Three-Dimensional Printed Stimulating Hybrid Smart Bandage
Abstract
1. Introduction
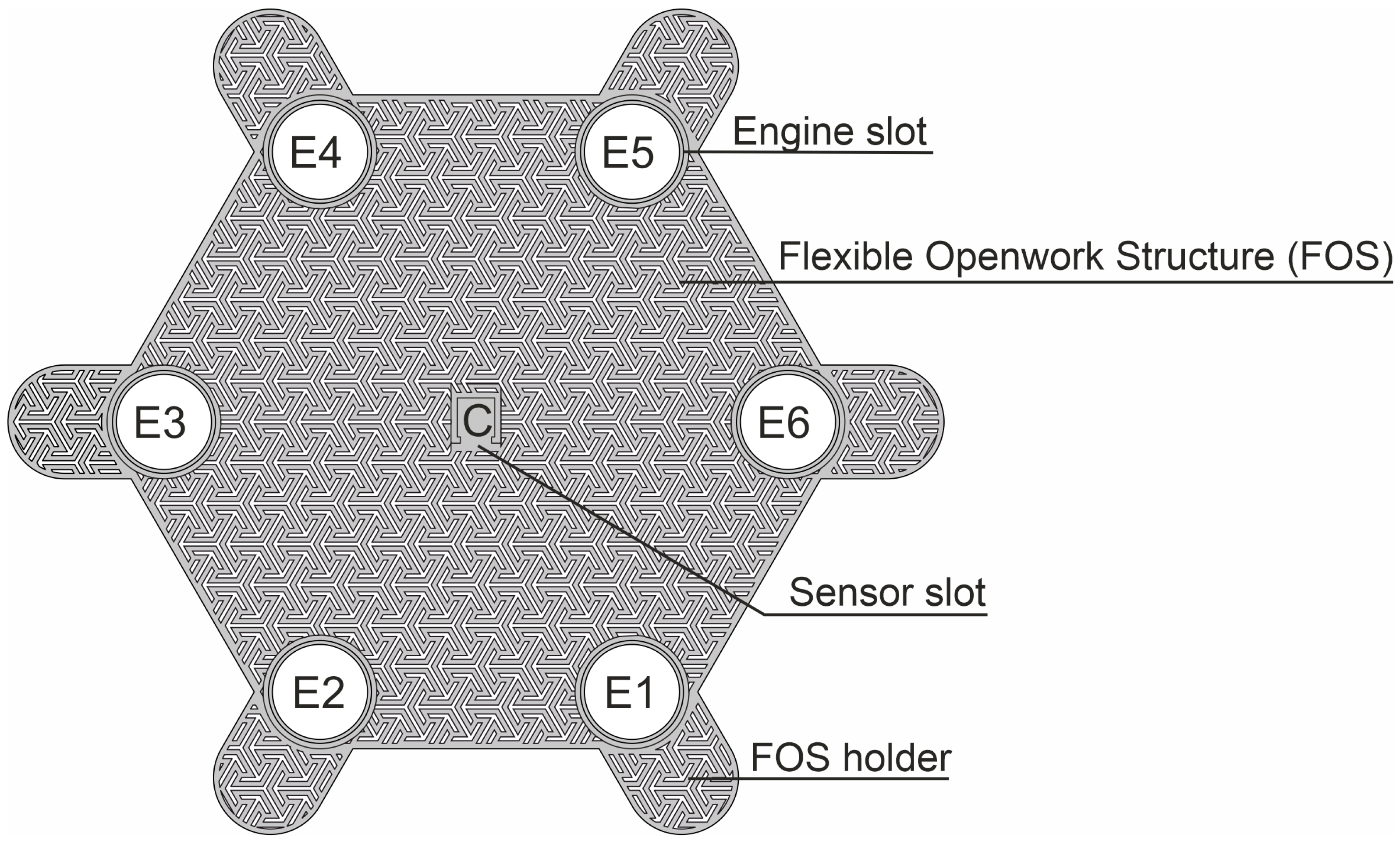
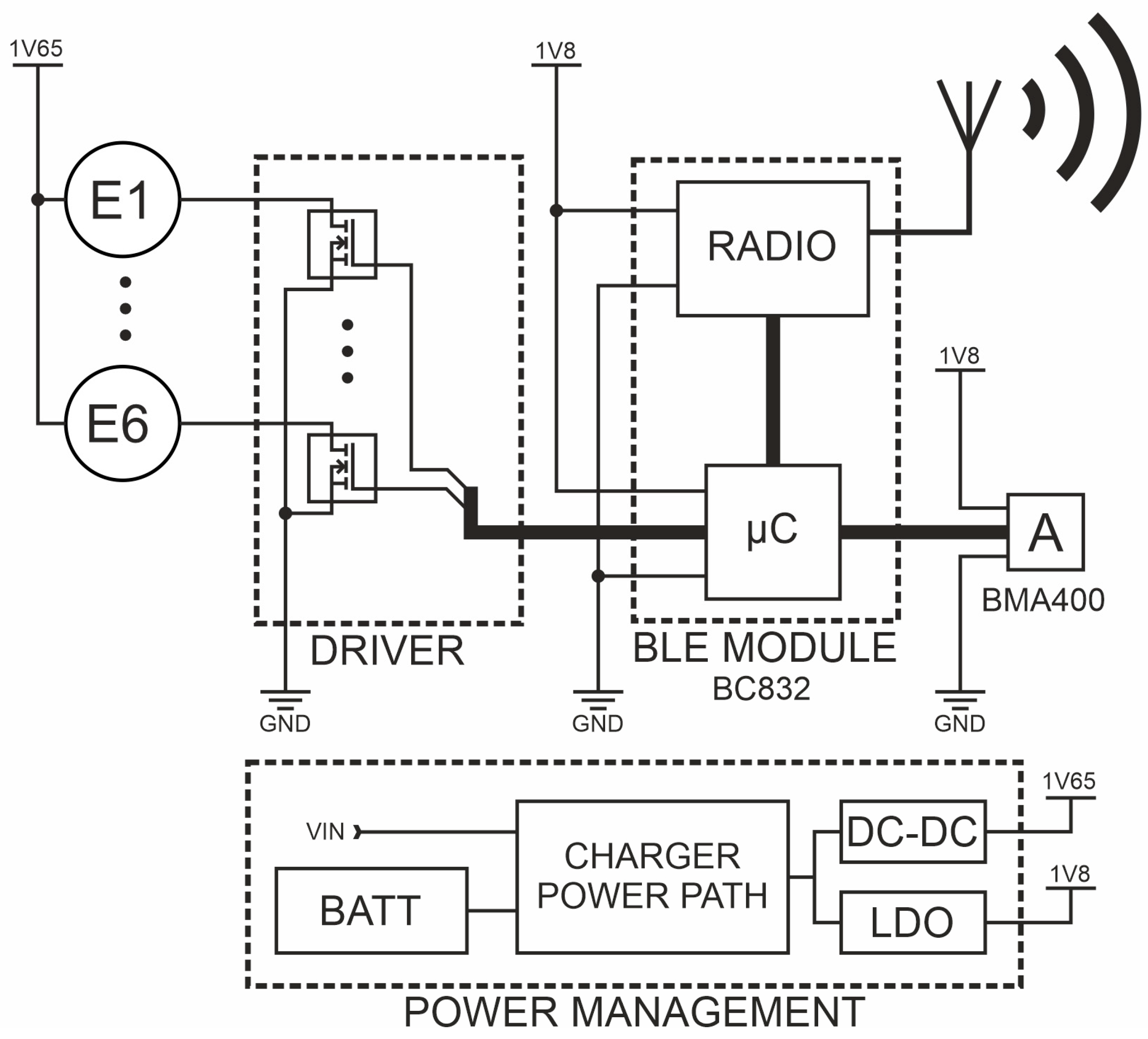
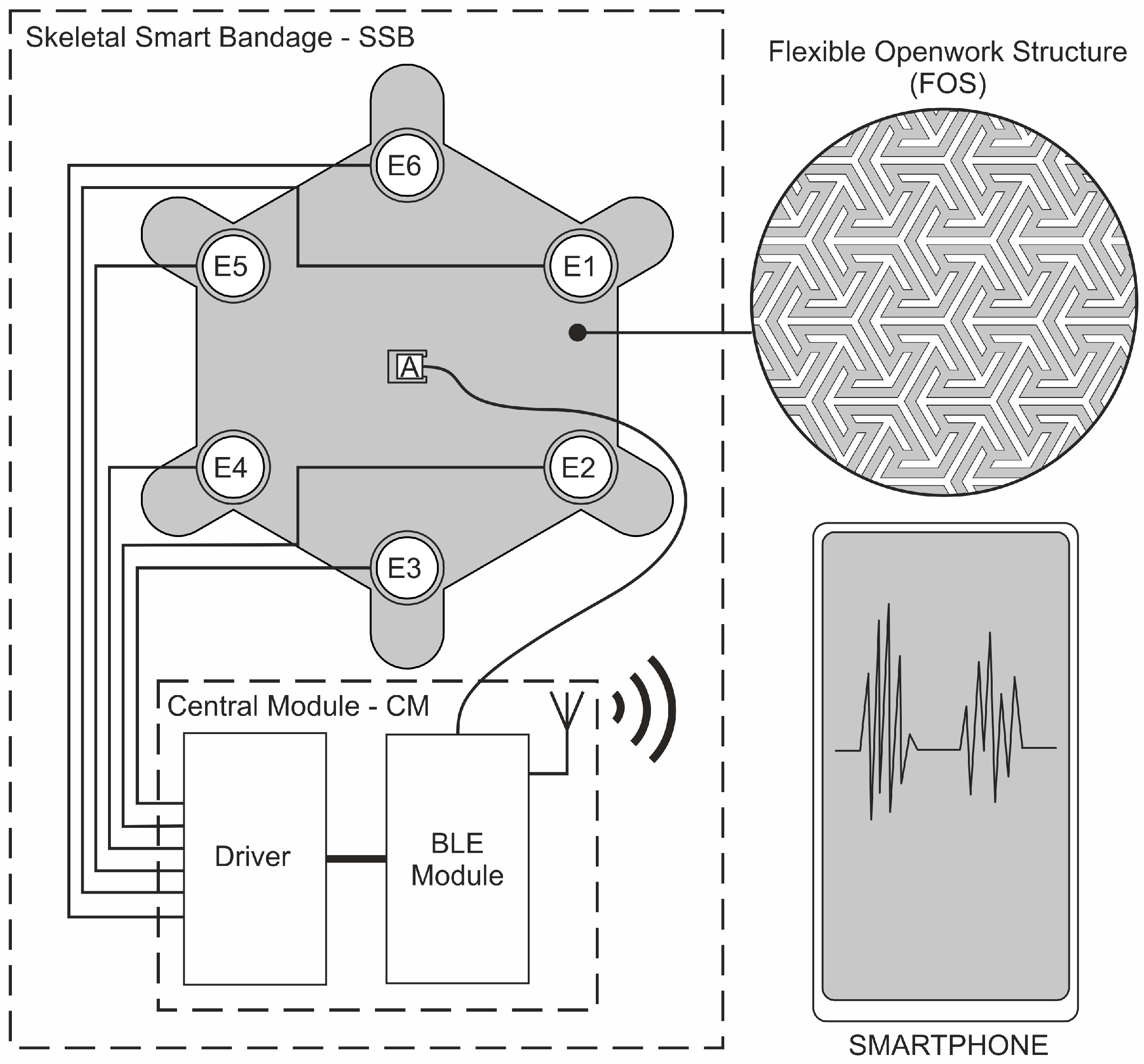
2. Materials and Methods
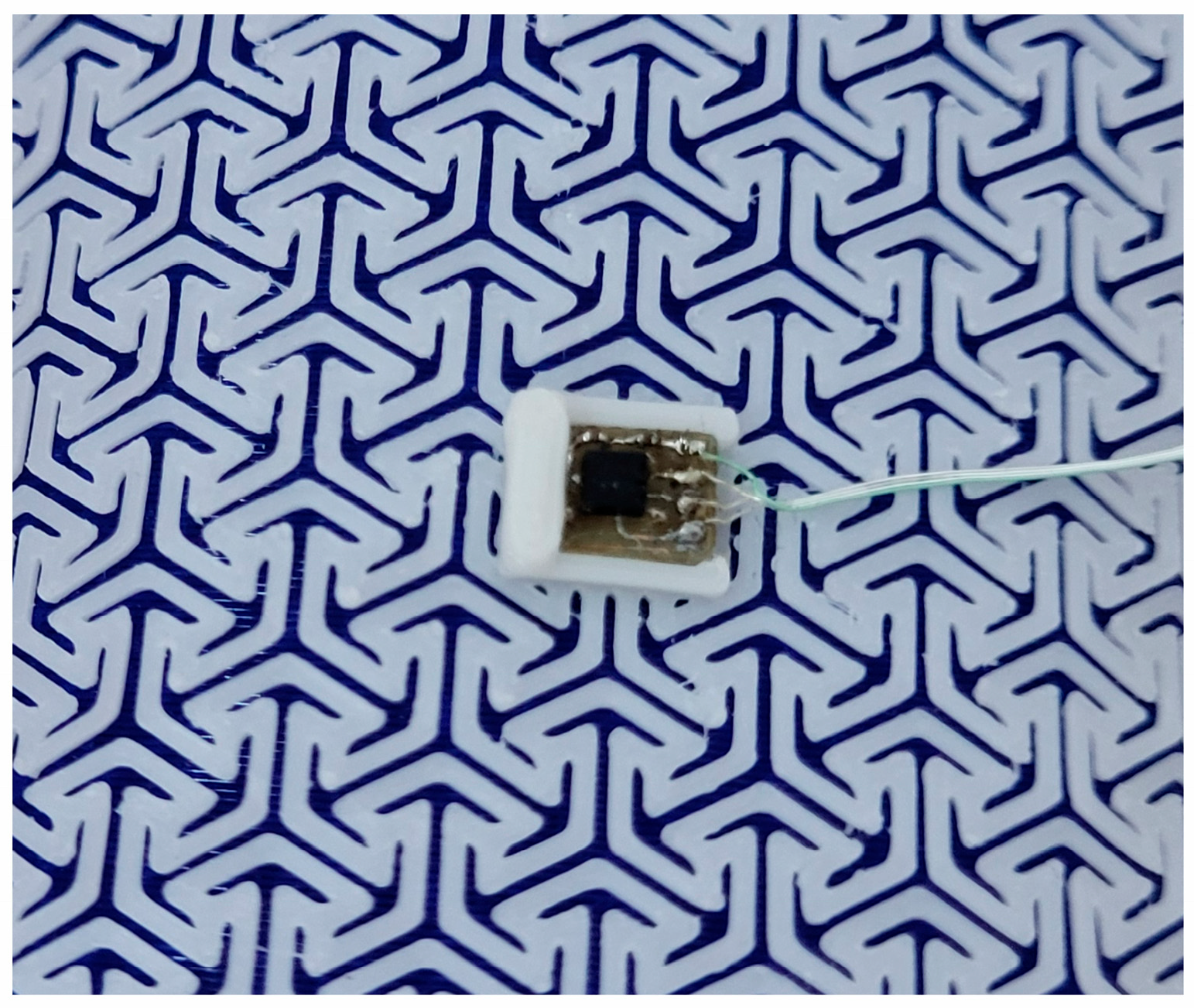
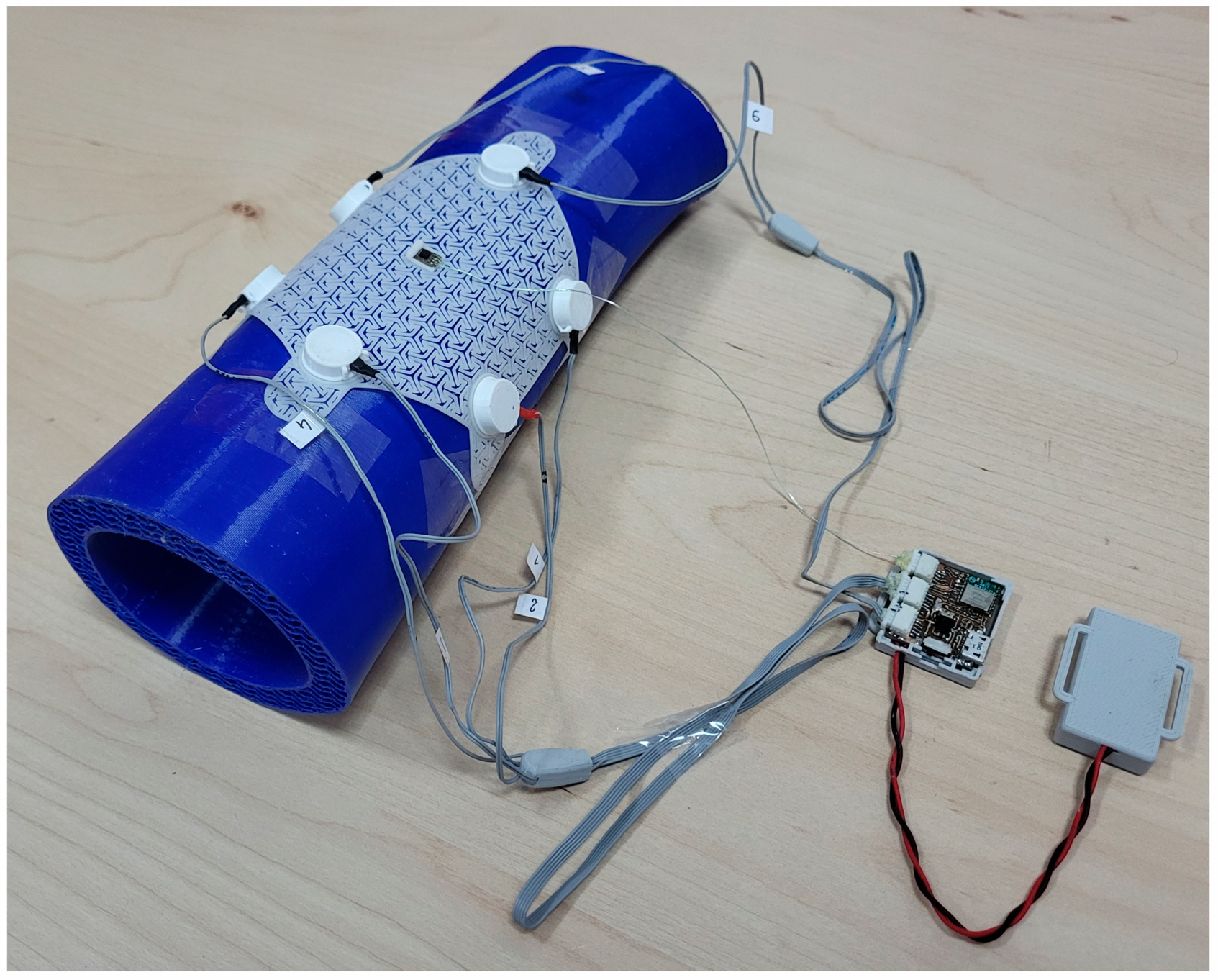
2.1. Measurement Equipment
2.2. Verification of Wearable System
- P—pressure;
- T—tension (applied force);
- n—number of bandage layers;
- r—radius of curvature;
- w—bandage width.
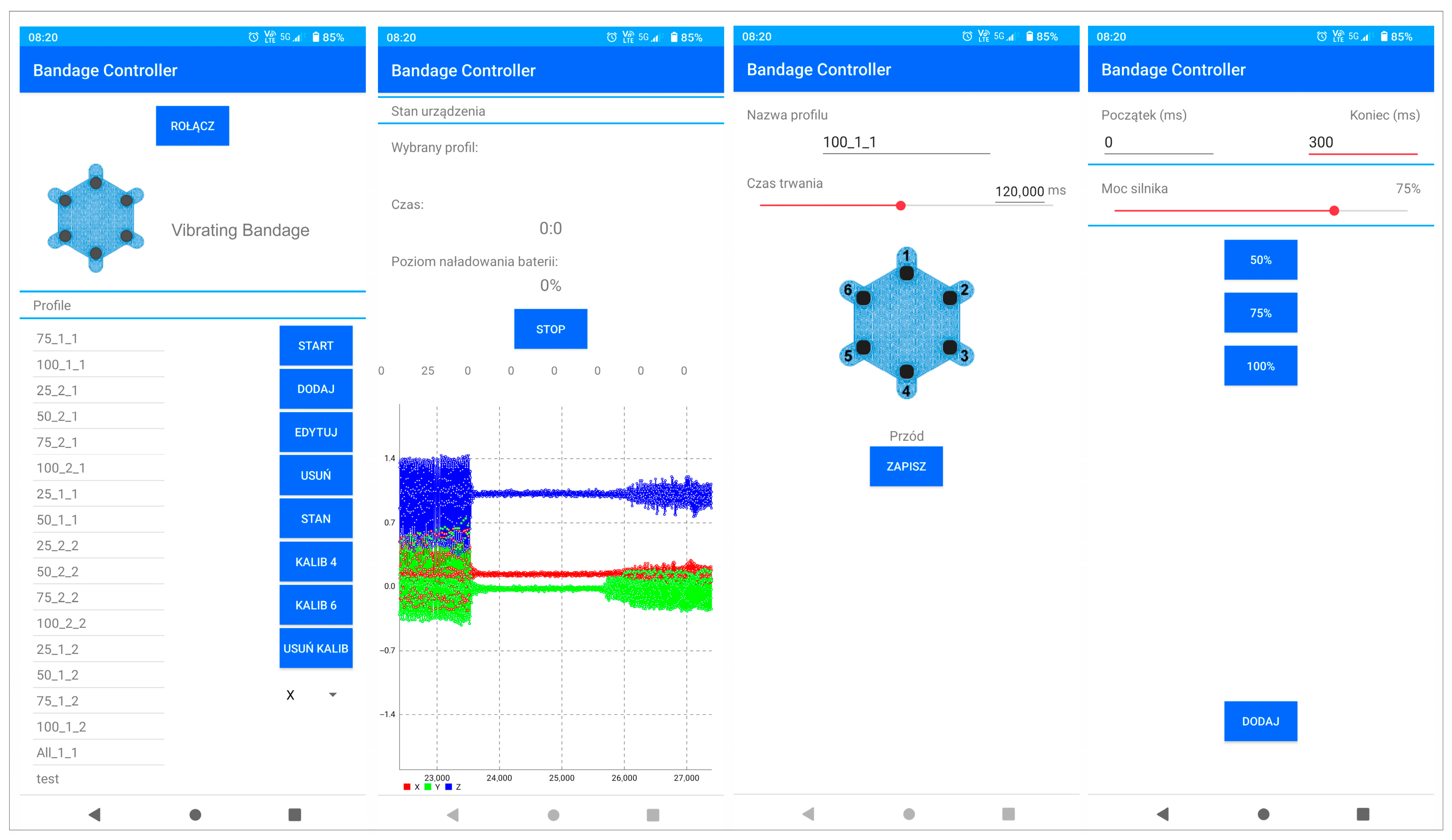
2.3. Smart Bandage Mobile Application
2.4. Calibration Procedure of Actuators
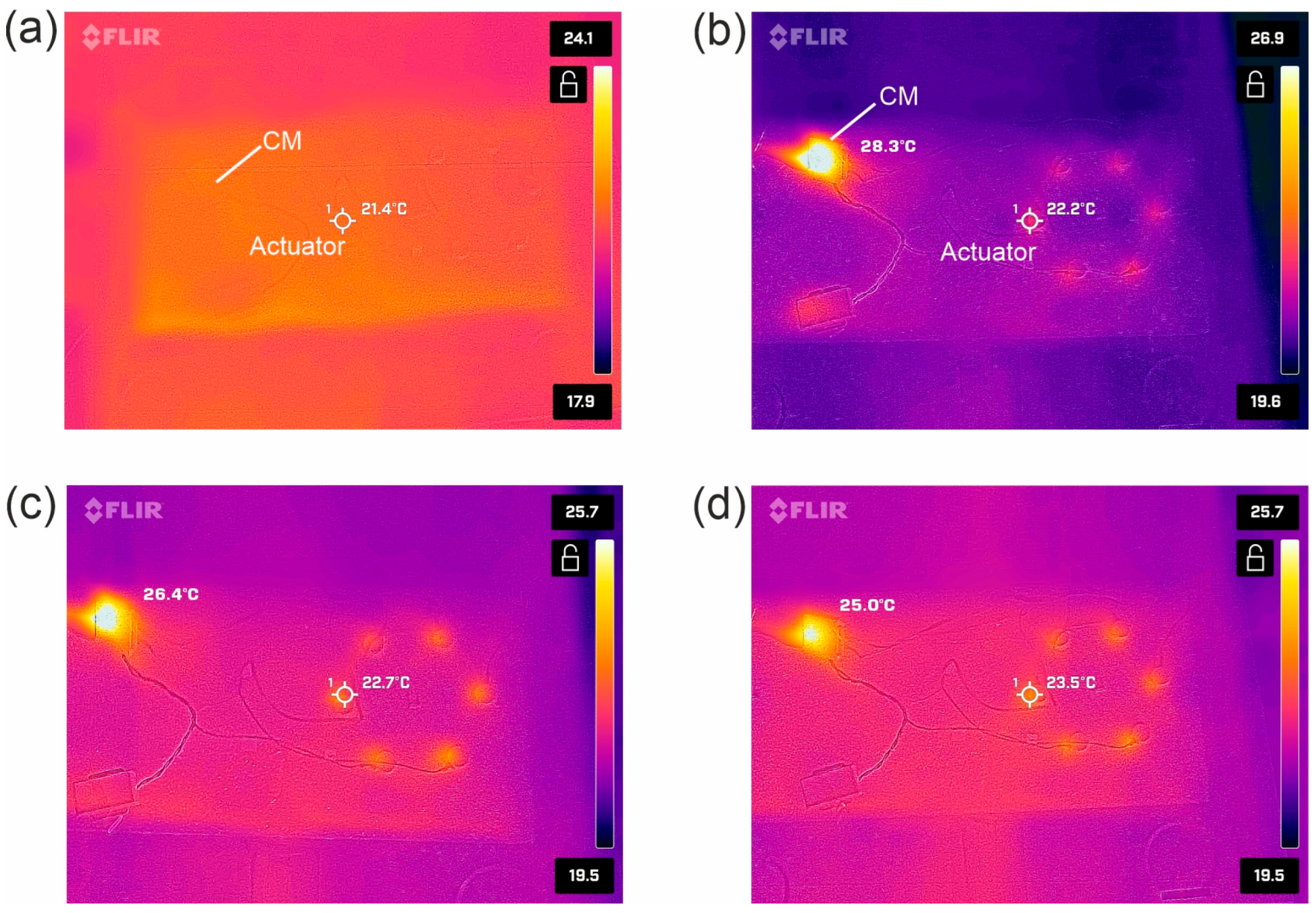
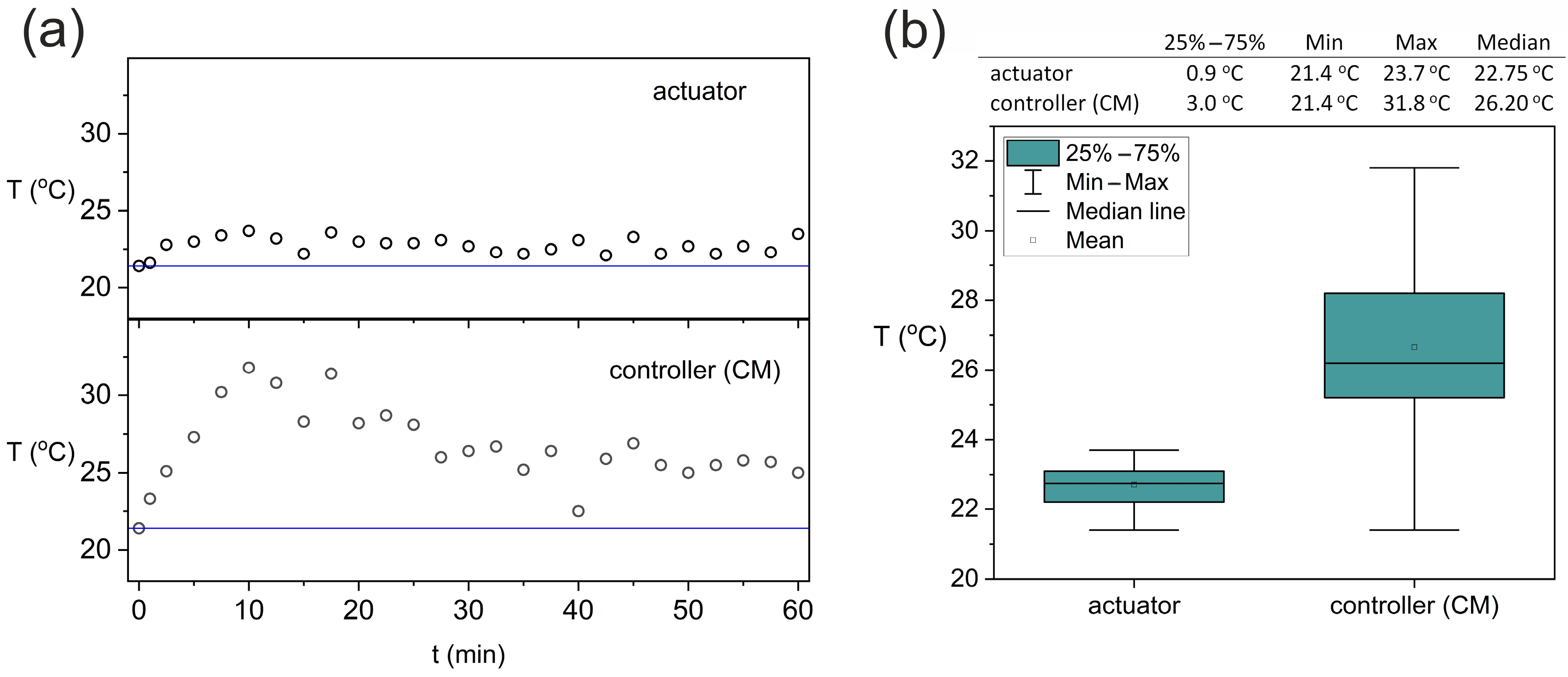
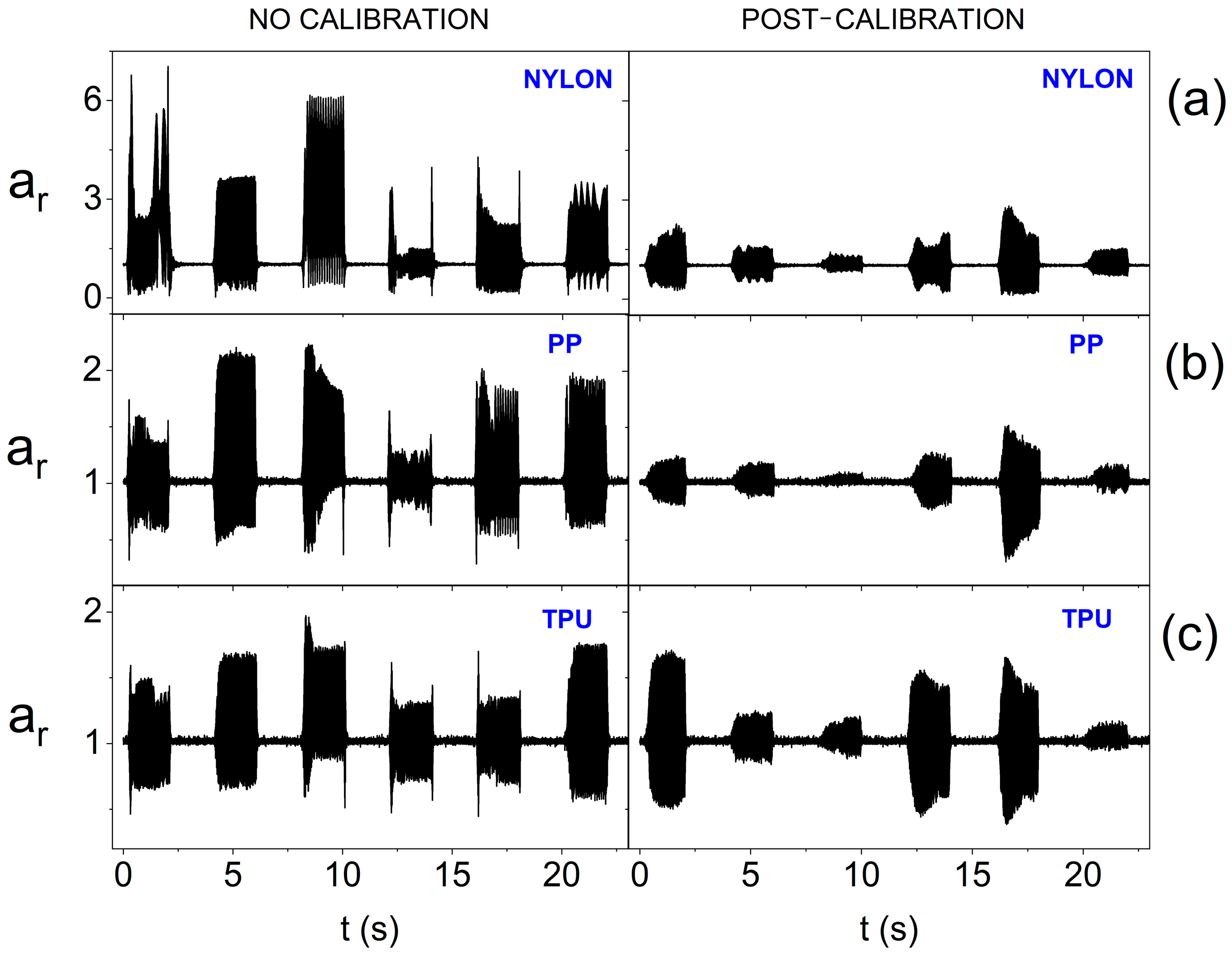
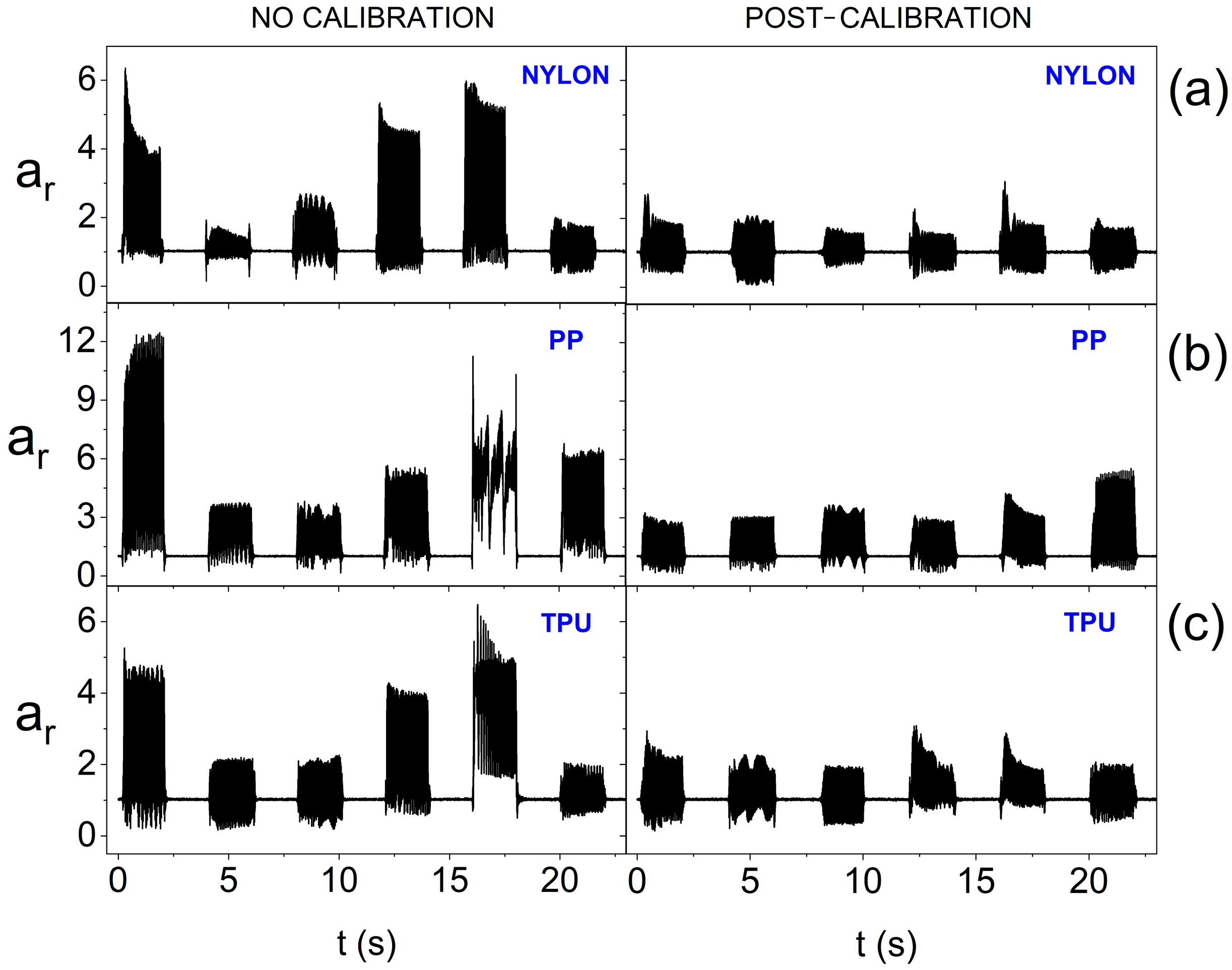
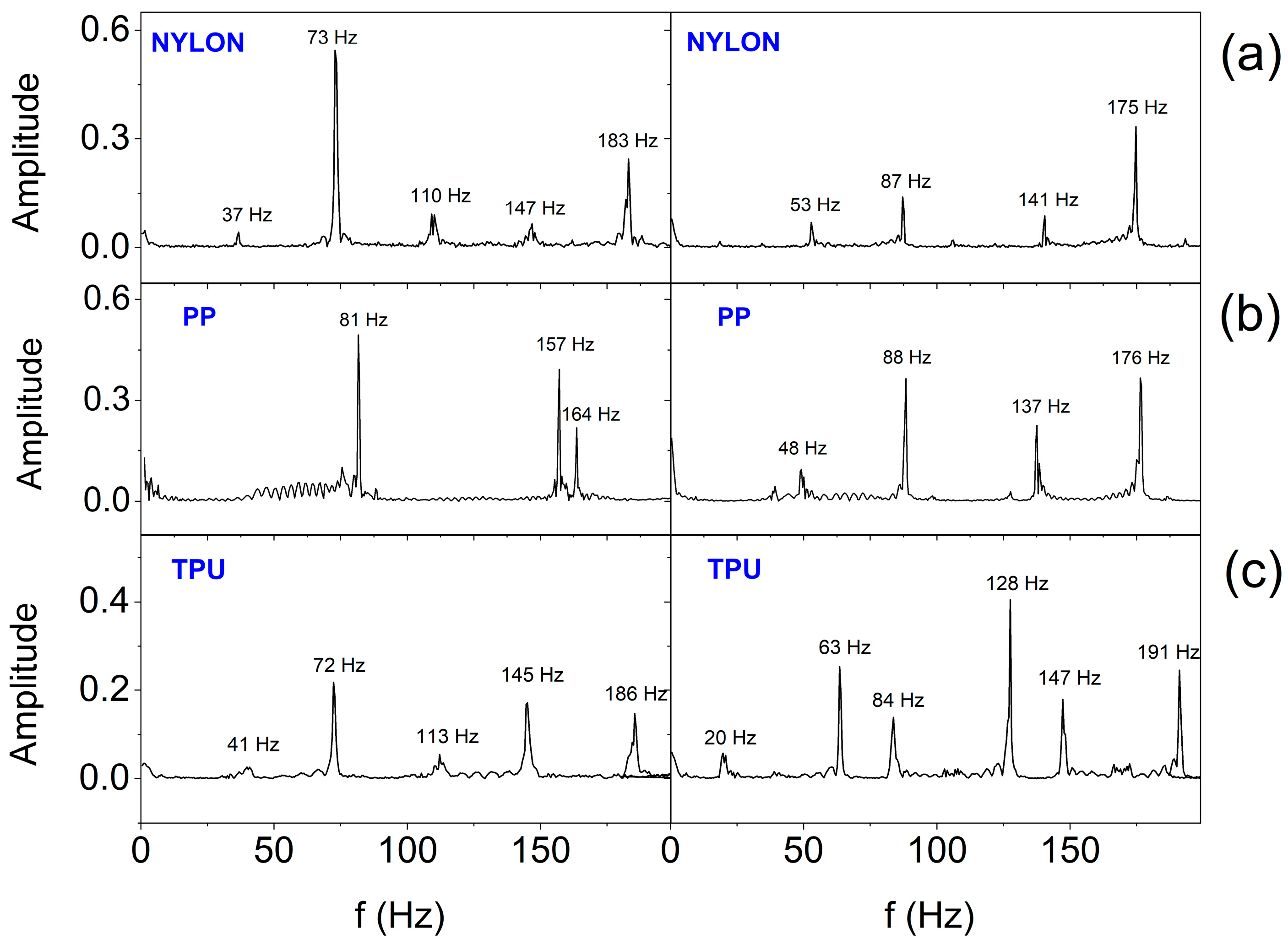
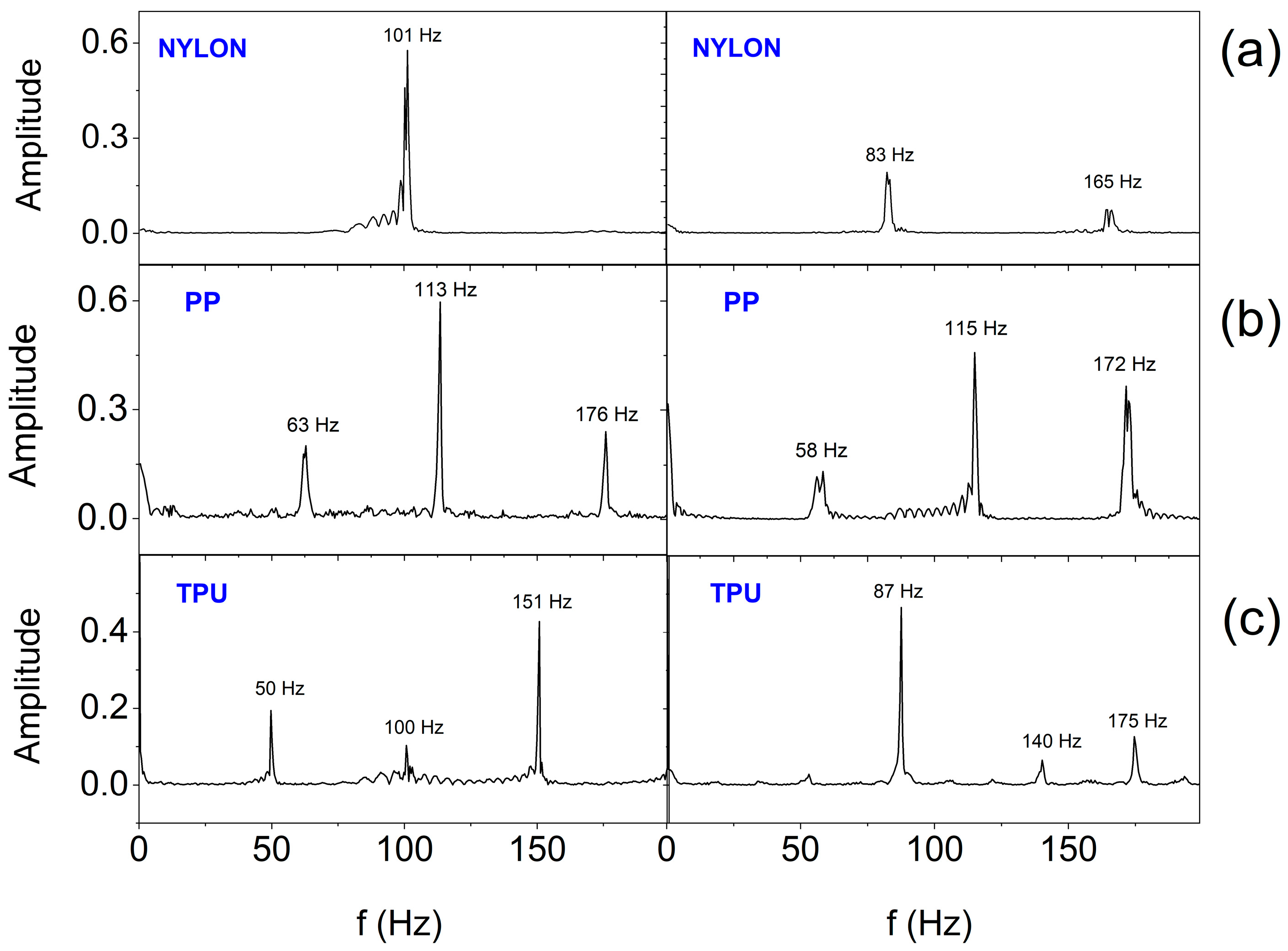
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagan, S.M.; Choi, S.B.; Seung-Boo, J.; Jong-Woong, K. Electronic textiles: New age of wearable technology for healthcare and fitness solutions. Mater. Today Bio 2023, 19, 100565. [Google Scholar] [CrossRef] [PubMed]
- Knulst, A.J.; Berger, S.; van den Boom, J.; Bosch, I.; Nicolai, N.; Maharjan, S.; Raaijmakers, E.; Tsai, C.-L.; van de Weerd, L.; Dankelman, J.; et al. The WOCA negative pressure wound therapy device designed for low resource settings. HardwareX 2025, 21, e00620. [Google Scholar] [CrossRef]
