Innovative Sensor-Based Approaches for Assessing Neurodegenerative Diseases: A Brief State-of-the-Art Review
Abstract
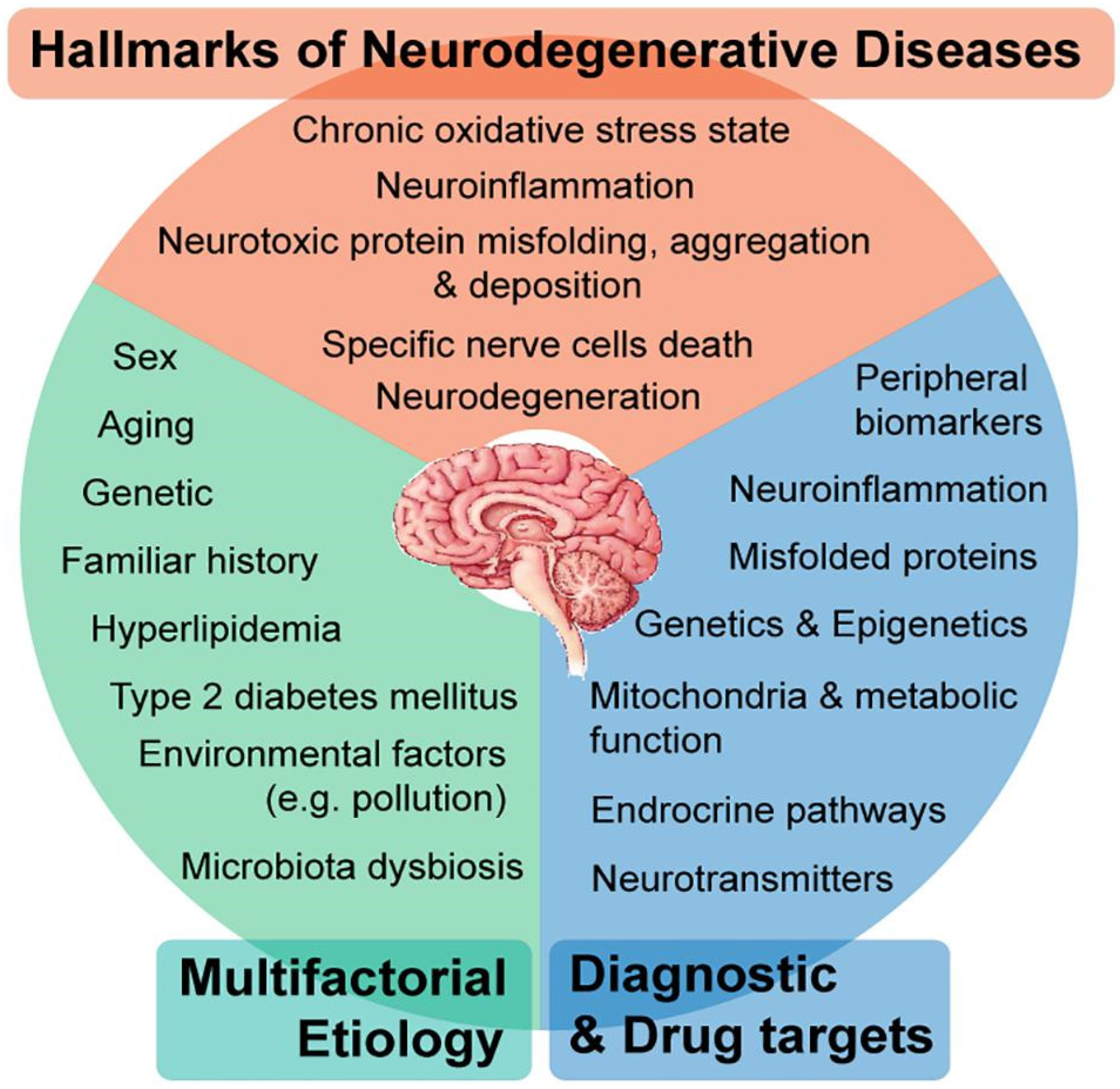
1. Introduction
| Approach/Component | Description/Role |
|---|---|
| Holistic Systems Biology | Integrates multi-layered biological data (e.g., genomics, metabolomics) to model the interplay between host and microbiome in neurodegeneration. |
| Microbiota–Gut–Brain Axis Pathways | Examines direct and indirect mechanisms (metabolites, immune modulators, barrier effects) through which the microbiome influences the CNS. |
| Microbial Dysbiosis Evidence | Surveys shift in gut microbial community—particularly in Alzheimer’s and Parkinson’s disease—and explores how these imbalances contribute to NDDs. |
| Dietary Modulation Strategies | Explores diet-based interventions to stimulate neuroprotective microbial metabolite production by modifying microbial composition. |
| Genome-Scale Metabolic Models (GEMs) | Uses GEMs to simulate microbe–microbe and host–microbe metabolic interactions, determining the microbiome’s impact on disease development or prevention. |
| Multi-Omics + GEMs for Personalized Diets | Proposes an integrative systems framework combining GEMs and omics data to design personalized, anti-inflammatory diets targeting gut microbiota in NDD prevention. |
2. Advances in Technology for NND Monitoring
2.1. Emerging Technological Innovations
2.2. Wearable and Sensor-Based Monitoring Technologies
2.3. Emerging Sensor Technologies
2.4. Digital Biomarkers
2.5. Artificial Intelligence and Machine Learning in NND Monitoring
2.6. Remote Patient Monitoring and Telemedicine
2.7. Vision-Based Approaches as Sensor-Based Technology
2.8. Clinical Implementation Progress
2.9. Real-World Applications
2.10. Integration Challenges
3. Challenges and Future Directions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviations | Meaning |
| NDDS | Neurodegenerative diseases |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| MS | Multiple Sclerosis |
| FTD | Frontotemporal Dementia |
| AI | Artificial intelligence |
| IoT | The internet of Things |
| DeFi | Decentralized finance |
| AR | Augmented reality |
| VR | Virtual reality |
| ML | Machine learning |
| GPS | Global positioning system |
| ECG | Electrocardiogram |
| CGMs | Glucose monitors |
| HIPAA | Health Insurance Portability and Accountability Act |
| AUC | Area under the curve |
| EEG | Electroencephalogram |
| IMU | Inertial Measurement Unit |
| RGB | Red, green, and blue |
| EMG | Electromyography |
References
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Temple, S. Advancing Cell Therapy for Neurodegenerative Diseases. Cell Stem Cell 2023, 30, 512–529. [Google Scholar] [CrossRef]
- Voigtlaender, S.; Pawelczyk, J.; Geiger, M.; Vaios, E.J.; Karschnia, P.; Cudkowicz, M.; Dietrich, J.; Haraldsen, I.R.J.H.; Feigin, V.; Owolabi, M.; et al. Artificial Intelligence in Neurology: Opportunities, Challenges, and Policy Implications. J. Neurol. 2024, 271, 2258–2273. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Martin, J.B. Molecular Basis of Neurodegenerative Disorders. N. Engl. J. Med. 1999, 340, 1970–1980. [Google Scholar] [CrossRef]
