jt. Surg. Am. 2015, 97, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Brinker, M.R.; Trivedi, A.; O’Connor, D.P. Debilitating Effects of Femoral Non-union on Health-Related Quality of Life. J. Orthop. Trauma 2017, 31, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.H.; de Steiger, R.; Richardson, M.; Gruen, R.; Balogh, Z.J. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 2014, 45, 1653–1658. [Google Scholar] [CrossRef]
- Zeckey, C.; Mommsen, P.; Andruszkow, H.; Macke, C.; Frink, M.; Stübig, T.; Hüfner, T.; Krettek, C.; Hildebrand, F. The aseptic femoral and tibial shaft non-union in healthy patients—An analysis of the health-related quality of life and the socioeconomic outcome. Open Orthop. J. 2011, 5, 193–197. [Google Scholar] [CrossRef]
- Antonova, E.; Le, T.K.; Burge, R.; Mershon, J. Tibial. shaft fractures: Costly burden of nonunions. BMC Musculoskelet. Disord. 2013, 14, 42. [Google Scholar] [CrossRef]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef]
- Maisenbacher, T.C. Socioeconomic Burden of Non-Union of the Long Tubular Bones of the Lower Extremity. Doctoral Dissertation, University of Tübingen, Tübingen, Germany, 2024. [Google Scholar]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef]
- Flores, M.J.; Brown, K.E.; O’Marr, J.M.; Adejuyigbe, D.; Rodarte, P.; Gomez-Alvarado, F.; Nwachuku, K.; Mayur Urva, M.; Shearer, D. The economic impact of infection and/or nonunion on long-bone shaft fractures: A systematic review. OTA Int. Open Access J. Orthop. Trauma 2024, 7, e337. [Google Scholar] [CrossRef]
- Abumunaser, L.A.; Al-Sayyad, M.J. Evaluation of the calori et Al nonunion scoring system in a retrospective case series. Orthopedics 2011, 34, 359. [Google Scholar] [CrossRef]
- Everding, J.; Roßlenbroich, S.; Raschke, M.J. Non-union of the long tubular bones. Surgeon 2018, 89, 73–88. [Google Scholar]
- Weber, B.G.; Cech, O. Pseudarthrosis: Pathophysiology, Biomechanics, Therapy, Results; Verlag Hans Huber: Bern, Switzerland, 1976; ISBN 3456801955. [Google Scholar]
- Bhandari, M.; Guyatt, G.H.; Swiontkowski, M.F.; Tornetta Iii, P.; Schemitsch, E.H. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J. Orthop. Trauma 2002, 16, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Roberts, P.J.; Moorcroft, C.I.; Brown, M.F.; Thomas, P.B.; Wade, R.H. Reliability of radiographs in defining union in intramedullary fixation. Injury 2004, 35, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Augat, P.; Faschingbauer, M.; Seide, K.; Tobita, K.; Callary, S.A.; Solomon, L.B.; Holstein, J.H. Biomechanical methods for the assessment of fracture repair. Injury 2014, 45S, S32–S38. [Google Scholar] [CrossRef]
- Warmerdam, E.; Orth, M.; Pohlemann, T.; Ganse, B. Gait Analysis to Monitor Fracture Healing of the Lower Leg. Bioengineering 2023, 10, 255. [Google Scholar] [CrossRef]
- Weinrich, N.; Seide, K.; Wendlandt, R.; Schümann, U.; Faschingbauer, M.; Wolter, D.; Jürgens Ch Müller, J. A System for Wireless Monitoring of Loads Acting on Internal Fixators In Vivo. Biomed. Technol. 2005, 50, 1535–1536. [Google Scholar]
- Faschingbauer, M. Telemetrisch Instrumentierter Fixateur Interne für die Osteosynthese, Biokompartibilitätsnachweis und Entwicklung des Klinischen Anwendungsverfahrens. Medical Doctoral Dissertation, Habilitationsschrift Universität zu Lübeck, Lübeck, Germany, 2009. [Google Scholar]