- Piper, A.; Öberg Månsson, I.; Khaliliazar, S.; Landin, R.; Hamedi, M.M. A disposable, wearable, flexible, stitched textile electrochemical biosensing platform. Biosens. Bioelectron. 2021, 191, 113604. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Haque, M.N.; Kabiraz, D.C.; Yeasin, A.; Rashid, H.A.; Sarker, A.C.; Hossain, G. A review on advanced nanocomposites materials based smart textile biosensor for healthcare monitoring from human sweat. Sens. Actuators A Phys. 2023, 350, 114093. [Google Scholar] [CrossRef]
- Kolipaka, T.; Pandey, G.; Abraham, N.; Srinivasarao, D.A.; Raghuvanshi, R.S.; Rajinikanth, P.S.; Tickoo, V.; Srivastava, S. Stimuli-responsive polysaccharide-based smart hydrogels for diabetic wound healing: Design aspects, preparation methods and regulatory perspectives. Carbohydr. Polym. 2024, 324, 121537. [Google Scholar] [CrossRef]
- Garraud, O.; Hozzein, W.N.; Badr, G. Wound healing: Time to look for intelligent, ‘natural’ immunological approaches? BMC Immunol. 2017, 18 (Suppl. 1), 23. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart wound dressing for advanced wound management: Real-time monitoring and on-demand treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Yang, C.; Yang, C.; Chen, Y.; Liu, J.; Liu, Z.; Chen, H.