Polyimide-Based Flexible Microelectrode Array for Non-Invasive Transcorneal Electrical Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
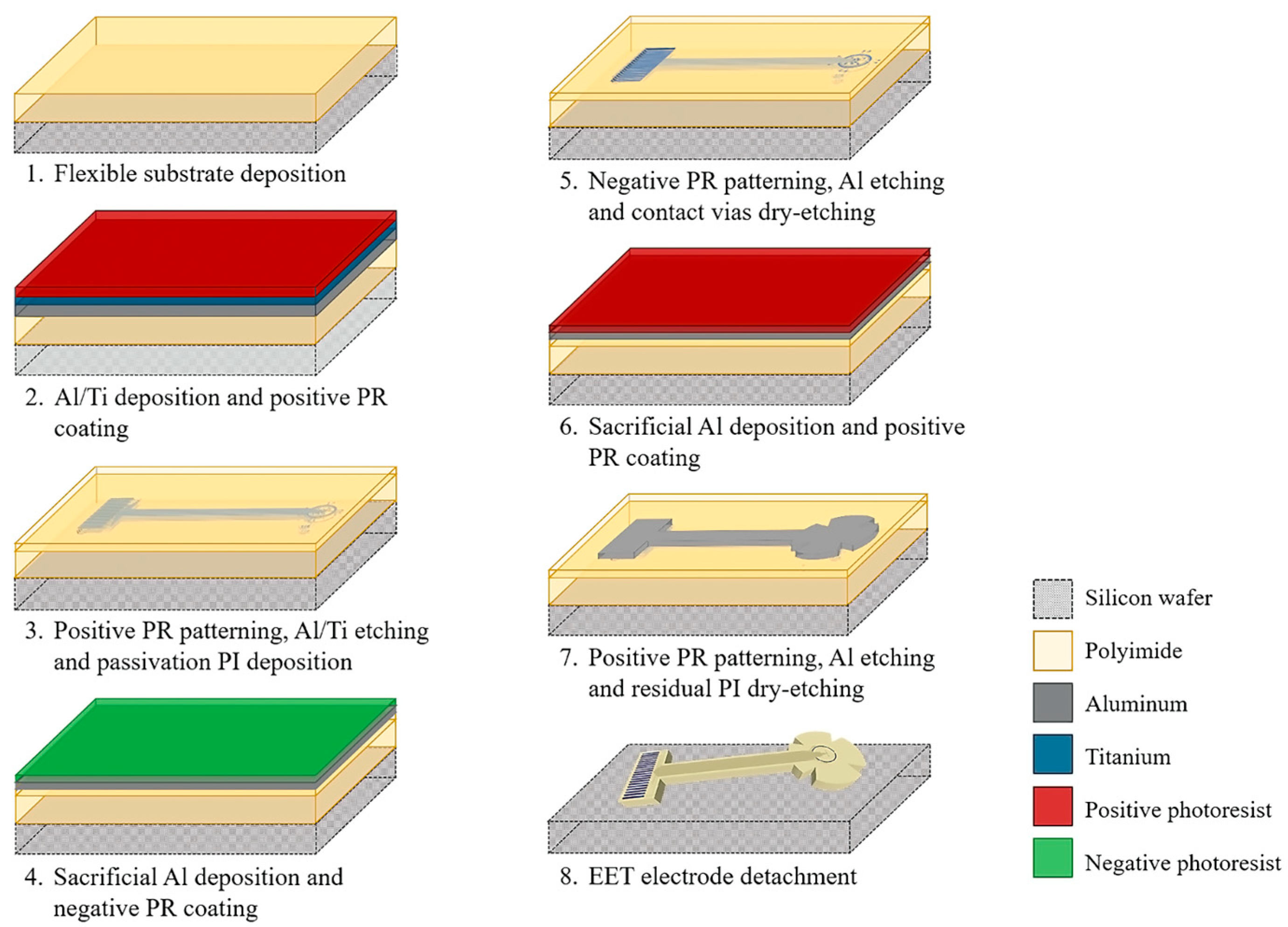
2.2. Fabrication Process
2.3. Electrode Characterization
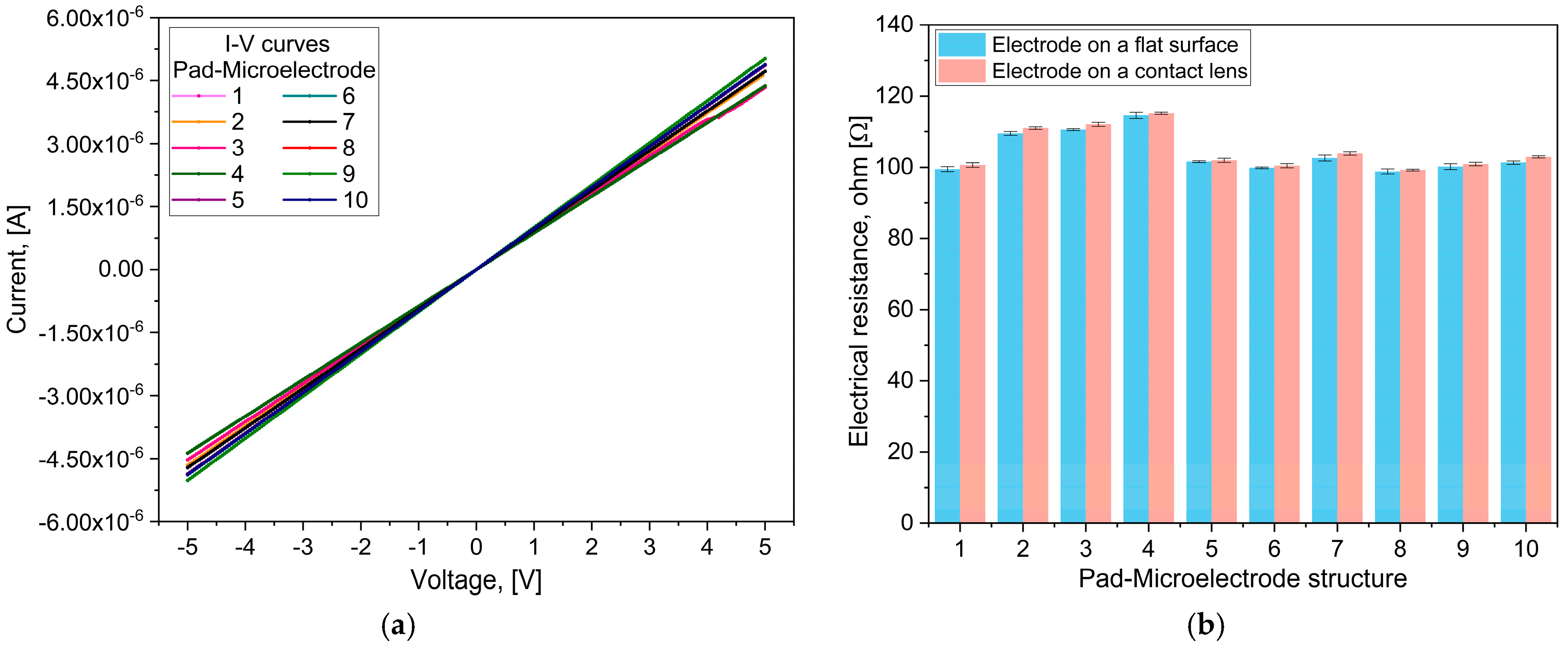
2.3.1. Electrical Testing
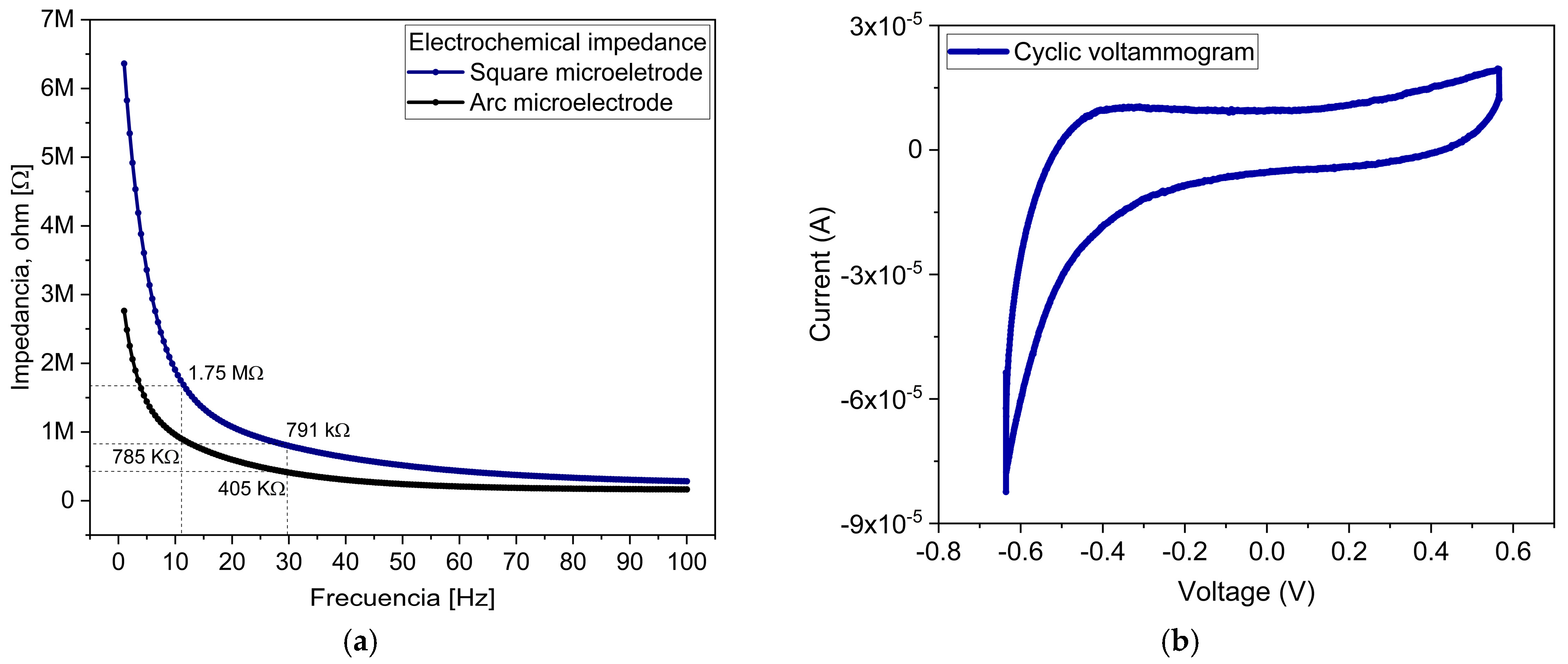
2.3.2. Electrochemical Characterization
2.3.3. Electrical Signal Transmission
2.3.4. Transmittance Spectra Measurement
3. Results and Discussion
3.1. Planar Electrode Array Fabrication
3.2. Electrical Testing
3.3. Electrochemical Characterization
3.4. Electrical Signals Transmission
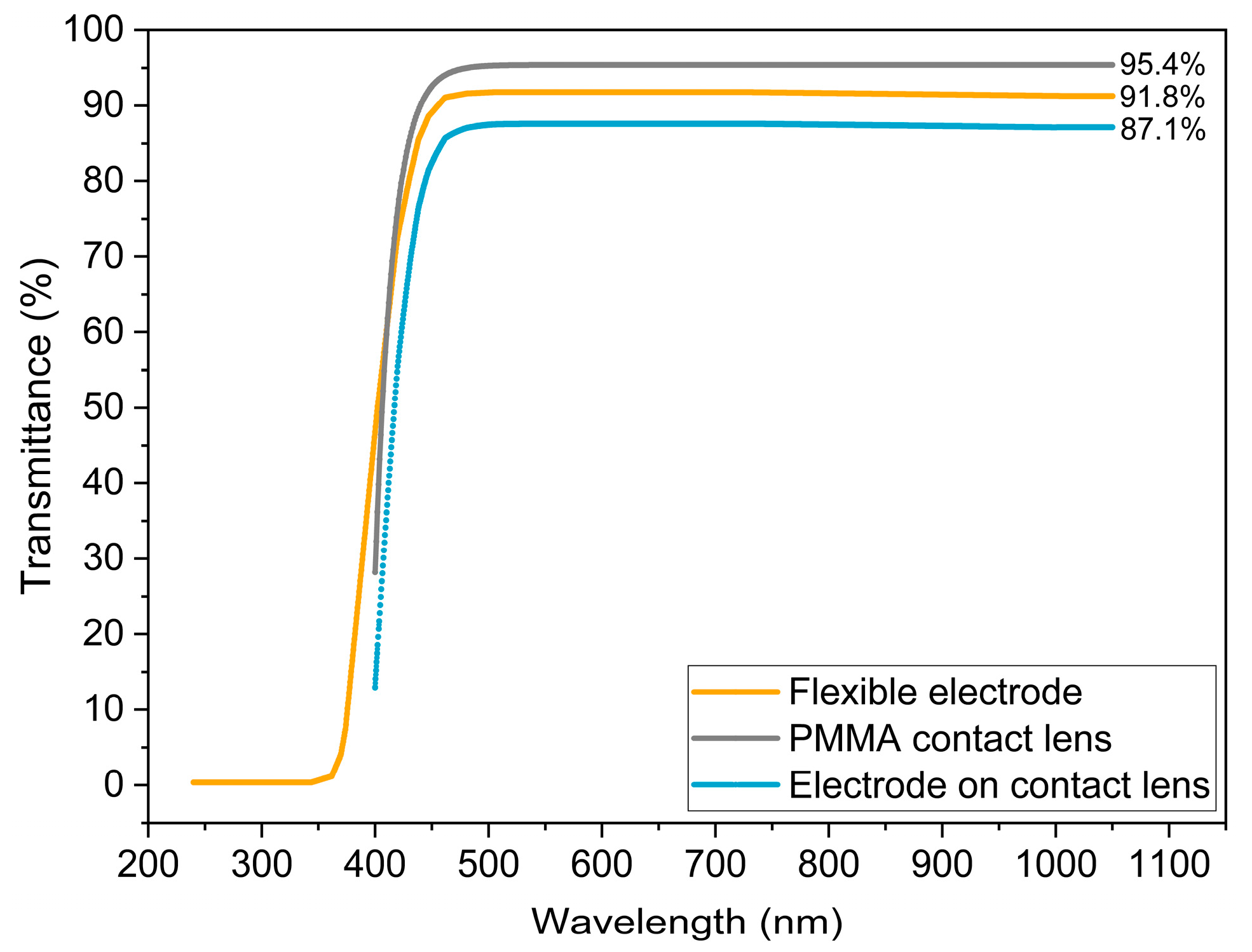
3.5. Transmittance Spectra Measure
3.6. Comparison Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TES | Transcorneal electrical stimulation |
| Al | Aluminum |
| Ti | Titanium |
| PI | Polyimide |
| rpm | revolutions per minute |
| ERG | Electroretinogram |
| RDDs | Retinal degenerative diseases |
| RPE | Retinal pigment epithelium |
| AMD | Age-related macular degeneration |
| DR | Diabetic retinopathy |
| RP | Retinitis pigmentosa |
| Anti-VEGF | Anti-vascular endothelial growth factor |
| ES | Electrical stimulation |
| IGF-1 | Insulin-like growth factor 1 |
| BDNF | Brain-derived neurotrophic factor |
| CNTF | Ciliary neurotrophic factor |
| DTL | Dawson–Trick–Litzkow |
| RIE | Reactive Ion Etch |
| PR | Photoresist |
| DI | Deionized water |
| HF | Hydrofluoric acid |
| HNO3 | Nitric acid |
| EIS | Electrochemical impedance spectroscopy |
| CV | Cyclic voltammetry |
| CE | Counter electrode |
| Pt | Platinum |
| Ag | Silver |
| AgCl | Silver chloride |
| RE | Reference electrode |
| WE | Working electrode |
| PBS | Phosphate-buffered saline solution |
| PMMA | Polymethyl methacrylate |
| Au | Gold |
| TiN | Titanium nitride |