- Moss, C. Near-Field Telemetry System for the Instrumentation of Passive Implants. Ph.D. Thesis, University of Technology Harburg, Hamburg, Germany, 2012. [Google Scholar]
- Seide, K.; Aljudaibi, M.; Weinrich, N.; Kowald, B.; Jürgens, C.; Müller, J.; Faschingbauer, M. Telemetric assessment of bone healing with an instrumented internal fixator. J. Bone Jt. Trauma Surg. 2012, 94-B, 398–404. [Google Scholar] [CrossRef]
- Kowald, B.; Seide, K.; Auerswald, M.; Faschingbauer, M. Slow-healing non-unions. Measurement of mechanical stability with the instrumented implant. 3 case reports. Trauma Berufskrankh. 2019, 21, 181–192. [Google Scholar] [CrossRef]
- Faschingbauer, M.; Seide, K.; Aljudaibi, M.; Kowald, B.; Münch, M.; Weinrich, N.; Jürgens, C.; Moss, C.; Kirchner, R. Intelligent implants for monitoring bone healing. Trauma Occup. Dis. 2013, 15, 240–248. [Google Scholar]
- Kienast, B.; Kowald, B.; Seide, K.; Aljudaibi, M.; Muench, M.; Faschingbauer, M.; Juergens, C.; Gille, J. An electronically instrumented internal fixator for the assessment of bone healing. Bone Jt. Res. 2016, 5, 191–197. [Google Scholar] [CrossRef]
- Abou-Khalil, R.; Colnot, C. Cellular and molecular bases of skeletal regeneration: What can we learn from genetic mouse models? Bone 2014, 64, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Brinker, M.R.; O’Connor, D.P. Nonunions: Evaluation and Treatment. In Skeletal Trauma: Basic Science, Management, and Reconstruction; Browner, B.D., Levine, A.M., Jupiter, J.B., Saunders, Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 615–708. [Google Scholar]
- Burny, F. Methods of evaluation of fracture consolidation. Acta Chir. Belg. 1970, 69, 658–681. [Google Scholar] [PubMed]
- Burny, F.; Moulart, F.; Bourgois, R. Determination of the deformation of in vivo implants. Results of a study of 10 patients treated with a nail-plate. Acta Orthop. Belg. 1976, 42, 52. [Google Scholar]
- Burny, F.; Donkerwolcke, M.; Bourgois, R.; Domb, M.; Saric, O. Twenty years experience in fracture healing measurement with strain gauges. Orthopaedics 1984, 7, 1823–1826. [Google Scholar] [CrossRef]
- Burny, F.; Donkerwolcke, M.; Moulart, F.; Bourgois, R.; Puers, R.; Van Schuylenbergh, K.; Barbosa, M.; Paiva, O.; Rodes, F.; Bégueret, J.B.; et al. Concept, design and fabrication of smart orthopedic implants. Med Eng. Phys. 2000, 22, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Claes, L. The measurement of bone healing in external fixator osteosynthesis with the FM 100 fractometer. Chirurg 1991, 62, 354–355. [Google Scholar]
- Cunningham, J.L.; Evans, M.; Harris, J.D.; Kenwright, J. The measurement of stiffness of fractures treated with external fixation. Eng. Med. 1987, 16, 229–232. [Google Scholar] [CrossRef]
- Seide, K.; Weinrich, N.; Wenzl, M.E.; Wolter, D.; Jürgens, C. Three-dimensional load measurements in an external fixator. J. Biomech. 2004, 37, 1361–1369. [Google Scholar] [CrossRef]
- Sommelet, J.; Hummer, J.; Ory, J.M. Méthode objective d’appréciation des déformations du complexe os-implant “in vivo” par télémesure. Acta Orthop. Belg. 1976, 42, 88–97. [Google Scholar]