-J. The trends in wound management: Sensing, therapeutic treatment, and “theranostics”. J. Sci. Adv. Mater. Devices 2023, 8, 100619. [Google Scholar] [CrossRef]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent advances in biosensors for real time monitoring of pH, temperature, and oxygen in chronic wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2022, 41, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Hossain, I.; Zahid, S.; Chowdhury, M.A.; Hossain, M.; Hossain, N.; Islam, A.; Mobarak, H. Smart bandage: A device for wound monitoring and targeted treatment. Results Chem. 2024, 7, 101292. [Google Scholar] [CrossRef]
- Xu, L.; Ding, L.; Sun, Y.; Zhang, T.; Zhu, Y.; Yan, B.; Yang, M.; Ramakrishna, S.; Zhang, J.; Long, Y.-Z. Stretchable, flexible and breathable polylactic acid/polyvinyl pyrrolidone bandage based on Kirigami for wounds monitoring and treatment. Int. J. Biol. Macromol. 2023, 237, 124204. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 28949. [Google Scholar] [CrossRef]
- Pal, A.; Goswami, D.; Cuellar, H.E.; Castro, B.; Kuang, S.; Martinez, R.V. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 2018, 117, 696–705. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Xu, C.; Wang, C.; Song, Y.; Min, J.; Tu, J.; Solomon, S.A.; Li, J.; Banks, J.L.; Armstrong, D.G.; et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 2023, 9, eadf7388. