3D Printing in the Design of Potentiometric Sensors: A Review of Techniques, Materials, and Applications
Abstract
1. Introduction
2. Fundamentals of Potentiometric Sensors
3. Rapid Prototyping—3D Printing
- Binder Jetting (BJ): A method in which a liquid binder is selectively applied to bond powdered materials;
- Directed Energy Deposition (DED): Techniques where thermal energy is used to melt materials layer by layer;
- Material Extrusion (ME): A method where material is extruded through a nozzle (also known as FDM—Fused Deposition Modeling);
- Material Jetting (MJ): Involves the selective deposition of material droplets, including photopolymers (Polyjet Process) and wax;
- Powder Bed Fusion (PBF): A 3D printing method where regions of a powder bed are fused using thermal energy; includes techniques like Selective Laser Sintering (SLS).
- Sheet Lamination (SL): An additive manufacturing process in which sheets of material are bonded to create the final product;
- Vat Photopolymerization (VP): A process in which an object is created from a liquid photopolymer in a vat and cured through light-activated polymerization; includes techniques like SLA–stereolitography [25].
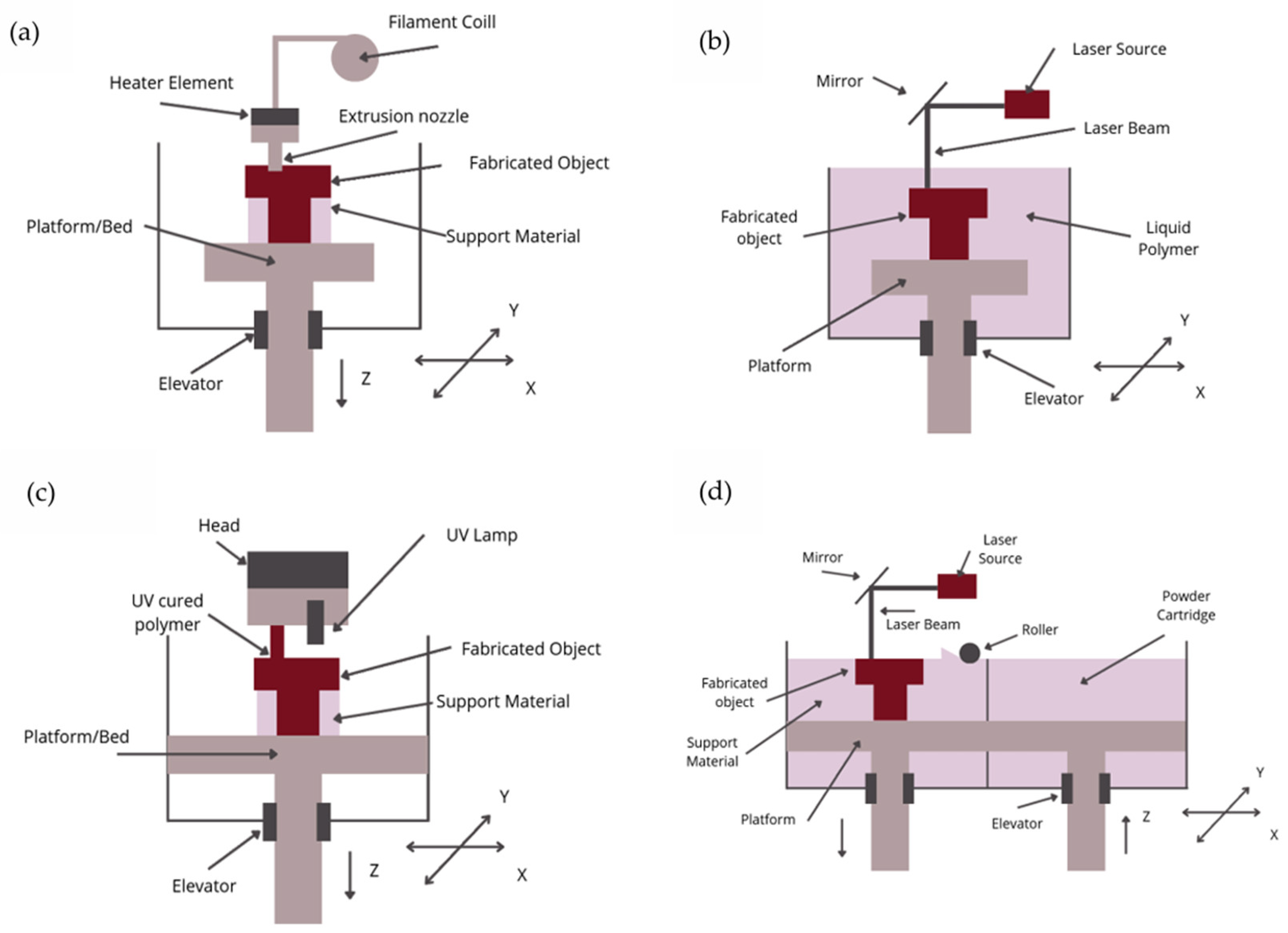
3.1. FDM—Fused Deposition Modeling
3.2. SLA—Stereolithography
3.3. Polyjet 3D Printing
3.4. LCM—Lithography-Based Ceramic Manufacturing
3.5. SLM—Selective Laser Melting
4. Application of 3D Printing Techniques in Manufacturing of Potentiometric Sensors
4.1. Application of FDM—Fused Deposition Modeling in Potentiometric Sensors


4.2. Application of SLA—Stereolithography Modeling in Potentiometric Sensors
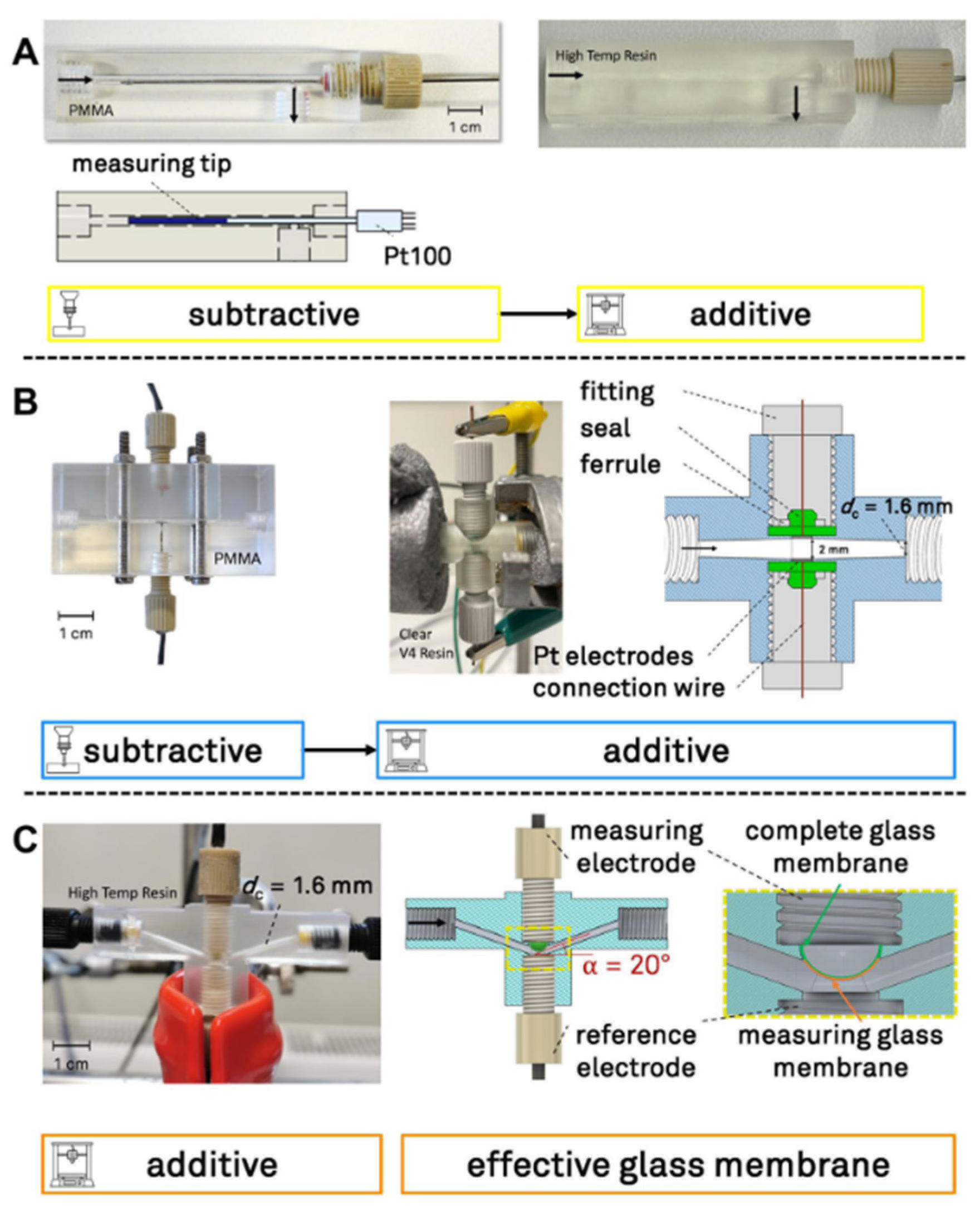
4.3. Application of LCM—Lithography-Based Ceramic Manufacturing in Potentiometric Sensors
4.4. PolyJet Printing in Potentiometric Sensors

4.5. Application of SLM—Selective Laser Melting in Potentiometric Sensors
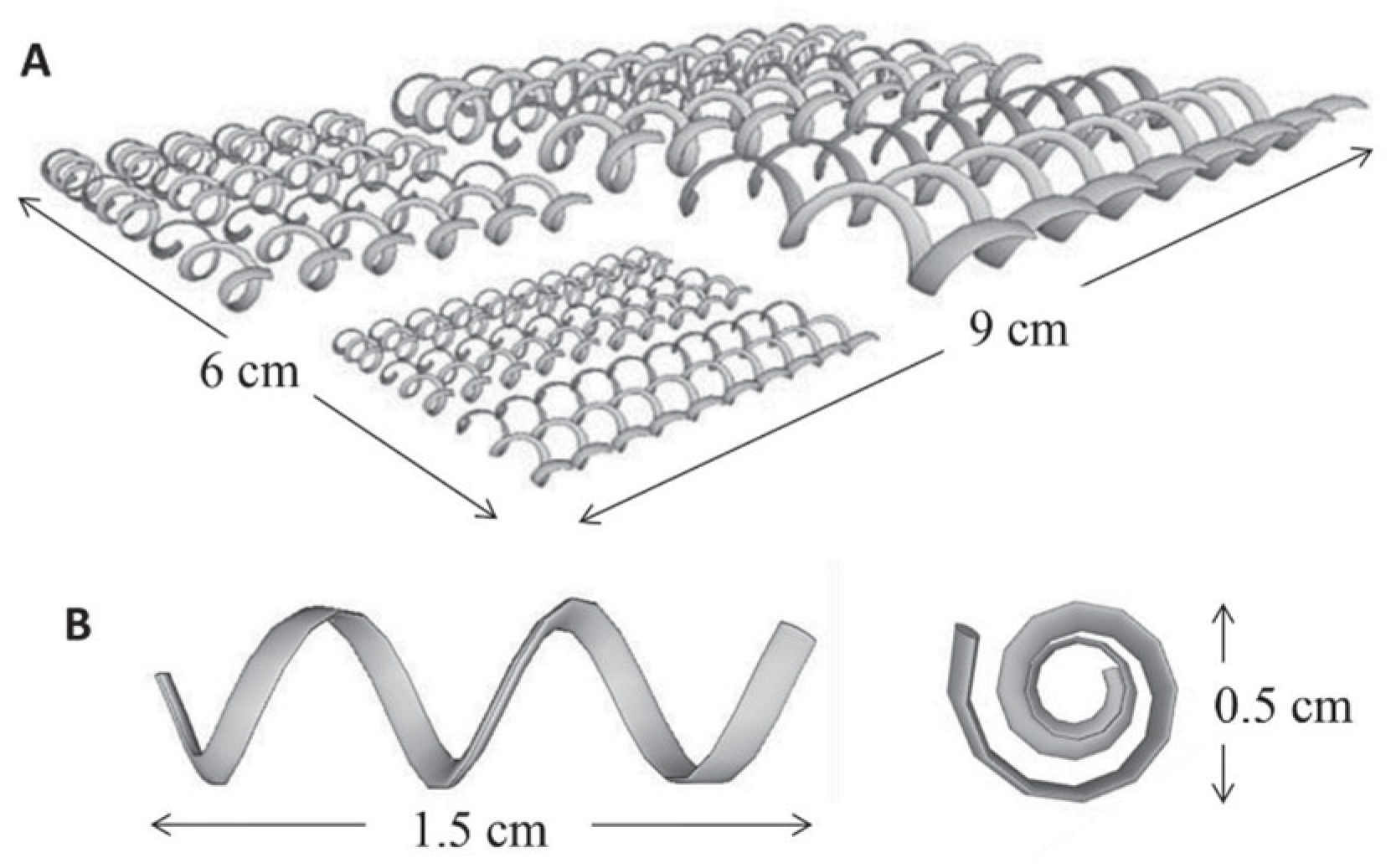
5. Discussion
| L.p | Printing Technique | Printing Material | Printed Part | Analyte | Reference |
|---|---|---|---|---|---|
| 1 | FDM | Carbon black infused PLA | Working Electrode | pH and potassium | [63] |
| 2 | FDM-3D pen | Carbon black infused PLA | Working Electrode | potassium, calcium, and chloride | [64] |
| 3 | FDM | Carbon black infused PLA-ProtoPasta | Working Electrode | Acetylocholine | [51] |