| CSC | Charge storage capacity |
| Cr | Chrome |
References
- Ruiz, J.; Arias, L. Manual de la Retina SERV, 2nd ed.; Elsevier: Barcelona, Spain, 2019. [Google Scholar]
- Yoon, E.; Koo, B.; Wong, J.; Elyahoodayan, S.; Weiland, J.D.; Lee, C.D.; Meng, E. An implantable microelectrode array for chronic in vivo epiretinal stimulation of the rat retina. J. Micromech. Microeng. 2020, 30, 124001. [Google Scholar] [CrossRef]
- Holan, V.; Palacka, K.; Hermankova, B. Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials. Cells 2021, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, N.K. The Role of Inflammation in Retinal Neurodegeneration and Degenerative Diseases. Int. J. Mol. Sci. 2022, 23, 386. [Google Scholar] [CrossRef]
- Yue, L.; Weiland, J.D.; Roska, B.; Humayun, M.S. Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog. Retin. Eye Res. 2016, 53, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, S.; Ye, L.; Chen, C.; Liu, S.; Yang, H.; Zhuang, P.; Liu, Z.; Jiang, H.; Han, J.; et al. Visual impairment and blindness caused by retinal diseases: A nationwide register-based study. J. Glob. Health 2023, 13, 04126. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, N.K. Inflammation and retinal degenerative diseases. Neural Regen. Res. 2023, 18, 513–518. [Google Scholar] [CrossRef]
- Maurya, M.; Bora, K.; Blomfield, A.K.; Pavlovich, M.C.; Huang, S.; Liu, C.-H.; Chen, J. Oxidative stress in retinal pigment epithelium degeneration: From pathogenesis to therapeutic targets in dry age-related macular degeneration. Neural Regen. Res. 2023, 18, 2173–2181. [Google Scholar] [CrossRef]
