Validity of AI-Driven Markerless Motion Capture for Spatiotemporal Gait Analysis in Stroke Survivors
Abstract
Highlights
- KinaTrax (HumanVersion 8.2, KinaTrax Inc., Boca Raton, FL, USA) markerless motion capture is a valid system for measuring spatiotemporal gait metrics after stroke during comfortable and fast walking speeds.
- Measures of stride width and single-limb support time should be interpreted with caution.
- KinaTrax is a promising sensor-free and streamlined gait analysis technology that can be integrated into gait rehabilitation after stroke.
Abstract
1. Introduction
2. Materials and Methods
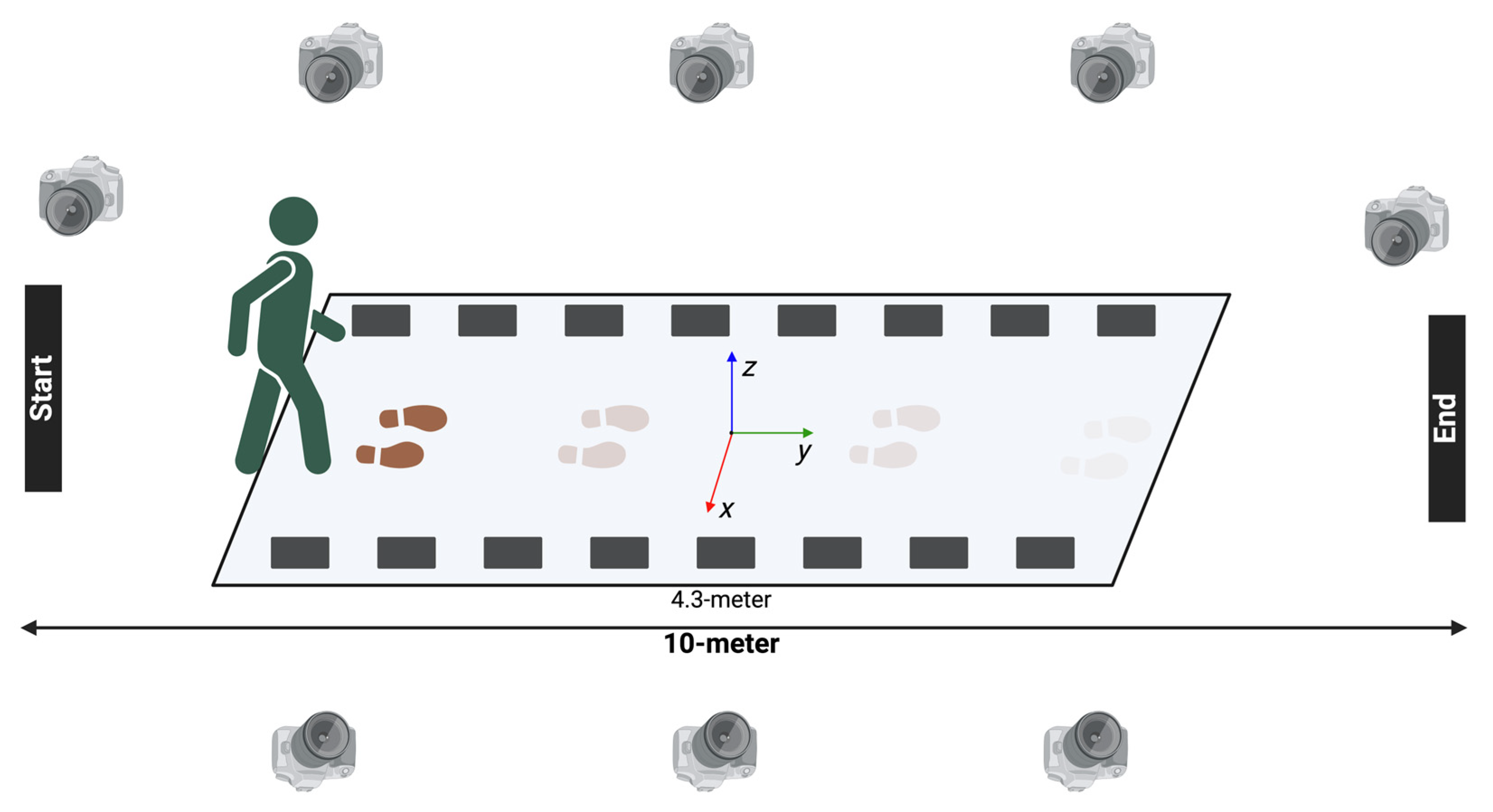
2.1. Study Cohort
2.2. Study Procedures
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
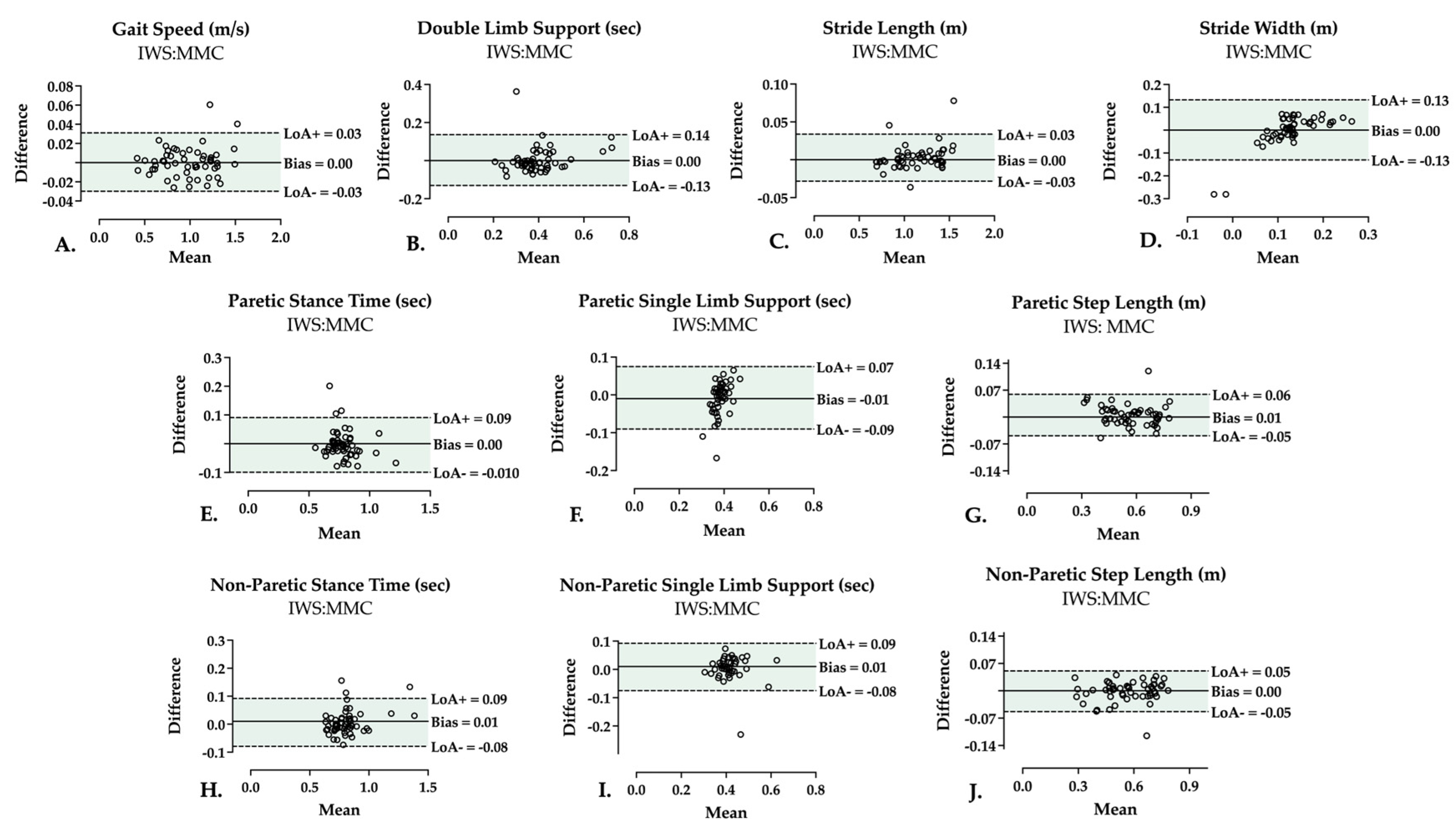
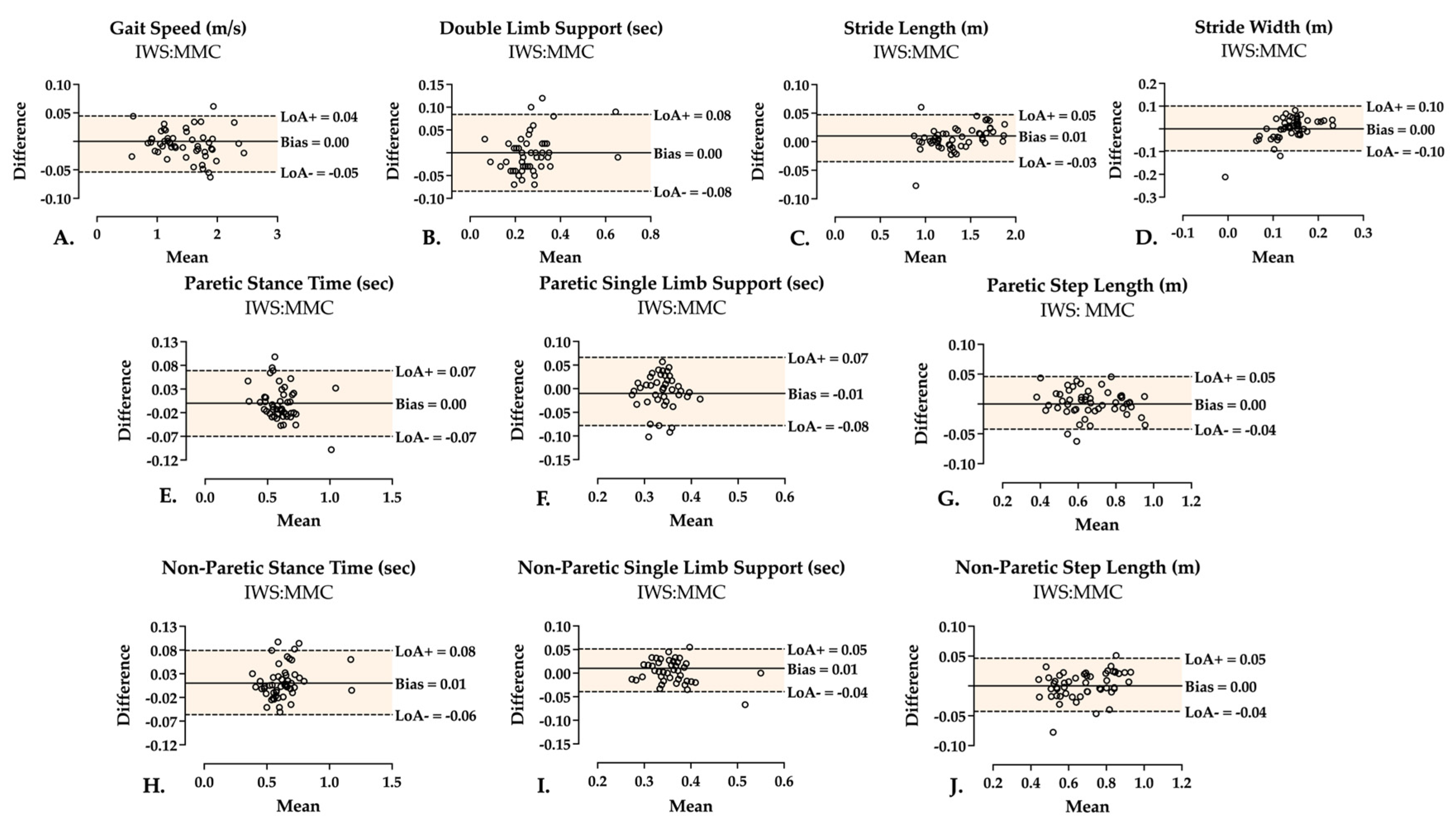
3.2. Two-Limb Parameters
3.3. Single-Limb Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 10MWT | 10-Meter Walk Test |
| IWS | Instrumented Walkway System |
| MMC | Markerless Motion Capture |
| 2D | 2-Dimensional |
| 3D | 3-Dimensional |
| CNN | Convolutional Neural Network |
| CS | Comfortable Speed |
| FS | Fastest Speed |
| DLS | Double Limb Support |
| SLS | Single Limb Support |
| HPE | Human Pose Estimation |
| HS | Heel Strike |
| TO | Toe Off |
| GSR | Gait Speed Reserve |
References
- GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Louie, D.R.; Simpson, L.A.; Mortenson, W.B.; Field, T.S.; Yao, J.; Eng, J.J. Prevalence of Walking Limitation After Acute Stroke and Its Impact on Discharge to Home. Phys. Ther. 2022, 102, pzab246. [Google Scholar] [CrossRef]
- Heshmatollah, A.; Darweesh, S.K.L.; Dommershuijsen, L.J.; Koudstaal, P.J.; Ikram, M.A.; Ikram, M.K. Quantitative Gait Impairments in Patients With Stroke or Transient Ischemic Attack: A Population-Based Approach. Stroke 2020, 51, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Tok, F. Gait Disturbances in Patients with Stroke. PM&R 2014, 6, 635–642. [Google Scholar] [CrossRef]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Randall, E.P. Reliability