Tapered Optical Fiber Optofluidics: Bridging In-Fiber and Outside-Fiber Architectures Toward Autonomous Lab-on-Fiber Biosensing
Abstract
1. Introduction
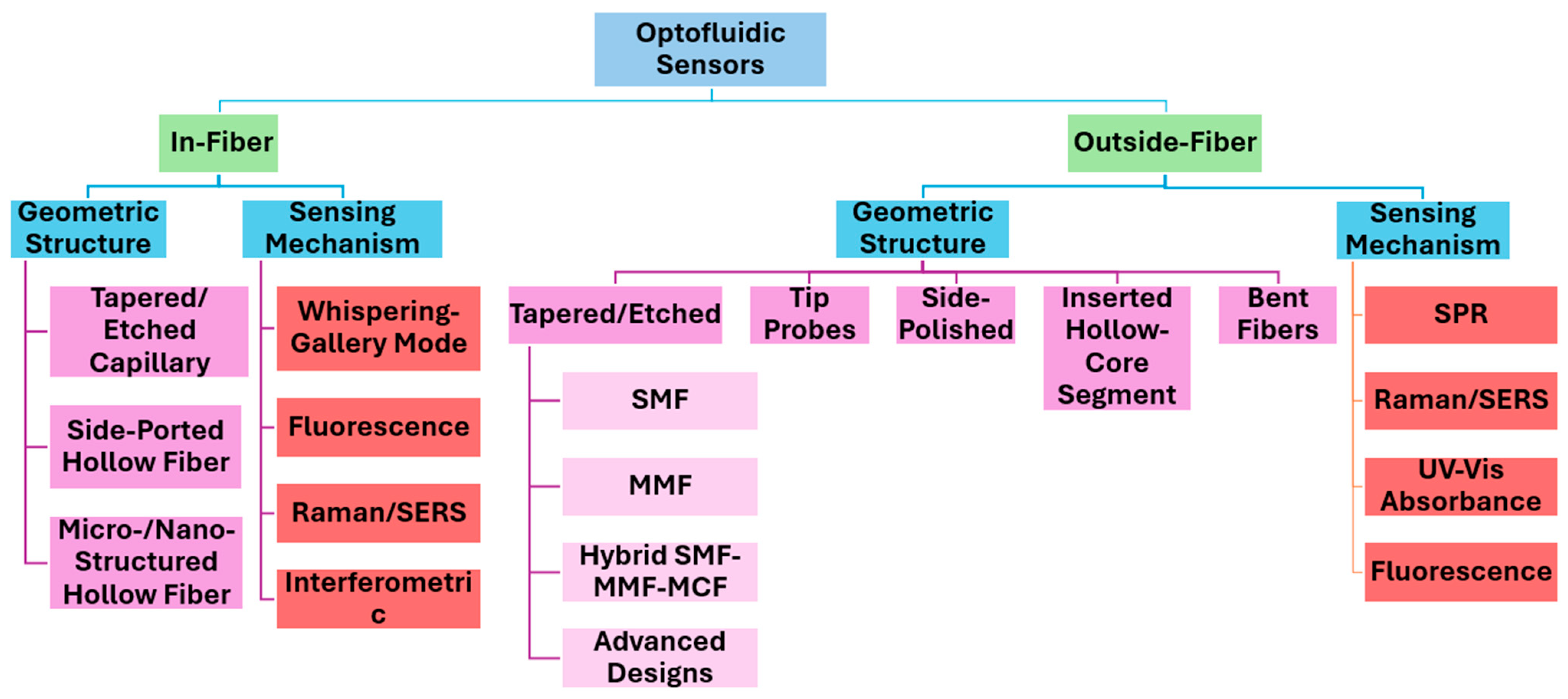
2. Optofluidic Integration Strategies: In-Fiber vs. Outside-Fiber Architectures
3. In-Fiber Optofluidics
3.1. Flow Principles and Materials
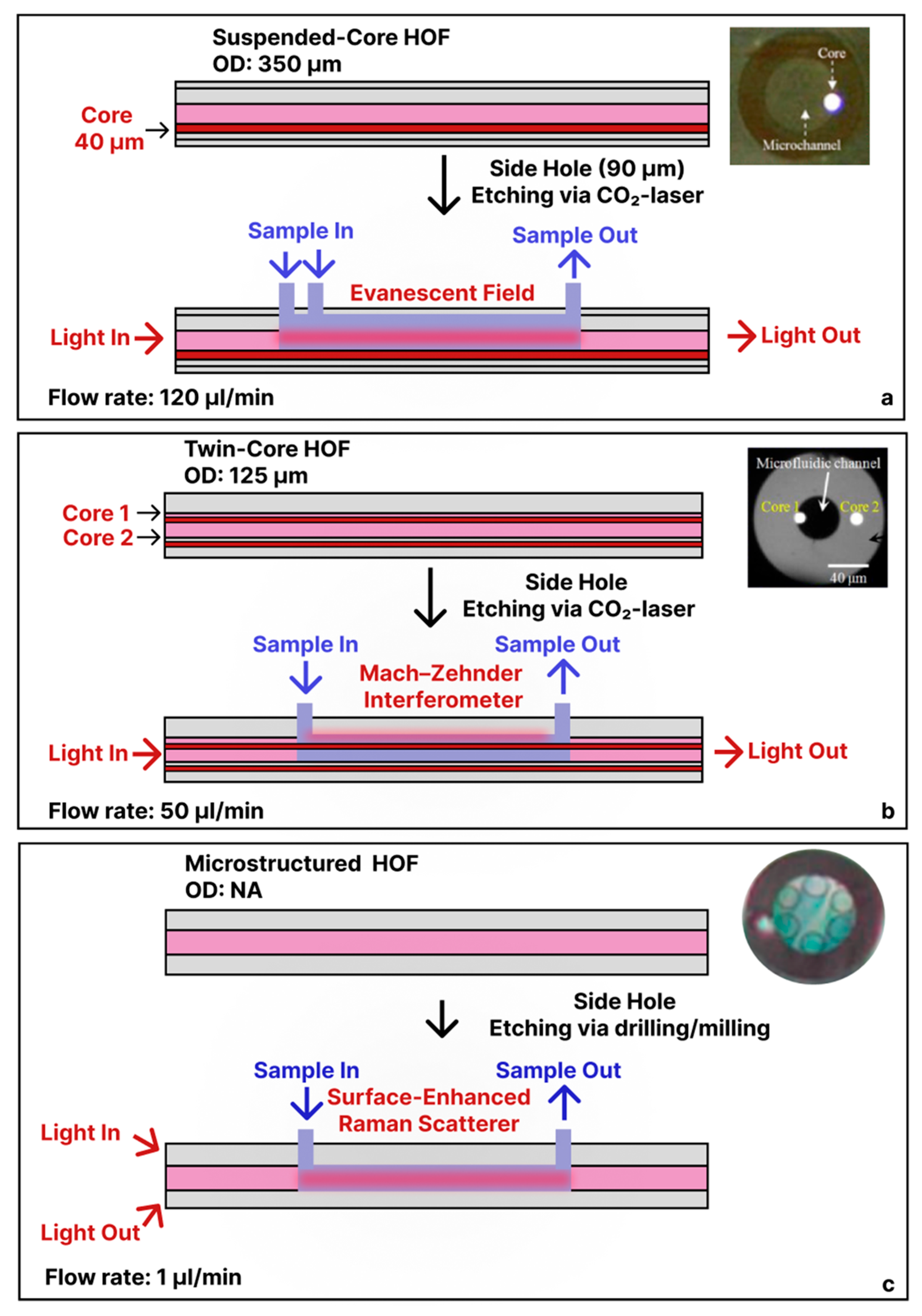
3.2. Tapered/Etched Capillary Sensors
3.3. Side-Ported Hollow-Fiber Sensors
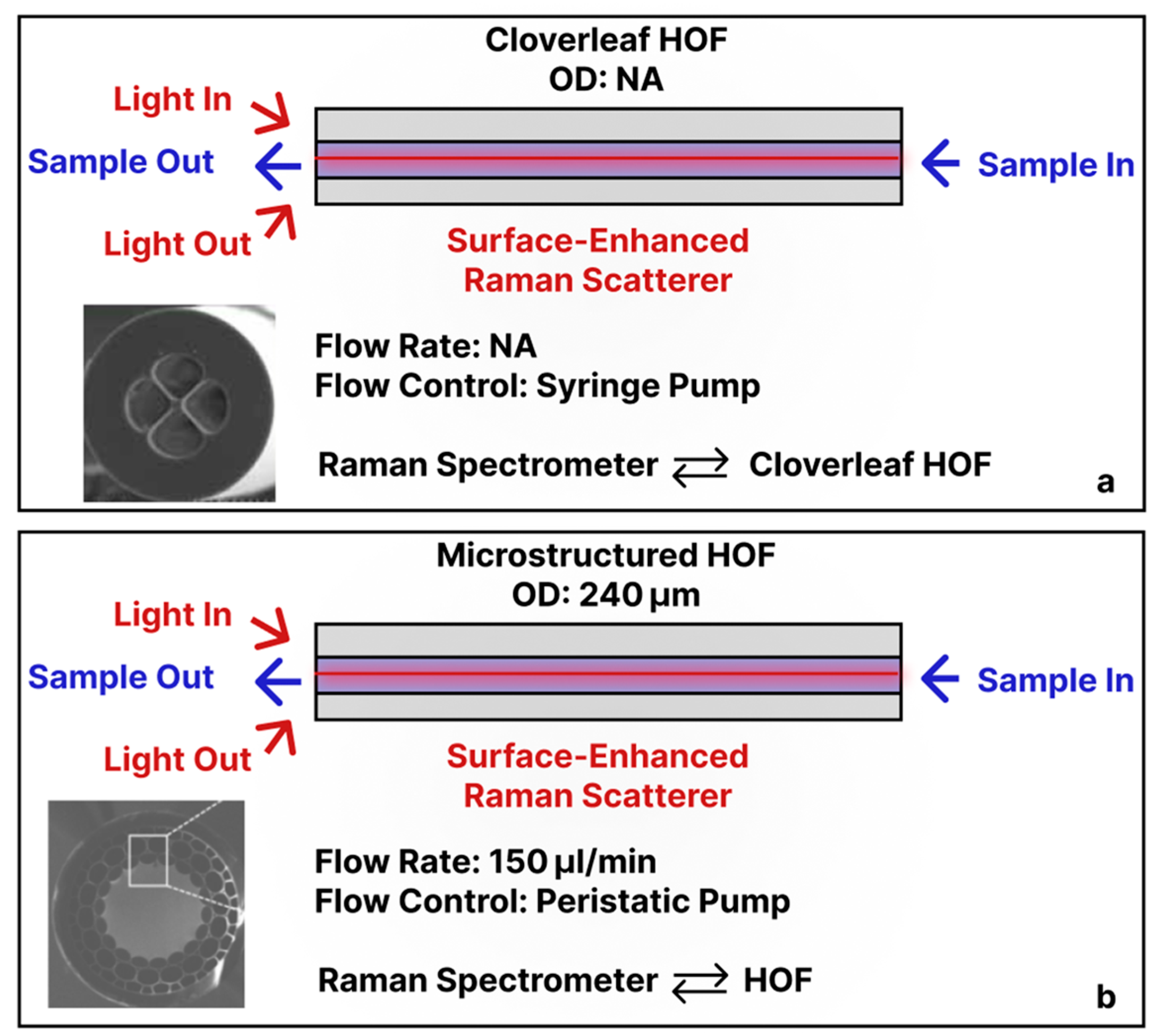
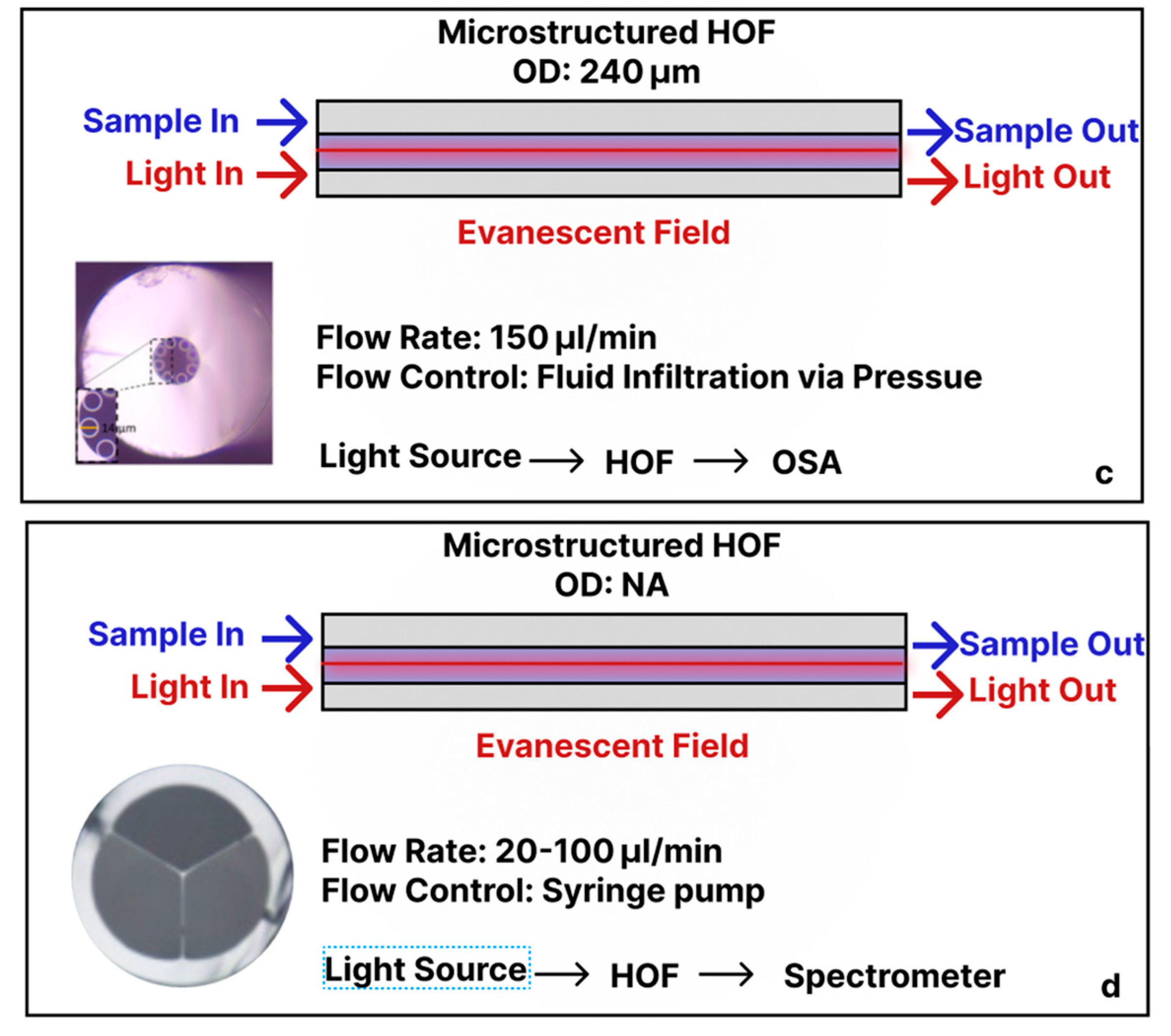
3.4. Micro-/Nano-Structured Hollow Fibers
4. Outside-Fiber Optofluidics
4.1. Flow Principles, Materials, and Optical Design Considerations
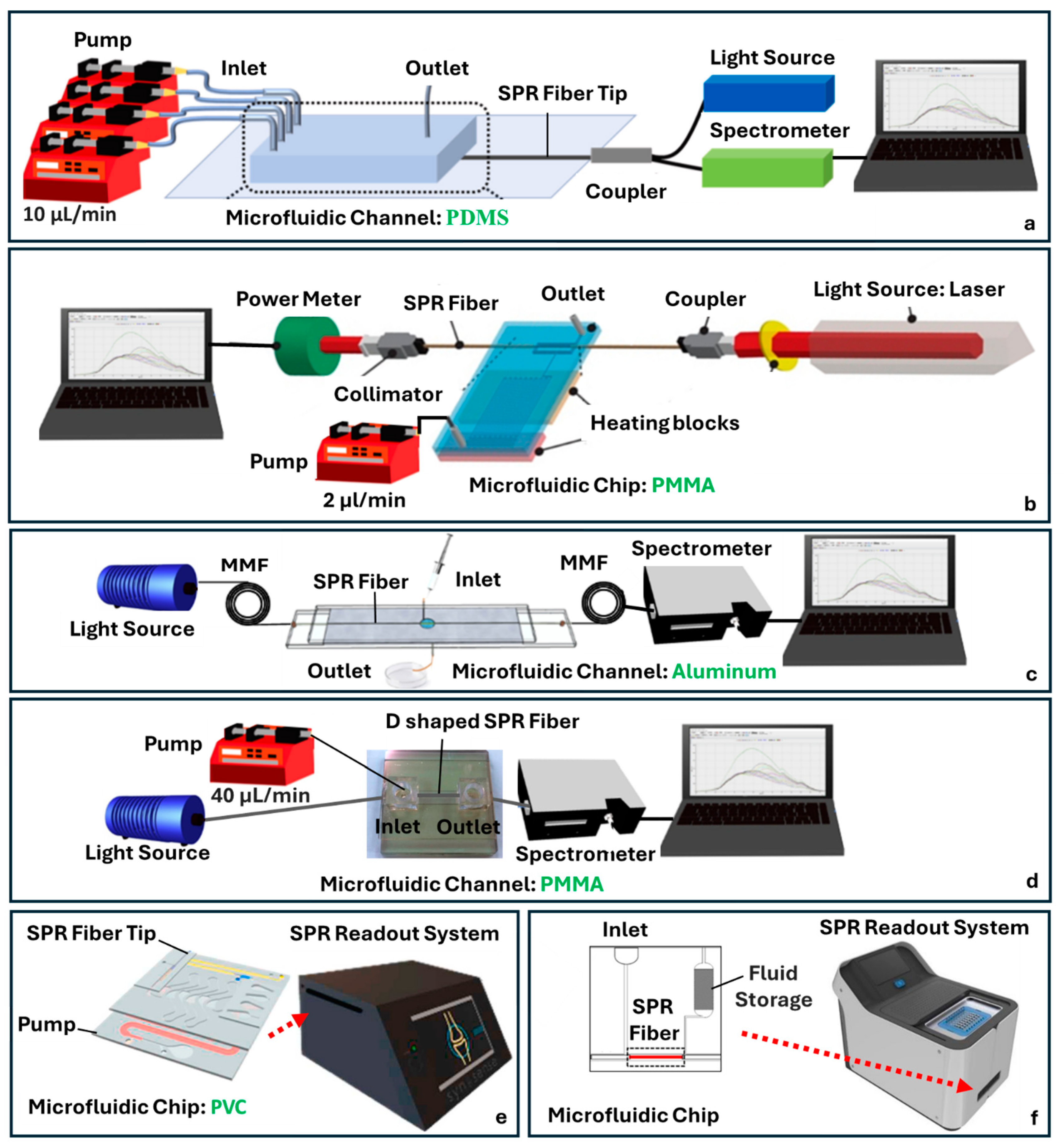
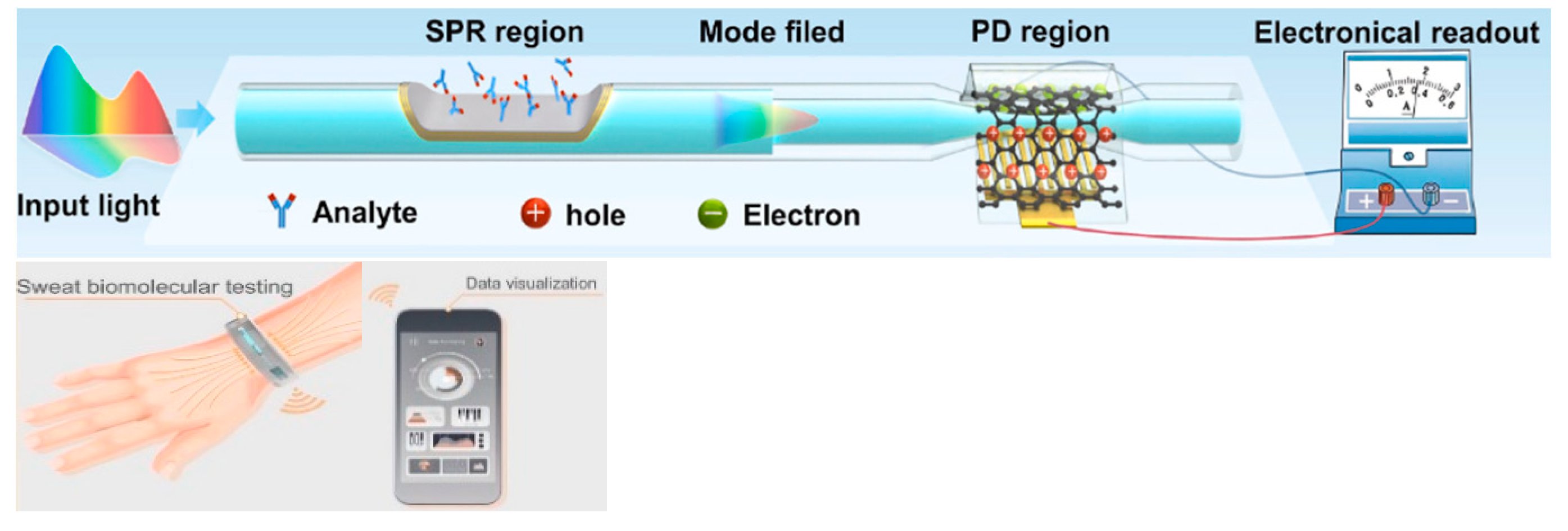
4.2. SPR-Based Fiber-Chip Platforms
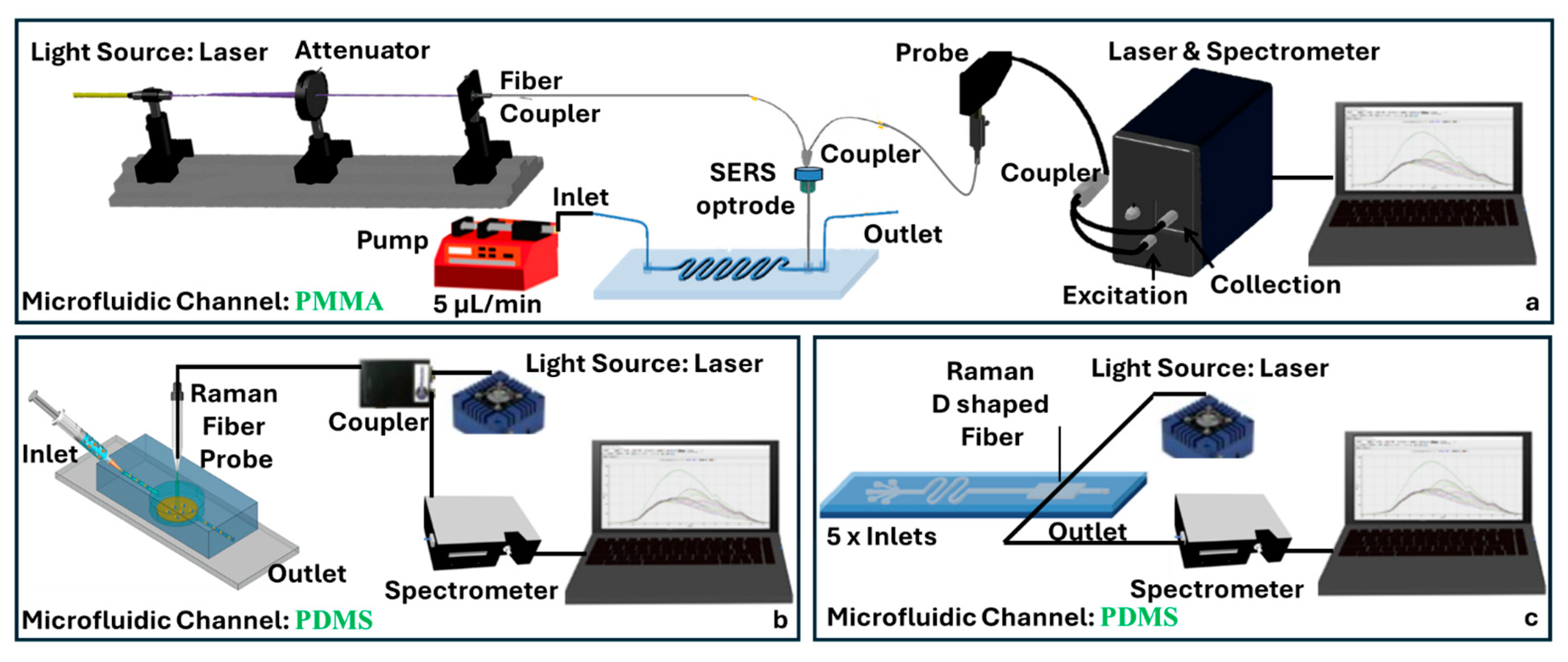
4.3. Raman/SERS Fiber-Chip Platforms
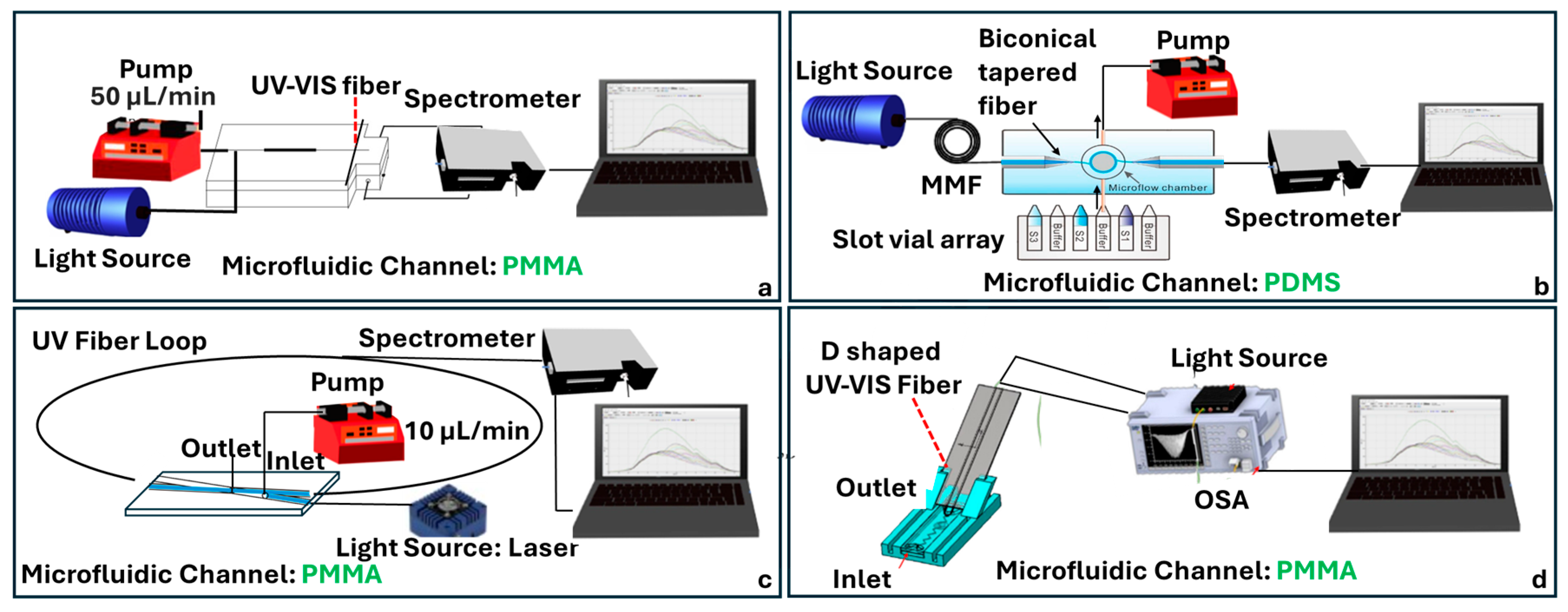
4.4. UV-Vis Absorbance Fiber-Chip Platforms
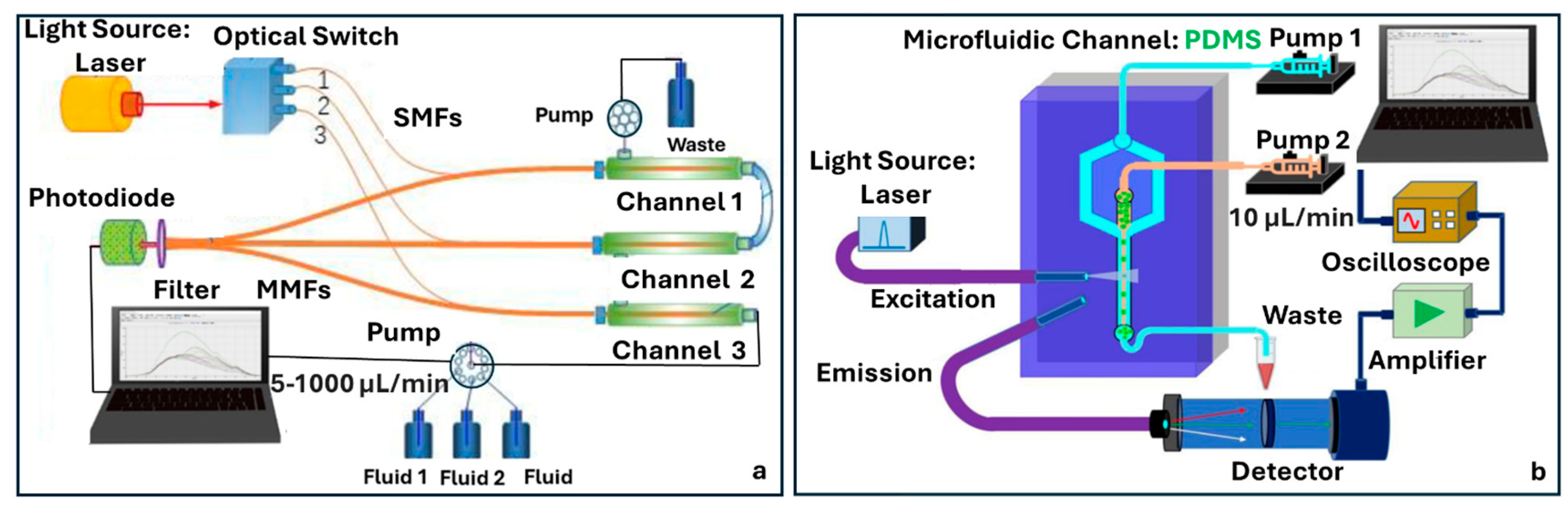
4.5. Fluorescence Fiber-Chip Platforms
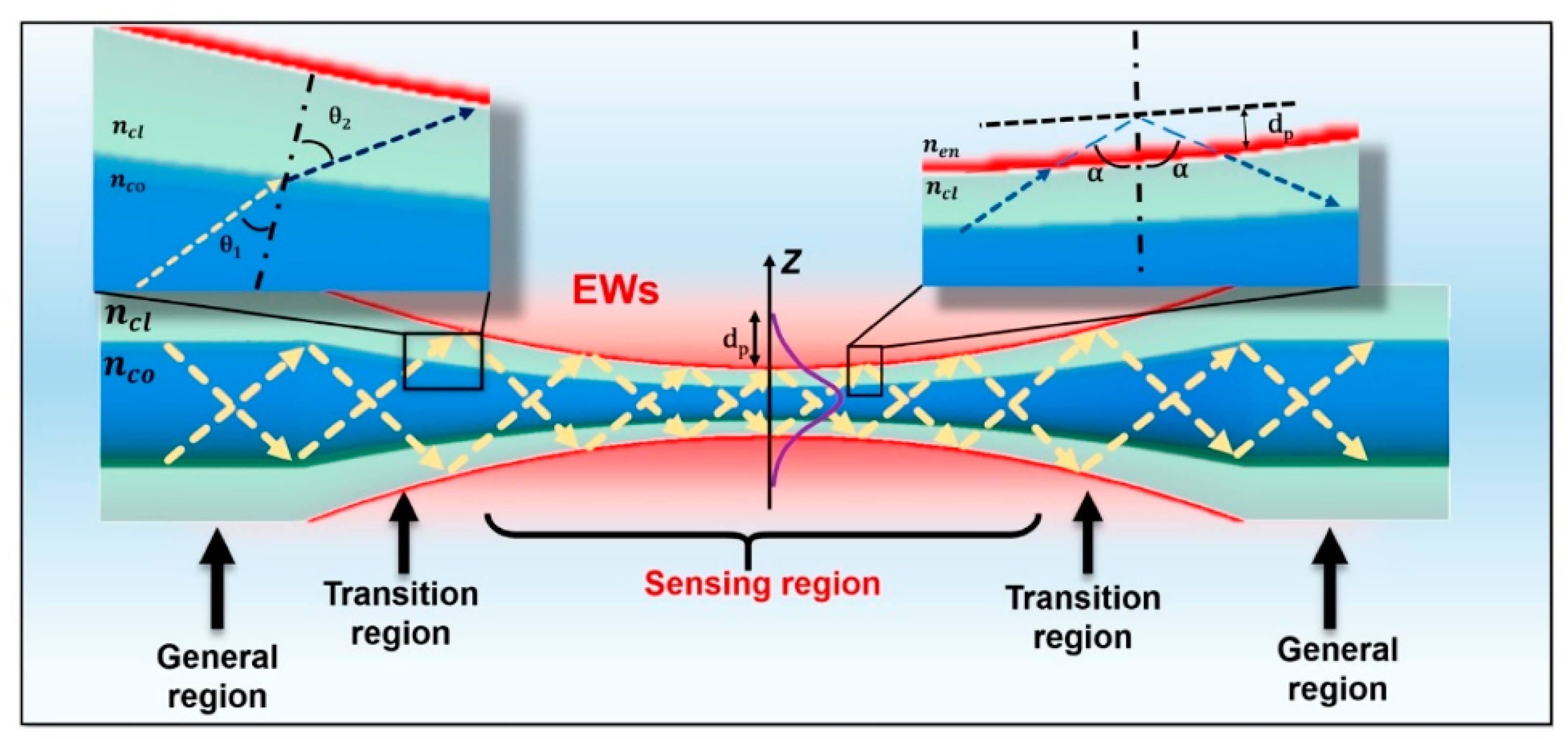
5. Tapered Optical Fibers
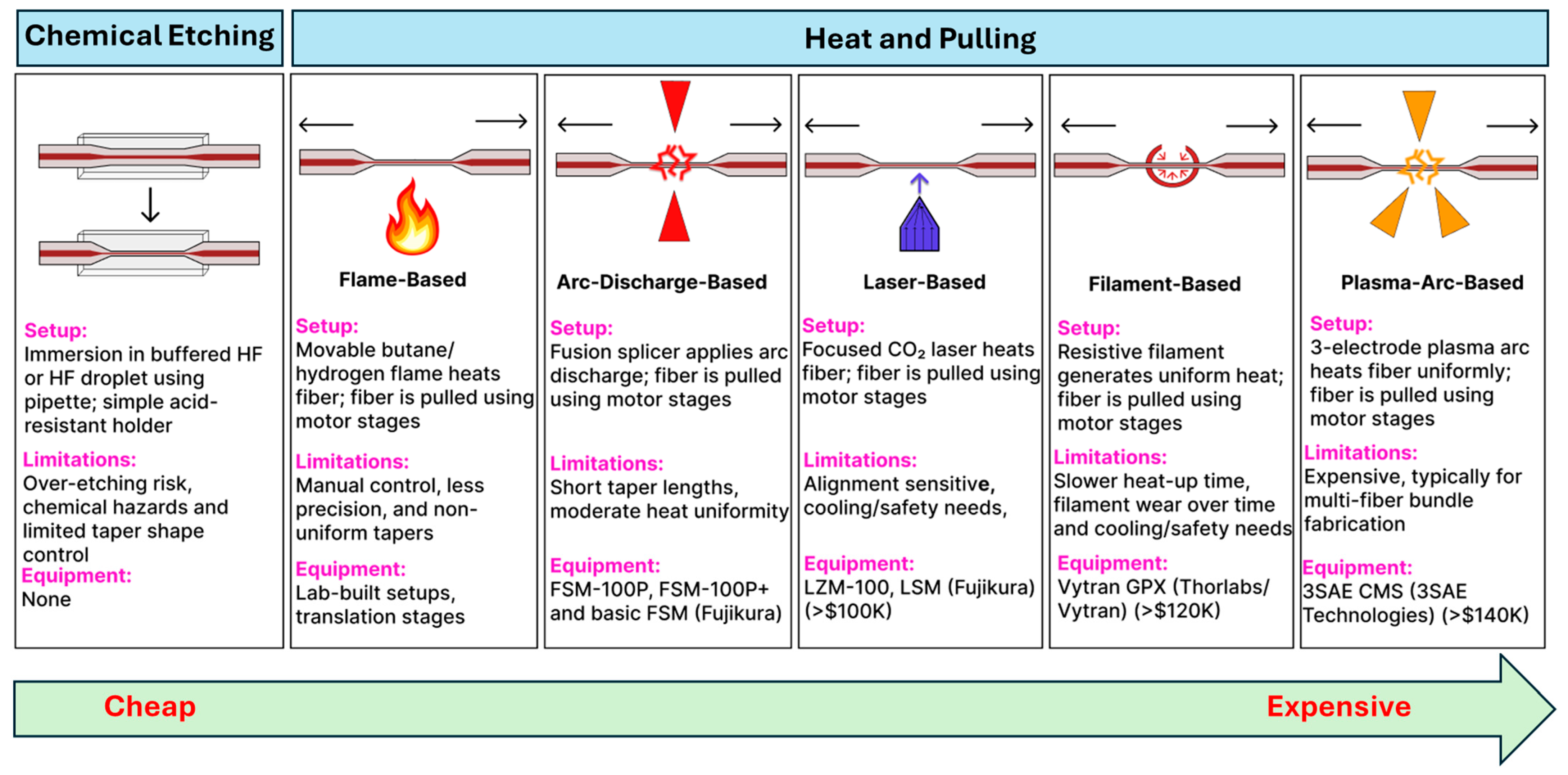
5.1. Fabrication Methods
5.2. Designs and Configurations of Tapered Optical Fiber Based Biosensors
5.2.1. Tapered SMF Geometries
5.2.2. Tapered MMF Geometries
5.2.3. Hybrid SMF–MMF–MCF Structures
5.2.4. Advanced Tapered Design
5.3. Outside-Fiber Optofluidics Utilizing Tapered Fibers
6. Challenges and Benefits of Fiber-Based Optofluidic Biosensors
7. Pathways to Self-Contained Fiber-Based Optofluidic Biosensors
8. Non-Fiber Microfluidic Sensing Platforms and Comparison with Fiber-Based Systems
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taha, B.A.; Ali, N.; Sapiee, N.M.; Fadhel, M.M.; Mat Yeh, R.M.; Bachok, N.N.; Al Mashhadany, Y.; Arsad, N. Comprehensive Review Tapered Optical Fiber Configurations for Sensing Application: Trend and Challenges. Biosensors 2021, 11, 253. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, X.; Li, X.; Zhang, J.; Wang, Y.; Li, D.; Hong, X.; Shao, Y.; Chen, Y. Enhanced Sensitivity Mach–Zehnder Interferometer-Based Tapered-in-Tapered Fiber-Optic Biosensor for the Immunoassay of C-Reactive Protein. Biosensors 2025, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, S.K.; Ansari, M.T.I.; Shadab, A. Analysis of Tapered Fiber-Optic Surface Plasmon Resonance (SPR) Bio-Sensing Probe With the Effect of Different Taper Profiles and Metal Choices. IEEE Trans. Plasma Sci. 2024, 52, 1295–1303. [Google Scholar] [CrossRef]
- Mansor, M.; Abu Bakar, M.H.; Omar, M.F.; Mustapha Kamil, Y.; Zainol Abidin, N.H.; Mustafa, F.H.; Mahdi, M.A. Taper biosensor in fiber ring laser cavity for protein detection. Opt. Laser Technol. 2020, 125, 106033. [Google Scholar] [CrossRef]
- Mumtaz, F.; Roman, M.; Zhang, B.; Huang, J. Assembly-free ultra-sensitive miniaturized strain sensor based on an asymmetric optical fiber taper. Measurement 2023, 211, 112655. [Google Scholar] [CrossRef]
- Liyanage, T.; Lai, M.; Slaughter, G. Label-free tapered optical fiber plasmonic biosensor. Anal. Chim. Acta 2021, 1169, 338629. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, H.; Zhang, H.; Hou, L.; Yang, J. Sensitivity-Enhanced Fiber-Optic Axial-Strain Sensor by Tapered-Microfiber-Assisted Micro-Open Cavity. IEEE Trans. Instrum. Meas. 2024, 73, 3450113. [Google Scholar] [CrossRef]
- Khalaf, A.L.; Mohamad, F.S.; Abdul Rahman, N.; Lim, H.N.; Paiman, S.; Yusof, N.A.; Mahdi, M.A.; Yaacob, M.H. Room temperature ammonia sensor using side-polished optical fiber coated with graphene/polyaniline nanocomposite. Opt. Mater. Express 2017, 7, 1858. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, S.; Liao, C.; Wang, Y.; Wang, G.; Xu, X.; Fu, C.; Xu, G.; Lian, J.; Wang, Y. Surface plasmon resonance refractive sensor based on silver-coated side-polished fiber. Sens. Actuators. B Chem. 2016, 230, 206–211. [Google Scholar] [CrossRef]
- Li, H.; Chu, R.; Cao, J.; Zhou, F.; Guo, K.; Zhang, Q.; Wang, H.; Liu, Y. Sensitive and reproducible on-chip SERS detection by side-polished fiber probes integrated with microfluidic chips. Measurement 2023, 218, 113203. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Zhang, D.; Tong, X. Hydrogen sensing performance comparison of Pd layer and Pd/WO3 composite thin film coated on side-polished single- and multimode fibers. Sens. Actuators B Chem. 2010, 149, 161–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, Z.; Shi, Y.; Liu, C.; Li, X.; Lv, J.; Yang, L.; Chu, P.K. Photonic fibre crystal sensor with a D-shape based on surface plasma resonance containing microfluidic channels for detection of a wide range of refractive indexes. J. Mod. Opt. 2022, 69, 1–11. [Google Scholar] [CrossRef]
- Lidiya, A.E.; Raja, R.V.J.; Pham, V.D.; Ngo, Q.M.; Vigneswaran, D. Detecting hemoglobin content blood glucose using surface plasmon resonance in D-shaped photonic crystal fiber. Opt. Fiber Technol. 2019, 50, 132–138. [Google Scholar] [CrossRef]