- Hague, S.M.; Klaffke, S.; Bandmann, O. Neurodegenerative disorders: Parkinson’s disease and Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1058–1063. [Google Scholar] [CrossRef]
- Harding, B.N.; Kariya, S.; Monani, U.R.; Chung, W.K.; Benton, M.; Yum, S.W.; Tennekoon, G.; Finkel, R.S. Spectrum of neuropathophysiology in spinal muscular atrophy type I. J. Neuropathol. Exp. Neurol. 2015, 74, 15–24. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, J.; Xie, J.; Liao, W.H.; Lei, Y.; Cao, H.; Qu, Q.; Bowen, C. Wearable sensors and features for diagnosis of neurodegenerative diseases: A systematic review. Digit Health 2023, 9, 20552076231173569. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Pei, J.; Alugoju, P.; Anthikapalli, N.V.A.; Jayaraman, S.; Veeraraghavan, V.P.; Gopathy, S.; Roy, J.R.; Janaki, C.S.; Thalamati, D.; et al. New strategies of Neurodegenerative Disease Treatment with Extracellular Vesicles (EVs) derived from Mesenchymal Stem Cells (MSCs). Theranostics 2023, 13, 4138–4165. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.; Bhuskute, K.R.; Varghese, N.R.; Buchanan, J.A.; Xu, Y.; McCutcheon, F.M.; Medcalf, R.L.; Jolliffe, K.A.; Sunde, M.; New, E.J.; et al. A Coumarin-Based Array for the Discrimination of Amyloids. ACS Sens. 2024, 9, 615–621. [Google Scholar] [CrossRef]
- Rosario, D.; Boren, J.; Uhlen, M.; Proctor, G.; Aarsland, D.; Mardinoglu, A.; Shoaie, S. Systems Biology Approaches to Understand the Host-Microbiome Interactions in Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Rane, N.; Choudhary, S.; Rane, J. Artificial Intelligence and Machine Learning in Business Intelligence, Finance, and E-Commerce: A Review. Finance and E-Commerce: A Review. Int. J. Res. Appl. Technol. 2024, 4, 211–223. [Google Scholar]
- Singh, P.K. Digital Transformation in Supply Chain Management: Artificial Intelligence (AI) and Machine Learning (ML) as Catalysts for Value Creation. Int. J. Supply Chain. Manag. 2023, 12, 57–63. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Chahal, P. Artificial intelligence and machine learning algorithms. In Challenges and Applications for Implementing Machine Learning in Computer Vision; IGI Global Scientific Publishing: Hershey, PA, USA, 2020; pp. 188–219. [Google Scholar]
- Higgins, O.; Short, B.L.; Chalup, S.K.; Wilson, R.L. Artificial Intelligence (AI) and Machine Learning (ML) based Decision Support Systems in Mental Health: An Integrative Review. Int. J. Ment. Health Nurs. 2023, 32, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V. Enhancing Transparency and Understanding in AI decision-Making Processes. Iconic Res. Eng. J. 2024, 8, 168–172. [Google Scholar]
- Wang, W.J.; Taylor, R.; Rees, J. Recent Advancement of Deep Learning Applications to Machine Condition Monitoring, Part 1: A Critical Review. Acoust. Aust. 2021, 49, 207–219. [Google Scholar] [CrossRef]