| 4 | FDM and Direct Ink Writing | Semiflex, ABS, Electrifil, PLA for Robot finger and Cellulose-based conductive Ink for the LC circuit | Robotic finger and LC circuit | Potassium, Calcium and Ammonium | [65] |
| 5 | FDM | PLA with Carbon Black and PETg | Fully insulated Working Electrode | Potassium | [33] |
| 6 | FDM | PETg | Insulating body for Microlectrodes | pH | [66] |
| 7 | FDM/DIM | Solid electrolytes with a perovskite structure-BaCe0.6Zr0.3Y0.1O3-α (BCZY) and Platinum Ink | Working Electrode | Hydrogen in high temperature | [32] |
| 8 | FDM | PLA | Robot for carbon layers and membrane deposition | Potassium and Sodium | [67] |
| 9 | SLA | Photocurable resin | Flow cell | pH | [81] |
| 10 | SLA | Photocurable resin | Electrode housing | pH | [82] |
| 11 | MJP and SLA | Photocurable polymer | Electrode housing and a filter for Cl interference ions | Ag+-detection of bacteria | [83] |
| 12 | SLA | Photocurable resin | Flow cell | Sodium, Potassium and Calcium | [85] |
| 13 | SLA | Photocurable resin | ISE housing | Sodium, Potassium and Calcium | [88] |
| 14 | SLA | Photocurable resin | Microneedles as Working Electrodes | pH | [50] |
| 15 | SLA | Photocurable resin with Ach+-selective ionophore or Benzalkonium Chloride Ionophore | ISM | Acetylocholine and benzalkonium chloride | [68] |
| 16 | SLA | Photocurable resin with Calcium Selective Ionophore | ISM | Calcium | [101] |
| 17 | LCM method | BaCe0.6Zr0.3Y0.1O3-α (BCZY)— | Working Electrode | Hydrogen in high temperature | [102] |
| 18 | PolyJet printing | Rigid polymer Veroblack, Flexible polymer TangoBlack | Housing of wearable sensor | Sodium and Potassium | [78] |
| 19 | SLM | Stainless steel | Working Electrode | pH | [105] |
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 3DPE | 3D-printed electrode |
| 3DP-SS | 3D-printed stainless-steel |
| ABS | Acrylonitrile butadiene |
| Ach+ | Acetylocholine |
| AOA/OA | antioxidant/oxidant activity |
| ASS-ISE | all-solid-state ion-selective electrode |
| BA+ | Benzalkonium |
| BCZY | BaCe0.6Zr0.3Y0.1O3-α |
| BJ | Binder Jetting |
| CAD | Computer Aided Design |
| CAM | Computer Aided Manufacturing |
| CB-PLA | Carbon Black with PLA |
| CRM | certified reference materials |
| CWE | Coated wire electrode |
| DED | Directed Energy Deposition |
| DLP | Digital Light Projector |
| FDM | Fused deposition modeling |
| FFF | Fused Filament Fabrication |