- O’Neal, T.B.; Luther, E.E. Retinitis pigmentosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gonzalez-Cortes, J.H.; Martinez-Pacheco, V.A.; Gonzalez-Cantu, J.E.; Bilgic, A.; de Ribot, F.M.; Sudhalkar, A.; Mohamed-Hamsho, J.; Kodjikian, L.; Mathis, T. Current Treatments and Innovations in Diabetic Retinopathy and Diabetic Macular Edema. Pharmaceutics 2022, 15, 122. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, M.; Xiong, G.; Han, X.; Lee, V.W.H.; So, K.-F.; Chiu, K.; Xu, Y. Trans-Sclera Electrical Stimulation Improves Retinal Function in a Mouse Model of Retinitis Pigmentosa. Life 2022, 12, 1917. [Google Scholar] [CrossRef]
- Hernández-Sebastián, N.; Carpio-Verdín, V.M.; Ambriz-Vargas, F.; Morales-Morales, F.; Benítez-Lara, A.; Buenrostro-Jáuregui, M.H.; Bojorges-Valdez, E.; Barrientos-García, B. Fabrication and Characterization of a Flexible Thin-Film-Based Array of Microelectrodes for Corneal Electrical Stimulation. Micromachines 2023, 14, 1999. [Google Scholar] [CrossRef]
- Chang, K.; Enayati, S.; Cho, K.S.; Utheim, T.P.; Chen, D.F. Non-invasive electrical stimulation as a potential treatment for retinal degenerative diseases. Neural Regen. Res. 2021, 16, 1558–1559. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, T.; Liu, B.; Wang, L.Q.; Peng, G.H.; Qin, L.M.; Yan, Z.J.; Huang, Y.F. The transcorneal electrical stimulation as a novel therapeutic strategy against retinal and optic neuropathy: A review of experimental and clinical trials. Int. J. Ophthalmol. 2016, 9, 914–919. [Google Scholar] [CrossRef]
- Yang, M.; Lennikov, A.; Chang, K.; Ashok, A.; Lee, C.; Cho, K.; Utheim, T.P.; Dartt, D.A.; Chen, D.F. Transcorneal but not transpalpebral electrical stimulation disrupts mucin homeostasis of the ocular surface. BMC Ophthalmol. 2022, 22, 490. [Google Scholar] [CrossRef]
- Agadagba, S.K.; Lim, L.W.; Chan, L.L.H. Advances in transcorneal electrical stimulation: From the eye to the brain. Front. Cell. Neurosci. 2023, 17, 1134857. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Kwon, J.; Kim, D.H.; Im, C. Multi-channel transorbital electrical stimulation for effective stimulation of posterior retina. Sci. Rep. 2021, 11, 9745. [Google Scholar] [CrossRef]