- Ahmed, K.; Paul, B.K.; Vasudevan, B.; Rashed, A.N.; Maheswar, R.; Amiri, I.S.; Yupapin, P. Design of D-shaped elliptical core photonic crystal fiber for blood plasma cell sensing application. Results Phys. 2019, 12, 2021–2025. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Zhang, J.; Zhang, Y.; Rao, X.; Chen, C.; Liu, H.; Deng, Y.; Liao, C.; Smietana, M.J.; et al. A D-Shaped Polymer Optical Fiber Surface Plasmon Resonance Biosensor for Breast Cancer Detection Applications. Biosensors 2023, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, R.A.; Salih, Q.M.; Hasan, A.D.; Alkhasraji, J.M.D.; Kalankesh, H.V. D-Shaped Microfluidic Channel Bimetallic with a Highly Sensitive SPR RI Sensor for a Large Detection Range. Plasmonics 2024, 19, 1383–1394. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Wu, Y.-C.; Liu, Y.; Cai, H.Y.; Liu, J. Highly sensitive dual-function sensor for refractive index and temperature using D-shaped microchannel photonic crystal fiber. Opt. Express. 2024, 32, 12405. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Tran, T.-B.; Nguyen, T.T.-V.; Le, H.-D.; Chiang, C.-C. Integration of a Microfluidics System With a U-Shaped Optical Fiber Sensor for Sensing Various Concentrations of Glucose. IEEE Sens. J. 2024, 24, 20617–20628. [Google Scholar] [CrossRef]
- Wang, W.; Xia, L.; Xiao, X.; Li, G. Recent Progress on Microfluidics Integrated with Fiber-Optic Sensors for On-Site Detection. Sensors 2024, 24, 2067. [Google Scholar] [CrossRef]
- Xia, L.; Li, G. Recent progress of microfluidics in surface-enhanced Raman spectroscopic analysis. J. Sep. Sci. 2021, 44, 1752–1768. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhou, Y.; Zheng, W.; Sun, Y.; Ma, G.; Zhao, Y. Optical Fiber Optofluidic Bio-Chemical Sensors: A Review. Laser Photon. Rev. 2021, 15, 2000526. [Google Scholar] [CrossRef]
- Blue, R.; Uttamchandani, D. Recent advances in optical fiber devices for microfluidics integration. J. Biophotonics 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Subrahmanyam, T.V.B.; Sharma, A.D.; Thyagarajan, K.; Pal, B.P.; Goyal, I.C. Novel refractometer using a tapered optical fibre. Electron. Lett. 1984, 20, 534–535. [Google Scholar] [CrossRef]
- Sidhik, S.; Ittiarah, J.V.; Gangopadhyay, T.K. Design and Analysis of Chemically Etched and Biconically Tapered Fiber for Chemical Sensing Application. In Advances in Optical Science and Engineering. Springer Proceedings in Physics; Lakshminarayanan, V., Bhattacharya, I., Eds.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, W.; Wu, N.; Zou, X.; Wang, X. Tapered optical fiber sensor for label-free detection of biomolecules. Sensors 2011, 11, 3780–3790. [Google Scholar] [CrossRef]
- Korposh, S.; James, S.W.; Lee, S.-W.; Tatam, R.P. Tapered Optical Fibre Sensors: Current Trends and Future Perspectives. Sensors 2019, 19, 2294. [Google Scholar] [CrossRef] [PubMed]
- Esteban, Ó.; González-Cano, A.; Díaz-Herrera, N.; Navarrete, M.-C. Absorption as a selective mechanism in surface plasmon resonance fiber optic sensors. Opt. Lett. 2006, 31, 3089–3091. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Peng, Y.; Chen, X.; Zhang, S.; Zhao, Y. Optimization of tapered optical fiber sensor based on SPR for high sensitivity salinity measurement. Opt. Fiber. Technol. 2023, 78, 103309. [Google Scholar] [CrossRef]
- Teng, C.; Maosen, L.; Min, R.; Shijie, D.; Ming, C.; Minmin, X.; Libo, Y.; Hongchang, D. A High-Sensitivity SPR Sensor Based on MMF-Tapered HCF-MMF Fiber Structure for Refractive Index Sensing. IEEE Sens. J. 2022, 22, 18517–18523. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, X.; Qin, Y.K.; Zhang, Y.; Mou, J.; Deng, Y.; Liu, W.; Zhang, Y.; Yuan, L. Refractive index SPR sensor with high sensitivity and wide detection range using tapered silica fiber and photopolymer coating. Opt. Express 2023, 31, 31768–31779. [Google Scholar] [CrossRef]
- Tang, J.L.; Cheng, S.F.; Hsu, W.T.; Chiang, T.Y.; Chau, L.K. Fiber-optic biochemical sensing with a colloidal gold-modified long period fiber grating. Sens. Actuators B Chem. 2006, 119, 105–109. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Jung, W.; Han, J.; Choi, J.W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Bai, S.; Gao, Y.; Metcalfe, G.; Cheng, W.; Zhu, Y. Multiplexed detection of cancer biomarkers using a microfluidic platform integrating single bead trapping and acoustic mixing techniques. Nanoscale 2018, 10, 20196–20206. [Google Scholar] [CrossRef]
- Essaouiba, A.; Okitsu, T.; Kinoshita, R.; Jellali, R.; Shinohara, M.; Danoy, M.; Legallais, C.; Sakai, Y.; Leclerc, E. Development of a pancreas-liver organ-on-chip coculture model for organ-to-organ interaction studies. Biochem. Eng. J. 2020, 164, 107783. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, T. Remarks of Optical Fibers and Devices for Microfluidic Sensing: Preparation and Processing. Photonic Sens. 2025, 15, 250208. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Song, B.; Zhang, C.; Wang, Q.; Gao, X.; Huang, K. Microstructured Optical Fiber Based on Surface Plasmon Resonance for Dual-Optofluidic-Channel Sensing. Plasmonics 2022, 17, 1965–1971. [Google Scholar] [CrossRef]