of Spatiotemporal Asymmetry During Overground Walking for Individuals Following Chronic Stroke. J. Neurol. Phys. Ther. 2011, 35, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Fulk, G.D.; He, Y.; Boyne, P.; Dunning, K. Predicting Home and Community Walking Activity Poststroke. Stroke 2017, 48, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Tasseel-Ponche, S.; Roussel, M.; Toba, M.N.; Sader, T.; Barbier, V.; Delafontaine, A.; Meynier, J.; Picard, C.; Constans, J.M.; Schnitzler, A.; et al. Dual-task versus single-task gait rehabilitation after stroke: The protocol of the cognitive-motor synergy multicenter, randomized, controlled superiority trial (SYNCOMOT). Trials 2023, 24, 172. [Google Scholar] [CrossRef]
- Park, J.; Kim, T.H. The effects of balance and gait function on quality of life of stroke patients. NeuroRehabilitation 2019, 44, 37–41. [Google Scholar] [CrossRef]
- Rudberg, A.S.; Berge, E.; Laska, A.C.; Jutterström, S.; Näsman, P.; Sunnerhagen, K.S.; Lundström, E. Stroke survivors’ priorities for research related to life after stroke. Top. Stroke Rehabil. 2020, 28, 153–158. [Google Scholar] [CrossRef]
- Baker, R. Gait analysis methods in rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 4. [Google Scholar] [CrossRef]
- Kesar, T. The Effects of Stroke and Stroke Gait Rehabilitation on Behavioral and Neurophysiological Outcomes: Challenges and Opportunities for Future Research. Del. J. Public Health 2023, 9, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Borzelli, D.; De Marchis, C.; Quercia, A.; De Pasquale, P.; Casile, A.; Quartarone, A.; Calabrò, R.S.; D’avella, A. Muscle Synergy Analysis as a Tool for Assessing the Effectiveness of Gait Rehabilitation Therapies: A Methodological Review and Perspective. Bioengineering 2024, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Grau-Pellicer, M.; Chamarro-Lusar, A.; Medina-Casanovas, J.; Ferrer, B.C.S. Walking speed as a predictor of community mobility and quality of life after stroke. Top. Stroke Rehabil. 2019, 26, 349–358. [Google Scholar] [CrossRef]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Alali, S.I.I.I.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 12, 650024. [Google Scholar] [CrossRef]