- Liu, J.; Ma, A.K.; So, K.F.; Lee, V.W.; Chiu, K. Mechanisms of electrical stimulation in eye diseases: A narrative review. Adv. Ophthalmol. Pract. Res. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Morimoto, T.; Miyoshi, T.; Matsuda, S.; Tano, Y.; Fujikado, T.; Fukuda, Y. Transcorneal electrical stimulation rescues axotomized retinal ganglion cells by activating endogenous retinal IGF-1 system. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Miura, G.; Fujiwara, T.; Iwase, T.; Ozawa, Y.; Shiko, Y.; Kawasaki, Y.; Yamamoto, S. Exploratory clinical trial to evaluate the efficacy and safety of transdermal electrical stimulation in patients with central retinal artery occlusion. PLoS ONE 2023, 18, e0282003. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, W.; Liang, L.; Li, Y.; Xu, K.; Li, X.; Huang, Z.; Jin, Y. Noninvasive electrical stimulation as a neuroprotective strategy in retinal diseases: A systematic review of preclinical studies. J. Transl. Med. 2024, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Kahle, N.; Peters, T.; Braun, A.; Franklin, J.; Michalik, C.; Gekeler, F.; Wilhelm, B. Transkorneale Elektrostimulation bei Retinitis pigmentosa: Prüfplan einer multizentrischen, prospektiven, randomisierten, kontrollierten und doppelblinden Studie im Auftrag des Gemeinsamen Bundesausschusses (G-BA-Erprobungsrichtlinie). Ophthalmologe 2021, 118, 512–516. [Google Scholar] [CrossRef]
- Jolly, J.K.; Wagner, S.K.; Martus, P.; MacLaren, R.E.; Wilhelm, B. Transcorneal electrical stimulation for the treatment of retinitis pigmentosa: A multicenter safety study of the OkuStim® system (TESOLA-Study). Ophthalmic Res. 2020, 63, 234–243. [Google Scholar] [CrossRef]
