The Usefulness of Wearable Sensors for Detecting Freezing of Gait in Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
2. Review Methodology
- Studies focusing on FoG detection in PD patients using wearable technology in clinical or real-life settings;
- Original peer-reviewed articles in English.
- Studies were excluded based on the following exclusion criteria:
- Studies that do not involve wearable technology;
- Studies that do not focus on FoG detection;
- Non-human studies;
- Studies that do not provide sufficient details about the study design and results;
- Conference or workshop articles.
3. Results
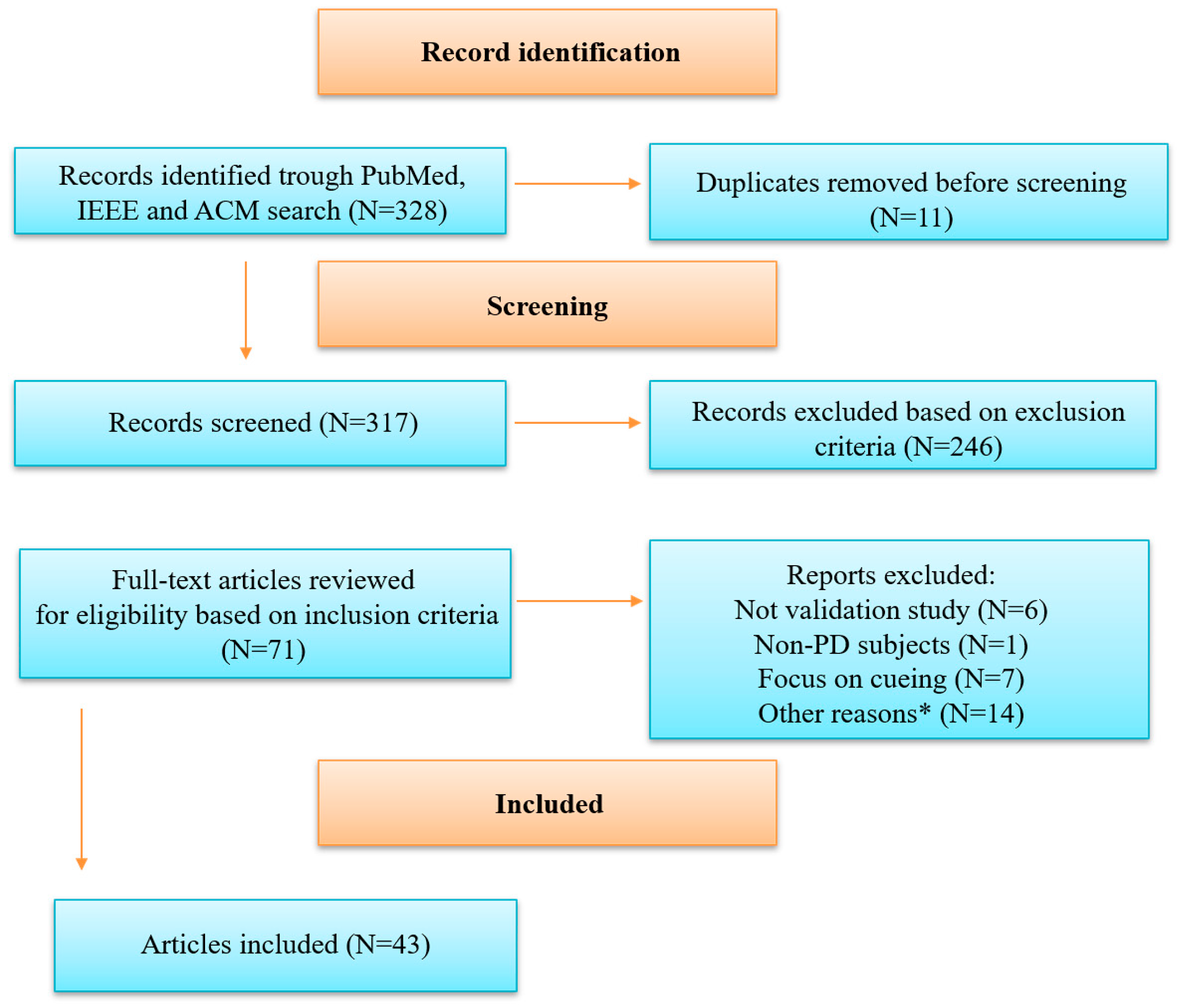
3.1. Article Selection Process
3.2. Demographic Data and Testing Environment
4. Sensor Types, Locations on the Body for Sensor Placement and Gait Tasks
4.1. Sensor Types and Combinations of Sensors
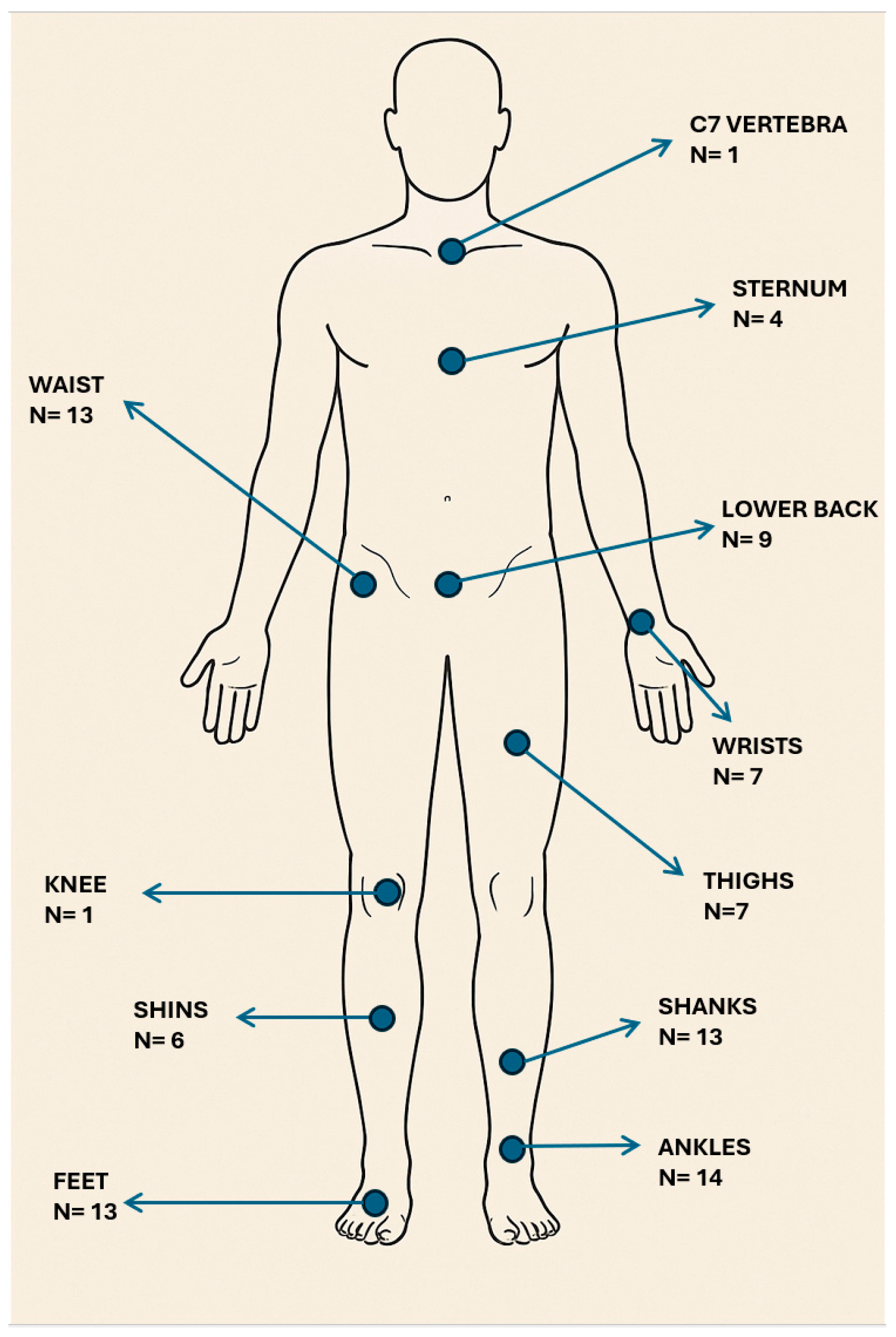
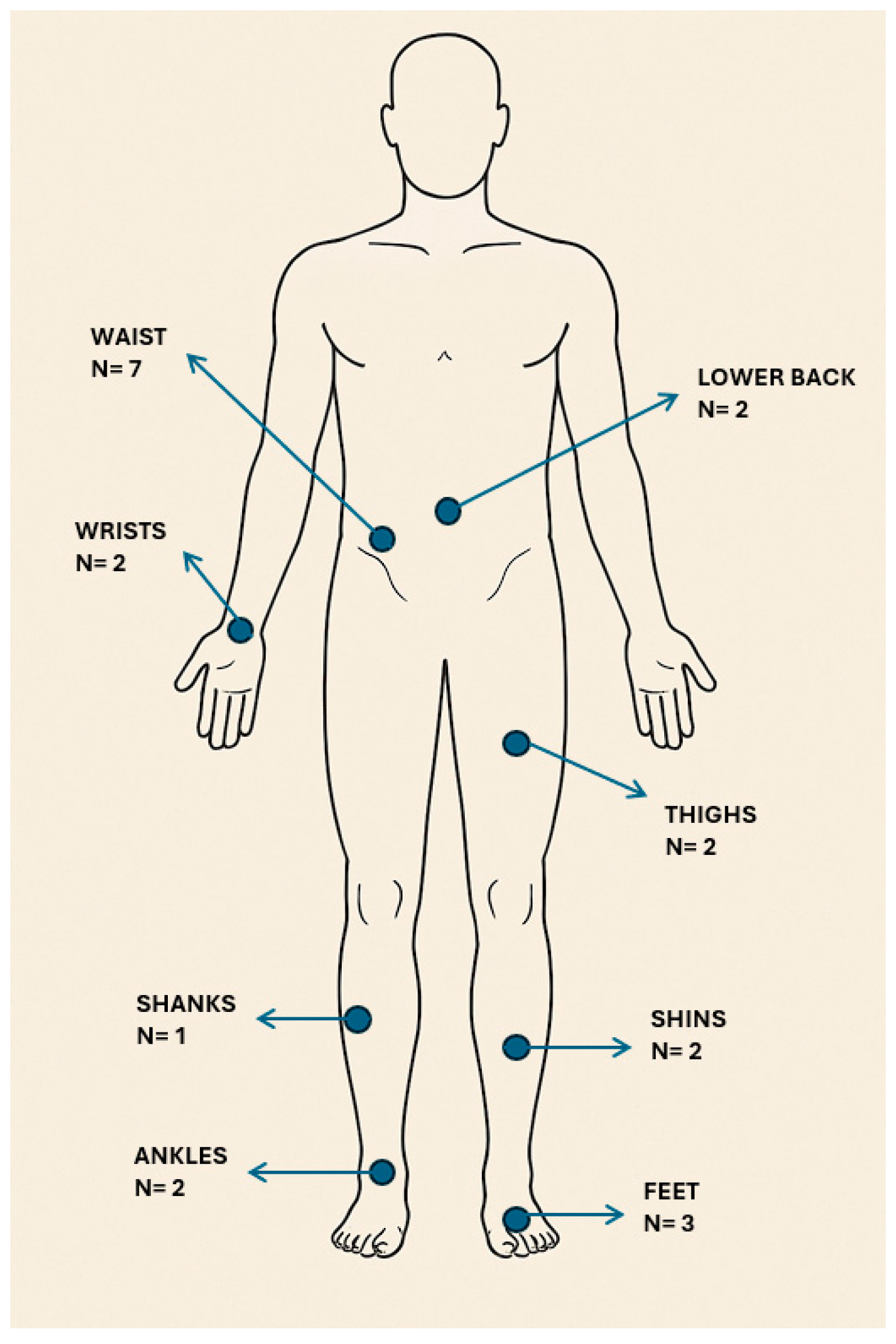
4.2. Locations on the Body for Sensor Placement
4.3. Gait Tasks
4.4. Performance Metrics for Sensors and Sensor Combinations
5. Data Analysis Algorithms
6. FoG Detection in a Real-Life Naturalistic Environment
7. Discussion
7.1. Sensor Types and Performance
7.2. Locations on the Body Where Sensors Were Placed
7.3. Gait Tasks
7.4. Medication State
7.5. Patient FoG Status
7.6. Data Analysis Algorithms
7.7. Potential Use of Sensors for FoG Detection in Real-Life Naturalistic Settings
7.8. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2MWT | 2-Minute Walk Test |
| 5Fold-CV | 5-Fold Cross-Validation |
| ADL | Activities of Daily Living |
| APA | Anticipatory postural adjustment |
| AP | Anteroposterior |
| AUC | Area Under the Curve |
| AUROC | Area Under the Receiver Operating Characteristic Curve |
| BiLSTM | Bidirectional Long Short-Term Memory |
| CNN | Convolutional Neural Network |
| CLT | Clinical Testing |
| COP | Centre of Pressure |
| CuPiD | Clinical Decision Support System and Patient Interaction Platform for PD |
| CWT | Continuous Wavelet Transform |
| ECG | Electrocardiography |
| EEG | Electroencephalography |
| EMG | Electromyography |
| FFT | Fast Fourier Transform |
| FoG | Freezing of Gait |
| HbO2 | Oxyhaemoglobin |
| HHb | Deoxyhaemoglobin |
| HRQoL | Health-Related Quality of Life |
| HTSAN | Hierarchical Temporal Spatiotemporal Attention Network |
| H&Y | Hoehn–Yahr |
| IADL | Instrumental Activities of Daily Living |
| IMU | Inertial Measurement Unit |
| k-LDA | Kernel Linear Discriminant Analysis |
| k-NN | Kernel Nearest Neighbours |
| k-PCA | Kernel Principal Component Analysis |
| LDA | Linear Discriminant Analysis |
| LOSO | Leave One Subject Out |
| ML | Machine Learning |
| MLa | Mediolateral |
| NFoFQ | New Freezing of Gait Questionnaire |
| NN | Neural Network |
| NR | Not Reported |
| ON | On Medication State |
| OFF | Off Medication State |
| PCA | Principal Component Analysis |
| PD | Parkinson’s Disease |
| PFC | Prefrontal Cortex |
| PNN | Probabilistic Neural Network |
| PP | Plantar Pressure |
| PS | Past Samples |
| RBF | Radial Basis Function |
| RL | Real Life |
| ROC | Receiver Operating Characteristic |
| RNN | Recurrent Neural Network |
| RQA | Recurrence Quantification Analysis |
| RUS | Random Under Sampling |
| SC | Skin Conductance |
| SLT | Sagittal Leg Tilt |
| SNpc | Substantia Nigra Pars Compacta |
| SVM | Support Vector Machine |
| sEMG | Surface Electromyography |
| TCNN | Temporal Convolutional Neural Network |
| TUG | Timed Up and Go Test |
| fNRIS | Functional Near-Infrared Spectroscopy |
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.C.; Shine, J.M.; Hall, J.M.; O’Callaghan, C.; Mowszowski, L.; Gilat, M.; Szeto, J.Y.Y.; Naismith, S.L.; Lewis, S.J.G. The major impact of freezing of gait on quality of life in Parkinson’s disease. J. Neurol. 2015, 262, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Grimbergen, Y.A.; Munneke, M.; Bloem, B.R. Falls in Parkinson’s disease. Curr. Opin. Neurol. 2004, 17, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Tosserams, A.; Fasano, A.; Gilat, M.; Factor, S.A.; Giladi, N.; Lewis, S.J.G.; Moreau, C.; Bloem, B.R.; Nieuwboer, A.; Nonnekes, J. Management of freezing of gait—mechanism-based practical recommendations. Nat. Rev. Neurol. 2025, 21, 327–344. Available online: https://www.nature.com/articles/s41582-025-01079-6 (accessed on 19 May 2025). [CrossRef]