- Mummolo, C.; Mangialardi, L.; Kim, J.H. Quantifying Dynamic Characteristics of Human Walking for Comprehensive Gait Cycle. J. Biomech. Eng. 2013, 135, 091006. [Google Scholar] [CrossRef]
- Sanders, O.; Wang, B.; Kontson, K. Concurrent Validity Evidence for Pressure-Sensing Walkways Measuring Spatiotemporal Features of Gait: A Systematic Review and Meta-Analysis. Sensors 2024, 24, 4537. [Google Scholar] [CrossRef]
- Parati, M.; Ambrosini, E.; DE Maria, B.; Gallotta, M.; Vecchia, L.A.D.; Ferriero, G.; Ferrante, S. The reliability of gait parameters captured via instrumented walkways: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2022, 58, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Mündermann, L.; Corazza, S.; Andriacchi, T.P. The evolution of methods for the capture of human movement leading to markerless motion capture for biomechanical applications. J. Neuroeng. Rehabil. 2006, 3, 6. [Google Scholar] [CrossRef]
- Stenum, J.; Cherry-Allen, K.M.; Pyles, C.O.; Reetzke, R.D.; Vignos, M.F.; Roemmich, R.T. Applications of Pose Estimation in Human Health and Performance across the Lifespan. Sensors 2021, 21, 7315. [Google Scholar] [CrossRef]
- Kidziński, Ł.; Yang, B.; Hicks, J.L.; Rajagopal, A.; Delp, S.L.; Schwartz, M.H. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat. Commun. 2020, 11, 4054. [Google Scholar] [CrossRef]
- Barzyk, P.; Boden, A.S.; Howaldt, J.; Stürner, J.; Zimmermann, P.; Seebacher, D.; Liepert, J.; Stein, M.; Gruber, M.; Schwenk, M. Steps to Facilitate the Use of Clinical Gait Analysis in Stroke Patients: The Validation of a Single 2D RGB Smartphone Video-Based System for Gait Analysis. Sensors 2024, 24, 7819. [Google Scholar] [CrossRef]
- Cerfoglio, S.; Ferraris, C.; Vismara, L.; Amprimo, G.; Priano, L.; Bigoni, M.; Galli, M.; Mauro, A.; Cimolin, V. Estimation of gait parameters in healthy and hemiplegic individuals using Azure Kinect: A comparative study with the optoelectronic system. Front. Bioeng. Biotechnol. 2024, 12, 1449680. [Google Scholar] [CrossRef]