- Della Volpe-Waizel, M.; Zuche, H.C.; Müller, U.; Rickmann, A.; Scholl, H.P.N.; Todorova, M.G. Metabolic monitoring of transcorneal electrical stimulation in retinitis pigmentosa. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Schatz, A.; Pach, J.; Gosheva, M.; Naycheva, L.; Willmann, G.; Wilhelm, B.; Gekeler, F. Transcorneal electrical stimulation for patients with retinitis pigmentosa: A prospective, randomized, sham-controlled follow-up study over 1 year. Investig. Ophthalmol. Vis. Sci. 2017, 58, 257–269. [Google Scholar] [CrossRef]
- Naycheva, L.; Gosheva, M.; Pach, J.; Schatz, A.; Zrenner, E.; Gekeler, F. Identifying High and Low Responders to Transcorneal Electrical Stimulation Treatment for Patients with Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2018, 56, 3804. [Google Scholar]
- Xie, J.; Wang, G.-J.; Yow, L.; J Cela, C.; Humayun, M.S.; Weiland, J.D.; Lazzi, G.; Jadvar, H. Modeling and percept of transcorneal electrical stimulation in humans. IEEE Trans. Biomed. Eng. 2011, 58, 1932–1939. [Google Scholar] [CrossRef]
- Yoo, Y.S.; Park, S.; Eun, P.; Park, Y.M.; Lim, D.H.; Chung, T.Y. Corneal Neuro-Regenerative Effect of Transcutaneous Electrical Stimulation in Rabbit Lamellar Keratectomy Model. Transl. Vis. Sci. Technol. 2022, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.-N.; Acar, U.; Sobacı, G.; Göksülük, D. Outcomes of transcorneal electrical stimulation therapy in the early stages of retinitis pigmentosa. Turk. J. Med. Sci. 2022, 52, 741–746. [Google Scholar] [CrossRef]
- Dizdar, Y.D.; Sevik, M.O.; Şahin, Ö. Transcorneal electrical stimulation therapy may have a stabilization effect on multifocal electroretinography for patients with retinitis pigmentosa. Retina 2022, 42, 923–933. [Google Scholar] [CrossRef]