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Jia, Z.; Song, X.; Wang, S.; Bai, B.; Wang, J.; Zhang, J.; Zhou, G.; Lei, D. Mechanically skin-like and water-resistant self-healing bioelastomer for high-tension wound healing. Bioact. Mater. 2024, 39, 443–455. [Google Scholar] [CrossRef]
- Vithalani, H.; Dave, H.; Singh, H.; Sharma, D.; Navale, A.; Dhanka, M. Mechanically robust, mouldable, dynamically crosslinked hydrogel flap with multiple functionalities for accelerated deep skin wound healing. Biomater. Adv. 2025, 169, 214195. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Yang, P.; He, H.; Yang, Y.; Lan, Z.; Guo, W.; Qin, Y.; Zhang, Q.; Li, S. A multifunctional electronic dressing with textile-like structure for wound pressure monitoring and treatment. J. Colloid Interface Sci. 2025, 679, 737–747. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Y.; Chang, J.; Hui, Y.; Ye, J.; Weng, G.; Li, M.; Wang, Y.; Wum, Q. Electrostimulation combined with biodegradable electroactive oriented nanofiber polycaprolactone/gelatin/carbon nanotube to accelerate wound healing. Mater. Today Bio 2025, 31, 101490. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Lu, W.; Hu, Y. Review on chitosan-based antibacterial hydrogels: Preparation, mechanisms, and applications. Int. J. Biol. Macromol. 2025, 255, 128080. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, H.; Aghabaglou, F.; McCarthy, A.; Mostafavi, A.; Wiseman, C.; Bonick, Z.; Ghanavati, I.; Harris, S.; Kreikemeier-Bower, C.; MousaviBasri, S.M.; et al. A Wirelessly Controlled Smart Bandage with 3D-Printed Miniaturized Needle Arrays. Adv. Funct. Mater. 2020, 30, 1905544. [Google Scholar] [CrossRef] [PubMed]
- Santos de Oliveira, R.; Fantaus, S.S.; Guillot, A.J.; Melero, A. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yi, C.; Lin, S.; Ye, H.; Xue, J.; Yin, J.; Zhong, R.; Yao, H.; Liao, H.; Zhang, Y.; et al. e-Bandage: Exploiting Smartphone as a Therapeutic Device for Cutaneous Wound Treatment. Adv. Intell. Syst. 2024, 6, 2300494. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Sun, T.; Zeng, D.; Yang, C.; Wang, H.; Yang, C.; Guo, J.; Wu, Q.; Chen, H.-J.; et al. Integrated Multiplex Sensing Bandage for In Situ Monitoring of Early Infected Wounds. ACS Sens. 2021, 6, 3112–3124. [Google Scholar] [CrossRef]
- Heck, I.; Lu, W.; Wang, Z.; Zhang, X.; Adak, T.; Cu, T.; Crumley, C.; Zhang, Y.; Wang, X.S. Wireless Pressure-Sensor-Integrated Smart Bandage for the Management of Diabetic Foot Ulcers. Adv. Mater. Technol. 2023, 8, 2200821. [Google Scholar] [CrossRef]
- Jose, M.; Vijjapu, M.T.; Neumaier, L.; Rauter, L.; Chakkunny, A.H.; Corzo, D.; Thoelen, R.; Picard, A.; Kosel, J.; Deferme, W. Convergence of biocompatible printed electronics and sensing in wound dressings: A leap forward in sustainable health monitoring. Flex. Electron. 2025, 9, 46. [Google Scholar] [CrossRef]