- Conde, C.I.; Lang, C.; Baumann, C.R.; Easthope, C.A.; Taylor, W.R.; Ravi, D.K. Triggers for freezing of gait in individuals with Parkinson’s disease: A systematic review. Front. Neurol. 2023, 14, 1326300. [Google Scholar] [CrossRef] [PubMed]
- Factor, S.A. The clinical spectrum of freezing of gait in atypical parkinsonism. Mov. Disord. 2008, 23 (Suppl. S2), S431–S438. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, J.; Tan, Y.; Chen, S. Freezing of gait in Parkinson’s disease: Pathophysiology, risk factors and treatments. Transl. Neurodegener. 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Silva De Lima, A.L.; Evers, L.J.W.; Hahn, T.; Bataille, L.; Hamilton, J.L.; Little, M.A.; Okuma, Y.; Bloem, B.R.; Faber, M.J. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: A systematic review. J. Neurol. 2017, 264, 1642–1654. [Google Scholar] [CrossRef]
- Di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sensors 2020, 20, 3529. [Google Scholar] [CrossRef]
- Huang, T.; Li, M.; Huang, J. Recent trends in wearable device used to detect freezing of gait and falls in people with Parkinson’s disease: A systematic review. Front. Aging Neurosci. 2023, 15, 1119956. [Google Scholar] [CrossRef] [PubMed]
- Glowinski, S.; Blazejewski, A.; Krzyzynski, T. Human Gait Feature Detection Using Inertial Sensors Wavelets. In Wearable Robotics: Challenges and Trends; González-Vargas, J., Ibáñez, J., Contreras-Vidal, J.L., Van Der Kooij, H., Pons, J.L., Eds.; Biosystems & Biorobotics; Springer International Publishing: Cham, Switzerland, 2017; Volume 16, pp. 397–401. ISBN 978-3-319-46531-9. Available online: http://link.springer.com/10.1007/978-3-319-46532-6_65 (accessed on 31 July 2025). [CrossRef]
- Goris, M.; Ginis, P.; Hansen, C.; Schlenstedt, C.; Hausdorff, J.M.; D’Cruz, N.; Vandenberghe, W.; Maetzler, W.; Nieuwboer, A.; Gilat, M. Is the freezing index a valid outcome to assess freezing of gait during turning in Parkinson’s disease? Front. Neurol. 2025, 15, 1508800. [Google Scholar] [CrossRef] [PubMed]
- Pardoel, S.; AlAkhras, A.; Jafari, E.; Kofman, J.; Lemaire, E.D.; Nantel, J. Real-Time Freezing of Gait Prediction and Detection in Parkinson’s Disease. Sensors 2024, 24, 8211. [Google Scholar] [CrossRef] [PubMed]
- Pardoel, S.; Shalin, G.; Nantel, J.; Lemaire, E.D.; Kofman, J. Early Detection of Freezing of Gait during Walking Using Inertial Measurement Unit and Plantar Pressure Distribution Data. Sensors 2021, 21, 2246. [Google Scholar] [CrossRef] [PubMed]
- Koltermann, K.; Jung, W.; Blackwell, G.; Pinney, A.; Chen, M.; Cloud, L.; Pretzer-Aboff, I.; Zhou, G. FoG-Finder: Real-time Freezing of Gait Detection and Treatment. In Proceedings of the 8th ACM/IEEE International Conference on Connected Health: Applications, Systems and Engineering Technologies, Orlando, FL, USA, 21–23 June 2023; pp. 22–33. [Google Scholar]
- Marcante, A.; Di Marco, R.; Gentile, G.; Pellicano, C.; Assogna, F.; Pontieri, F.E.; Spalletta, G.; Macchiusi, L.; Gatsios, D.; Giannakis, A.; et al. Foot Pressure Wearable Sensors for Freezing of Gait Detection in Parkinson’s Disease. Sensors 2020, 21, 128. [Google Scholar] [CrossRef]
- Krasovsky, T.; Heimler, B.; Koren, O.; Galor, N.; Hassin-Baer, S.; Zeilig, G.; Plotnik, M. Bilateral Leg Stepping Coherence as a Predictor of Freezing of Gait in Patients With Parkinson’s Disease Walking With Wearable Sensors. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. 2023, 31, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Slemenšek, J.; Geršak, J.; Bratina, B.; van Midden, V.M.; Pirtošek, Z.; Šafarič, R. Wearable Online Freezing of Gait Detection and Cueing System. Bioengineering 2024, 11, 1048. [Google Scholar] [CrossRef]
- Chomiak, T.; Xian, W.; Pei, Z.; Hu, B. A novel single-sensor-based method for the detection of gait-cycle breakdown and freezing of gait in Parkinson’s disease. J. Neural Transm. Vienna Austria 1996 2019, 126, 1029–1036. [Google Scholar] [CrossRef]
- Park, J.-M.; Moon, C.-W.; Lee, B.C.; Oh, E.; Lee, J.; Jang, W.-J.; Cho, K.H.; Lee, S.-H. Detection of freezing of gait in Parkinson’s disease from foot-pressure sensing insoles using a temporal convolutional neural network. Front. Aging Neurosci. 2024, 16, 1437707. [Google Scholar] [CrossRef]
