Secure Integration of Sensor Networks and Distributed Web Systems for Electronic Health Records and Custom CRM
Abstract
1. Introduction
2. Related Work
Bibliometric Landscape of Research Actors and Geographical Distribution
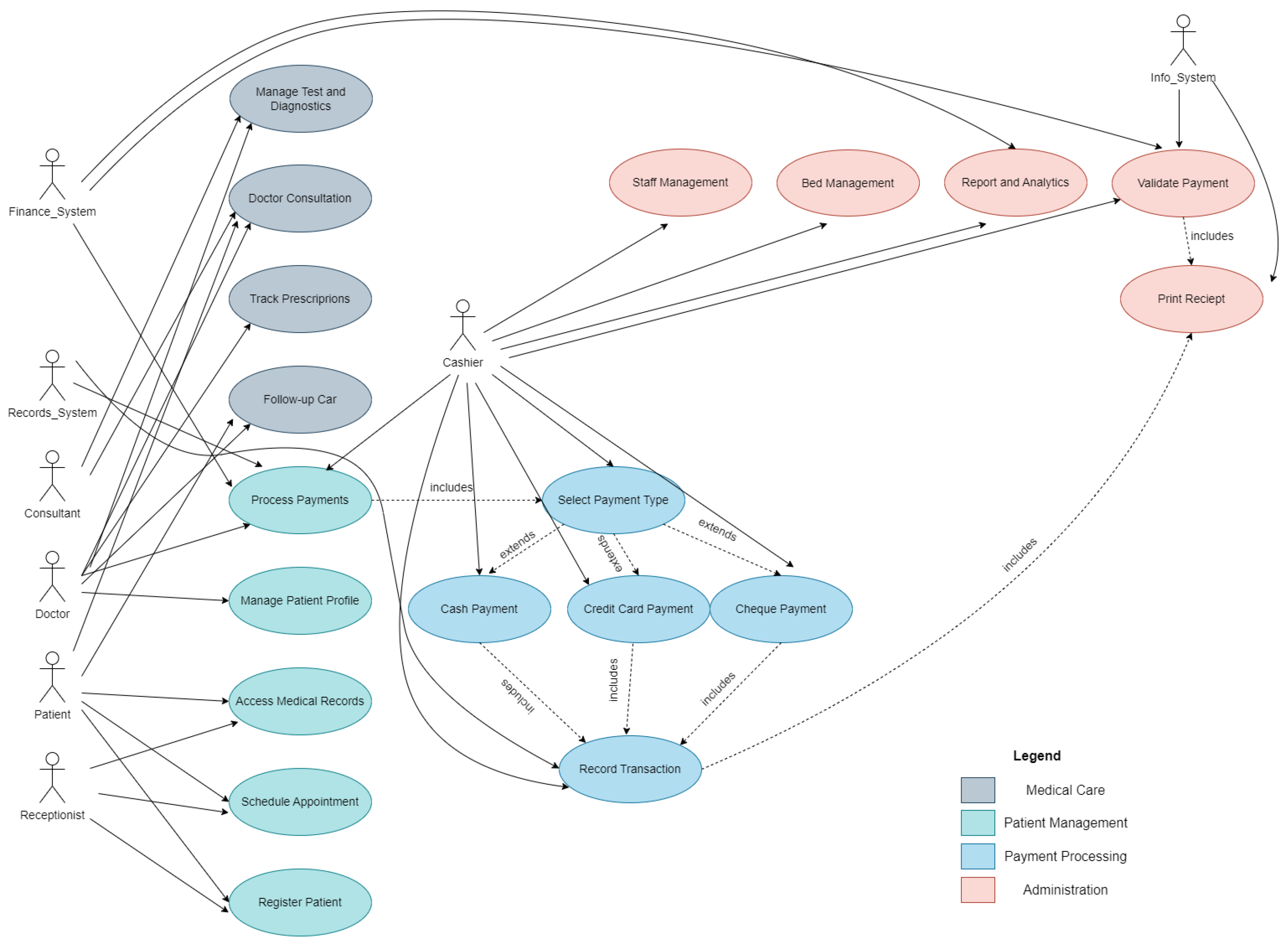
3. Materials and Methods
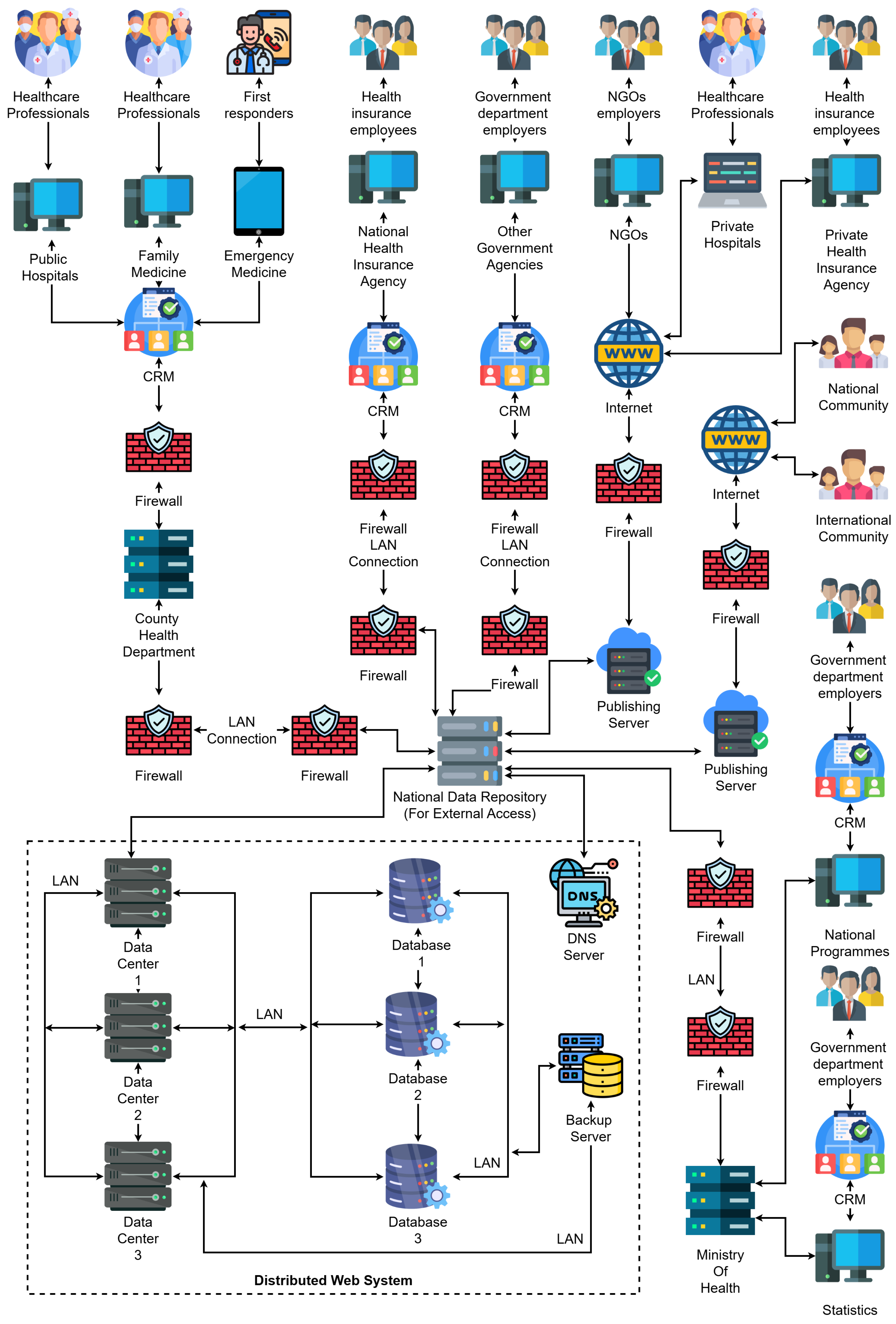
3.1. System Architecture Overview
- Stakeholder Interaction Layer—At the top of the diagram, we identify the major contributors to healthcare information flows, namely, public and private hospitals, family medicine units, emergency responders, health insurance employees, government departments, and NGOs. These actors are equipped with digital terminals and systems connected to local CRM platforms, enabling the digitalization of interactions with patients and healthcare service management.