- Li, B.-L.; Li, D.-R.; Chen, J.-H.; Liu, Z.-Y.; Wang, G.-H.; Zhang, X.-P.; Xu, F.; Lu, Y. Hollow core micro-fiber for optical wave guiding and microfluidic manipulation. Sens. Actuators B Chem. 2018, 262, 953–957. [Google Scholar] [CrossRef]
- Wan, H.; Chen, J.; Wan, C.; Zhou, Q.; Wang, J.; Zhang, Z. Optofluidic microcapillary biosensor for label-free, low glucose concentration detection. Biomed. Opt. Express 2019, 10, 3929–3937. [Google Scholar] [CrossRef]
- Zhang, H.; Han, B.; Li, X.; Zhao, Y.; Zhang, Y.N. An Optical Fiber Optofluidic Laser Biosensor for Rapid Hemoglobin Detection Using Organic Dye. J. Light. Technol. 2024, 42, 399–405. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, T.; Yue, G.; Li, E.; Yuan, L. Optofluidic integrated in-fiber fluorescence online optical fiber sensor. Sens. Actuators B Chem. 2015, 215, 345–349. [Google Scholar] [CrossRef]
- Yang, X.; Yu, W.; Liu, Z.; Yang, J.; Zhang, Y.; Kong, D.; Long, Q.; Yuan, T.; Cao, J.; Yuan, L.; et al. Optofluidic twin-core hollow fiber interferometer for label-free sensing of the streptavidin-biotin binding. Sens. Actuators B Chem. 2018, 277, 353–359. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Gao, S.; Wang, Y.; Wang, P.; Ebendorff-Heidepriem, H.; Ruan, Y. Microfluidic raman sensing using a single ring negative curvature hollow core fiber. Biosensors 2021, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.I.; Wang, Y.; Correa, R.A.; Bang, O.; Markos, C. Low-loss micro-machining of anti-resonant hollow-core fiber with focused ion beam for optofluidic application. Opt. Mater. Express 2021, 11, 338. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhang, Y.; Zhu, Z.; Wang, R.; Li, X.; Guan, A.; Tian, F.; Teng, P.; Gao, S.; et al. Optofluidic In-Fiber Surface-Enhanced Raman Detection Based on Cloverleaf Hollow Optical Fiber. IEEE Sens. J. 2024, 25, 2746–2752. [Google Scholar] [CrossRef]
- Merdalimova, A.A.; Rudakovskaya, P.G.; Ermatov, T.I.; Smirnov, A.S.; Kosolobov, S.S.; Skibina, J.S.; Demina, P.A.; Khlebtsov, B.N.; Yashchenok, A.M.; Gorin, D.A. SERS Platform Based on Hollow-Core Microstructured Optical Fiber: Technology of UV-Mediated Gold Nanoparticle Growth. Biosensors 2022, 12, 19. [Google Scholar] [CrossRef]
- Khozeymeh, F.; Melli, F.; Capodaglio, S.; Corradini, R.; Benabid, F.; Vincetti, L.; Cucinotta, A. Hollow-Core Fiber-Based Biosensor: A Platform for Lab-in-Fiber Optical Biosensors for DNA Detection. Sensors 2022, 22, 5144. [Google Scholar] [CrossRef]
- Moeglen-Paget, B.; Perumal, J.; Humbert, G.; Olivo, M.; Dinish, U.S. Optofluidic photonic crystal fiber platform for sensitive and reliable fluorescence based biosensing. Biomed. Opt. Express 2024, 15, 4281. [Google Scholar] [CrossRef]
- Gao, R.; Lu, D.F.; Cheng, J.; Jiang, Y.; Jiang, L.; Xu, J.D.; Qi, Z.M. Fiber optofluidic biosensor for the label-free detection of DNA hybridization and methylation based on an in-line tunable mode coupler. Biosens. Bioelectron. 2016, 86, 321–329. [Google Scholar] [CrossRef]
- Testa, G.; Persichetti, G.; Bernini, R. Optofluidic biosensing: Devices, strategies, and applications. TrAC Trends Anal. Chem. 2024, 178, 117865. [Google Scholar] [CrossRef]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of glass-based microfluidic devices: A review on its fabrication and biologic applications. Mater. Des. 2023, 225, 111517. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluid. 2021, 25, 99. [Google Scholar] [CrossRef]
- Lin, L.; Chung, C.-K. PDMS Microfabrication and Design for Microfluidics and Sustainable Energy Application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Niu, P.; Jiang, J.; Liu, K.; Wang, S.; Jing, J.; Xu, T.; Wang, T.; Liu, Y.; Liu, T. Fiber-integrated WGM optofluidic chip enhanced by microwave photonic analyzer for cardiac biomarker detection with ultra-high resolution. Biosens. Bioelectron. 2022, 208, 114238. [Google Scholar] [CrossRef]
- Qu, J.-H.; Ordutowski, H.; Van Tricht, C.; Verbruggen, R.; Barcenas Gallardo, A.; Bulcaen, M.; Ciwinska, M.; Gutierrez Cisneros, C.; Devriese, C.; Guluzade, S.; et al. Point-of-care therapeutic drug monitoring of adalimumab by integrating a FO-SPR biosensor in a self-powered microfluidic cartridge. Biosens. Bioelectron. 2022, 206, 114125. [Google Scholar] [CrossRef]
- Kim, H.-M.; Jeong, D.H.; Lee, H.-Y.; Park, J.-H.; Lee, S.-K. Design and validation of fiber optic localized surface plasmon resonance sensor for thyroglobulin immunoassay with high sensitivity and rapid detection. Sci. Rep. 2021, 11, 15985. [Google Scholar] [CrossRef]
- Yin, M.; Huang, B.; Gao, S.; Zhang, A.P.; Ye, X. Optical fiber LPG biosensor integrated microfluidic chip for ultrasensitive glucose detection. Biomed. Opt. Express 2016, 7, 2067. [Google Scholar] [CrossRef]