- Stett, A.; Schatz, A.; Gekeler, F.; Franklin, J. Transcorneal electrical stimulation dose-dependently slows the visual field loss in retinitis pigmentosa. Transl. Vis. Sci. Technol. 2023, 12, 29. [Google Scholar] [CrossRef]
- Tew, B.Y.; Gooden, G.C.; Lo, P.A.; Pollalis, D.; Ebright, B.; Kalfa, A.J. Transcorneal electrical stimulation restores DNA methylation changes in retinal degeneration. Front. Mol. Neurosci. 2024, 17, 1484964. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Paknahad, J.; Pollalis, D.; Kosta, P.; Thomas, B.; Tew, B.Y. An extraocular electrical stimulation approach to slow down the progression of retinal degeneration in an animal model. Sci. Rep. 2023, 13, 15924. [Google Scholar] [CrossRef]
- Cui, H.; Xie, X.; Xu, S.; Chan, L.-L.; Hu, Y. Electrochemical characteristics of microelectrode designed for electrical stimulation. Biomed. Eng. OnLine 2019, 18, 86. [Google Scholar] [CrossRef]
- Trada, H.-V.; Vendra, V.; Tinney, J.-P.; Yuan, F.; Jackson, D.-J.; Walsh, K.-M.; Keller, B.-B. Implantable thin-film porous microelectrode array (P-MEA) for electrical stimulation of engineered cardiac tissues. BioChip J. 2015, 9, 85–94. [Google Scholar] [CrossRef]
- Rodrigues, F.; Ribeiro, J.-F.; Anacleto, P.-A.; Fouchard, A.; David, O.; Sarro, P.-M.; Mendes, P.-M. Fabrication and characterization of polyimide-based ‘smooth’ titanium nitride microelectrode arrays for neural stimulation and recording. J. Neural Eng. 2019, 17, 016010. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, T.; Cai, Z.; Cao, Y.; Yang, H.; Duan, Y.-Y. Anodically electrodeposited iridium oxide films microelectrodes for neural microstimulation and recording. Sens. Actuators B Chem. 2009, 137, 334–339. [Google Scholar] [CrossRef]