- Institutional CRM layer—Each healthcare-related institution (e.g., hospitals, agencies) operates its own CRM node, which acts as an intelligent interface for managing interactions, patient records, appointment scheduling, billing, and communication. These CRM nodes are protected by dedicated firewalls and are connected through secure LANs to the back-end data infrastructure. Notably, the architecture supports both public-sector CRMs (e.g., county health departments, national health insurance agencies) and private-sector CRMs (e.g., private hospitals, private insurance).
- Distributed Web Infrastructure Layer—The bottom part of the diagram showcases the distributed data backbone. This layer includes:
- Multiple data centers, each with its own processing and storage capacities, redundantly connected to support fail-over and load balancing.
- Replicated databases that synchronize EHRs across institutions and regions.
- A DNS server, which resolves services and institutional addresses.
- Backup servers that ensure fault-tolerant storage of health records and institutional metadata.
- Publishing servers, which allow external access to anonymized or publicly relevant health data for national and international reporting (e.g., academic research, pandemic tracking).
- Integration with international communities through open publishing platforms.
- Data feedback to national programmes and ministries of health for policy-making, budgeting, and forecasting.
- Real-time synchronization and analytics between CRM nodes and distributed databases.
3.2. Data Synchronization and Communication Flow
3.3. Technological Stack and Deployment Model
3.4. Simulation Environment and Experimental Setup
| Algorithm 1: Monitoring and Collection |
| Input: Docker metrics, service logs |
| Output: Metrics in Prometheus-compatible format |
| While simulation is running do |
| For each container in service_cluster do |
| Collect CPU usage, memory usage, network I/O |
| Export metrics to Prometheus endpoint |
| End For |
| wait monitoring_interval (e.g., 10s) |
| End While |
| Algorithm 2: Input Data CRM |
| Input: Incoming JSON messages from Gateway |
| Output: Normalized values stored in patient record |
| For each received message do |
| Parse sensor values and timestamp |
| Validate value ranges and data format |
| If values within clinical thresholds then |
| Store in CRM database with status “normal” |
| Else |
| Tag as “alert” and trigger notification to medical staff |
| End If |
| End For |
4. Results and Discussion
Limitations of the Study
5. Conclusions and Future Work
- Integration of AI-driven decision support systems for early diagnosis, leveraging generative models and federated learning for privacy-preserving medical analytics.
- Expansion of real-time analytics modules for anomaly detection and adaptive resource allocation using stream processing frameworks.
- Deployment of blockchain-based audit trails to strengthen data immutability, traceability, and trust in inter-institutional collaborations.
- Evaluation of interoperability with legacy systems in low-resource environments to ensure global applicability.
- Incorporation of sensor fusion algorithms and contextual awareness to improve monitoring accuracy in remote and mobile healthcare scenarios. The evaluations planned in future work will focus on three core dimensions. First, in terms of scalability, the behavior of the system will be analyzed under increasing workloads by simulating up to ten distributed CRM instances and 500 concurrent patients. We expect to observe stable response times and efficient load distribution, which would validate the scalability of the proposed deployment model [37,43].
- −
- From the sensor to the gateway node.
- −
- From the CRM to an EHR module within the institution.