- Barletta, V.S.; Caivano, D.; Gigante, D.; Ragone, A. A Rapid Review of Responsible AI Frameworks: How to Guide the Development of Ethical AI. In Proceedings of the 27th International Conference on Evaluation and Assessment in Software Engineering, Oulu, Finland, 14–16 June 2023; pp. 358–367. [Google Scholar] [CrossRef]
- Oyeniran, O.C.; Adewusi, A.O.; Adeleke, A.G.; Akwawa, L.A.; Azubuko, C.F. AI-driven DevOps: Leveraging Machine Learning for Automated Software Deployment and Maintenance. Eng. Sci. Technol. 2023, 4, 728–740. [Google Scholar] [CrossRef]
- Rachakatla, S.K.; Ravichandran, P.; Kumar, N. Scalable Machine Learning Workflows in Data Warehousing: Automating Model Training and Deployment with AI. Aust. J. AI Data Sci. 2022, 1, 262–286. [Google Scholar]
- Barh, D. Biotechnology in Healthcare, Volume 2: Applications and Initiatives; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Pham, P.V. Medical biotechnology: Techniques and Applications. In Omics Technologies and Bio-Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 449–469. [Google Scholar]
- Anyanwu, E.C.; Arowoogun, J.O.; Odilibe, I.P.; Akomolafe, O.; Onwumere, C.; Ogugua, J.O. The Role of Biotechnology in Healthcare: A review of global trends. World J. Adv. Res. Rev. 2024, 21, 2740–2752. [Google Scholar] [CrossRef]
- Salama, R.; Al-Turjman, F.; Chaudhary, P.; Yadav, S.P. (Benefits of internet of things (IoT) applications in health care-an overview). In Proceedings of the 2023 International Conference on Computational Intelligence, Communication Technology and Networking (CICTN), Ghaziabad, India, 20–21 April 2023; pp. 778–784. [Google Scholar]
- Perwej, Y.; Haq, K.; Parwej, F.; Mumdouh, M.; Hassan, M. The Internet of Things (IoT) and its application domains. Int. J. Comput. Appl. 2019, 975, 182. [Google Scholar] [CrossRef]
- Mouha, R.A.R.A. Internet of Things (IoT). J. Data Anal. Inf. Process. 2021, 9, 77. [Google Scholar] [CrossRef]
- Hassan, W.H. Current research on Internet of Things (IoT) security: A Survey. Comput. Netw. 2019, 148, 283–294. [Google Scholar]
- Singh, D.S. Decentralized finance (DeFi): Exploring the Role of Blockchain and Cryptocurrency in Financial Ecosystems. Int. Res. J. Mod. Eng. Technol. Sci. 2024, 5. [Google Scholar] [CrossRef]
- Ghosh, A.; Gupta, S.; Dua, A.; Kumar, N. Security of Cryptocurrencies in Blockchain Technology: State-of-art Challenges and Future Prospects. J. Netw. Comput. Appl. 2020, 163, 102635. [Google Scholar] [CrossRef]
- Hashemi Joo, M.; Nishikawa, Y.; Dandapani, K. Cryptocurrency, a Successful Application of Blockchain Technology. Manag. Financ. 2020, 46, 715–733. [Google Scholar] [CrossRef]
- Corbet, S.; Urquhart, A.; Yarovaya, L. Cryptocurrency and Blockchain Technology; Walter de Gruyter GmbH & Co., KG: Berlin, Germany, 2020; p. 196. [Google Scholar]
- Kaur, N.; Sharma, A. Robotics and automation in manufacturing processes. In Intelligent Manufacturing; CRC Press: Boca Raton, FL, USA, 2025; pp. 97–109. [Google Scholar]
- Sostero, M. Automation and Robots in Services: Review of Data and Taxonomy. 2020. Available online: https://econstor.eu/bitstream/10419/231346/1/jrc-wplet202014.pdf (accessed on 7 July 2025).