| HPM | Hybrid Potentiometric Measurements |
| IoT | Internet of Things |
| ISE | ion-selective electrode |
| ISF | interstitial fluid |
| ISM | ion-selective membrane |
| ISME | Ion-selective Membrane Electrode |
| LCD | Liquid Crystal Display |
| LC-ISE | Liquid-Contact ISE |
| LCM | Lithography-Based Ceramic Manufacturing |
| ME | Material Extrusion |
| MJ | Material Jetting |
| MN | Microneedles |
| OD-MWCNT | multi-walled carbon nanotubes functionalized with octadecylamine |
| PBF | Powder Bed Fusion |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| PET | polyethylene terephthalate |
| PETg | Polyethylene Terephthalate Glyco |
| PGA | Porous Graphene Gel |
| PLA | Polylactide |
| POC | Point of care |
| POT | poly(3-octylthiophene-2,5-diyl) (POT). |
| POU | Point of use |
| PPy | Polypyrolle |
| PU | Polyurethane |
| PVC | polyvinyl chloride |
| QRE | Quasi Reference Electrode |
| RE | Reference Electrode |
| RGO | Reduced graphene oxide |
| SC-ISE | Screen Printed Ion Selective Electrode |
| SL | Sheet Lamination |
| SLA | Stereolithography |
| SLM | Selective Laser Melting |
| SLS | Selective Laser Sintering |
| SPE | Screen Printed Electrode |
| THF | tetrahydrofuran |
| TPU | polyurethane |
| VP | Vat Photopolymerization |
| WE | Working Electrode |
References
- Harris, D.C. Quantitative Chemical Analysis, 7th ed.; Freeman: New York, NY, USA, 2007; ISBN 978-0-7167-7041-1. [Google Scholar]
- Glasco, D.L.; Sheelam, A.; Ho, N.H.B.; Mamaril, A.M.; King, M.; Bell, J.G. Editors’ Choice—Review—3D Printing: An Innovative Trend in Analytical Sensing. ECS Sens. Plus 2022, 1, 010602. [Google Scholar] [CrossRef]
- Hassan, S.A.; ElDin, N.B.; Zaazaa, H.E.; Moustafa, A.A.; Mahmoud, A.M. Point-of-care diagnostics for drugs of abuse in biological fluids: Application of a microfabricated disposable copper potentiometric sensor. Microchim. Acta 2020, 187, 491. [Google Scholar] [CrossRef]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; Wiley: Chichester, UK, 2012; ISBN 978-0-470-71066-1. [Google Scholar]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. TrAC Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Cadogan, A.; Gao, Z.; Lewenstam, A.; Ivaska, A.; Diamond, D. All-solid-state sodium-selective electrode based on a calixarene ionophore in a poly(vinyl chloride) membrane with a polypyrrole solid contact. Anal. Chem. 1992, 64, 2496–2501. [Google Scholar] [CrossRef]