- −
- Inter-institutional data synchronization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathore, N.; Kumari, A.; Patel, M. Synergy of AI and Blockchain to Secure Electronic Healthcare Records. Secur. Priv. 2025, 8, e463. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kapse, M. Transitioning from CRM to Social CRM in the Healthcare Industry. In Social Media Marketing; Springer: Berlin/Heidelberg, Germany, 2025; pp. 171–184. [Google Scholar]
- Sharma, V.K.; Sharma, V.; Kumar, D. Customer relationship management in healthcare: Strategies for adoption in a public health system. J. Market. Theory Pract. 2024, 1–26. [Google Scholar] [CrossRef]
- Hung, S.Y.; Hung, W.H.; Tsai, C.A.; Jiang, S.C. Critical Factors of Hospital Adoption on CRM System: Organizational and Information System Perspectives. Decis. Support Syst. 2010, 48, 592–603. [Google Scholar] [CrossRef]
- Sfat, R.; Marin, I.; Goga, N.; Popa, R.; Darla, I.C.; Marian, C.V. Conceptualization of an Intelligent HL7 Application Based on Questionnaire Generation and Editing. In Proceedings of the 2021 IEEE International Black Sea Conference on Communications and Networking (BlackSeaCom), Bucharest, Romania, 24–28 May 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–4. [Google Scholar]
- Hölbl, M.; Kompara, M.; Kamišalić, A.; Nemec Zlatolas, L. A Systematic Review of the Use of Blockchain in Healthcare. Symmetry 2018, 10, 470. [Google Scholar] [CrossRef]
- Dăscălescu, A.C.; Boriga, R.E.; Priescu, I. A New Keyed Hash Function Based on Compounded Chaotic Maps. IEEE Access 2025, 13, 75363–75376. [Google Scholar] [CrossRef]
- Dias, D.; Paulo Silva Cunha, J. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [PubMed]
- Vicoveanu, D.; Gherman, O.; Șoldănescu, I.; Lavric, A. Patient Health Record Smart Network Challenges and Trends for a Smarter World. Sensors 2025, 25, 3710. [Google Scholar] [CrossRef]