- Macrorie, R.; Marvin, S.; While, A. Robotics and automation in the city: A research agenda. Urban Geogr. 2021, 42, 197–217. [Google Scholar] [CrossRef]
- Djuric, A.M.; Urbanic, R.J.; Rickli, J.L. A framework for Collaborative Robot (CoBot) Integration in Advanced Manufacturing Systems. SAE Int. J. Mater. Manuf. 2016, 9, 457–464. [Google Scholar] [CrossRef]
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging Renewable and Sustainable Energy Technologies: State of the art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Randolph, J.; Masters, G.M. Energy for Sustainability: Technology, Planning, Policy; Island Press: Washington, DC, USA, 2008. [Google Scholar]
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Kalogirou, S. Renewable Energy for Sustainable Development. Renew. Energy 2022, 199, 1145–1152. [Google Scholar] [CrossRef]
- Moskowitz, S.L. The Advanced Materials Revolution: Technology and Economic Growth in the Age of Globalization; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Skiba, R. Advanced Materials Engineering Fundamentals; After Midnight Publishing: London, UK, 2025. [Google Scholar]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards Safe and Sustainable Innovation in Nanotechnology: State-of-play for Smart Nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Hsiang, E.; He, Z.; Zhan, T.; Wu, S. Augmented Reality and Virtual Reality Displays: Emerging Technologies and Future Perspectives. Light Sci. Appl. 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Jaboob, A.M.M.; Garad, A.; Al-Ansi, A. Analyzing Augmented Reality (AR) and Virtual Reality (VR) Recent Development in Education. Soc. Sci. Humanit. Open 2023, 8, 100532. [Google Scholar] [CrossRef]
- Memon, Q.A.; Al Ahmad, M.; Pecht, M. Quantum Computing: Navigating the Future of Computation, Challenges, and Technological Breakthroughs. Quantum Rep. 2024, 6, 627–663. [Google Scholar] [CrossRef]
- Hidary, J.D. Quantum Computing: An Applied Approach; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Jadhav, A.; Jadhav, V.S. A review on 3D printing: An Additive Manufacturing Technology. Mater. Today Proc. 2022, 62, 2094–2099. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, R.; Ranjan, K.; Pandey, D.K. Artificial Intelligence in Biotechnology. In Artificial Intelligence and Biological Sciences; CRC Press: Boca Raton, FL, USA, 2025; Volume 192. [Google Scholar]
- Ali, M.; Shabbir, K.; Mohsin, S.M.; Kumar, A.; Aziz, M.; Zubair, M.; Sultan, H.M. A New Era of Discovery: How Artificial Intelligence has Revolutionized the Biotechnology. Nepal J. Biotechnol. 2024, 12, 1–11. [Google Scholar] [CrossRef]
- Izankar, S.V.; Kumar, P.; Waghmare, G. Evolution of Artificial Intelligence in Biotechnology: From Discovery to Ethical and Beyond. AIP Conf. Proc. 2024, 3188, 080037. [Google Scholar] [CrossRef]
- Das, S. Applications of Sensor Technology in Healthcare. In Revolutionizing Healthcare Treatment with Sensor Technology; IGI Global: Hershey, PA, USA, 2024; pp. 79–99. [Google Scholar]
- Khan, A.O.R.; Islam, S.M.; Islam, A.T.; Paul, R.; Bari, M.S. Real-time Predictive Health Monitoring using AI-driven Wearable Sensors: Enhancing Early Detection and Personalized Interventions in Chronic Disease Management. Int. J. Multidiscip. Res. 2024, 6, 1–21. [Google Scholar] [CrossRef]
- Singh, B.; Kaunert, C.; Vig, K.; Gautam, B.K. Wearable Sensors Assimilated with Internet of Things (IoT) for Advancing Medical Imaging and Digital Healthcare: Real-time scenario. In Inclusivity and Accessibility in Digital Health; IGI Global: Hershey, PA, USA, 2024; pp. 275–297. [Google Scholar]