- Li, T.; Sun, B.; Xia, K.; Zeng, Q.; Wu, T.; Humayun, M.S. Design and fabrication of a high-density flexible microelectrode array. In Proceedings of the 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017; pp. 299–302. [Google Scholar] [CrossRef]
- Jiang, X.; Sui, X.; Lu, Y.; Yan, Y.; Zhou, C.; Li, L.; Ren, Q.; Chai, X. In Vitro and In Vivo evaluation of a photosensitive polyimide thin-film microelectrode array suitable for epiretinal stimulation. J. Neuroeng. Rehabil. 2013, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-J.; Kim, J.-H.; Yang, W.-J.; Han, D.-J.; Park, J.; Park, D.-W. Parylene-Based Flexible Microelectrode Arrays for the Electrical Recording of Muscles and the Effect of Electrode Size. Appl. Sci. 2020, 10, 7364. [Google Scholar] [CrossRef]
- Biswas, S.; Sikdar, D.; Das, D.; Mahadevappa, M.; Das, S. PDMS based multielectrode arrays for superior In-Vitro retinal stimulation and recording. Biomed. Microdevices 2017, 19, 75. [Google Scholar] [CrossRef]
- Bard, A.-J.; Faulkner, L.R.; White, H.-S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Song, X.; Guo, T.; Ma, S.; Zhou, F.; Tian, J.; Liu, Z.; Li, L. Spatially selective retinal ganglion cell activation using low invasive extraocular temporal interference stimulation. Int. J. Neural Syst. 2025, 35, 2450066. [Google Scholar] [CrossRef]
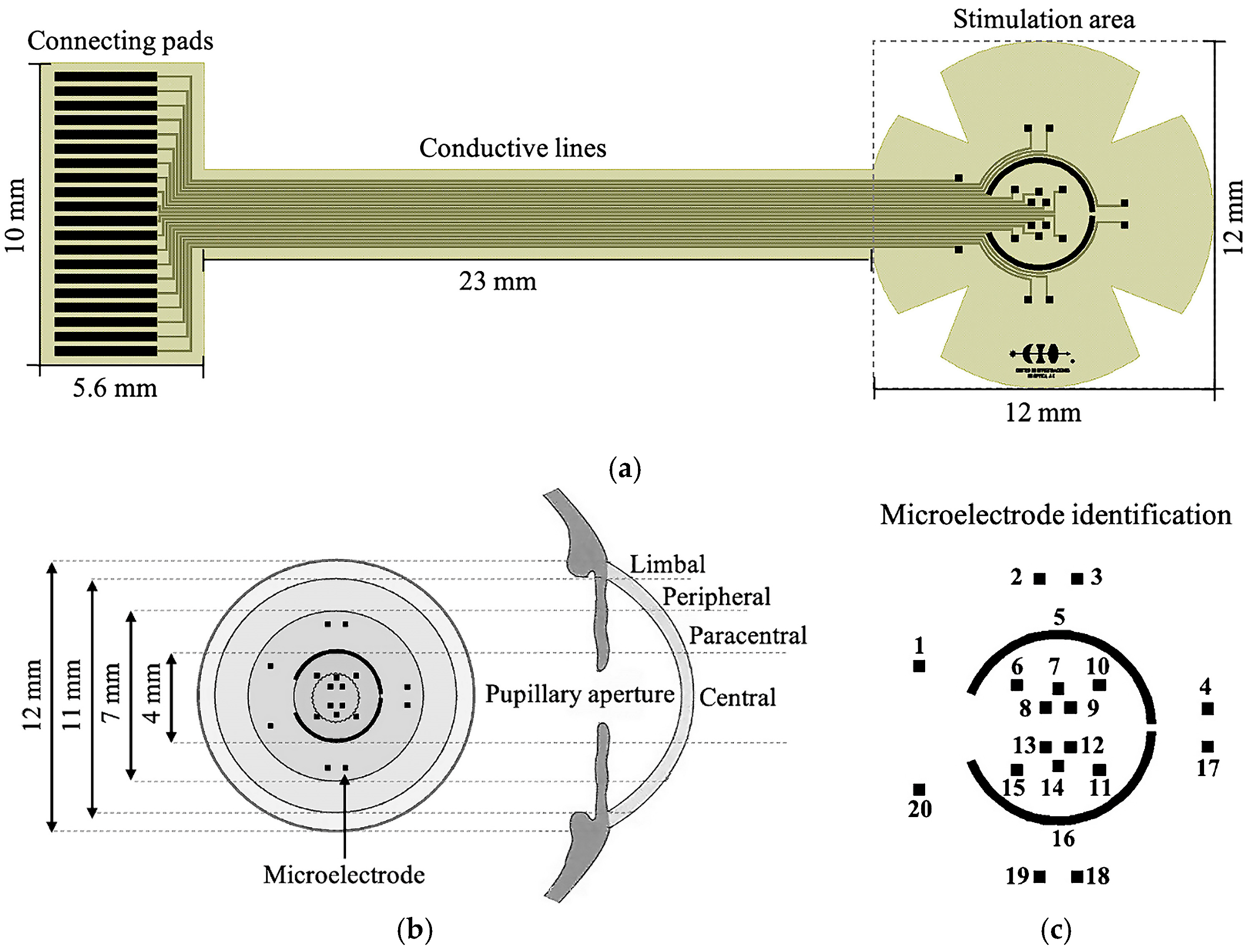




| Reference | Electrode Type | Application | TES Parameters | Fabrication Technology | Stimulation Corneal Regions |
|---|---|---|---|---|---|
| This work | Microelectrode array | Selective TES | 11 Hz, 10 ms, and 500 µA | MEMS and flexible electronics | Central and paracentral |
| [22,23] | DTL | TES | Bipolar rectangular pulses, 5 ms, 20 Hz, and 600 µA | Conventional | Inferior |
| [34] | DTL | TES | Biphasic rectangular pulses, 1 ms, 20 Hz, and 200 to 400 µA | Conventional | Inferior |