- Saragih, I.D.; Susanto, H.; Lin, H.-C.; Lee, B.-O. Vibration therapy for patients with hard-to-heal wounds: A systematic review and meta-analysis of experimental studies. J. Tissue Viability 2025, 34, 100852. [Google Scholar] [CrossRef]
- Johnson-Love, O.; Salmeron-Sanchez, M.; Reid, S.; Childs, P.G.; Dalby, M.J. Vibration-based cell engineering. Nat. Rev. Bioeng. 2025, 3, 408–429. [Google Scholar] [CrossRef]
- Yu, C.O.; Leung, K.S.; Jiang, J.L.; Wang, T.B.; Chow, S.K.; Cheung, W.H. Low-Magnitude High-Frequency Vibration Accelerated the Foot Wound Healing of n5-streptozotocin-induced Diabetic Rats by Enhancing Glucose Transporter 4 and Blood Microcirculation. Sci. Rep. 2017, 7, 11631. [Google Scholar] [CrossRef]
- Syabariyah, S.; Nurachmah, E.; Widjojo, B.D.; Prasetyo, S.; Sanada, H.; Irianto; Nakagami, G.; Suriadi; Kardiatun, T.; Hisan, U.K. The Effect of Vibration on the Acceleration of Wound Healing of Diabetic Neuropathic Foot Ulcer: A Prospective Experimental Study on Human Patients. Healthcare 2023, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Pongkitwitoon, S.; Weinheimer-Haus, E.M.; Koh, T.J. Low-intensity vibrations accelerate proliferation and alter macrophage phenotype in vitro. J. Biomech. 