- Christian, G.D.; Dasgupta, P.K.; Schug, K.A.; Schug, K. Analytical Chemistry, 7th ed.; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-0-470-88757-8. [Google Scholar]
- Harvey, D. Modern Analytical Chemistry; McGraw-Hill: Boston, MA, USA, 2000; ISBN 978-0-07-237547-3. [Google Scholar]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; Dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Su, A.; Al’Aref, S.J. History of 3D Printing. In 3D Printing Applications in Cardiovascular Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–10. ISBN 978-0-12-803917-5. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Pretsch, E. Potentiometric sensors for trace-level analysis. TrAC Trends Anal. Chem. 2005, 24, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Biosensors; Scheller, F., Schubert, F., Eds.; Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-08-087559-0. [Google Scholar]
- Düzgün, A.; Imran, H.; Levon, K.; Rius, F.X. Protein Detection with Potentiometric Aptasensors: A Comparative Study between Polyaniline and Single-Walled Carbon Nanotubes Transducers. Sci. World J. 2013, 2013, 282756. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes. Part I. Inorganic Cations (Technical Report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Nandhakumar, R.; Venkatesan, K. A process parameters review on selective laser melting-based additive manufacturing of single and multi-material: Microstructure, physical properties, tribological, and surface roughness. Mater. Today Commun. 2023, 35, 105538. [Google Scholar] [CrossRef]
- Remaggi, G.; Zaccarelli, A.; Elviri, L. 3D Printing Technologies in Biosensors Production: Recent Developments. Chemosensors 2022, 10, 65. [Google Scholar] [CrossRef]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef]
- Wohlers, T.; Gornet, T. History of Additive Manufacturing; Wohlers Associates, Inc.: Fort Collins, CO, USA, 2014. [Google Scholar]
- Kristiawan, R.B.; Imaduddin, F.; Ariawan, D.; Ubaidillah; Arifin, Z. A review on the fused deposition modeling (FDM) 3D printing: Filament processing, materials, and printing parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- What’s the Ideal Filament for FDM 3D Printing? 3D Printing Materials Compared. PROTOLABS NETWORK. Available online: https://www.hubs.com/knowledge-base/fdm-3d-printing-materials-compared/ (accessed on 17 July 2025).