- Hu, X.-G.; Zhao, Y.; Peng, Y.; Chen, X.-M.; Wang, L.-F.; Lin, Z.-T.; Hu, S. In-situ label-free temperature-compensated DNA hybridization detection with a fiber-optic interferometer and a fiber Bragg grating for microfluidic chip. biosens. Bioelectron. 2023, 242, 115703. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, Z.; Wu, J.; Li, S.; Li, W.; Zhang, H.; Li, X. A Raman immunosensor based on SERS and microfluidic chip for all-fiber detection of brain natriuretic peptide. Infrared Phys. Technol. 2022, 125, 104252. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, H.-J.; Park, J.-H.; Lee, S.-K. High-performance biosensor using a sandwich assay via antibody-conjugated gold nanoparticles and fiber-optic localized surface plasmon resonance. Anal. Chim. Acta 2022, 1213, 339960. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Trinh, K.T.L.; Yoon, W.J.; Lee, N.Y.; Ju, H. Integration of a microfluidic polymerase chain reaction device and surface plasmon resonance fiber sensor into an inline all-in-one platform for pathogenic bacteria detection. Sens. Actuators B Chem. 2017, 242, 1–8. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, L.; Li, S.; Qi, R.; Li, X.C.W. Highly sensitive refractive index detection based on compact HSC-SPR structure in a microfluidic Chip. Sens. Actuators A Phys. 2019, 297, 111558. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Li, C.-J.; Hsu, J.-C. Integration of Curved D-Type Optical Fiber Sensor with Microfluidic Chip. Sensors 2016, 17, 63. [Google Scholar] [CrossRef]
- Chang, T.-C.; Sun, A.Y.; Huang, Y.-C.; Wang, C.-H.; Wang, S.-C.; Chau, L.-K. Integration of Power-Free and Self-Contained Microfluidic Chip with Fiber Optic Particle Plasmon Resonance Aptasensor for Rapid Detection of SARS-CoV-2 Nucleocapsid Protein. Biosensors 2022, 12, 785. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Wang, H.; Chen, G.; Cong, M.; Song, W.; Jia, Q.; Xu, S.; Xu, W.A. A Surface-Enhanced Raman Scattering Optrode Prepared by in Situ Photoinduced Reactions and Its Application for Highly Sensitive On-Chip Detection. ACS Appl. Mater. Interfaces 2014, 6, 11706–11713. [Google Scholar] [CrossRef]
- Bo, H.; Ke, Y.; Yong, Z.; Jie, Z. Microfluidic integrated D-shaped optical fiber SERS probe with high sensitivity and ability of multi-molecule detection. Opt. Express 2023, 31, 27304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Tian, M.; Li, R.; Quan, X.; Jia, Q. A novel organic–inorganic hybrid monolithic column prepared in-situ in a microchip and its application for the determination of 2-amino-4-chlorophenol in chlorzoxazone tablets. Talanta 2013, 115, 801–805. [Google Scholar] [CrossRef]
- Mei, H.; Pan, J.; Zhang, Z.; Zhang, L.; Tong, L. Coiled Optical Nanofiber for Optofluidic Absorbance Detection. ACS Sens. 2019, 4, 2267–2271. [Google Scholar] [CrossRef]
- Waechter, H.; Bescherer, K.; Dürr, C.J.; Oleschuk, R.D.; Loock, H.-P. 405 nm Absorption Detection in Nanoliter Volumes. Anal. Chem. 2009, 81, 9048–9054. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Yang, R.; Song, D.; Long, F.; Zhu, A. Integrated multichannel all-fiber optofluidic biosensing platform for sensitive and simultaneous detection of trace analytes. Anal. Chim. Acta 2018, 1040, 112–119. [Google Scholar] [CrossRef]
- Fattah, M.A.A.; Abouali, H.; Hosseini, S.A.; Yin, J.; Khan, A.A.; Aghamohammadi, H.; Poudineh, M.; Ban, D. An Optofluidic System for Monitoring Fluorescently Activated Protein Biomarkers. Anal. Sens. 2024, 4, e202300064. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, X.; Liu, X.; Li, G.; Singh, R.; Zhang, B.; Kumar, S. Advances in Tapered Optical Fiber Sensor Structures: From Conventional to Novel and Emerging. Biosensors 2023, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Raham, H.S.; Nida, M.H. Tapered single-mode no-core optical fiber sensor coated with layer gold for hemoglobin sensing. J. Opt. 2024, 53, 4886–4892. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Q.; Chen, H.; Ling, Q.; Ren, Z.; Peng, B.; Chen, D. Ultra-high sensitive refractive index sensor based on etched SNS fiber structure and self-imaging. Opt. Commun. 2024, 570, 130893. [Google Scholar] [CrossRef]
- Son, G.; Jung, Y.; Yu, K. Tapered Optical Fiber Couplers Fabricated by Droplet-Based Chemical Etching. IEEE Photonics J. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Ascorbe, J.; Corres, J.M.; Matias, I.R.; Arregui, F.J. High sensitivity humidity sensor based on cladding-etched optical fiber and lossy mode resonances. Sens. Actuators B Chem. 2016, 233, 7–16. [Google Scholar] [CrossRef]
- Borjikhani, P.; Granpayeh, N.; Zibaii, M.I. High sensitivity tapered fiber refractive index biosensor using hollow gold nanoparticles. Sci. Rep. 2025, 15, 1458. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Gao, M.; Li, Y.; Yang, H.; Sun, C.; Guo, Q.; Yu, Y. A highly sensitive tapered fiber biosensor modified by PDMS combustion product and graphene oxide for MUC1 detection. Sens. Actuators Rep. 2025, 9, 100287. [Google Scholar] [CrossRef]