2016, 49, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer-Haus, E.M.; Judex, S.; Ennis, W.J.; Koh, T.J. Low-Intensity Vibration Improves Angiogenesis and Wound Healing in Diabetic Mice. PLoS ONE 2014, 9, e91355. [Google Scholar] [CrossRef]
- Janik, P.; Pielka, M.; Janik, M.A.; Wróbel, Z. Respiratory monitoring system using Bluetooth Low Energy. Sens. Actuators A Phys. 2019, 286, 152–162. [Google Scholar] [CrossRef]
- Janik, P.; Janik, M.A.; Pielka, M. Power saving by a smart breath sensor working in non-connectable advertising mode. Sens. Actuators A Phys. 2020, 315, 112324. [Google Scholar] [CrossRef]
- Janik, P.; Janik, M.A.; Pielka, M. Monitoring Breathing and Heart Rate Using Episodic Broadcast Data Transmission. Sensors 2022, 22, 6019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pielka, M.; Janik, P.; Janik, M.A.; Wróbel, Z. Adaptive Data Transmission Algorithm for the System of Inertial Sensors for Hand Movement Acquisition. Sensors 2022, 22, 9866. [Google Scholar] [CrossRef]
- Bluetooth Low Energy (BLE) 5 Micro Module BC832, Version 2.02; Fanstel Corp.: Scottsdale, AZ, USA, 2020. Available online: www.fanstel.com (accessed on 11 June 2025).
- nRF52832 Product Specification v1.9, Nordic Semiconductor ASA: Trondheim, Norway, 2023. Available online: www.nordicsemi.com (accessed on 11 June 2025).