- Miotto, R.; Li, L.; Kidd, B.A.; Dudley, J.T. Deep Patient: An Unsupervised Representation to Predict the Future of Patients from the Electronic Health Records. Sci. Rep. 2016, 6, 26094. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Nkenyereye, L.; Kwon, J.; Choi, Y.H. Secure and Lightweight Cloud-Assisted Video Reporting Protocol over 5G-Enabled Vehicular Networks. Sensors 2017, 17, 2191. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, D.; Li, M.; Yin, D.; Wang, S.; Wang, J. Ag@Au Core–Shell Porous Nanocages with Outstanding SERS Activity for Highly Sensitive SERS Immunoassay. Sensors 2019, 19, 1554. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.R.; Kwak, D.; Kabir, M.H.; Hossain, M.; Kwak, K.S. The Internet of Things for Health Care: A Comprehensive Survey. IEEE Access 2015, 3, 678–708. [Google Scholar] [CrossRef]
- Pahl, C. Containerization and the PaaS Cloud. IEEE Cloud Comput. 2015, 2, 24–31. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y.; Li, Y.; Yang, B.; Cao, L. Digital Twinning for Smart Hospital Operations: Framework and Proof of Concept. Technol. Soc. 2023, 74, 102317. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Righi, R.D.R.; da Costa, C.A.; Antunes, R.S. Smart Hospitals and IoT Sensors: Why Is QoS Essential Here? J. Sens. Actuator Netw. 2022, 11, 33. [Google Scholar] [CrossRef]
- Topol, E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again; Hachette: London, UK, 2019. [Google Scholar]
- Ileana, M.; Petrov, P.; Milev, V. Advancing Education Management through Integrated CRM Solutions Leveraging the Scalability and Efficiency of a Distributed Web Systems Architecture. In Proceedings of the 20th International Conference on Virtual Learning—Virtual Reality (ICVL 2025), Bucharest, Romania, 10 April 2025. [Google Scholar] [CrossRef]
- Ileana, M. Optimizing Performance of Distributed Web Systems. Inform. Econ. 2023, 27, 78–87. [Google Scholar] [CrossRef]
- Rospricilia, T.A. Goals of Customer Relationship Management in Hospitals Based on the Customer Life Cycle: A Systematic Literature Review. In Proceedings of the 2022 International Seminar on Application for Technology of Information and Communication (iSemantic), Semarang, Indonesia, 17–18 September 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 89–94. [Google Scholar]
- Pascot, D.; Bouslama, F.; Mellouli, S. Architecturing Large Integrated Complex Information Systems: An Application to Healthcare. Knowl. Inf. Syst. 2011, 27, 115–140. [Google Scholar] [CrossRef]