| [35] | DTL | TES | 1 ms, 20 Hz, and 0.1–1 mA | Conventional | Inferior |
| [36] | ERG-JET | TES | 6 Hz, 20–150 µA, and 10 ms | Conventional | Peripheral |
| [37] | ERG-JET | TES | Biphasic stimulus pulses, 6 Hz, 100–200 µA, and 10 ms | Conventional | Peripheral |
| [43] | Microelectrode array | Selective TES | Sinusoidal signal, 1–8 kHz and 1–100 mA | Numerical simulation | Several regions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpio-Verdín, V.M.; Hernández-Sebastián, N.; Barrientos-García, B.; Solis-Ortiz, S.; Bojorges-Valdez, E.R.; López-Huerta, F.; Mares-Castro, C.I.; Calleja-Arriaga, W. Polyimide-Based Flexible Microelectrode Array for Non-Invasive Transcorneal Electrical Stimulation. Sensors 2025, 25, 5198. https://doi.org/10.3390/s25165198
Carpio-Verdín VM, Hernández-Sebastián N, Barrientos-García B, Solis-Ortiz S, Bojorges-Valdez ER, López-Huerta F, Mares-Castro CI, Calleja-Arriaga W. Polyimide-Based Flexible Microelectrode Array for Non-Invasive Transcorneal Electrical Stimulation. Sensors. 2025; 25(16):5198. https://doi.org/10.3390/s25165198
Chicago/Turabian StyleCarpio-Verdín, Víctor Manuel, Natiely Hernández-Sebastián, Bernardino Barrientos-García, Silvia Solis-Ortiz, Erik R. Bojorges-Valdez, Francisco López-Huerta, Carlos Ismael Mares-Castro, and Wilfrido Calleja-Arriaga. 2025. "Polyimide-Based Flexible Microelectrode Array for Non-Invasive Transcorneal Electrical Stimulation" Sensors 25, no. 16: 5198. https://doi.org/10.3390/s25165198
APA StyleCarpio-Verdín, V. M., Hernández-Sebastián, N., Barrientos-García, B., Solis-Ortiz, S., Bojorges-Valdez, E. R., López-Huerta, F., Mares-Castro, C. I., & Calleja-Arriaga, W. (2025). Polyimide-Based Flexible Microelectrode Array for Non-Invasive Transcorneal Electrical Stimulation. Sensors, 25(16), 5198. https://doi.org/10.3390/s25165198