- Chu, C.; Yang, X.; Jiang, H.; Yang, X.; Ma, M.; Teng, P.; Wang, S.; Wang, R.; Wen, X.; Li, K.; et al. Parallel tapered optical fiber biosensor for highly sensitive detection of Mucin 1. IEEE Sens. J. 2025, 25, 13027–13032. [Google Scholar] [CrossRef]
- Kahlet, N.A.; Isa, N.M.; Alauddin, S.M.; Burham, N.; Saad, H. Enhancement of Sensing Performance for Alcohol in Aqueous Solution using Tapered Optical Fiber Coated with Polyaniline via Air-Brushing Technique. Int. J. Integr. Eng. 2024, 16, 280–289. [Google Scholar] [CrossRef]
- Wang, H.; Zhen, R.; Wen, Z.; Bao, X.; Cai, Y.; Gao, S. Analysis and fabrication of dual-core tapered fiber for high-sensitivity and high-accuracy multiparameter sensing. Opt. Laser Technol. 2024, 177, 111163. [Google Scholar] [CrossRef]
- Aziz, M.S.; Shamsudin, M.S.; Fahri, M.A.S.A.; Syuhada, A.; Ibrahim, R.K.R.; Bakhtiar, H.; Harun, S.W. Glucose oxidase-based enzyme immobilised on tapered optical fibre for reliability improvement in selective glucose sensing. Optik 2022, 259, 168970. [Google Scholar] [CrossRef]
- Yang, X.; Guo, J.; Yang, F.; Yang, G.; Wu, Y.; Li, Z.; Liu, Y.; Yang, X.; Yao, J. Tapered optical fiber LRSPR biosensor based on gold nanoparticle amplification for label-free BSA detection. Sens. Actuators B Chem. 2025, 426, 136986. [Google Scholar] [CrossRef]
- Xiao, P.; Sun, Z.; Huang, Y.; Lin, W.; Ge, Y.; Xiao, R.; Li, K.; Li, Z.; Lu, H.; Yang, M.; et al. Development of an optical microfiber immunosensor for prostate specific antigen analysis using a high-order-diffraction long period grating. Opt. Express 2020, 28, 15783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Singh, R.; Zhang, B.; Kumar, S.; Li, G. WaveFlex biosensor based on S-tapered and waist-expanded technique for detection of glycosylated hemoglobin. Biomed. Opt. Express 2023, 14, 6100. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.; Wei, D.; Liu, B.; Hu, Y.; Liu, J.; Wan, S.; Wu, T.; He, X.-D.; Jiang, J.; et al. Ultrahigh sensitivity fiber laser integrated biosensor for TNF-α detection in human blood sample. Sens. Actuators B Chem. 2025, 422, 136561. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, D.; Li, Z.; Xu, Z.; Ran, Y.; Guan, B. Assembly tapered fiber Bragg grating tip with gold nanostars for heat generation and gradient temperature sensing. Opt. Laser Technol. 2024, 175, 110759. [Google Scholar] [CrossRef]
- Hidayat, N.; Safwan Abd Aziz, M.; Nur, H.; Taufiq, A.; Mufti, N.; Rakhmata Mukti, R.; Bakhtiar, H. Sensitivity enhancement of gold nanospheres assisted CO2 laser tapered optical fiber for refractive index sensor. Opt. Fiber Technol. 2023, 77, 103275. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, L.; Zhu, G.; Dong, M.; Zeng, Z. ZIF-8/Lipase Coated Tapered Optical Fiber Biosensor for the Detection of Triacylglycerides. IEEE Sens. J. 2020, 20, 14173–14180. [Google Scholar] [CrossRef]
- Shaimerdenova, M.; Ayupova, T.; Ashikbayeva, Z.; Bekmurzayeva, A.; Blanc, W.; Tosi, D. Reflector-Less Shallow-Tapered Optical Fiber Biosensors for Rapid Detection of Cancer Biomarkers. J. Light. Technol. 2023, 41, 4114–4122. [Google Scholar] [CrossRef]
- Kang, X.; Wang, R.; Jiang, M.; Li, E.; Li, Y.; Wang, T.; Ren, Z. A label-free biosensor for pepsin detection based on graphene oxide functionalized micro-tapered long period fiber grating. Sens. Actuators Rep. 2023, 5, 100139. [Google Scholar] [CrossRef]
- Wang, R.; Ren, Z.; Kong, D.; Hu, B.; He, Z. Highly sensitive label-free biosensor based on graphene-oxide functionalized micro-tapered long period fiber grating. Opt. Mater. 2020, 109, 110253. [Google Scholar] [CrossRef]
- Li, Y.; Du, M.; He, S.; Wang, R.; Zhang, Z.; Wang, Q. Sensitive label-free hemoglobin detection based on polydopamine functionalized graphene oxide coated micro-tapered long-period fiber grating. Optik 2023, 275, 170626. [Google Scholar] [CrossRef]
- Kamil, Y.M.; Al-Rekabi, S.H.; Yaacob, M.H.; Syahir, A.; Chee, H.Y.; Mahdi, M.A.; Abu Bakar, M.H. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019, 9, 13483. [Google Scholar] [CrossRef]
- Zulkeflee, N.N.; Kamil, Y.M.; Mashohor, S.; Bakar, M.H.A. Functionalized cascaded tapered optical fiber sensor for simultaneous detection of dengue II E and SARS-CoV-2 S proteins. Biosens. Bioelectron. 2025, 277, 117200. [Google Scholar] [CrossRef]
- Idris, S.; Azeman, N.H.; Azmy, N.A.N.; Ratnam, C.T.; Mahdi, M.A.; Bakar, A.A.A. Gamma irradiated Py/PVA for GOx immobilization on tapered optical fiber for glucose biosensing. Sens. Actuators B Chem. 2018, 273, 1404–1412. [Google Scholar] [CrossRef]
- Gangal, A.; Choudhary, K.; Duseja, M.; Shukla, R.K.; Kumar, S. Green synthesis of Silver nanoparticles from plant oil for enzyme-functionalized optical fiber biosensor: Improved sensitivity and selectivity in ascorbic acid detection. Opt. Laser Technol. 2025, 186, 112635. [Google Scholar] [CrossRef]