- Eltoukhy, M.; Oh, J.; Kuenze, C.; Signorile, J. Improved kinect-based spatiotemporal and kinematic treadmill gait assessment. Gait Posture 2017, 51, 77–83. [Google Scholar] [CrossRef]
- Eltoukhy, M.; Kuenze, C.; Oh, J.; Jacopetti, M.; Wooten, S.; Signorile, J. Microsoft Kinect can distinguish differences in over-ground gait between older persons with and without Parkinson’s disease. Med. Eng. Phys. 2017, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.E.; Sheikh, Y. OpenPose: Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 43, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Kanko, R.M.; Laende, E.K.; Strutzenberger, G.; Brown, M.; Selbie, W.S.; DePaul, V.; Scott, S.H.; Deluzio, K.J. Assessment of spatiotemporal gait parameters using a deep learning algorithm-based markerless motion capture system. J. Biomech. 2021, 122, 110414. [Google Scholar] [CrossRef] [PubMed]
- McGuirk, T.E.; Perry, E.S.; Sihanath, W.B.; Riazati, S.; Patten, C. Feasibility of Markerless Motion Capture for Three-Dimensional Gait Assessment in Community Settings. Front. Hum. Neurosci. 2022, 16, 867485. [Google Scholar] [CrossRef]
- Ripic, Z.; Signorile, J.F.; Best, T.M.; Jacobs, K.A.; Nienhuis, M.; Whitelaw, C.; Moenning, C.; Eltoukhy, M. Validity of artificial intelligence-based markerless motion capture system for clinical gait analysis: Spatiotemporal results in healthy adults and adults with Parkinson’s disease. J. Biomech. 2023, 155, 111645. [Google Scholar] [CrossRef]
- Schoenwether, B.; Ripic, Z.; Nienhuis, M.; Signorile, J.F.; Best, T.M.; Eltoukhy, M.; Mentiplay, B.F. Reliability of artificial intelligence-driven markerless motion capture in gait analyses of healthy adults. PLoS ONE 2025, 20, e0316119. [Google Scholar] [CrossRef] [PubMed]
- Ripic, Z.; Signorile, J.F.; Kuenze, C.; Eltoukhy, M. Concurrent validity of artificial intelligence-based markerless motion capture for over-ground gait analysis: A study of spatiotemporal parameters. J. Biomech. 2022, 143, 111278. [Google Scholar] [CrossRef]