- BMA400 3-Axes Ultra-Low Power Accelerometer. Doc. Rev. 2.2. January 2025. Available online: https://www.bosch-sensortec.com/products/motion-sensors/accelerometers/bma400/ (accessed on 11 June 2025).
- Aboalasaad, A.R.R.; Kolcavova Sircova, B.; Ahmad, Z. Influence of tensile stress on woven compression bandage structure and porosity. AUTEX Res. J. 2020, 20, 263–273. [Google Scholar] [CrossRef]
- Wano, N.; Sanguanrungsirikul, S.; Keelawat, S.; Somboonwong, J. The effects of whole-body vibration on wound healing in a mouse pressure ulcer model. Heliyon 2021, 7, e06893. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, M.A.; Pielka, M.; Kovalchuk, P.; Mierzwa, M.; Janik, P. Three-Dimensional Printed Stimulating Hybrid Smart Bandage. Sensors 2025, 25, 5090. https://doi.org/10.3390/s25165090
Janik MA, Pielka M, Kovalchuk P, Mierzwa M, Janik P. Three-Dimensional Printed Stimulating Hybrid Smart Bandage. Sensors. 2025; 25(16):5090. https://doi.org/10.3390/s25165090
Chicago/Turabian StyleJanik, Małgorzata A., Michał Pielka, Petro Kovalchuk, Michał Mierzwa, and Paweł Janik. 2025. "Three-Dimensional Printed Stimulating Hybrid Smart Bandage" Sensors 25, no. 16: 5090. https://doi.org/10.3390/s25165090
APA StyleJanik, M. A., Pielka, M., Kovalchuk, P., Mierzwa, M., & Janik, P. (2025). Three-Dimensional Printed Stimulating Hybrid Smart Bandage. Sensors, 25(16), 5090. https://doi.org/10.3390/s25165090