- Suriya, S. Design of UML Diagrams for WEBMED-Healthcare Service System Services. EAI Endors. Trans. E-Learn. 2022, 8, 1. [Google Scholar] [CrossRef]
- Roy, T.; Bertaux, A.; Narsis, O.L.; Didier, J.P.; Laroche, D. Unified Modeling Language for Patient-Centered Telerehabilitation: A Comprehensive Framework Integrating Medical and Biopsychosocial Pathways. Int. J. Med. Inform. 2025, 199, 105882. [Google Scholar] [CrossRef]
- Samoila, C.G.; Predescu, M.; Slusanschi, E.I. Performance Analysis of Medical Imaging Workflows. U.P.B. Sci. Bull. Ser. C 2024, 86, 41–50. [Google Scholar]
- Cezar, D.; Gheorghe, G.; Gabriel, P.; Mariuca-Roxana, G. Device for Securing IoT in the Wireless Environment. In Proceedings of the 2024 16th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Bucharest, Romania, 27–28 June 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–6. [Google Scholar]
- Wager, K.A.; Lee, F.W.; Glaser, J.P. Health Care Information Systems: A Practical Approach for Health Care Management; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Popescu, D.A. An Enhanced Genetic Algorithm for Optimized Educational Assessment Test Generation Through Population Variation. Big Data Cogn. Comput. 2025, 9, 98. [Google Scholar] [CrossRef]
- Marian, C.V.; Croitoru, V.; Pavaloiu, I.B. Automatic Allocation Mechanism for Virtual Machines Interface Addresses IP Address Interfaces Provisioning. In Proceedings of the 2018 International Conference on Communications (COMM), Bucharest, Romania, 20–24 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 403–406. [Google Scholar]
- Popescu, D.A.; Bold, N.; Domșa, O. The Optimisation of Genetic Assessment Test Generation Based on Fuzzy Scoring. In Proceedings of the International Conference on Breaking Barriers with Generative Intelligence, Cluj-Napoca, Romania, 10 June 2024; Springer Nature: Cham, Switzerland, 2024; pp. 93–101. [Google Scholar]
- Reddy, S.; Fox, J.; Purohit, M.P. Artificial Intelligence-Enabled Healthcare Delivery. J. R. Soc. Med. 2019, 112, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial Intelligence in Healthcare: Past, Present and Future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef]