- Reches, T.; Dagan, M.; Herman, T.; Gazit, E.; Gouskova, N.A.; Giladi, N.; Manor, B.; Hausdorff, J.M. Using Wearable Sensors and Machine Learning to Automatically Detect Freezing of Gait during a FOG-Provoking Test. Sensors 2020, 20, 4474. [Google Scholar] [CrossRef] [PubMed]
- May, D.S.; Tueth, L.E.; Earhart, G.M.; Mazzoni, P. Using Wearable Sensors to Assess Freezing of Gait in the Real World. Bioengineering 2023, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Mazzetta, I.; Zampogna, A.; Suppa, A.; Gumiero, A.; Pessione, M.; Irrera, F. Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson’s Disease Using Electromyography and Inertial Signals. Sensors 2019, 19, 948. [Google Scholar] [CrossRef] [PubMed]
- Arami, A.; Poulakakis-Daktylidis, A.; Tai, Y.F.; Burdet, E. Prediction of Gait Freezing in Parkinsonian Patients: A Binary Classification Augmented With Time Series Prediction. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Borzì, L.; Mazzetta, I.; Zampogna, A.; Suppa, A.; Olmo, G.; Irrera, F. Prediction of Freezing of Gait in Parkinson’s Disease Using Wearables and Machine Learning. Sensors 2021, 21, 614. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Chen, Z.; Ling, Y.; Zhao, J. Recognition of freezing of gait in Parkinson’s disease based on combined wearable sensors. BMC Neurol. 2022, 22, 229. [Google Scholar] [CrossRef]
- Zampogna, A.; Borzì, L.; Rinaldi, D.; Artusi, C.A.; Imbalzano, G.; Patera, M.; Lopiano, L.; Pontieri, F.; Olmo, G.; Suppa, A. Unveiling the Unpredictable in Parkinson’s Disease: Sensor-Based Monitoring of Dyskinesias and Freezing of Gait in Daily Life. Bioengineering. 2024, 11, 440. [Google Scholar] [CrossRef]
- Antonini, A.; Reichmann, H.; Gentile, G.; Garon, M.; Tedesco, C.; Frank, A.; Falkenburger, B.; Konitsiotis, S.; Tsamis, K.; Rigas, G.; et al. Toward objective monitoring of Parkinson’s disease motor symptoms using a wearable device: Wearability and performance evaluation of PDMonitor(®). Front. Neurol. 2023, 14, 1080752. [Google Scholar] [CrossRef]
- Delgado-Terán, J.D.; Hilbrants, K.; Mahmutović, D.; Silva de Lima, A.L.; Wezel, R.J.v.; Heida, T. Ankle Sensor-Based Detection of Freezing of Gait in Parkinson’s Disease in Semi-Free Living Environments. Sensors 2025, 25, 1895. [Google Scholar] [CrossRef]
- Borzì, L.; Sigcha, L.; Rodríguez-Martín, D.; Olmo, G. Real-time detection of freezing of gait in Parkinson’s disease using multi-head convolutional neural networks and a single inertial sensor. Artif. Intell. Med. 2023, 135, 102459. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, L.; Rocchi, L.; Mazilu, S.; Gazit, E.; Hausdorff, J.M.; Chiari, L. Identification of Characteristic Motor Patterns Preceding Freezing of Gait in Parkinson’s Disease Using Wearable Sensors. Front. Neurol. 2017, 8, 394. [Google Scholar] [CrossRef]
- Mancini, M.; Shah, V.V.; Stuart, S.; Curtze, C.; Horak, F.B.; Safarpour, D.; Nutt, J.G. Measuring freezing of gait during daily-life: An open-source, wearable sensors approach. J. Neuroeng. Rehabil. 2021, 18, 1. [Google Scholar] [CrossRef]
- Caballol, N.; Bayés, À.; Prats, A.; Martín-Baranera, M.; Quispe, P. Feasibility of a wearable inertial sensor to assess motor complications and treatment in Parkinson’s disease. PLoS ONE 2023, 18, e0279910. [Google Scholar] [CrossRef] [PubMed]
- Demrozi, F.; Bacchin, R.; Tamburin, S.; Cristani, M.; Pravadelli, G. Toward a Wearable System for Predicting Freezing of Gait in People Affected by Parkinson’s Disease. IEEE J. Biomed. Health Inform. 2020, 24, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
- Tzallas, A.T.; Tsipouras, M.G.; Rigas, G.; Tsalikakis, D.G.; Karvounis, E.C.; Chondrogiorgi, M.; Psomadellis, F.; Cancela, J.; Pastorino, M.; Waldmeyer, M.T.A.; et al. PERFORM: A system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors 2014, 14, 21329–21357. [Google Scholar] [CrossRef] [PubMed]
- Tripoliti, E.E.; Tzallas, A.T.; Tsipouras, M.G.; Rigas, G.; Bougia, P.; Leontiou, M.; Konitsiotis, S.; Chondrogiorgi, M.; Tsouli, S.; Fotiadis, D.I. Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput. Methods Programs Biomed. 2013, 110, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.; O’Day, J.; Kehnemouyi, Y.; Burnett, G.; Bronte-Stewart, H. Gait Parameters Measured from Wearable Sensors Reliably Detect Freezing of Gait in a Stepping in Place Task. Sensors 2021, 21, 2661. [Google Scholar] [CrossRef] [PubMed]