- Wang, X.; Yu, H.; Kold, S.; Rahbek, O.; Bai, S. Wearable Sensors for Activity Monitoring and Motion Control: A review. Biomim. Intell. Robot. 2023, 3, 100089. [Google Scholar] [CrossRef]
- George, A.H.; Shahul, A.; George, A.S. Wearable Sensors: A new way to track health and Wellness. Partn. Univers. Int. Innov. J. 2023, 1, 15–34. [Google Scholar]
- Liao, Y.; Thompson, C.; Peterson, S.; Mandrola, J.; Beg, M.S. The future of Wearable Technologies and Remote Monitoring in Health Care. In American Society of Clinical Oncology Educational Book; Annual Meeting; American Society of Clinical Oncology: Alexandria, VA, USA, 2019; p. 115. [Google Scholar]
- Henriksen, A.; Mikalsen, M.H.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; Hopstock, L.A.; Grimsgaard, S. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-worn Wearables. J. Med. Internet Res. 2018, 20, e110. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Shandhi, M.M.H.; Master, H.; Dunn, J.; Brittain, E. Wearable Devices in Cardiovascular Medicine. Circ. Res. 2023, 132, 652–670. [Google Scholar] [CrossRef]
- Sun, X. A comprehensive engineering Design Analysis of Apple Watch as a Smart Wearable Device. Appl. Comput. Eng. 2024, 71, 52–57. [Google Scholar] [CrossRef]
- Salamone, F.; Masullo, M.; Sibilio, S. Wearable Devices for Environmental Monitoring in the Built Environment: A Systematic Review. Sensors 2021, 21, 4727. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable Sensors for Monitoring the Physiological and Biochemical Profile of the Athlete. npj Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef]
- Yang, G.; Hong, J.; Park, S. Wearable Device for Continuous Sweat Lactate Monitoring in Sports: A Narrative Review. Front. Physiol. 2024, 15, 1376801. [Google Scholar] [CrossRef]
- Khatiwada, P.; Yang, B.; Lin, J.; Blobel, B. Patient-Generated Health Data (PGHD): Understanding, Requirements, Challenges, and Existing Techniques for Data Security and Privacy. J. Pers. Med. 2024, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Thacharodi, A.; Singh, P.; Meenatchi, R.; Ahmed, Z.H.T.; Kumar, R.R.S.; V, N.; Kavish, S.; Maqbool, M.; Hassan, S. Revolutionizing Healthcare and Medicine: The Impact of Modern Technologies for a Healthier Future—A Comprehensive Review. Health Care Sci. 2024, 3, 329–349. [Google Scholar] [CrossRef]
- Vidyarthi, A. Monitoring and Diagnosis of Neurodegenerative Diseases through Advanced Sensor Integration and Machine Learning Techniques. Int. J. Eng. Artif. Intell. Manag. Decis. Support Policies 2024, 1, 33–41. [Google Scholar] [CrossRef]
- Johnson, S.; Kantartjis, M.; Severson, J.; Dorsey, R.; Adams, J.; Kangarloo, T.; Kostrzeb, M.; Best, A.; Merickel, M.; Amato, D.; et al. Wearable Sensor-Based Assessments for Remotely Screening Early-Stage Parkinson’s Disease. Sensors 2024, 24, 5637. [Google Scholar] [CrossRef]
- Raknim, P.; Lan, K. Gait Monitoring for Early Neurological Disorder Detection Using Sensors in a Smartphone: Validation and a Case Study of Parkinsonism. Telemed. J. e-Health 2016, 22, 75–81. [Google Scholar] [CrossRef]
- Sica, M.; Tedesco, S.; Crowe, C.; Kenny, L.; Moore, K.; Timmons, S.; Barton, J.; O’Flynn, B.; Komari, D. Continuous Home Monitoring of Parkinson’s Disease Using Inertial Sensors: A Systematic Review. PLoS ONE 2021, 16, e0246528. [Google Scholar] [CrossRef] [PubMed]
- Shcherbak, A.; Kovalenk, E.; Somov, A. Detection and Classification of Early Stages of Parkinson’s Disease Through Wearable Sensors and Machine Learning. IEEE Trans. Instrum. Meas. 2023, 72, 1–9. [Google Scholar] [CrossRef]