- Li, M.; Yan, M.; Xu, B.; Zhao, C.; Wang, D.; Wang, Y.; Chen, H. A dual-mode optical fiber sensor for SERS and fluorescence detection in liquid. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2023, 290, 122267. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Soares, M.S.; Marques, C.; Zhang, B.; Kumar, S. Convex fiber-tapered seven core fiber-convex fiber (CTC) structure-based biosensor for creatinine detection in aquaculture. Opt. Express 2022, 30, 13898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xie, Y.; Singh, R.; Zhang, B.; Kumar, S. Smart Fiber-Optic WaveFlex Biosensor With Nano-Interface for Acrylamide Monitoring in Food Safety Applications. IEEE Sens. J. 2025, 25, 8339–8346. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, Z.; Singh, R.; Marques, C.; Wu, Q.; Kaushik, B.K.; Jha, R.; Zhang, B.; Kumar, S. Localized Plasmon-Based Multicore Fiber Biosensor for Acetylcholine Detection. IEEE Trans. Instrum. Meas. 2022, 71, 3133335. [Google Scholar] [CrossRef]
- Wang, Z.; Singh, R.; Marques, C.; Jha, R.; Zhang, B.; Kumar, S. Taper-in-taper fiber structure-based LSPR sensor for alanine aminotransferase detection. Opt. Express 2021, 29, 43793. [Google Scholar] [CrossRef]
- Zhang, W.; Singh, R.; Wang, Z.; Li, G.; Xie, Y.; Jha, R.; Marques, C.; Zhang, B.; Kumar, S. Humanoid shaped optical fiber plasmon biosensor functionalized with graphene oxide/multi-walled carbon nanotubes for histamine detection. Opt. Express 2023, 31, 11788. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Li, X.; Singh, R.; Min, R.; Zhang, B.; Kumar, S. Ultra-sensitive fiber optic LSPR biosensor with enhanced enzyme functionalization for real-time putrescine detection. Opt. Express 2025, 33, 238. [Google Scholar] [CrossRef]
- Zhu, G.; Agrawal, N.; Singh, R.; Kumar, S.; Zhang, B.; Saha, C.; Kumar, S. A novel periodically tapered structure-based gold nanoparticles and graphene oxide—Immobilized optical fiber sensor to detect ascorbic acid. Opt. Laser Technol. 2020, 127, 106156. [Google Scholar] [CrossRef]
- Mizuno, Y.; Ujihara, H.; Lee, H.; Hayashi, N.; Nakamura, K. Polymer optical fiber tapering using hot water. Appl. Phys. Express 2017, 10, 062502. [Google Scholar] [CrossRef]
- Gao, Q.; Wei, D.; Yao, Y.; Liu, B.; Liu, J.; Hu, Y.; Yang, H.; Fu, Y.; Zhang, Y.; Wan, S.; et al. Ultrahigh-Sensitivity Fiber Biosensor for C-Reactive Protein Detection in Blood Sample. IEEE Sens. J. 2025, 25, 8309–8316. [Google Scholar] [CrossRef]
- Li, X.; Singh, R.; Zhang, B.; Kumar, S.; Li, G. Ultralow-limit of detection optical fiber LSPR biosensor based on a ring laser for des-γ-carboxy prothrombin detection. Photonics Res. 2025, 13, 698. [Google Scholar] [CrossRef]
- Zhang, Y.; Singh, R.; Marques, C.; Malathi, S.; Li, Q.; Vyoma, W.; Kumar, S.; Zhang, B. Plasmon-Based Tapered-in-Tapered Fiber Structure for p-Cresol Detection: From Human Healthcare to Aquaculture Application. IEEE Sens. J. 2022, 22, 18493–18500. [Google Scholar] [CrossRef]
- Shen, C.; Huang, J.; Hu, S.; Chen, Y.; Zhang, L.; Yi, C.; Hu, X.; Chen, Y.; Chen, L.; Liu, G.; et al. Direct electronical readout of surface plasmon resonance biosensor enabled by on-fiber Graphene/PMMA photodetector. Biosens. Bioelectron. 2025, 271, 116992. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Mu, J.; Fang, W.; Tong, L. Femtoliter-scale optical nanofiber sensors. Opt. Express 2015, 23, 28408. [Google Scholar] [CrossRef]
- González-León, K.; Delgado-Macuil, R.J.; Vertti-Cervantes, B.; Muñoz-Aguirre, S.; Castillo-Mixcóatl, J.; García-Juárez, M.; Montes-Narvaez, O.; Ramírez-Sánchez, E.; Beltrán-Pérez, G. Application of support vector machine technique to optical fiber biosensors for neuroprotector (IL-10) detection in serum samples of murine model. Opt. Laser Technol. 2025, 186, 112629. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, W.; Zhang, Y.; Qiu, S.; Wang, W.; Guo, Y.; Han, Y.; Zu, L.; Jiang, L.; Yuan, J.; et al. Label-free microfiber biosensor for ultrahigh sensitivity detection of TNF-α protein. Opt. Express 2025, 33, 7546. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Chen, H.; Wang, D.N.; Wang, Y.; Zhao, C. A Microfluid Fiber Device for Trace Detection of Aggregation Induced Emission Molecules. IEEE Sens. J. 2022, 22, 5688–5694. [Google Scholar] [CrossRef]
- Sun, D.; Guo, T.; Ran, Y.; Huang, Y.; Guan, B.-O. In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 2014, 61, 541–546. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, W.; Lang, X.; Liu, X.; Singh, R.; Li, G.; Xie, Y.; Zhang, B.; Kumar, S. Development of Taper-in-Taper-Based Optical Fiber Sensors for Chemical and Biological Sensing. Photonics 2023, 10, 567. [Google Scholar] [CrossRef]
- Ma, W.; Li, G.; Li, X.; Singh, R.; Zhang, B.; Kumar, S. Signal-Enhanced Fiber-Optic LSPR Sensor With Hybrid Nanointerface for Ultrasensitive Detection of Putrescine in Low Concentrations. IEEE Sens. J. 2025, 25, 6388–6395. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Fang, W.; Tong, L.; Zhang, L. Ultra-sensitive nanofiber fluorescence detection in a microfluidic chip. Sensors 2015, 15, 4890–5898. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yang, R.; Fang, S.; Liu, Y.; Liu, J.; Xu, W.; Long, F.; Zhu, A. A novel dual-color total internal reflection fluorescence detecting platform using compact optical structure and silicon-based photodetector. Talanta 2019, 196, 78–84. [Google Scholar] [CrossRef]