- Ashfaque Mostafa, T.; Soltaninejad, S.; McIsaac, T.L.; Cheng, I. A Comparative Study of Time Frequency Representation Techniques for Freeze of Gait Detection and Prediction. Sensors 2021, 21, 6446. [Google Scholar] [CrossRef]
- Al-Adhaileh, M.H.; Wadood, A.; Aldhyani, T.H.H.; Khan, S.; Uddin, M.I.; Al-Nefaie, A.H. Deep learning techniques for detecting freezing of gait episodes in Parkinson’s disease using wearable sensors. Front. Physiol. 2025, 16, 1581699. [Google Scholar] [CrossRef]
- Bächlin, M.; Plotnik, M.; Roggen, D.; Giladi, N.; Hausdorff, J.M.; Tröster, G. A wearable system to assist walking of Parkinson s disease patients. Methods Inf. Med. 2010, 49, 88–95. [Google Scholar] [CrossRef]
- Jovanov, E.; Wang, E.; Verhagen, L.; Fredrickson, M.; Fratangelo, R. deFOG--A real time system for detection and unfreezing of gait of Parkinson’s patients. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 5151–5154. [Google Scholar] [CrossRef]
- Naghavi, N.; Miller, A.; Wade, E. Towards Real-Time Prediction of Freezing of Gait in Patients With Parkinson’s Disease: Addressing the Class Imbalance Problem. Sensors 2019, 19, 3898. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Yen, S.C.; Tay, A.; Tan, D.M.L.; Chia, N.S.Y.; Au, W.L. Convolutional Neural Network for Freezing of Gait Detection Leveraging the Continuous Wavelet Transform on Lower Extremities Wearable Sensors Data. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Montreal, QC, Canada, 20–24 July 2020; pp. 5410–5415. [Google Scholar] [CrossRef]
- Seuthe, J.; Heinzel, A.; Hulzinga, F.; Ginis, P.; Zeuner, K.E.; Deuschl, G.; D’Cruz, N.; Nieuwboer, A.; Schlenstedt, C. Towards a better understanding of anticipatory postural adjustments in people with Parkinson’s disease. PLoS ONE 2024, 19, e0300465. [Google Scholar] [CrossRef] [PubMed]
- Belluscio, V.; Stuart, S.; Bergamini, E.; Vannozzi, G.; Mancini, M. The Association between Prefrontal Cortex Activity and Turning Behavior in People with and without Freezing of Gait. Neuroscience 2019, 416, 168–176. [Google Scholar] [CrossRef]
- Hu, K.; Mei, S.; Wang, W.; Martens, K.A.E.; Wang, L.; Lewis, S.J.G.; Feng, D.D.; Wang, Z. Multi-Level Adversarial Spatio-Temporal Learning for Footstep Pressure Based FoG Detection. IEEE J. Biomed. Health Inform. 2023, 27, 4166–4177. [Google Scholar] [CrossRef]
- Mazilu, S.; Calatroni, A.; Gazit, E.; Mirelman, A.; Hausdorff, J.M.; Troster, G. Prediction of Freezing of Gait in Parkinson’s From Physiological Wearables: An Exploratory Study. IEEE J. Biomed. Health Inform. 2015, 19, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Mikos, V.; Heng, C.-H.; Tay, A.; Yen, S.-C.; Chia, N.S.Y.; Koh, K.M.L.; Tan, D.M.L.; Au, W.L. A Wearable, Patient-Adaptive Freezing of Gait Detection System for Biofeedback Cueing in Parkinson’s Disease. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 503–515. [Google Scholar] [CrossRef]
- Murtaza, G.; Hammoud, M.; Somov, A. Multi-Modal Feature Set-Based Detection of Freezing of Gait in Parkinson’s Disease Patients Using SVM. IEEE Access 2025, 13, 114798–114811. [Google Scholar] [CrossRef]
- Noor, M.H.M.; Nazir, A.; Wahab, M.N.A.; Ling, J.O.Y. Detection of Freezing of Gait Using Unsupervised Convolutional Denoising Autoencoder. IEEE Access 2021, 9, 115700–115709. [Google Scholar] [CrossRef]
- Pierleoni, P.; Belli, A.; Bazgir, O.; Maurizi, L.; Paniccia, M.; Palma, L. A Smart Inertial System for 24h Monitoring and Classification of Tremor and Freezing of Gait in Parkinson’s Disease. IEEE Sens. J. 2019, 19, 11612–11623. [Google Scholar] [CrossRef]
- Prado, A.; Kwei, K.; Vanegas-Arroyave, N.; Agrawal, S.K. Identification of Freezing of Gait in Parkinson’s Patients Using Instrumented Shoes and Artificial Neural Networks. In Proceedings of the 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), New York, NY, USA, 29 November–1 December 2020; IEEE: New York, NY, USA, 2020; pp. 68–73. Available online: https://ieeexplore.ieee.org/document/9224357/ (accessed on 6 August 2025).