- Chakilam, C. Leveraging AI, ML, and Big Data for Precision Patient Care in Modern Healthcare Systems. Eur. J. Anal. Artif. Intell. (EJAAI) 2024, 2, 1. [Google Scholar]
- Amato, F.; López, A.; Peña-Méndez, E.M.; Vaňhara, P.; Hampl, A.; Havel, J. Artificial Neural Networks in Medical Diagnosis. J. Appl. Biomed. 2013, 11, 47–58. [Google Scholar] [CrossRef]
- Ileana, M.; Petrov, P.; Milev, V. Optimizing CRM Platforms with Distributed Cloud Architectures for Scalable Performance and Security. In Proceedings of the 2024 8th International Symposium on Innovative Approaches in Smart Technologies (ISAS), Istanbul, Turkey, 6–7 December 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–6. [Google Scholar]
- Rauniyar, A.; Hagos, D.H.; Jha, D.; Håkegård, J.E.; Bagci, U.; Rawat, D.B.; Vlassov, V. Federated Learning for Medical Applications: A Taxonomy, Current Trends, Challenges, and Future Research Directions. IEEE Internet Things J. 2023, 11, 7374–7398. [Google Scholar] [CrossRef]
- Shen, G.; Fu, Z.; Gui, Y.; Susilo, W.; Zhang, M. Efficient and Privacy-Preserving Online Diagnosis Scheme Based on Federated Learning in E-Healthcare System. Inf. Sci. 2023, 647, 119261. [Google Scholar] [CrossRef]
- Ileana, M.; Petrov, P.; Milev, V. Optimizing Customer Experience by Exploiting Real-Time Data Generated by IoT and Leveraging Distributed Web Systems in CRM Systems. IoT 2025, 6, 24. [Google Scholar] [CrossRef]
- Pannu, H.S.; Gupta, S.; Kumar, M. Fuzzy Logic for Medical Diagnosis. Expert Syst. Appl. 2022, 201, 117067. [Google Scholar]
- Li, Z.; Li, M.; Li, A.; Lin, Z. Blockchain-Based Collaborative Data Analysis Framework for Distributed Medical Knowledge Extraction. Comput. Ind. Eng. 2024, 190, 110099. [Google Scholar] [CrossRef]
- Niu, S.; Song, M.; Fang, L.; Yu, F.; Han, S.; Wang, C. Keyword Search over Encrypted Cloud Data Based on Blockchain in Smart Medical Applications. Comput. Commun. 2022, 192, 33–47. [Google Scholar] [CrossRef]
- Bhowmik, T.; Banerjee, I. EEPPDA—Edge-Enabled Efficient Privacy-Preserving Data Aggregation in Smart Healthcare Internet of Things Network. Int. J. Netw. Manag. 2023, 33, e2216. [Google Scholar] [CrossRef]