- What is CAD Modeling? Comparing Design Software for 3D Printing. PROTOLABS NETWORK. Available online: https://www.hubs.com/knowledge-base/3d-modeling-cad-software/ (accessed on 17 July 2025).
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D Printing of Polymers Containing Natural Fillers: A Review of their Mechanical Properties. Polymers 2019, 11, 1094. [Google Scholar] [CrossRef]
- Hinojo, A.; Lujan, E.; Nel-lo, M.; Abella, J.; Colominas, S. Potentiometric Hydrogen Sensor with 3D-Printed BaCe0.6Zr0.3Y0.1O3-α Electrolyte for High-Temperature Applications. Sensors 2022, 22, 9707. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.; Torricelli, D.; Cuartero, M.; Crespo, G.A. 3D-Printed Transducers for Solid Contact Potentiometric Ion Sensors: Improving Reproducibility by Fabrication Automation. Anal. Chem. 2024, 96, 15572–15580. [Google Scholar] [CrossRef] [PubMed]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2023, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- What is SLA 3D Printing? Sinterit. Available online: https://sinterit.com/3d-printing-guide/3d-printing-technologies/what-is-sla-3d-printing/ (accessed on 17 July 2025).
- Swainson, W.K. Method, Medium and Apparatus for Producing Three-Dimensional Figure Productd. Google Patent US4041476, 9 August 1977. [Google Scholar]
- Beaman, J.J.; Barlow, D.L.; Crawford, R.H.; Marcus, H.L.; McAlea, K.P. Solid Freeform Fabrication: A New Direction in Manufacturing; Springer: Boston, MA, USA, 1997. [Google Scholar]
- Kodama, H. Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev. Sci. Instrum. 1981, 52, 1770–1773. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; Lim, C.S. Rapid Prototyping: Principles and Applications; World Scientific: Singapore, 2010; ISBN 9789814281735. [Google Scholar]
- Wohlers, T.; Gornet, T. History of Additive Manufacturing. 2015. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4474824 (accessed on 17 July 2025).
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Fijoł, N.; Aguilar-Sánchez, A.; Mathew, A.P. 3D-printable biopolymer-based materials for water treatment: A review. Chem. Eng. J. 2022, 430, 132964. [Google Scholar] [CrossRef]
- SLA 3D Printing Materials Compared. 3DS—Dassaults Systems. Available online: https://www.3ds.com/make/solutions/blog/sla-3d-printing-materials-compared (accessed on 17 July 2025).
- White Biocompatible 3D Printing Resin. B9Creations Digital Manufacturing Technology. Available online: https://shop.b9c.com/biores-white (accessed on 17 July 2025).
- Guttridge, C.; Shannon, A.; O’Sullivan, A.; O’Sullivan, K.J.; O’Sullivan, L.W. Biocompatible 3D printing resins for medical applications: A review of marketed intended use, biocompatibility certification, and post-processing guidance. Ann. 3D Print. Med. 2022, 5, 100044. [Google Scholar] [CrossRef]
- Barbosa, I.S.O.; Manrique, Y.A.; Paiva, D.; Faria, J.L.; Santos, R.J.; Silva, C.G. Efficient photocatalytic reactors via 3D printing: SLA fabrication and TiO2 hybrid materials. RSC Adv. 2025, 15, 2275–2286. [Google Scholar] [CrossRef]
- Vishal, F.; Sushi Kumar, S.; Raksha, G.B.; Yash, H.T.; Vaishanavi, S.G.; Swaraj, P.F. Adaptation of 3D Printing Technology for Fabrication of Economical Upper Limb Prostheses. In Lecture Notes in Mechanical Engineering; Springer: Singapore, 2021; pp. 861–868. ISBN 978-981-334-319-1. [Google Scholar] [CrossRef]
- Agrawal, S.; Ray, H.; Kulat, A.; Garhekar, Y.; Jibhakate, R.; Singh, S.K.; Bisaria, H. Evaluation of tensile property of SLA 3D printed NextDent biocompatible Class I material for making surgical guides for implant surgery. Mater. Today Proc. 2023, 72, 1231–1235. [Google Scholar] [CrossRef]
- Liang, X.; Li, Q.; Qu, B.; Qiu, Y.; Liu, S.; Wang, Q.; Liang, J.; Tan, H.; Liu, Y.; Li, J. High-precision and high-strength SiC ceramic green body by stereolithography: Slurry design and defect control. J. Alloys Compd. 2025, 1035, 180860. [Google Scholar] [CrossRef]
- Parrilla, M.; Vanhooydonck, A.; Johns, M.; Watts, R.; De Wael, K. 3D-printed microneedle-based potentiometric sensor for pH monitoring in skin interstitial fluid. Sens. Actuators B Chem. 2023, 378, 133159. [Google Scholar] [CrossRef]
- Ho, N.H.B.; Glasco, D.L.; Sopp, R.N.; Bell, J.G. Multiplexed Electrochemical Device for the Detection of Biomarkers of Parkinson’s Disease Using 3D Printing. ECS Trans. 2022, 109, 29–37. [Google Scholar] [CrossRef]
- Udroiu, R.; Braga, I.C. Polyjet technology applications for rapid tooling. MATEC Web Conf. 2017, 112, 03011. [Google Scholar] [CrossRef]
- Emiliani, N.; Porcaro, R.; Pisaneschi, G.; Bortolani, B.; Ferretti, F.; Fontana, F.; Campana, G.; Fiorini, M.; Marcelli, E.; Cercenelli, L. Post-printing processing and aging effects on Polyjet materials intended for the fabrication of advanced surgical simulators. J. Mech. Behav. Biomed. Mater. 2024, 156, 106598. [Google Scholar] [CrossRef]
- PolyJetTM Technology. Stratasys. Available online: https://www.stratasys.com/en/guide-to-3d-printing/technologies-and-materials/polyjet-technology/ (accessed on 17 July 2025).