- Ricciardi, A.; Crescitelli, A.; Vaiano, P.; Quero, G.; Consales, M.; Pisco, M.; Esposito, E.; Cusano, A. Lab-on-fiber technology: A new vision for chemical and biological sensing. Analyst 2015, 140, 8068–8079. [Google Scholar] [CrossRef]
- Hassan, S.U.; Tariq, A.; Noreen, A.; Donia, H.; Zaidi, S.Z.J.; Bokhari, H.; Zhang, X. Capillary-Driven Flow Microfluidics Combined with Smartphone Detection: An Emerging Tool for Point-of-Care Diagnostics. Diagnostics 2020, 10, 509. [Google Scholar] [CrossRef]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An outlook on microfluidics: The promise and the challenge. Lab. Chip. 2022, 22, 530–536. [Google Scholar] [CrossRef]
- Choi, K.; Mudrik, J.M.; Wheeler, A.R. A guiding light: Spectroscopy on digital microfluidic devices using in-plane optical fibre waveguides. Anal. Bioanal. Chem. 2015, 407, 7467–7475. [Google Scholar] [CrossRef]
- Hu, Y.; Muhammad, T.; Wu, B.; Wei, A.; Yang, X.; Chen, L. A simple on-line detection system based on fiber-optic sensing for the realtime monitoring of fixed bed adsorption processes of molecularly imprinted polymers. J. Chromatogr. A 2020, 1622, 461112. [Google Scholar] [CrossRef]
- Yuan, Y.; Jia, H.; Xu, D.; Wang, J. Novel method in emerging environmental contaminants detection: Fiber optic sensors based on microfluidic chips. Sci. Total Environ. 2023, 857, 159563. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.M.; Shi, Y.; Zhu, X.Q.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Han, X.T. Optofluidic marine phosphate detection with enhanced absorption using a Fabry–Pérot resonator. Lab. Chip. 2017, 17, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Li, Y.; Liu, S.; Li, W.; Tao, L.; Chen, C.; Qian, Z.; Yang, Y. On-chip spectroscopic monitoring of erythrocyte oxygenation under hematocrit and oxygen gradients. J. Sci. Adv. Mater. Devices 2022, 7, 100515. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Hu, S.; Peng, Y. Applications of fiber-optic biochemical sensor in microfluidic chips: A review. Biosens. Bioelectron. 2020, 166, 112447. [Google Scholar] [CrossRef]
- Hunt, H.K.; Armani, A.M. Recycling microcavity optical biosensors. Opt. Lett. 2011, 36, 1092–1094. [Google Scholar] [CrossRef]
- Leitão, C.; Pereira, S.O.; Marques, C.; Cennamo, N.; Zeni, L.; Shaimerdenova, M.; Ayupova, T.; Tosi, D. Cost-Effective Fiber Optic Solutions for Biosensing. Biosensors 2022, 12, 575. [Google Scholar] [CrossRef]
- Henriksson, A.; Neubauer, P.; Birkholz, M. Dielectrophoresis: An Approach to Increase Sensitivity, Reduce Response Time and to Suppress Nonspecific Binding in Biosensors? Biosensors 2022, 12, 784. [Google Scholar] [CrossRef]
- Casabella, S.; Scully, P.; Goddard, N.; Gardner, P. Automated analysis of single cells using Laser Tweezers Raman Spectroscopy. Analyst 2016, 141, 689–696. [Google Scholar] [CrossRef]
- Zaman, M.A.; Wu, M.; Ren, W.; Jensen, M.A.; Davis, R.W.; Hesselink, L. Spectral tweezers: Single sample spectroscopy using optoelectronic tweezers. Appl. Phys. Lett. 2024, 124, 071104. [Google Scholar] [CrossRef]
- Chen, Y.S.; Huang, C.H.; Pai, P.C.; Seo, J.; Lei, K.F. A Review on Microfluidics-Based Impedance Biosensors. Biosensors 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, B.; Zhu, Y.; Zhao, J. Microcantilever sensors for biochemical detection. J. Semicond. 2023, 44, 023105. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Wang, S.; Chu, C.; Teng, P.; Tian, F.; Zhang, Y.; Liu, Z.; Yang, X. Review of Optical Fiber Optofluidic Chemical Sensors and Biosensors. Photonic Sens. 2024, 15, 250134. [Google Scholar] [CrossRef]




| Taper Type | Cladding/Core Diameter (μm) | Taper Waist Diameter (μm) Taper Waist Length (mm) | Optical Path Setup | Fluidic Flow Setup | Refs. | |
|---|---|---|---|---|---|---|
| a | Tapered SMF | 125 μm/ 8–9 μm | 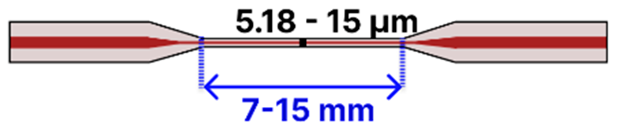 | Light Source → Tapered Fiber → OSA/Spectrometer | Drop-casting, immersion/ Microfluidic Platform | [4,79,96,99,113,114,115] |
| b | Tapered Asymmetric Doped SMF | 125 μm/ 15 μm | 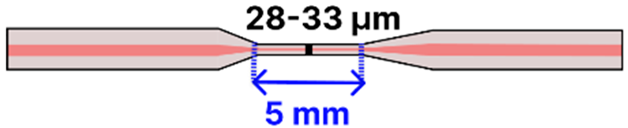 | Backscatter Reflectometer ↔ Tapered Fiber | Drop-casting, immersion | [92] |
| c | Tapered PDMS Coated SMF | 125 μm/ 9 μm |  | Light Source → Tapered Fiber → OSA | Drop-casting, immersion | [80] |
| d | Dual-Tapered SMF | 125 μm/ 8.2 μm | 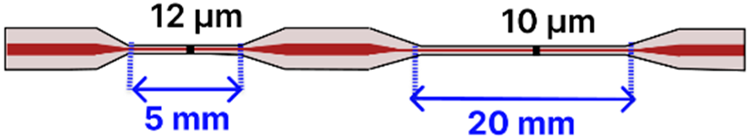 | Light Source → Tapered Fiber → OSA | Drop-casting, immersion | [97] |
| e | Tapered SMF Tip | 125 μm/ NA | 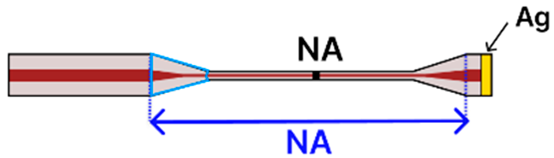 | Light Source → Tapered Fiber → OSA | Dipping, immersion | [81] |