- Rohlmann, A.; Graichen, F.; Weber, U.; Bergmann, G. 2000 Volvo Award winner in biomechanical studies: Monitoring in vivo implant loads with a telemeterised internal spinal fixation device. Spine 2000, 25, 2981–2986. [Google Scholar] [CrossRef]
- Schneider, E.; Michel, M.C.; Genge, M.; Zuber, K.; Ganz, R.; Perren, S.M. Loads acting in an intramedullary nail during fracture healing in the human femur. J. Biomech. 2001, 34, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Richards, R.G.; Windolf, M. Smart implants in fracture care—Only buzzword or real opportunity? Injury 2021, 52 (Suppl. S2), S101–S105. [Google Scholar] [CrossRef] [PubMed]
- Windolf, M.; Varjas, V.; Gehweiler, D.; Schwyn, R.; Arens, D.; Constant, C.; Zeiter, S.; Richards, R.G.; Ernst, M. Continuous implant load monitoring to assess bone healing status—Evidence from animal testing. Medicina 2022, 58, 858. [Google Scholar] [CrossRef] [PubMed]
- Bizzoca, D.; Vicenti, G.; Caiaffa, V.; Abate, A.; De Carolis, O.; Carrozzo, M.; Solarino, G.; Moretti, B. Assessment of fracture healing in orthopaedic trauma. Injury 2023, 54 (Suppl. S1), S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, J.; Song, J.; Li, Z. The application of impantable sensors in the musculoskeletal system: A review. Front. Bioeng. Biotechnol. 2024, 12, 1270237. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, J.; Liu, S.; Huo, T.; He, J.; Xue, M.; Fang, Y.; Wang, H.; Xie, Y.; Xie, M.; et al. Application of artificial intelligence technology in the field of orthopedics: A narrative review. Artif. Intell. Rev. 2024, 57, 13. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Zhang, Y.; Zhu, H. Medical image analysis using deep learning algorithms. Front. Public Health 2023, 11, 1273253. [Google Scholar] [CrossRef]
- Aldelemy, A.; Adjei, E.; Siaw, P.O.; Al-Dulaimi, A.; Doychinov, V.; Ali, N.T.; Qahwaji, R.; Buckley, J.G.; Twigg, P.; Abd-Alhameed, R.A. Monitoring bone healing: Integrating RF sensing with AI. IEEE Access 2024, 13, 11114–11135. [Google Scholar] [CrossRef]
- Available online: https://horizon-smile.eu/ (accessed on 13 March 2025).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, A.P.; Kowald, B.; Münch, M.; Seide, K.; Weinrich, N.; Barth, T.; Kienast, B. Long-Term Evaluation of Bone Healing Monitoring Using an Instrumented Plate with Measurement Sensors (Smart Implant) over 10 Years. Sensors 2025, 25, 5779. https://doi.org/10.3390/s25185779
Schulz AP, Kowald B, Münch M, Seide K, Weinrich N, Barth T, Kienast B. Long-Term Evaluation of Bone Healing Monitoring Using an Instrumented Plate with Measurement Sensors (Smart Implant) over 10 Years. Sensors. 2025; 25(18):5779. https://doi.org/10.3390/s25185779
Chicago/Turabian StyleSchulz, Arndt P., Birgitt Kowald, Matthias Münch, Klaus Seide, Nils Weinrich, Tobias Barth, and Benjamin Kienast. 2025. "Long-Term Evaluation of Bone Healing Monitoring Using an Instrumented Plate with Measurement Sensors (Smart Implant) over 10 Years" Sensors 25, no. 18: 5779. https://doi.org/10.3390/s25185779
APA StyleSchulz, A. P., Kowald, B., Münch, M., Seide, K., Weinrich, N., Barth, T., & Kienast, B. (2025). Long-Term Evaluation of Bone Healing Monitoring Using an Instrumented Plate with Measurement Sensors (Smart Implant) over 10 Years. Sensors, 25(18), 5779. https://doi.org/10.3390/s25185779