- Jayaprakasam, B.S.; Thanjaivadivel, M. Integrating Deep Learning and EHR Analytics for Real-Time Healthcare Decision Support and Disease Progression Modeling. Int. J. Manag. Res. Rev. 2021, 11, 1–15. [Google Scholar]



| Parameter | Value/Description |
|---|---|
| Number of sensors | 200 virtual devices |
| Measurement interval | 5 s (pulse, temperature, SpO2) |
| Simulation duration | 30 min (real time) |
| Topology | 1 gateway node, 3 institutional servers, 1 CRM module |
| Technologies used | Docker Swarm, Prometheus, Node Exporter, Python 3.10 |
| Execution environment | Ubuntu 22.04, Intel Core i7 (Intel Corporation, Santa Clara, CA, USA), 16 GB RAM |
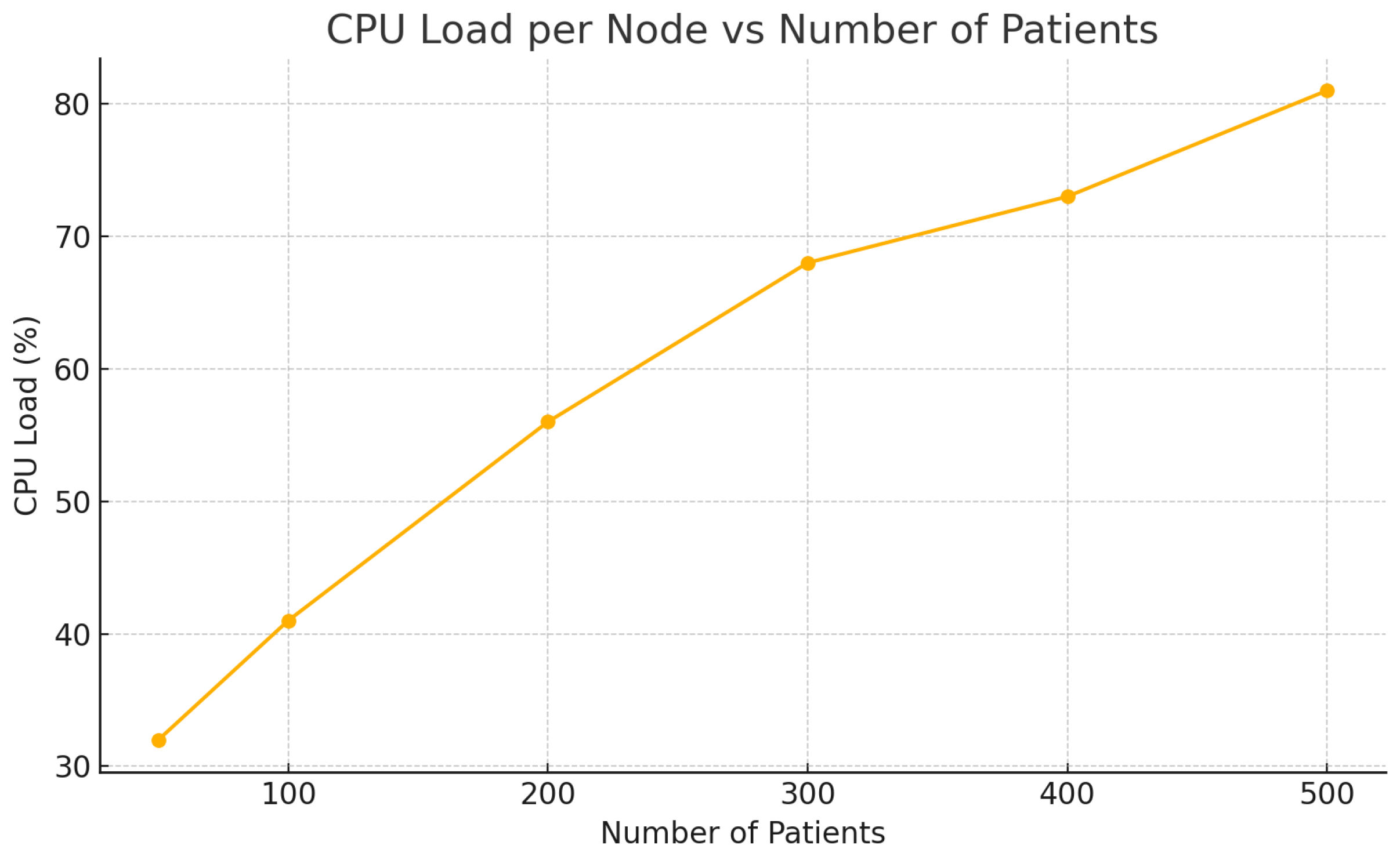
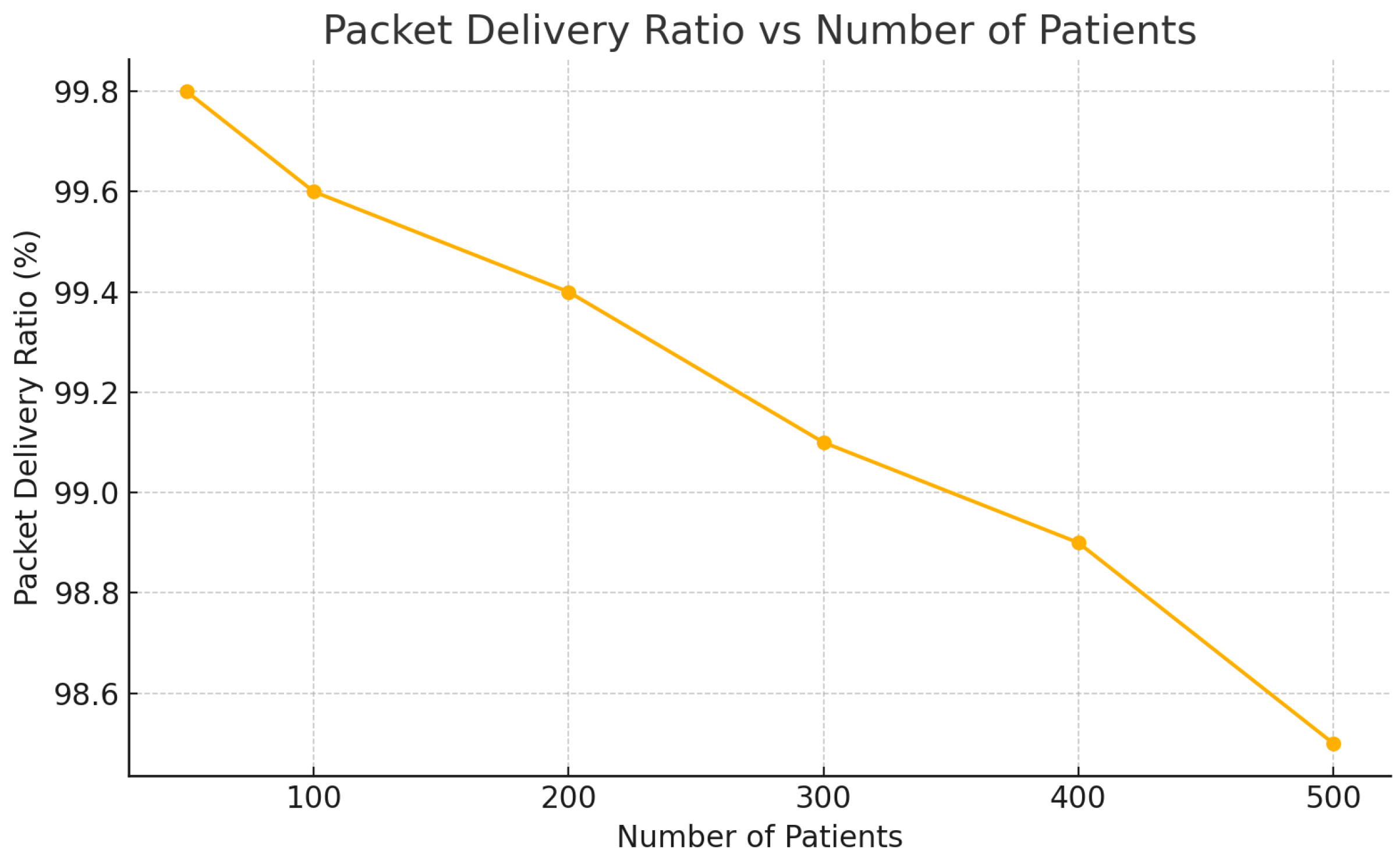
| Patients | Latency (ms) | CPU Load (%) | PDR (%) |
|---|---|---|---|
| 50 | 95 | 32 | 99.8 |
| 100 | 110 | 41 | 99.6 |
| 200 | 130 | 56 | 99.4 |
| 300 | 155 | 68 | 99.1 |
| 400 | 170 | 73 | 98.9 |
| 500 | 180 | 81 | 98.5 |
| Level of Latency | Average Value (ms) | Median (ms) | Standard Deviation (ms) |
|---|---|---|---|
| Sensor—Gateway | 55 | 53 | 7.1 |
| CRM—EHR | 65 | 62 | 9.8 |
| Between institutions | 92 | 88 | 12.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ileana, M.; Petrov, P.; Milev, V. Secure Integration of Sensor Networks and Distributed Web Systems for Electronic Health Records and Custom CRM. Sensors 2025, 25, 5102. https://doi.org/10.3390/s25165102
Ileana M, Petrov P, Milev V. Secure Integration of Sensor Networks and Distributed Web Systems for Electronic Health Records and Custom CRM. Sensors. 2025; 25(16):5102. https://doi.org/10.3390/s25165102
Chicago/Turabian StyleIleana, Marian, Pavel Petrov, and Vassil Milev. 2025. "Secure Integration of Sensor Networks and Distributed Web Systems for Electronic Health Records and Custom CRM" Sensors 25, no. 16: 5102. https://doi.org/10.3390/s25165102
APA StyleIleana, M., Petrov, P., & Milev, V. (2025). Secure Integration of Sensor Networks and Distributed Web Systems for Electronic Health Records and Custom CRM. Sensors, 25(16), 5102. https://doi.org/10.3390/s25165102