- LCM Technology: How to 3D Print Ceramics. 2024. Available online: https://www.lithoz.com/en/technology/lcm-technology/ (accessed on 17 July 2025).
- Hinojo, A.; Lujan, E.; Abella, J.; Colominas, S. A novel solution for hydrogen monitoring in fusion processes: 3D printed BaCe0.6Zr0.3Y0.1O3-α sensors. Nucl. Mater. Energy 2024, 39, 101661. [Google Scholar] [CrossRef]
- Lithography-Based Ceramics Manufacturing (LCM). 2024. Available online: https://www.voxelmatters.com/additive-manufacturing/am-technologies/what-is-lcm-technology/ (accessed on 17 July 2025).
- Stampfl, J.; Schwentenwein, M.; Homa, J.; Prinz, F.B. Lithography-based additive manufacturing of ceramics: Materials, applications and perspectives. MRS Commun. 2023, 13, 786–794. [Google Scholar] [CrossRef]
- What is SLM 3D Printing. JLC3DP 2025. Available online: https://jlc3dp.com/help/article/What-is-SLM-3D-Printing (accessed on 17 July 2025).
- Mukalay, T.A.; Trimble, J.A.; Mpofu, K.; Muvunzi, R. Selective laser melting: Evaluation of the effectiveness and reliability of multi-scale multiphysics simulation environments. Heliyon 2024, 10, e25706. [Google Scholar] [CrossRef]
- Lu, J.; Zhuo, L. Additive manufacturing of titanium alloys via selective laser melting: Fabrication, microstructure, post-processing, performance and prospect. Int. J. Refract. Met. Hard Mater. 2023, 111, 106110. [Google Scholar] [CrossRef]
- Veloso, W.B.; Paixão, T.R.L.C.; Meloni, G.N. The Current Shortcomings and Future Possibilities of 3D Printed Electrodes. Anal. Chem. 2024, 96, 14315–14319. [Google Scholar] [CrossRef]
- McCole, M.; Bradley, M.; McCaul, M.; McCrudden, D. A low-cost portable system for on-site detection of soil pH and potassium levels using 3D printed sensors. Results Eng. 2023, 20, 101564. [Google Scholar] [CrossRef]
- Kalisz, J.; Wȩgrzyn, K.; Maksymiuk, K.; Michalska, A. 3D-Drawn Supports for Ion-Selective Electrodes. Anal. Chem. 2022, 94, 3436–3440. [Google Scholar] [CrossRef]
- Kim, T.; Kaur, M.; Kim, W.S. Humanoid Robot Actuation through Precise Chemical Sensing Signals. Adv. Mater. Technol. 2019, 4, 1900570. [Google Scholar] [CrossRef]
- Helú, M.A.B.; Liu, L. Fused deposition modeling (FDM) based 3D printing of microelectrodes and multi-electrode probes. Electrochim. Acta 2021, 365, 137279. [Google Scholar] [CrossRef]
- Ozer, T.; Agir, I.; Henry, C.S. Rapid prototyping of ion-selective electrodes using a low-cost 3D printed internet-of-things (IoT) controlled robot. Talanta 2022, 247, 123544. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.H.B. 3D Printing Technology in Low-Cost Diagnostic Sensors for Neurological Disorders; Washington State University: Pullman, WA, USA, 2023; ISBN 9798379913533. [Google Scholar]
- Elhassan, M.M.; Glasco, D.L.; Sheelam, A.; Mahmoud, A.M.; Hegazy, M.A.; Mowaka, S.; Bell, J.G. Potentiometric detection of apomorphine in human plasma using a 3D printed sensor. Biosens. Bioelectron. 2024, 248, 115971. [Google Scholar] [CrossRef]
- Ho, N.H.B.; Glasco, D.L.; Bell, J.G. Potentiometric Analysis of Benzalkonium Chloride with 3D Printed Ion-Selective Membranes. ECS Sens. Plus 2022, 1, 020601. [Google Scholar] [CrossRef]
- Kim, T. Three-Dimensional Printing of Conductive Composite for Wireless Chemical Sensor Systems. Available online: https://summit.sfu.ca/item/19686 (accessed on 17 July 2025).