- Shi, B.; Tay, A.; Au, W.L.; Tan, D.M.L.; Chia, N.S.Y.; Yen, S.-C. Detection of Freezing of Gait Using Convolutional Neural Networks and Data From Lower Limb Motion Sensors. IEEE Trans. Biomed. Eng. 2022, 69, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Tahafchi, P.; Judy, J.W. Freezing-of-Gait Detection Using Wearable Sensor Technology and Possibilistic K-Nearest-Neighbor Algorithm. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Berlin, Germany, 23–27 July 2019; pp. 4246–4249. [Google Scholar] [CrossRef]
- Giladi, N.; Shabtai, H.; Simon, E.S.; Biran, S.; Tal, J.; Korczyn, A.D. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat. Disord. 2000, 6, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hsu, Y.-L. A Review of Accelerometry-Based Wearable Motion Detectors for Physical Activity Monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef]
- Mesin, L.; Porcu, P.; Russu, D.; Farina, G.; Borzì, L.; Zhang, W.; Guo, Y.; Olmo, G. A Multi-Modal Analysis of the Freezing of Gait Phenomenon in Parkinson’s Disease. Sensors 2022, 22, 2613. [Google Scholar] [CrossRef]
- Hellmers, S.; Krey, E.; Gashi, A.; Koschate, J.; Schmidt, L.; Stuckenschneider, T.; Hein, A.; Zieschang, T. Comparison of machine learning approaches for near-fall-detection with motion sensors. Front. Digit. Health 2023, 5, 1223845. [Google Scholar] [CrossRef] [PubMed]
- Borzì, L.; Demrozi, F.; Bacchin, R.A.; Turetta, C.; Sigcha, L.; Rinaldi, D.; Fazzina, G.; Balestro, G.; Picelli, A.; Pravadelli, G.; et al. Freezing of gait detection: The effect of sensor type, position, activities, datasets, and machine learning model. J. Park. Dis. 2025, 15, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.K.; Lewis, S.J.G. Future Therapeutic Strategies for Freezing of Gait in Parkinson’s Disease. Front. Hum. Neurosci. 2021, 15, 741918. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, S.; Gaztanaga, W.; Yadav, A.P.; Chang, S.J.; Krucoff, M.O.; Cajigas, I.; Turner, D.A.; Wang, D.D. Freezing of Gait in Parkinson’s Disease: Invasive and Noninvasive Neuromodulation. Neuromodulation Technol. Neural Interface 2021, 24, 829–842. [Google Scholar] [CrossRef]
- Hulzinga, F.; Nieuwboer, A.; Dijkstra, B.W.; Mancini, M.; Strouwen, C.; Bloem, B.R.; Ginis, P. The New Freezing of Gait Questionnaire: Unsuitable as an Outcome in Clinical Trials? Mov. Disord. Clin. Pract. 2020, 7, 199–205. [Google Scholar] [CrossRef]
- Cockx, H.M.; Lemmen, E.M.; Van Wezel, R.J.A.; Cameron, I.G.M. The effect of doorway characteristics on freezing of gait in Parkinson’s disease. Front. Neurol. 2023, 14, 1265409. [Google Scholar] [CrossRef]
- Haussler, A.M.; Tueth, L.E.; May, D.S.; Earhart, G.M.; Mazzoni, P. Refinement of an Algorithm to Detect and Predict Freezing of Gait in Parkinson Disease Using Wearable Sensors. Sensors 2024, 25, 124. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ji, J.; Zhu, Y.; Dell, T.; Liu, X. Flexible Gel-Free Multi-Modal Wireless Sensors With Edge Deep Learning for Detecting and Alerting Freezing of Gait Symptom. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Moreno Arostegui, J.M.; Cabestany, J.; Bayés, À.; Alcaine, S.; Mestre, B.; Prats, A.; et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS ONE 2017, 12, e0171764. [Google Scholar] [CrossRef] [PubMed]
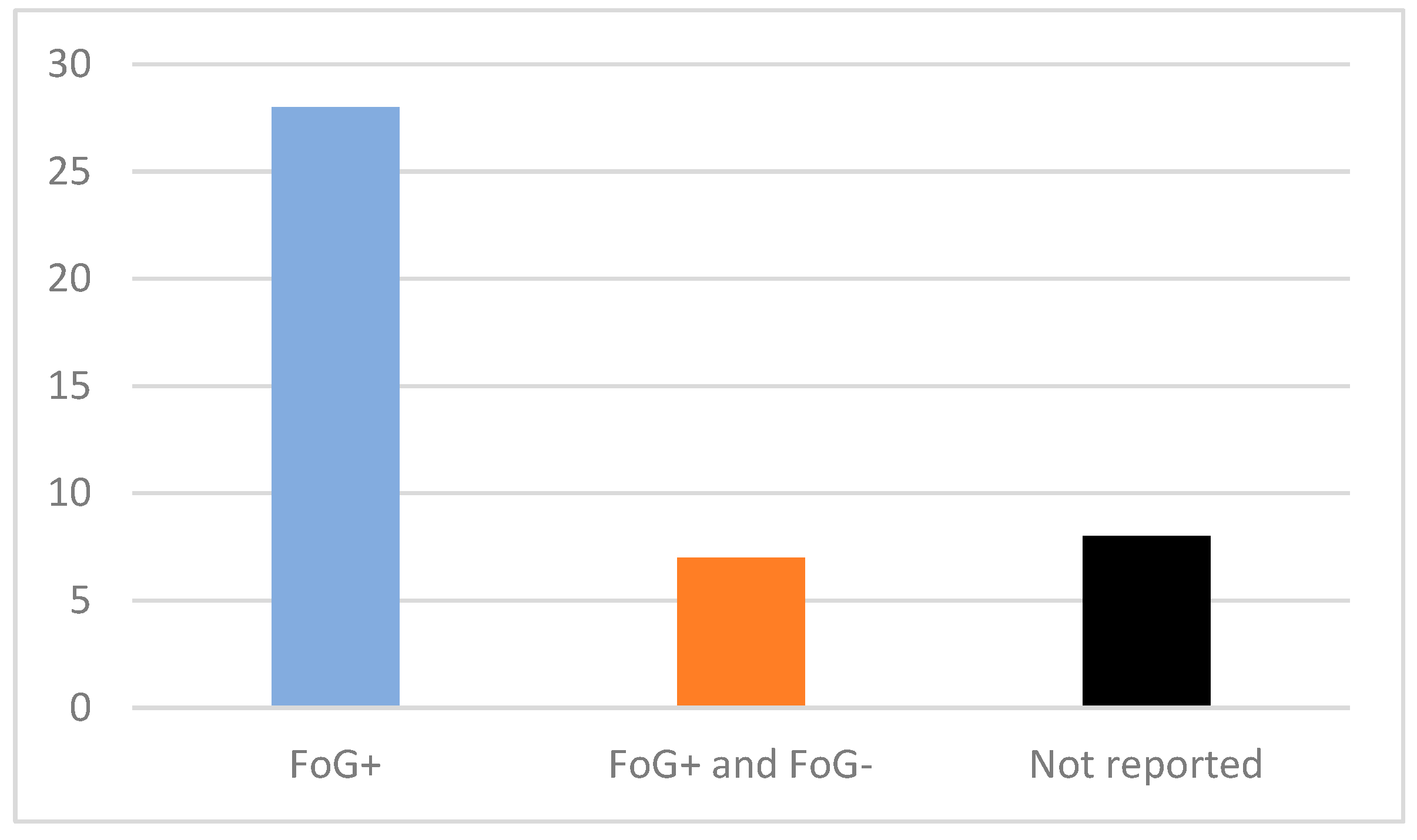
| Database | Search String | No. of Records |
|---|---|---|
| PubMed | ((“freezing of gait”[Title/Abstract] OR “FoG”[Title/Abstract]) AND (“wearable sensor”[Title/Abstract] OR “wearable sensors”[Title/Abstract] OR “wearable device”[Title/Abstract] OR “wearable devices”[Title/Abstract]) AND (“Parkinson’s disease”[Title/Abstract] OR “Parkinson disease”[Title/Abstract])) | 101 |
| IEEE Explore | (“freezing of gait” OR “FoG”) AND (“wearable sensor” OR “wearable sensors” OR “wearable device” OR “wearable devices”) AND (“Parkinson’s disease” OR “Parkinson disease”) | 91 |
| ACM digital library | (“freezing of gait” OR “FoG”) AND (“wearable sensor” OR “wearable sensors” OR “wearable device” OR “wearable devices”) AND (“Parkinson’s disease” OR “Parkinson disease”) | 136 |
| Author, Year | Demographic and Clinical Data | Sensor Type and Model | Sensor Location | Test | Main Results | ON/ OFF | CLT/ RL | Algorithm |
|---|---|---|---|---|---|---|---|---|
| Ren et al. [28], 2022 | 12 PD-FoG+ Mean age: 66.75 Mean H&Y: 2.67 | Accelerometers, gyroscopes, force sensing resistors Commercial BMX055 | Waist, thighs, shanks, feet, insoles—1 sensor per body part | Random gait test, TUG | Sensitivity: 78.39% Specificity: 91.66% Accuracy: 88.09% Precision: 77.58% F-score: 77.98% | ON | CLT | ML: random forest |
| Zampogna et al. [29], 2024 | 71 PD, 33 PD-FoG+, 29 PD-FoG- Mean age: 69 Mean H&Y: 2 | Accelerometer Commercial STAT-ONTM | Waist (left side)—1 sensor | Daily activities for 5–8 days | Sensitivity: 0.82 Specificity: 0.79 Accuracy: 0.81 | ON | RL | ML: support vector machine |
| Pardoel et al. [15], 2024 | 21PD-FoG+ Dataset: Pardoel et al. 2022. Mean age: 72.4 Mean H&Y: NR | Plantar pressure insoles Commercial Tekscan | Both feet—1 sensor per foot | Walking on freeze-inducing path with cognitive dual-tasking | Sensitivity: 77.68%, Specificity: 79.99%, FOG identification: 86.84% Predicted FoG: 0.94s before onset | OFF | CLT | ML: RUS boost ensemble of decision trees |
| Pardoel et al. [16], 2021 | 11 PD-FoG+ Mean age: 72.7 Mean H&Y: NR | Accelerometer, gyroscope and plantar pressure sensor Commercial Tekscan and Shimmer3 | Insoles in shoes—1 sensor per shoe Shanks—1 sensor per shank | FoG provoking walking path with dual-tasking, narrow passages, turns, stops and starts | Sensitivity: 76.4% Specificity: 86.2% FOG-only detection: 93.4% | OFF | CLT | ML: RUS-boosted decision tree ensemble |
| Koltermann et al. [17], 2023 | 11 PD-FoG Mean age: NR Mean H&Y: NR | Accelerometer and gyroscope Commercial Ultigesture IMU | Both ankles—2 sensors per ankle | Walking tests designed to trigger FoG | F1 score: +13.4% accuracy +10.7% FPR reduced by 85.8% | ON/ OFF | CLT | ML: multi-input convolutional neural network |
| Marcante et al. [18], 2020 | 20 PD Mean age: 68.6 FoG status: NR Mean H&Y: >3 | Plantar pressure sensors and accelerometer Commercial Moticon GmbH | Both feet (in-shoe insoles with 13 sensors and 1 accelerometer) | TUG, 360° turn, 2MWT, door opening, drinking task, standing and walking under various conditions | Sensitivity: 96%; specificity: 94% FPR: 6% FNR: 4% | ON/ OFF | CLT | Threshold-based detection algorithm using force, COP, vertical acceleration signals |