- Crenna, F.; Rossi, G.B.; Berardengo, M. Filtering Biomechanical Signals in Movement Analysis. Sensors 2021, 21, 4580. [Google Scholar] [CrossRef]
- Zeni, J.A., Jr.; Richards, J.G.; Higginson, J.S. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 2008, 27, 710–714. [Google Scholar] [CrossRef]
- Liu, J.; Tang, W.; Chen, G.; Lu, Y.; Feng, C.; Tu, X.M. Correlation and agreement: Overview and clarification of competing concepts and measures. Shanghai Arch. Psychiatry 2016, 28, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Lonini, L.; Moon, Y.; Embry, K.; Cotton, R.J.; McKenzie, K.; Jenz, S.; Jayaraman, A. Video-Based Pose Estimation for Gait Analysis in Stroke Survivors during Clinical Assessments: A Proof-of-Concept Study. Digit. Biomark. 2022, 6, 9–18. [Google Scholar] [CrossRef]
- Aung, N.; Bovonsunthonchai, S.; Hiengkaew, V.; Tretriluxana, J.; Rojasavastera, R.; Pheung-Phrarattanatrai, A. Concurrent validity and intratester reliability of the video-based system for measuring gait poststroke. Physiother. Res. Int. 2019, 25, e1803. [Google Scholar] [CrossRef]
- Chow, J.W.; Stokic, D.S. Longitudinal Changes in Temporospatial Gait Characteristics during the First Year Post-Stroke. Brain Sci. 2021, 11, 1648. [Google Scholar] [CrossRef]
- Cimolin, V.; Vismara, L.; Ferraris, C.; Amprimo, G.; Pettiti, G.; Lopez, R.; Galli, M.; Cremascoli, R.; Sinagra, S.; Mauro, A.; et al. Computation of Gait Parameters in Post Stroke and Parkinson’s Disease: A Comparative Study Using RGB-D Sensors and Optoelectronic Systems. Sensors 2022, 22, 824. [Google Scholar] [CrossRef]
- Ferraris, C.; Cimolin, V.; Vismara, L.; Votta, V.; Amprimo, G.; Cremascoli, R.; Galli, M.; Nerino, R.; Mauro, A.; Priano, L. Monitoring of Gait Parameters in Post-Stroke Individuals: A Feasibility Study Using RGB-D Sensors. Sensors 2021, 21, 5945. [Google Scholar] [CrossRef]