- Ricci, M.; Di Lazzaro, G.; Pisani, A.; Mercuri, N.; Giannini, F.; Saggio, G. Assessment of Motor Impairments in Early Untreated Parkinson’s Disease Patients: The Wearable Electronics Impact. IEEE J. Biomed. Health Inform. 2020, 24, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.C.; Motin, M.A.; Pah, N.D.; Drotár, P.; Kempster, P.; Kumar, D. Computerized Analysis of Speech, and Voice for Parkinson’s Disease: A Systematic Review. Comput. Methods Programs Biomed. 2022, 226, 107133. [Google Scholar] [CrossRef]
- Chudzik, A.; Sledzianowski, A.; Przybyszewski, A.W. Machine Learning and Digital Biomarkers Can Detect Early Stages of Neurodegenerative Diseases. Sensors 2024, 24, 1572. [Google Scholar] [CrossRef]
- Wal, A.; Singh, H.M.N.; Vig, M.K.K.; Prakash, B. Artificial Intelligence and Machine Learning-Based Advances in the Management of Neurodegenerative Diseases. In Molecular Targets and Therapeutic Interventions Against Neurodegenerative Diseases; CDC Press: Boca Raton, FL, USA, 2025; p. 102. [Google Scholar]
- Paradis, L.; Han, Q. A Data Collection Protocol for Real-Time Sensor Applications. Pervasive Mob. Comput. 2009, 5, 369–384. [Google Scholar] [CrossRef]
- Anjum, M.; Shahab, S.; Yu, Y. Syndrome Pattern Recognition Method Using Sensed Patient Data for Neurodegenerative Disease Progression Identification. Diagnostics 2023, 13, 887. [Google Scholar] [CrossRef]
- Dhankhar, S.; Mujwar, S.; Garg, N.; Chauhan, S.; Saini, M.; Sharma, P.; Kumar, S.; Sharma, S.K.; Rani, M.A.; Kamal, N. Artificial Intelligence in the Management of Neurodegenerative Disorders. CNS Neurol. Disord.-Drug Targets 2024, 23, 931–940. [Google Scholar] [CrossRef]
- Andriopoulos, E. The Rise of AI in Telehealth. In Emerging Practices in Telehealth; Elsevier: Amsterdam, The Netherlands, 2023; pp. 183–207. [Google Scholar]
- Ali, H. AI in Neurodegenerative Disease Research: Early Detection, Cognitive Decline Prediction, and Brain Imaging Biomarker Identification. Int. J. Eng. Technol. Res. Manag. 2022, 6, 71. [Google Scholar]
- Rajaraman, S. Artificial Intelligence in Neurodegenerative Diseases: Opportunities and Challenges. In AI and Neurodegenerative Diseases: Insights and Solutions; Gaur, L., Abraham, A., Ajith, R., Eds.; Springer: Cham, Switzerland, 2024; pp. 133–153. [Google Scholar] [CrossRef]
- Kumar, A.V.; Kumar, S.; Garg, V.K.; Goel, N.; Hoang, V.T.; Kashyap, D. Future perspectives for Automated Neurodegenerative Disorders Diagnosis: Challenges and Possible Research Directions. In Data Analysis for Neurodegenerative Disorders; Springer: Berlin/Heidelberg, Germany, 2023; pp. 255–267. [Google Scholar]
- Broza, Y.; Har-Shai, L.; Jeries, R.; Cancilla, J.C.; Glass-Marmor, L.; Lejbkowicz, I.; Torrecilla, J.; Yao, X.; Feng, X.; Narita, A.; et al. Exhaled Breath Markers for Nonimaging and Noninvasive Measures for Detection of Multiple Sclerosis. ACS Chem. Neurosci. 2017, 8, 2402–2413. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Schlesinger, I.; Ionescu, R.; Nassar, M.; Axelrod, N.; Robertman, D.; Yael, T.; Azar, F.; Marmur, A.; Aharon-Peretz, J.; et al. Detection of Alzheimer’s and Parkinson’s Disease from Exhaled Breath Using Nanomaterial-Based Sensors. Nanomedicine 2013, 8, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, K.; Chmiel, F.; Arora, S.; Gunter, K.; Husain, M.; Hu, M. Neu Health: Smartphone-Based Remote Digital Monitoring for Parkinson’s Disease and Dementia. Neu Health 2024, 102, S49–S53. [Google Scholar]
- Lussier, M.; Lavoie, M.; Giroux, S.; Consel, C.; Guay, M.; Macoir, J.; Hudon, C.; Lorrain, D.; Talbot, L.; Langlois, F.; et al. Early Detection of Mild Cognitive Impairment With In-Home Monitoring Sensor Technologies Using Functional Measures: A Systematic Review. IEEE J. Biomed. Health Inform. 2019, 23, 838–847. [Google Scholar] [CrossRef]