| Antonini et al. [30], 2023 | 65 PD Mean age: 65.8 FoG status: NR Mean H&Y: NR | Accelerometer, gyroscope and magnetometer Commercial PD-Monitor® | Wrists, ankles, waist—1 sensor per body part | Phase I: supervised tasks in hospital Phase II: free-living activities for up to 3 days | Accuracy: 96% Specificity: 98% Sensitivity: 83% | ON/ OFF | CLT/ RL | ML: naive Bayes classifier, ROC-based thresholding |
| Delgado-Terán et al. [31], 2025 | 21 PD-FoG+; mean age: 74 Mean H&Y: 0.5 | Accelerometer and gyroscope Custom IMUs | Right ankle—1 sensor | Walking, turning, sitting, standing, household tasks | AUROC: 0.89–0.96 (5Fold-CV), 0.90–0.93 (LOSO) Sensitivity: 96.5%; F1-score: 92.1% | ON/ OFF | Semi RL, VV | ML: convolutional neural network (machine learning) |
| Borzì et al. [32], 2023 | 21 PD-FoG+; mean age: 69.3 Mean H&Y: NR Dataset used: FP7 REMPARK (main test set) | Accelerometer Commercial IMUs in REMPARK Project | Waist—1 sensor | Free living activities | Main test set: 50% FoG predicted 3.1s before onset, 50% FoG detected with 0.8s delay Sensitivity: 0.877; specificity: 0.883 | ON/ OFF | RL | ML: multi-head convolutional neural network |
| Palmerini et al. [33], 2017 | 11 PD-FoG+ Mean age: 67.7 Mean H&Y: 3.1 Dataset used: CuPiD | Accelerometer and gyroscope Commercial IMUs in CuPiD project | Left and right ankles; lower back—1 sensor per body part | Multiple walking conditions turns, dual tasks, narrow corridors | Mean AUC: 0.76, sensitivity: 0.83, specificity: 0.67 | ON | CLT | ML: linear discriminant analysis |
| Krasovsky et al. [19], 2023 | 14 PD-FoG+ Mean age: 65.1 Mean H&Y: NR | Accelerometer, gyroscope and magnetometer Commercial Mobility Lab OpalTM | Waist and both shanks—1 sensor per body part | Walking tasks with turns, dual-tasking, figure-eight patterns, voluntary stops | Sensitivity: 98%, specificity: 42%, balanced accuracy: 70.2% SLT occurred ~1.8s before FoG onset | OFF | CLT, VV | Wavelet coherence analysis (threshold) |
| Slemenšek et al. [20], 2024 | 9 PD-FoG+; mean age: 67 Mean H&Y: 2.7 | Accelerometers, gyroscope and muscle activity sensors Custom IMUs | Below both knees—multisensory strip per knee | 10–15 min gait trials with walking, turning, door crossing | Sensitivity: 2.7% Specificity: 97.2% Accuracy: 95.0% F1-score: 0.023 Mean detection delay: 261 ms | ON | CLT | ML: NN+RNN+PS model |
| Chomiak et al. [21], 2019 | 21 PD, 9 HC Mean age: NR FoG status: NR Mean H&Y: NR | Gyroscope and accelerometer in iPod Touch Commercial device | Thigh—1 sensor in pocket | Walking or stepping in place with turning, dual-tasking and cup carrying | Model B: <5% mean error rate, 0% mode error rate, ~100% sensitivity and specificity | NR | CLT | ML: RQA + SVM with Monte Carlo cross-validation |
| Borzì et al. [27], 2021 | 11 PD-FoG+ patients Mean age: 73 Mean H&Y: 2.7 | Accelerometer, gyroscope and magnetometer Custom IMUs | Both shins—2 sensors per body part | Timed Up and Go test in free-living-like setting with narrow corridor and door | Pre-FOG detection in LOSO; sensitivity: 84.1–85.5%, specificity: 85.9–86.3%, accuracy: 85.5–86.1% | ON/ OFF | Semi-CLT, sim. RL, VV | ML: wrapper feature selection + SVM and LDA classifiers |
| Mancini et al. [34], 2021 | Study I: 45 PD (27 FoG+ and 18 FoG-) and 21 HC Mean age: PD 70.1 Study II: 48 PD (23 FoG+ and 25 FOG-) Mean age PD 68.6 Mean H&Y: 2–4 | Accelerometer, gyroscope and magnetometer Commercial Mobility Lab OpalTM | Study I: feet, shins, wrists, sternum, lower back—1 sensor per body part Study II: feet and lower back—1 sensor per body part | Study I: 2-minute walk, 1-minute dual-task walk Study II: 7 days of unsupervised daily living monitoring | Study I: Accuracy: 85–88% Sensitivity: 80–89% Specificity: 87–88% Study II: less time spent freezing between people with and without FoG (p < 0.05) | Study I: OFF Study II: ON | Study I: CLT Study II: RL | Open-source threshold-based: freezing ratio (threshold) |
| Mazzetta et al. [25], 2019 | 7 PD-FoG+ Age range: 65–79 Mean H&Y: 2–3 | Accelerometer, gyroscope and surface EMG Commercial IMUs | Shins and shanks—1 sensor per leg | TUG with obstacles, turning, door crossing | FOG detection: 2% false negatives 5% false positives | ON/ OFF | CLT, RL, VV | Gyro and sEMG fusion; custom real-time FOG index (threshold) |
| Caballol et al. [35], 2023 | 39 PD Mean age: 69 FoG status: NR Mean H&Y: NR | Accelerometer Commercial STAT-ONTM | Waist (left)—1 sensor | 12-hour/day wear for 7 days; normal ADLs | Detected: FoG (23%) Kappa for FoG = 0.481 | ON/ OFF | RL | ML: support vector machine |
| Demrozi et al. [36], 2020 | 10 PD (8 PD-FoG+; 2 PD-FoG-) patients from dataset; mean age: 66.5 Mean H&Y: 2.7 Dataset used: DAPHNET | Accelerometer Custom IMUs | Lower back, waist, ankle | Gait tasks with FoG, no-FoG and pre-FoG segments; labelled via video annotation | Pre-FoG detection: Sensitivity: 94.1% Specificity: 97.1% device latency: ~100–120 ms | OFF | Sim. RL | ML: k-NN classifier with PCA, LDA, kPCA, kLDA |
| Park et al. [22], 2024 | 14 PD-FoG+ patients Mean age: NR Mean H&Y: NR | Foot pressure sensors Commercial Pedar system | Both feet—multiple sensors per foot | Standardized 140 m walking path with dual-tasking, narrow corridors, turning, tray carrying | TCNN: Accuracy: 0.99 Precision: 0.68, sensitivity: 0.88, specificity: 0.99 F1-score: 0.76 | ON | CLT, RL, VV | ML: temporal convolutional neural network |
| Tzallas et al. [37], 2014 | Short term: 24 PD Long term: 20 PD Mean age: NR FoG status: NR Mean H&Y: NR | Accelerometer and gyroscope Commercial PERFORM IMUs | Wrists, ankles, waist—1 sensor per body part | Bed-to-chair walking, door opening, drinking; free-living for 5 days (~4 h/day) | FoG detection: 79% accuracy (short-term) | Mixed ON/ OFF | RL | ML: random forest |
| Tripoliti et al. [38], 2013 | 11PD-FoG+ 5 HC Mean age: 63 Mean H&Y: NR | Accelerometer and gyroscope Commercial ANCO IMUs | Accelerometers: both legs, both wrists, chest, waist—6 sensors; Gyroscopes: chest, waist—2 sensors | Standardized motor protocol: rising, walking, door crossing, water drinking | Random Forest: 96.11% accuracy Sensitivity: 81.94% Specificity: 98.74% | ON/OFF | Semi RL, VV | ML. naive Bayes, random forests, decision tree, random tree |