| f | Tapered MMF | 125 μm/ 60 μm | 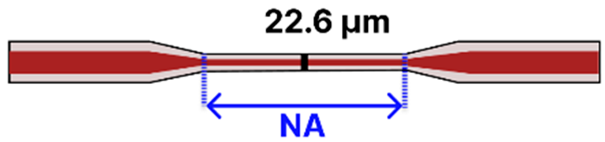 | Light Source → Tapered Fiber → Spectrometer | Drop-casting, immersion | [84] |
| g | Tapered MMF Tip | 125 μm/ 62.5–105 μm | 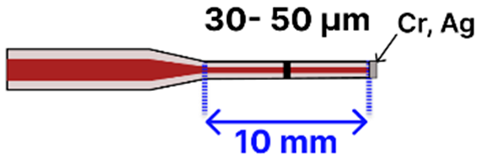 | Light Source → Tapered Fiber → Power Meter/Raman Spectroscopy/Spectrometer | Dipping, immersion | [98,100] |
| h | Tapered MMF Ball Tip | 125 μm/ 105 μm | 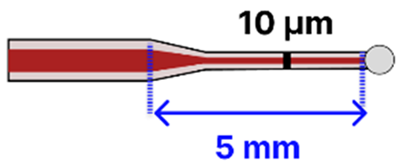 | Light Source → Tapered Fiber → Spectrometer | Microfluidic Platform | [116] |
| i | Tapered MMF Tip Grating Inscribed | 125 μm/ 62.5 μm | 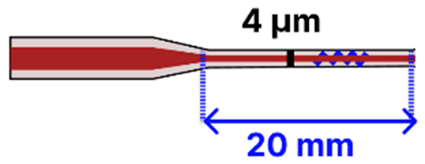 | Light Source/Spectrometer ↔ Tapered Fiber | Microfluidic Platform | [117] |
| Taper Type | Cladding/Core Diameter (μm) | Taper Waist Diameter (μm) Taper Waist Length (mm) | Optical Path Setup | Fluidic Flow Setup | Refs. | |
|---|---|---|---|---|---|---|
| a | MMF-Tapered SMF-MMF | 125 μm/ SMF: 8 μm MMF: 62.5 μm | 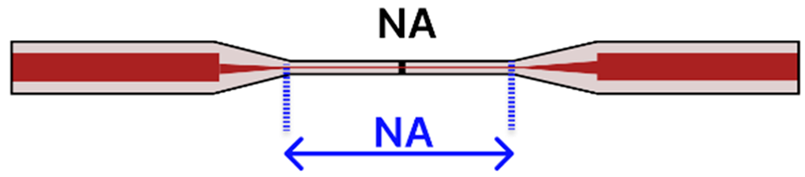 | Light Source → Tapered Fiber → Spectrometer | Microfluidic Platform | [85] |
| b | SMF—Tapered MCF—SMF | 125 μm/ SMF: 8–9 μm MCF: 6.1–7.5 μm | 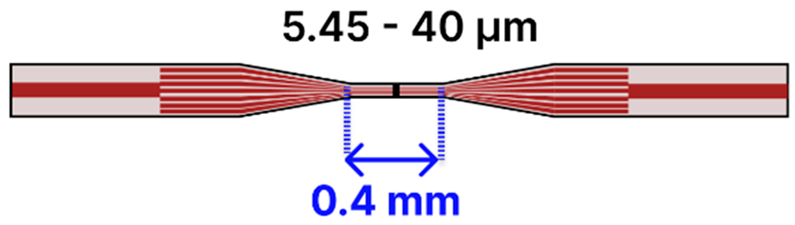 | Light Source → Tapered Fiber → OSA/Spectrometer | Drop-casting, immersion | [88,101,109] |
| c | MMF—Tapered MCF—MMF | 125 μm/ MMF: 62.5 μm MCF: 6.1 μm |  | Light Source → Tapered Fiber → Spectrometer | Drop-casting, immersion | [103,110] |
| d | MMF—3 Tapered MCF—MMF | 125 μm/ MMF: 62.5 μm MCF: 6.1 μm | 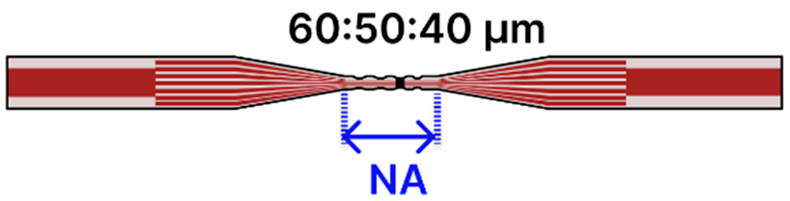 | Light Source → Tapered Fiber → OSA/Spectrometer | Drop-casting, immersion | [102] |
| e | Taper-in-Taper | 125 μm/ 9 μm | 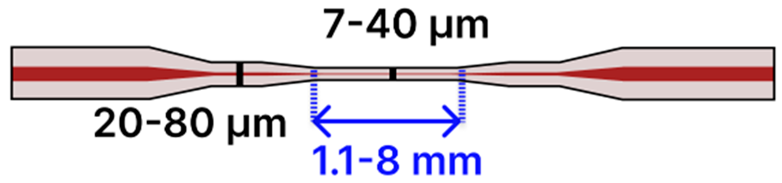 | Light Source → Tapered Fiber → OSA/Spectrometer | Drop-casting, immersion | [2,104,111,118] |
| f | SMF Tapered Humanoid | 125 μm/ 8.2 μm | 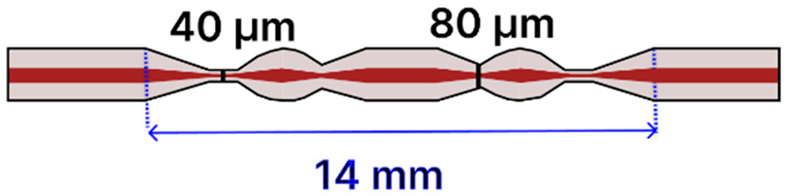 | Light Source → Tapered Fiber → Spectrometer | Drop-casting, immersion | [105] |
| g | SMF Ball: S-Shaped Tapered MMF | 125 μm/ SMF: 8.2 μm; MMF: 62.5 μm | 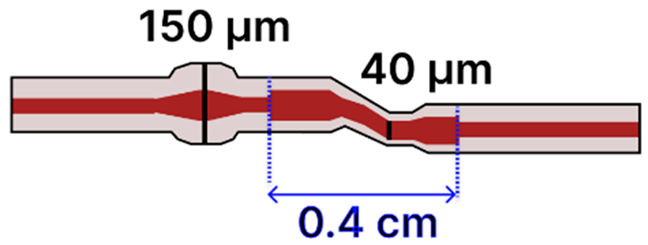 | Light Source → Tapered Fiber → Spectrometer | Drop-casting, immersion | [106,119] |
| h | Periodically Tapered SMF | 125 μm/ ~8–10 μm | 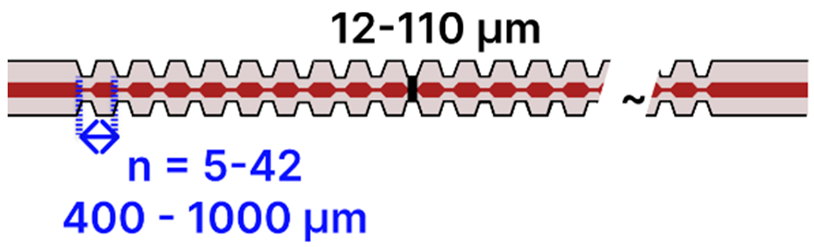 | Light Source → Tapered Fiber → OSA | Drop-casting, immersion | [86,93,95,107] |
| Fiber Type and Taper | Microfluidic Platform | Sample Volume | Analytes | LOD | Optical Setup | Key Features | Ref. |
|---|---|---|---|---|---|---|---|
| Tapered SMF, ~0.8 µm waist (flame-based tapering) | PDMS chip, 5 µm wide detection channel | ~1.0 fL | Fluorescein RI sensing | Fluorescence: 10−7 M RI sensing: 2.8 × 10−4 RIU | Fluorescence: 473 nm laser → Tapered SMF → CCD microscope RI sensing: Broadband unpolarized tungsten-halogen lamp → Tapered SMF → Spectrometer | Femtoliter detection, fast response (~600 ms) | [113] |
| Tapered MMF Tip (125 µm/62.5 µm), ~4 µm waist (flame-based tapering + Bragg grating) | PDMS chip, Width 200 µm × Height 150 µm microchannel | ~50 µL | Target DNA (hybridization) | 0.5 µM | Broadband light source/Spectrometer ↔ Tapered MMF Tip | Bragg grating, label-free DNA sensing, and partial reusability | [117] |
| Tapered MMF Ball Tip (125 µm/105 µm), ~10 µm waist (plasma-arc-based tapering) | Teflon AF2400 capillary (229 µm ID) | 0.649 µL | TPE-COOH | 0.316 µM | UV LED (340 nm)/CCD-type spectrometer ↔ Tapered MMF ball tip | Ball tip fiber, laser-ablated flow port, and optimized analyte delivery | [116] |
| Tapered SMF, ~7.2 µm waist (flame-based tapering) | PDMS chip, Width 125 µm × Height 150 µm × Length 5 cm | ~500 nL | Rhodamine 6G, QD-Streptavidin | 100 pM (R6G), 10 pM (QD-Strep) | 532 nm laser/Spectrometer ↔ Tapered SMF | Soft lithography, controlled flow, and minimized nonspecific binding | [120] |
| Tapered MMF, 200 µm waist (HF etching) | Custom microfluidic cell (35 mm × 800 µm × 800 µm) | N/A | Cy5.5, Pacific Blue | 2 aM (Cy5.5), 20 aM (PB) | Laser diode (405 nm/635nm) → Tapered MMF → Silicon-based photodetector | Dual-color, fiber optical switch, and low-cost SOP-1000 photodetector | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lako, A.; Sypabekova, M. Tapered Optical Fiber Optofluidics: Bridging In-Fiber and Outside-Fiber Architectures Toward Autonomous Lab-on-Fiber Biosensing. Sensors 2025, 25, 5229. https://doi.org/10.3390/s25175229
Lako A, Sypabekova M. Tapered Optical Fiber Optofluidics: Bridging In-Fiber and Outside-Fiber Architectures Toward Autonomous Lab-on-Fiber Biosensing. Sensors. 2025; 25(17):5229. https://doi.org/10.3390/s25175229
Chicago/Turabian StyleLako, Alba, and Marzhan Sypabekova. 2025. "Tapered Optical Fiber Optofluidics: Bridging In-Fiber and Outside-Fiber Architectures Toward Autonomous Lab-on-Fiber Biosensing" Sensors 25, no. 17: 5229. https://doi.org/10.3390/s25175229
APA StyleLako, A., & Sypabekova, M. (2025). Tapered Optical Fiber Optofluidics: Bridging In-Fiber and Outside-Fiber Architectures Toward Autonomous Lab-on-Fiber Biosensing. Sensors, 25(17), 5229. https://doi.org/10.3390/s25175229