- Menz, H.B.; Latt, M.D.; Tiedemann, A.; Mun San Kwan, M.; Lord, S.R. Reliability of the GAITRite® walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture 2004, 20, 20–25. [Google Scholar] [CrossRef]
- Commandeur, D.; Klimstra, M.; Yoshida, K.; Hundza, S. The Minimum Number of Strides Required for Reliable Gait Measurements in Older Adult Fallers and Non-Fallers. Sensors 2024, 24, 7666. [Google Scholar] [CrossRef] [PubMed]
- Hollman, J.H.; Childs, K.B.; McNeil, M.L.; Mueller, A.C.; Quilter, C.M.; Youdas, J.W. Number of strides required for reliable measurements of pace, rhythm and variability parameters of gait during normal and dual task walking in older individuals. Gait Posture 2010, 32, 23–28. [Google Scholar] [CrossRef]
- Enzinger, C.; Johansen-Berg, H.; Dawes, H.; Bogdanovic, M.; Collett, J.; Guy, C.; Ropele, S.; Kischka, U.; Wade, D.; Fazekas, F.; et al. Functional MRI Correlates of Lower Limb Function in Stroke Victims With Gait Impairment. Stroke 2008, 39, 1507–1513. [Google Scholar] [CrossRef]
- Xu, X.; McGorry, R.W.; Chou, L.-S.; Lin, J.-H.; Chang, C.C. Accuracy of the Microsoft Kinect™ for measuring gait parameters during treadmill walking. Gait Posture 2015, 42, 145–151. [Google Scholar] [CrossRef]
- Wang, S.; Pitts, J.; Purohit, R.; Shah, H. The Influence of Motion Data Low-Pass Filtering Methods in Machine-Learning Models. Appl. Sci. 2025, 15, 2177. [Google Scholar] [CrossRef]
- Vive, S.; Elam, C.; Bunketorp-Käll, L. Comfortable and Maximum Gait Speed in Individuals with Chronic Stroke and Community-Dwelling Controls. J. Stroke Cerebrovasc. Dis. 2021, 30, 106023. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.W.; Stokic, D.S. The contribution of walking speed versus recent stroke to temporospatial gait variability. Gait Posture 2022, 100, 216–221. [Google Scholar] [CrossRef]