| Diep et al. [39], 2021 | 10 PD-FoG+ patients Mean age: 62.5 years Mean H&Y: NR | Accelerometer and gyroscope Commercial Mobility Lab OpalTM | Lateral shanks—1 sensor per body part | Stepping-in-place task for 100 seconds | General logistic model: AUC 0.81 Accuracy: 0.84, sensitivity: 0.86, specificity: 0.81 | OFF | CLT | ML: binomial logistic regression |
| Reches et al. [23], 2020 | 71 PD-FoG+; mean age: 69.9 Used dataset: multicentric Mean H&Y: NR | Accelerometer, gyroscope and magnetometer Commercial Mobility Lab OpalTM | Lower back and both ankles—3 sensors per body part | FOG-provoking test in lab under 3 difficulty levels (single, dual motor, dual motor–cognitive) | SVM with RBF kernel: 86.6% accuracy, sensitivity: 80%, specificity: 82.5% | ON/ OFF | CLT | ML: support vector machine |
| Ashfaque et al. [40], 2021 | 10 PD-FoG+ Mean age: 66.4 Mean H&Y: 2.6 Dataset: DAHPNET | Accelerometer Commercial Mobility Lab OpalTM | Ankle, thigh, lower back—3 sensors per body part | Daily activities; annotated FOG and PreFOG (237 FOG events) | Best ensemble (M9): Accuracy: ~98.5% Precision: ~98%, sensitivity: ~98.5%, specificity: ~97.9% | NR | Sim. RL | ML: CNN, BiLSTM, ensemble models (machine learning) |
| Al-Adhaileh et al. [41], 2025 | Multiple datasets: tDCS FoG (50 PD-FoG+), DeFOG (60 PD-FoG+), daily living (65 total incl. 45 PD-FoG+, 20 negative controls), Hantao’s (30 PD-FoG+); mean age: NR Mean H&Y: NR | Accelerometers, gyroscope, magnetometers, EMG and EEG (varies by dataset) Commercial IMUs, varies by dataset | Lower limbs (shins, thighs, ankles), waist; EMG on lower limb muscles, EEG on scalp Location and number of sensor placements varies by dataset | Controlled lab (FoG-provoking), home walking, week-long daily life monitoring | HTSAN model: AUC: 0.88–0.96 F1-score: 0.84–0.94 Accuracy: 85–98% | Mixed ON/ OFF | CLT, RL | ML: HTSAN |
| Bächlin et al. [42], 2010 | 10 PD-FoG+; mean age: 66.4 years Mean H&Y: 2.6 | Accelerometer Custom IMUs | Shank, thigh, waist—3 sensor per body part | Straight walking, 360° turns, ADL simulations (e.g., fetching water) | Online detection: 73.1% sensitivity; 81.6% specificity | 8 pts. OFF, 2 ON | CLT and RL | Proprietary FOG detection algorithm |
| Jovanov et al. [43], 2009 | 1 PD FoG status: NR 4 “simulated” PD-FoG+ Mean age: NR Mean H&Y: NR | Accelerometer and gyroscope Commercial Bosch SMB380 | Right knee—1 sensor | Simulated FOG paths with sit-to-stand transitions and walking | Average detection latency of 332 ms, max latency of 580 ms; 0 false positives in 5 trials | NR | CLT | Rule-based algorithm with FFT (threshold) |
| Naghavi et al. [44], 2019 | 18 PD Mean age: 70.0 FoG status: NR Mean H&Y: NR | Accelerometer Commercial Mobility Lab OpalTM | Right and left ankles—1 sensor per body part | Obstacle-triggering path with narrow corridors, turns, stops | Best model ensemble): 97.4% FoG detection, 66.7% prediction, F1-score of 90.7% Sensitivity: 90.8%, specificity: 95% | NR | CLT | ML: ensemble: support vector machines, k-nearest neighbours, multi-layer perceptron |
| Shi et al. [45], 2020 | 67 PD-FoG+ Mean age: 69 Mean H&Y: NR | Accelerometer and gyroscope Custom IMUs | Both ankles and C7 vertebra—1 sensor per body part | 7m TUG and simulated real-life setting | 2D CNN (best ensemble) Accuracy: 89.2%, sensitivity: 82.1, specificity: 96% | NR | CLT, VV | ML: 2D CNN |
| May et al. [24], 2023 | 19 PD-FoG+ Mean age: 71.95 Mean H&Y: 2.7 | Accelerometer and gyroscope Commercial Physilog and ActiGraph | Both feet and left side of waist—1 sensor per body part | Laboratory FoG tasks, simulated IADL tasks, 3-day unsupervised home monitoring | Mean detection accuracy > 90% (IADL tasks); strong correlation with video review (ρ = 0.77); home and lab sensor data correlation (ρ = 0.72) | ON | CLT and RL | ML: 2D CNN with continuous wavelet transform |
| Seuthe et al. [46], 2024 | 50 PD (22 PD-FoG+ 28 PD-FoG) Mean age: NR Mean H&Y: 2.1 | Accelerometer Commercial Mobility Lab OpalTM | Gait initiation test: feet and lower back—1 sensor per body part For FoG detection: both shanks—1 sensor per body part | Gait initiation, overground walking, turning in place; single-task and dual-task conditions | Neither ML APA size nor APAPA size was significantly correlated with any FOG-related outcomes | ON | Sim. RL, RL | Modified pFOG algorithm, threshold: pFOG > 0.7 |
| Belluscio et al. [47], 2019 | 32 PD (15PD-FoG+ 17 PD- FoG- and 8 HC Mean age: 67 Mean H&Y: NR | Accelerometer, gyroscope, magnetometer and fNRIS Commercial Mobility Lab OpalTM | IMUs: sternum, pelvis, wrists, shanks, both feet—1 sensor per body part fNRIS—forehead | 360° turning-in-place for 2 minutes under single-task and dual-task conditions | Higher PFC activity is correlated with worse FOG in PD-FOG+ patients (p.0.048) and smaller number of turns in PD-FOG+ (P 0.02) | OFF | CLT | Signal preprocessing of fNIRS (HbO2/HHb). IMU-derived FoG ratio (threshold) |
| Goris et al. [14], 2025 | 177 PD (54 PD-FoG-, 22 PD-FoG+ (aware), 82 PD-FoG+ (unaware) Mean age: 62.56 Mean H&Y: 1–3 | Accelerometer and gyroscope Commercial Mobility Lab OpalTM | Both shins, lower back—1 sensor per body part Feet-mounted sensors were excluded from final analysis | 1-minute 360° alternating turn with cognitive dual-task | Best AUC = 0.65, sensitivity: 68.3%, specificity: 61.7% | ON | CLT | ML: FOG index derived from FOG ratio; frequency domain analysis; ROC analysis (threshold) |
| Arami et al. [26], 2019 | 10 PD-FoG+ Mean age: 66.5 Mean H&Y: 2.6 Dataset: DAPHNET | Accelerometer Custom IMUs | Lower back, shank, thigh—3 sensors | Walking trials with turns and simulated ADLs | Sensitivity: 93% Specificity: 91% | ON | CLT, RL | ML: SVM and PNN |
| Hu et al. [48], 2023 | 21 PD FoG status: NR Mean age: NR Mean H&Y: NR | Foot pressure sensor—1 sensor Commercial Zeno Walkway | Feet—1 sensor (walkway) | TUG trials with FoG provoking elements | Sensitivity: 83.4% Specificity: 72.9% Accuracy: 75.7% AUC: 0.85 | O/OFF | CLT | ML: adversarial spatial–temporal network |
| Mazilu et al. [49], 2015 | 18 PD-FoG+ Mean age: 68.9 Mean H&Y: 2-4 Dataset: CuPiD | ECG and SC Commercial Shimmer sensors | Sternum and wrist—2 sensors | Walking trials with FoG provoking elements | 71.3% of FoG episodes predicted with SC sensor | ON | CLT | Anomaly-based algorithm (threshold) |