- Sigcha, L.; Borzì, L.; Amato, F.; Rechichi, I.; Ramos-Romero, C.; Cárdenas, A.; Gascó, L.; Olmo, G. Deep Learning and Wearable Sensors for the Diagnosis and Monitoring of Parkinson’s Disease: A Systematic Review. Expert Syst. Appl. 2023, 229, 120541. [Google Scholar] [CrossRef]
- Aggarwal, J.K.; Xia, L. Human activity recognition from 3D data: A review. Pattern Recognit. Lett. 2014, 48, 70–80. [Google Scholar] [CrossRef]
- Liu, W.; Lin, X.; Chen, X.; Wang, Q.; Wang, X.; Yang, B.; Cai, N.; Chen, R.; Chen, G.; Lin, Y. Vision-based estimation of MDS-UPDRS scores for quantifying Parkinson’s disease tremor severity. Med. Image Anal. 2023, 85, 102754. [Google Scholar] [CrossRef]
- Sibai, F.N.; Alqahtani, A.; Alanazi, M.; Alhassan, A.; Ahmed, A. Vision-based human activity recognition in healthcare: A comprehensive review. Sensors 2023, 25, 1357. [Google Scholar]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef]
- Zhang, H.; Ho, E.S.; Zhang, F.X.; Shum, H.P. Pose-based tremor classification for Parkinson’s disease diagnosis from video. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2022; Springer: Cham, Switzerland, 2022; pp. 489–499. [Google Scholar] [CrossRef]
- Sapienza, S.; Tsurkalenko, O.; Giraitis, M.; Mejia, A.C.; Zelimkhanov, G.; Schwaninger, I.; Klucken, J. Assessing the clinical utility of inertial sensors for home monitoring in Parkinson’s disease: A comprehensive review. npj Park. Dis. 2024, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Culicetto, L.; Cardile, D.; Marafioti, G.; Lo Buono, V.; Ferraioli, F.; Massimino, S.; Di Lorenzo, G.; Sorbera, C.; Brigandì, A.; Vicario, C.M.; et al. Recent advances (2022–2024) in eye-tracking for Parkinson’s disease: A promising tool for diagnosing and monitoring symptoms. Front. Aging Neurosci. 2025, 17, 1534073. [Google Scholar] [CrossRef] [PubMed]
- Koerner, J.; Zou, E.; Karl, J.A.; Poon, C.; Metman, L.V.; Sodini, C.G.; Sze, V.; David, F.J.; Heldt, T. Towards scalable screening for the early detection of Parkinson’s disease: Validation of an iPad-based eye movement assessment system against a clinical-grade eye tracker. npj Park. Dis. 2025, 11, 233. [Google Scholar] [CrossRef] [PubMed]
| Technology Platform | Detection Accuracy | Early Detection Capability | Clinical Validation Status |
|---|---|---|---|
| Wearable inertial sensors | Approximately 95% accuracy for early Parkinson’s disease detection; over 90% accuracy for Parkinson’s disease symptom detection in free-living environments. | Demonstrated capability for detecting early-stage Parkinson’s disease. | Validated in multiple studies, including real-world settings. |
| Smartphone-based sensors | Approximately 98% accuracy in step length estimation, 94% accuracy in identifying gait changes for Parkinson’s disease. | Showed potential for early Parkinson’s disease detection through gait analysis. | Limited clinical validation, mostly proof-of-concept studies. |
| Breath analysis | Up to 90% accuracy for Multiple Sclerosis detection; 85% accuracy for Alzheimer’s disease vs. healthy, up to 78% for Parkinson’s disease vs. healthy. | Demonstrated potential for early-stage detection. | Limited clinical validation, mostly experimental studies. |
| Blood-based biomarkers (ultrasensitive detection) | High sensitivity and specificity were reported, but specific metrics were not provided. | Showed potential for early Alzheimer’s disease detection. | Promising results, but limited large-scale clinical validation. |