- Wang, Y.; Mukaino, M.; Ohtsuka, K.; Otaka, Y.; Tanikawa, H.; Matsuda, F.; Tsuchiyama, K.; Yamada, J.; Saitoh, E. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int. J. Rehabil. Res. 2020, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Supiot, A.; Pradon, D.; Do, M.C.; Zory, R.; Roche, N. Minimal detectable change of kinematic and spatiotemporal parameters in patients with chronic stroke across three sessions of gait analysis. Hum. Mov. Sci. 2019, 64, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kesar, T.M.; Binder-Macleod, S.A.; Hicks, G.E.; Reisman, D.S. Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke. Gait Posture 2011, 33, 314–317. [Google Scholar] [CrossRef] [PubMed]
| Total = 19 | Median (IQR) or n (%) |
|---|---|
| Participants Demographics | |
| Age, years | 63 (55–65) |
| Sex, male | 10 (53%) |
| Race/Ethnicity | |
| Non-Hispanic White | 4 (21%) |
| Non-Hispanic Black | 7 (37%) |
| Hispanic | 8 (42%) |
| Stroke Characteristics | |
| Time since stroke, months | 33 (14–109) |
| Hemiparetic side, right | 10 (53%) |
| NHISS | |
| NIHSS (0–5) | 14 (74%) |
| NIHSS (6–15) | 5 (26%) |
| FAC | |
| FAC = 3 | 2 (11%) |
| FAC = 4 | 8 (42%) |
| FAC = 5 | 9 (47%) |
| MoCA | 23 (20–25) |
| Parameter | Correlation Coefficient | Absolute Agreement | Relative Consistency | |
|---|---|---|---|---|
| r, p | ICC (95%CI) | ICC (95%CI) | ||
| Two-limb | Gait speed (m/s) | 0.996, p < 0.001 | 0.999 (0.997–0.999) | 0.998 (0.997–0.999) |
| DLS (s) | 0.768, p < 0.001 | 0.804 (0.684–0.881) | 0.801 (0.680–0.880) | |
| Stride length (m) | 0.997, p < 0.001 | 0.998 (0.997–0.999) | 0.998 (0.997–0.999) | |
| Stride width (m) | 0.554, p < 0.001 | 0.442 (0.198–0.634) | 0.438 (0.195–0.630) | |
| Single-limb | Paretic | |||
| Stance time (s) | 0.858, p < 0.001 | 0.912 (0.854–0.948) | 0.911 (0.852–0.948) | |
| SLS (s) | 0.431, p < 0.001 | 0.314 (0.057–0.533) | 0.317 (0.057–0.538) | |
| Step length (m) | 0.973, p < 0.001 | 0.976 (0.959–0.986) | 0.976 (0.959–0.986) | |
| Non-Paretic | ||||
| Stance time (s) | 0.867, p < 0.001 | 0.958 (0.928–0.975) | 0.958 (0.928–0.975) | |
| SLS (s) | 0.691, p < 0.001 | 0.731 (0.578–0.834) | 0.734 (0.582–0.837) | |
| Step length (m) | 0.969, p < 0.001 | 0.982 (0.968–0.989) | 0.981 (0.968–0.989) |
| Parameter | Correlation Coefficient | Absolute Agreement | Relative Consistency | |