| Mikos et al. [50], 2019 | 63 PD-FoG+ Mean age: 68.9 Mean H&Y: 2.5 | Accelerometer, gyroscope and magnetometer Commercial | Ankle—1 sensor | Walking trials with turns and narrow spaces | Sensitivity: 95.6% Specificity: 90.2% | NR | CLT | ML: neural network |
| Murtaza et al. [51], 2025 | 12 PD-FoG+ patients Mean age: 69.1 Mean H&Y: not reported | Accelerometers, gyroscopes, EEG, EMG and SC Commercial IMUs | Waist, both shanks (IMUs), left wrist finger (SC), mastoid process (EEG), shins (EMG) | Walking trials with turns, stops, avoiding obstacles | EMG and IMUs data (best combination) F1 score: 98.82% | OFF | CLT | ML: SVM |
| Noor et al. [52], 2021 | 10 PD-FoG+ Mean age: NR Mean H&Y: NR Dataset: DAPHNET | Accelerometer Custom IMUs | Shank, thigh, lower back—3 sensors | Three walking trials with ADLs simulation | Sensitivity: 90.94% Specificity: 67.04% | NR | CLT | ML: naïve Bayes, SVM with RBF kernel, SVM with polynomial kernel, random forest, ensemble voting |
| Pierleoni et al. [53], 2019 | 10 PD-FoG+ Mean age: 67-7 Mean H&Y: NR | Accelerometer, gyroscope and magnetometer Commercial | Feet—1 sensor on each foot | TUG, walking through narrow spaces | 99.7% accuracy for FoG detection | ON | CLT | Freeze index (threshold) |
| Prado et al. [54], 2020 | 8 PD-FoG+ and 2 FoG- Mean age: 67.9 Mean H&Y: 2.8 | accelerometer, gyroscope, foot pressure sensors Commercial DeepSole system | Feet—12 sensors per foot | 7-meter Zeno Walkway | Sensitivity: 96.0% Specificity: 99.6% Accuracy: 99.5% | NR | CLT | ML: CNN |
| Shi et al. [55], 2022 | 63 PD-FoG+ Mean age: 69.4 Mean H&Y: NR | Accelerometer, gyroscope and magnetometer Custom IMUs | Ankle—1 sensor on each ankle | TUG and second walking trial with FoG provoking elements | F1 score: −91.5% | NR | CLT | ML: CNN |
| Tahafchi et al. [56], 2019 | 4-PD-FoG+ Mean age: NR Mean: H&Y: NR | Accelerometer, gyroscope and EMG Commercial Shimmer IMUs | Feet, shanks—2 sensors on each side | Walking trials designed to trigger FoG | AUC: 0.906-0.963 | NR | CLT | ML: CNN |
| Sensor Type for FoG Detection | Number of Studies | Percentage of Total Number of Studies |
|---|---|---|
| Accelerometer | 10 | 23.2% |
| Accelerometer + gyroscope | 11 | 25.5% |
| Accelerometer + gyroscope + magnetometer | 9 | 20.9% |
| Accelerometer + plantar pressure sensor | 1 | 2.3% |
| Accelerometer + gyroscope + plantar pressure sensor | 2 | 4.7% |
| Accelerometer + gyroscope + force sensing resistors | 1 | 2.3% |
| Accelerometer + gyroscope +muscle activity sensors | 1 | 2.3% |
| Accelerometer + gyroscope + sEMG | 2 | 4.7% |
| Accelerometer + gyroscope + magnetometer + sEMG | 1 | 2.3% |
| Accelerometer + gyroscope + EMG + SC sensor | 1 | 2.3% |
| Plantar pressure sensor | 3 | 7.0% |
| ECG + SC sensor | 1 | 2.3% |
| Sensor Type for FoG Detection | No. of Articles | Sensitivity (%) | Specificity (%) | Accuracy (%) | Other Performance Metrics | Algorithm | Author of Article with Best Performance |
|---|---|---|---|---|---|---|---|
| Accelerometer | 10 | 98.5 | 97.9 | 98.5 | - | ML | Ashfaque et al. [40] |
| Accelerometer + gyroscope | 11 | ~100 | ~100 | - | Avg. error rate: < 5% | ML | Chomiak et al. [21] |
| Accelerometer + gyroscope + magnetometer | 9 | 83 | 98 | 96 | - | M/ Threshold | Antonini et al. [30] |
| Accelerometer + plantar pressure sensor | 1 | 96 | 94 | - | - | Threshold | Marcante et al. [18] |
| Accelerometer + gyroscope + plantar pressure sensor | 2 | 96.0 | 99.6 | 99.5 | - | ML | Prado et al. [54] |
| Accelerometer + gyroscope + force sensing resistors | 1 | 78.4 | 91.7 | 88.1 | F1 score: 0.78 | ML | Ren et al. [28] * |
| Accelerometer + gyroscope + muscle activity sensors | 1 | 2.7 | 97.2 | 95.0 | F1 score: 0.023 | ML | Slemenšek et al. [20] |
| Accelerometer + gyroscope + magnetometer + sEMG | 1 | - | - | 85-97 | HTSAN model: AUC 0.88–0.96 F1-score: 0.84–0.94 | ML | Al-Adhaileh et al. [41] |
| Accelerometer + gyroscope + sEMG | 2 | - | - | - | FoG detection: 2% false negatives and 5% false positives | Threshold | Mazzetta et al. [25] |
| Accelerometer + gyroscope + EMG + SC sensor | 1 | - | - | - | F1 score: 0.99 (EMG and IMUs data) | ML | Murtaza et al. [51] |
| Plantar pressure sensor | 3 | 88 | 99 | 99 | F1 score: 0.76 | ML | Park et al. [22] |
| ECG + SC | 1 | - | - | - | 71.3% of FoG episodes predicted with SC sensor | Threshold | Mazilu et al. [49] |
| Sensor Type for FoG Detection | No. of Articles | Sensitivity (%) | Specificity (%) | Accuracy (%) | Other Performance Metrics | Sensor Integration Method and Placement | Algorithm | Author of Article with Best Performance |
|---|---|---|---|---|---|---|---|---|
| Accelerometer | 5 | 87.7 | 88.3 | - | 50% predicted FoG 3.1s before onset, 50% detected with 0.8 s delay | STAT-ONTM—single device worn on waist | ML | Borzi et al. [32] |
| Accelerometer + gyroscope + magnetometer | 1 | - | - | - | Time spent freezing differentiated FoG+ and FoG- | The Opal V2RTM—1 sensor on each foot and 1 sensor on lower back | Threshold | Mancini et al. (study II) [34] |
| Accelerometer + gyroscope | 2 | - | - | >90% | Strong correlation with video review (ρ = 0.77) Home and laboratory sensor data correlation (ρ = 0.72) | 1 sensor on each foot and 1 sensor on waist | ML | May et al. [24] |
| Accelerometer + gyroscope + magnetometer (DeFoG dataset) Accelerometer + gyroscope (Daily Living dataset) | 1 | - | - | DeFOG: 87% Daily living: 85% | DeFOG: AUC: 0.91 F1 score: 0.88 Daily: AUC: 0.88 F1 score: 0.84 | DeFOG dataset: 1 sensor on each ankle Daily living: 1 sensor on each ankle and 1 sensor on waist | ML | Al-Adhaileh et al. [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorčič, M.; Georgiev, D. The Usefulness of Wearable Sensors for Detecting Freezing of Gait in Parkinson’s Disease: A Systematic Review. Sensors 2025, 25, 5101. https://doi.org/10.3390/s25165101
Gregorčič M, Georgiev D. The Usefulness of Wearable Sensors for Detecting Freezing of Gait in Parkinson’s Disease: A Systematic Review. Sensors. 2025; 25(16):5101. https://doi.org/10.3390/s25165101
Chicago/Turabian StyleGregorčič, Matic, and Dejan Georgiev. 2025. "The Usefulness of Wearable Sensors for Detecting Freezing of Gait in Parkinson’s Disease: A Systematic Review" Sensors 25, no. 16: 5101. https://doi.org/10.3390/s25165101
APA StyleGregorčič, M., & Georgiev, D. (2025). The Usefulness of Wearable Sensors for Detecting Freezing of Gait in Parkinson’s Disease: A Systematic Review. Sensors, 25(16), 5101. https://doi.org/10.3390/s25165101