- Zhu, Z.; Ye, Z.; Zhang, Q.; Zhang, J.; Cao, F. Novel dual Pt-Pt/IrO ultramicroelectrode for pH imaging using SECM in both potentiometric and amperometric modes. Electrochem. Commun. 2018, 88, 47–51. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J. Iridium oxide fabrication and application: A review. J. Energy Chem. 2020, 46, 152–172. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Xu, B. A solid-state pH sensor based on WO3-modified vertically aligned multiwalled carbon nanotubes. Electrochem. Commun. 2009, 11, 1038–1041. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, X. Characterization and application of a metallic tungsten electrode for potentiometric pH measurements. J. Electroanal. Chem. 2014, 714–715, 45–50. [Google Scholar] [CrossRef]
- Kava, A.A.; Henry, C.S. Exploring carbon particle type and plasma treatment to improve electrochemical properties of stencil-printed carbon electrodes. Talanta 2021, 221, 121553. [Google Scholar] [CrossRef]
- Zahran, E.M.; New, A.; Gavalas, V.; Bachas, L.G. Polymeric plasticizer extends the lifetime of PVC-membrane ion-selective electrodes. Analyst 2014, 139, 757–763. [Google Scholar] [CrossRef]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaul, M. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef] [PubMed]
- Gioiello, A.; Moroni, G.; Cerra, B. Integrated Systems for Continuous Synthesis and Biological Screenings. In Methods and Principles in Medicinal Chemistry; Alza, E., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 159–197. ISBN 978-3-527-34689-9. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Dinter, R.; Helwes, L.; De Vries, S.; Jegatheeswaran, K.; Jibben, H.; Kockmann, N. 3D-printed open-source sensor flow cells for microfluidic temperature, electrical conductivity, and pH value determination. J. Flow. Chem. 2024, 14, 469–479. [Google Scholar] [CrossRef]
- Lonsdale, W.; Shylendra, S.P.; Wajrak, M.; Alameh, K. Application of all solid-state 3D printed pH sensor to beverage samples using matrix matched standard. Talanta 2019, 196, 18–21. [Google Scholar] [CrossRef]
- Zhang, T.; Monia Kabandana, G.K.; Ratajczak, A.M.; Chen, C. A quantitative sensing system based on a 3D-printed ion-selective electrode for rapid and sensitive detection of bacteria in biological fluid. Talanta 2022, 238, 123040. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [CrossRef]
- Dębosz, M.; Kozma, J.; Porada, R.; Wieczorek, M.; Paluch, J.; Gyurcsányi, R.E.; Migdalski, J.; Kościelniak, P. 3D-printed manifold integrating solid contact ion-selective electrodes for multiplexed ion concentration measurements in urine. Talanta 2021, 232, 122491. [Google Scholar] [CrossRef]
- Monia Kabandana, G.K.; Zhang, T.; Chen, C. Emerging 3D printing technologies and methodologies for microfluidic development. Anal. Methods 2022, 14, 2885–2906. [Google Scholar] [CrossRef]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon Nanotube-Based Ion Selective Sensors for Wearable Applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef]
- Dębosz, M.; Wieczorek, M.; Paluch, J.; Migdalski, J.; Baś, B.; Kościelniak, P. 3D-printed flow manifold based on potentiometric measurements with solid-state ion-selective electrodes and dedicated to multicomponent water analysis. Talanta 2020, 217, 121092. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Mahato, K.; Teymourian, H.; Longardner, K.; Litvan, I.; Wang, J. Wearable electrochemical microneedle sensing platform for real-time continuous interstitial fluid monitoring of apomorphine: Toward Parkinson management. Sens. Actuators B Chem. 2022, 354, 131234. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; Altura, B.M.; Altura, B.T.; Siggaard-Andersen, O. Composition of interstitial fluid. Clin. Chem. 1995, 41, 1522–1525. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.P. Theory and Principles of Membrane Electrodes. In Ion-Selective Electrodes in Analytical Chemistry; Freiser, H., Ed.; Springer: Boston, MA, USA, 1978; pp. 1–141. ISBN 978-1-4684-2594-9. [Google Scholar] [CrossRef]
- Jackson, D.T.; Nelson, P.N. Preparation and properties of some ion selective membranes: A review. J. Mol. Struct. 2019, 1182, 241–259. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Polymer Membrane Ion-Selective Electrodes-What are the Limits? Electroanalysis 1999, 11, 915–933. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Peer Reviewed: Tracing the History of Selective Ion Sensors. Anal. Chem. 2001, 73, 88A–97A. [Google Scholar] [CrossRef]
- Mikhelson, K.N. Ion-selective electrodes in PVC matrix. Sens. Actuators B Chem. 1994, 18, 31–37. [Google Scholar] [CrossRef]
- Cha, G.S.; Liu, D.; Meyerhoff, M.E.; Cantor, H.C.; Midgley, A.R.; Goldberg, H.D.; Brown, R.B. Electrochemical performance, biocompatibility, and adhesion of new polymer matrixes for solid-state ion sensors. Anal. Chem. 1991, 63, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, M.E.; Arbor, A. In viva blood-gas and electrolyte sensors: Progress and challenges. TrAC Trends Anal. Chem. 1993, 12, 257–266. [Google Scholar] [CrossRef]