|---|---|---|---|---|
| r, p | ICC (95%CI) | ICC (95%CI) | ||
| Two-limb | Gait speed (m/s) | 0.996, p < 0.001 | 0.998 (0.997–0.999) | 0.998 (0.997–0.999) |
| DLS (s) | 0.843, p < 0.001 | 0.917 (0.859–0.952) | 0.916 (0.857–0.951) | |
| Stride length (m) | 0.997, p < 0.001 | 0.997 (0.995–0.998) | 0.997 (0.995–0.998) | |
| Stride width (m) | 0.508, p < 0.001 | 0.476 (0.230–0.663) | 0.471 (0.227–0.659) | |
| Single-limb | Paretic | |||
| Stance time (s) | 0.928, p < 0.001 | 0.960 (0.931–0.977) | 0.959 (0.929–0.976) | |
| SLS (s) | 0.446, p < 0.001 | 0.480 (0.240–0.665) | 0.481 (0.239–0.667) | |
| Step length (m) | 0.985, p < 0.001 | 0.989 (0.981–0.994) | 0.989 (0.980–0.994) | |
| Non-Paretic | ||||
| Stance time (s) | 0.942, p < 0.001 | 0.969 (0.944–0.983) | 0.972 (0.951–0.984) | |
| SLS (s) | 0.846, p < 0.001 | 0.886 (0.807–0.934) | 0.890 (0.815–0.936) | |
| Step length (m) | 0.977, p < 0.001 | 0.987 (0.978–0.993) | 0.987 (0.977–0.993) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alammari, B.J.; Schoenwether, B.; Ripic, Z.; Kirk-Sanchez, N.; Eltoukhy, M.; Bishop, L. Validity of AI-Driven Markerless Motion Capture for Spatiotemporal Gait Analysis in Stroke Survivors. Sensors 2025, 25, 5315. https://doi.org/10.3390/s25175315
Alammari BJ, Schoenwether B, Ripic Z, Kirk-Sanchez N, Eltoukhy M, Bishop L. Validity of AI-Driven Markerless Motion Capture for Spatiotemporal Gait Analysis in Stroke Survivors. Sensors. 2025; 25(17):5315. https://doi.org/10.3390/s25175315
Chicago/Turabian StyleAlammari, Balsam J., Brandon Schoenwether, Zachary Ripic, Neva Kirk-Sanchez, Moataz Eltoukhy, and Lauri Bishop. 2025. "Validity of AI-Driven Markerless Motion Capture for Spatiotemporal Gait Analysis in Stroke Survivors" Sensors 25, no. 17: 5315. https://doi.org/10.3390/s25175315
APA StyleAlammari, B. J., Schoenwether, B., Ripic, Z., Kirk-Sanchez, N., Eltoukhy, M., & Bishop, L. (2025). Validity of AI-Driven Markerless Motion Capture for Spatiotemporal Gait Analysis in Stroke Survivors. Sensors, 25(17), 5315. https://doi.org/10.3390/s25175315