| Eye-tracking | Receiver operating characteristic area under the curve of 0.88 for differentiating Parkinson’s disease patients from controls. | Demonstrated potential for early cognitive decline detection. | Limited clinical validation, mostly experimental studies. |
| Smart home sensors | Although specific accuracy metrics were not reported, the system showed potential for monitoring long-term mild cognitive impairment. | Demonstrated capability for detecting early signs of cognitive decline. | Limited clinical validation, mostly proof-of-concept studies. |
| Multi-modal systems | Up to 80% accuracy reported for combined sensor approaches. | Showed potential for comprehensive early detection. | Limited clinical validation, mostly experimental studies. |
| Disease and Setting | Challenges | Limitations | Future Directions | Research Opportunities |
|---|---|---|---|---|
| Alzheimer’s (Clinic) | Subtle cognitive/motor decline is challenging to capture due to variability in testing environments. | Cognitive tests are often episodic, rather than continuous; imaging is also costly. | Multi-modal in-clinic sensors (eye-tracking, digital pen, EEG). | Early detection of mild cognitive impairment via digital biomarkers. |
| Alzheimer’s (Home) | Adherence to wearables and noise from daily routines. | Smart-home monitoring is costly and raises privacy concerns. | Passive monitoring (speech, mobility, sleep) using IoT. | Digital phenotyping of early memory and language decline. |
| Parkinson’s (Clinic) | Tremor/rigidity fluctuates; stress and meds alter readings. | Single-time-point measurements miss variability. | Digital gait labs and wearable sensors are available in the clinic. | Sensor-validated motor scoring aligned with MDS-UPDRS. |
| Parkinson’s (Home) | Continuous tremor and gait monitoring → large, noisy datasets. | Device heterogeneity, patient compliance. | Smartphone-based tapping/voice apps; continuous gait sensors. | Prodromal PD detection: real-world treatment–response biomarkers. |
| ALS (Clinic) | Rapid progression, heterogeneity of symptoms. | Clinical measures (ALSFRS-R) are subjective and infrequent. | Sensor-based speech and respiratory testing. | Digital endpoints for respiratory decline detection. |
| ALS (Home) | Difficulty in sustained sensor use as the function declines. | Limited accessibility/adapted devices. | Voice analysis apps and respiratory wearables for home use. | Longitudinal tracking of speech/motor decline for trials. |
| Huntington’s (Clinic) | Movements are variable; psychiatric symptoms are less quantifiable. | Imaging/clinical tests are limited in frequency. | Motion-capture systems; digital cognitive tests. | Quantifying subtle motor/cognitive onset before diagnosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbue, N.D.; Tabei, F.; Williams, K.; Olanrewaju, K. Innovative Sensor-Based Approaches for Assessing Neurodegenerative Diseases: A Brief State-of-the-Art Review. Sensors 2025, 25, 5476. https://doi.org/10.3390/s25175476
Mbue ND, Tabei F, Williams K, Olanrewaju K. Innovative Sensor-Based Approaches for Assessing Neurodegenerative Diseases: A Brief State-of-the-Art Review. Sensors. 2025; 25(17):5476. https://doi.org/10.3390/s25175476
Chicago/Turabian StyleMbue, Ngozi D., Fatemeh Tabei, Karen Williams, and Kazeem Olanrewaju. 2025. "Innovative Sensor-Based Approaches for Assessing Neurodegenerative Diseases: A Brief State-of-the-Art Review" Sensors 25, no. 17: 5476. https://doi.org/10.3390/s25175476
APA StyleMbue, N. D., Tabei, F., Williams, K., & Olanrewaju, K. (2025). Innovative Sensor-Based Approaches for Assessing Neurodegenerative Diseases: A Brief State-of-the-Art Review. Sensors, 25(17), 5476. https://doi.org/10.3390/s25175476