- Nam, H.; Cha, G.S. Alternative Polymer Matrices for Potentiometric Chemical Sensors. In Biosensors and Their Applications; Yang, V.C., Ngo, T.T., Eds.; Springer: Boston, MA, USA, 2000; pp. 311–332. ISBN 978-1-4613-6875-5. [Google Scholar] [CrossRef]
- Cosofret, V.; Erdosy, M.; Raleigh, J.; Johnson, T.; Neuman, M.; Buck, R. Aliphatic polyurethane as a matrix for pH sensors: Effects of native sites and added proton carrier on electrical and potentiometric properties. Talanta 1996, 43, 143–151. [Google Scholar] [CrossRef]
- Mamaril, A.M.; Glasco, D.L.; Leal Yepes, F.A.; Bell, J.G. Identifying Hypocalcemia in Dairy Cattle by Combining 3D Printing and Paper Diagnostics. ECS Sens. Plus 2022, 1, 040601. [Google Scholar] [CrossRef]
- Hinojo, A.; Lujan, E.; Nel-lo, M.; Colominas, S.; Abella, J. BaCe0.6Zr0.3Y0.1O3-α electrochemical hydrogen sensor for fusion applications. Fusion Eng. Des. 2023, 188, 113452. [Google Scholar] [CrossRef]
- Fox, A.R. Standard Method Performance Requirements. Accred. Qual. Assur. 2011, 16, 561–566. [Google Scholar] [CrossRef]
- McCaul, M.; Porter, A.; Barrett, R.; White, P.; Stroiescu, F.; Wallace, G.; Diamond, D. Wearable Platform for Real-time Monitoring of Sodium in Sweat. ChemPhysChem 2018, 19, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Moo, J.G.S.; Pumera, M. Helical 3D-Printed Metal Electrodes as Custom-Shaped 3D Platform for Electrochemical Devices. Adv. Funct. Mater. 2016, 26, 698–703. [Google Scholar] [CrossRef]
- Gutiérrez Pineda, E.; Alcaide, F.; Rodríguez Presa, M.J.; Bolzán, A.E.; Gervasi, C.A. Electrochemical Preparation and Characterization of Polypyrrole/Stainless Steel Electrodes Decorated with Gold Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 2677–2687. [Google Scholar] [CrossRef]
- Girija, T.C.; Sangaranarayanan, M.V. Investigation of polyaniline-coated stainless steel electrodes for electrochemical supercapacitors. Synth. Met. 2006, 156, 244–250. [Google Scholar] [CrossRef]
- Patake, V.D.; Lokhande, C.D.; Joo, O.S. Electrodeposited ruthenium oxide thin films for supercapacitor: Effect of surface treatments. Appl. Surf. Sci. 2009, 255, 4192–4196. [Google Scholar] [CrossRef]
- Khalil, M.; Liu, N.; Lee, R. Super-Nernstian Potentiometric pH Sensor based on the Electrodeposition of Iridium Oxide Nanoparticles. IJTech 2018, 9, 446. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Z.; Cao, Y.; Yang, H.; Duan, Y.Y. Activated iridium oxide films fabricated by asymmetric pulses for electrical neural microstimulation and recording. Electrochem. Commun. 2008, 10, 778–782. [Google Scholar] [CrossRef]
- Cândido, T.C.D.O.; Silva, D.N.D.; Borges, M.M.C.; Barbosa, T.G.; Trindade, S.O.D.D.; Pereira, A.C. 3D-Printed Electrochemical Sensors: A Comprehensive Review of Clinical Analysis Applications. Analytica 2024, 5, 552–575. [Google Scholar] [CrossRef]
- Silva, A.L.; Salvador, G.M.D.S.; Castro, S.V.F.; Carvalho, N.M.F.; Munoz, R.A.A. A 3D Printer Guide for the Development and Application of Electrochemical Cells and Devices. Front. Chem. 2021, 9, 684256. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Zhao, Z.; Zhang, D.; Feng, J.; Yu, L.; Lin, Z.; Guo, Q.; Huang, J.; Mao, J.; et al. 3D printing of micro-nano devices and their applications. Microsyst. Nanoeng. 2025, 11, 35. [Google Scholar] [CrossRef]
- Sun, Y.; Li, D.; Shi, Y.; Wang, Z.; Okeke, S.I.; Yang, L.; Zhang, W.; Zhang, Z.; Shi, Y.; Xiao, L. Application of 3D Printing Technology in Sensor Development for Water Quality Monitoring. Sensors 2023, 23, 2366. [Google Scholar] [CrossRef]
- Rajendran, J.; Esfandyarpour, R. Revolutionizing Personalized Health: The Frontier of Wearable Biomolecule Sensors Through 3D Printing Innovation. Biomed. Mater. Devices 2025, 3, 818–834. [Google Scholar] [CrossRef]
- Farahani, S.; Glasco, D.L.; Elhassan, M.M.; Sireesha, P.; Bell, J.G. Integration of 3D printed Mg2+ potentiometric sensors into microfluidic devices for bioanalysis. Lab Chip 2024, 24, 4096–4104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewska, A.; Lenar, N.; Paczosa-Bator, B. 3D Printing in the Design of Potentiometric Sensors: A Review of Techniques, Materials, and Applications. Sensors 2025, 25, 4986. https://doi.org/10.3390/s25164986
Zalewska A, Lenar N, Paczosa-Bator B. 3D Printing in the Design of Potentiometric Sensors: A Review of Techniques, Materials, and Applications. Sensors. 2025; 25(16):4986. https://doi.org/10.3390/s25164986
Chicago/Turabian StyleZalewska, Aleksandra, Nikola Lenar, and Beata Paczosa-Bator. 2025. "3D Printing in the Design of Potentiometric Sensors: A Review of Techniques, Materials, and Applications" Sensors 25, no. 16: 4986. https://doi.org/10.3390/s25164986
APA StyleZalewska, A., Lenar, N., & Paczosa-Bator, B. (2025). 3D Printing in the Design of Potentiometric Sensors: A Review of Techniques, Materials, and Applications. Sensors, 25(16), 4986. https://doi.org/10.3390/s25164986







