A Highly Sensitive Low-Temperature N-Butanol Gas Sensor Based on a Co-Doped MOF-ZnO Nanomaterial Under UV Excitation
Abstract
1. Introduction
2. Experimental Section
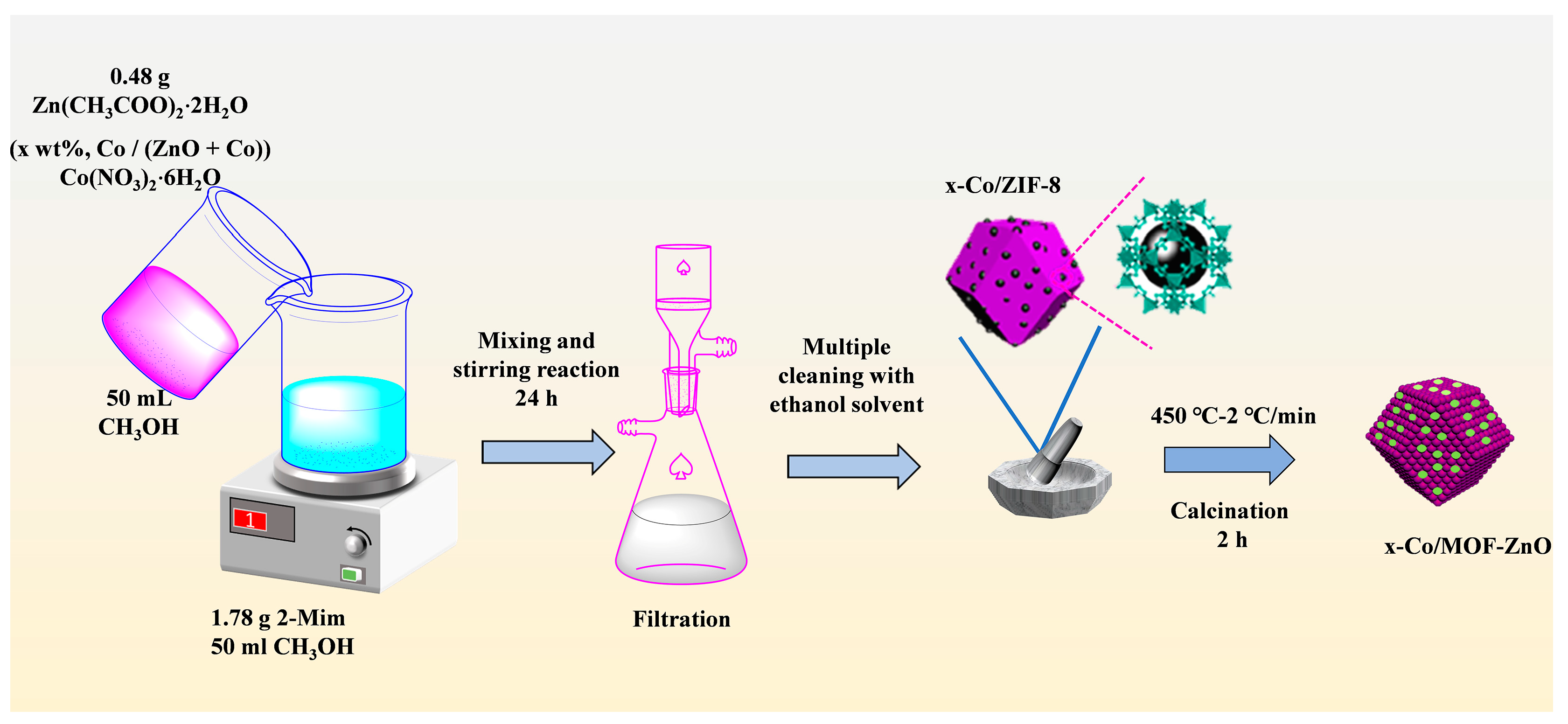
2.1. Materials and Synthesis
2.2. Preparation of Pure MOF-ZnO and x-Co/MOF-ZnO Materials
2.3. Characterization
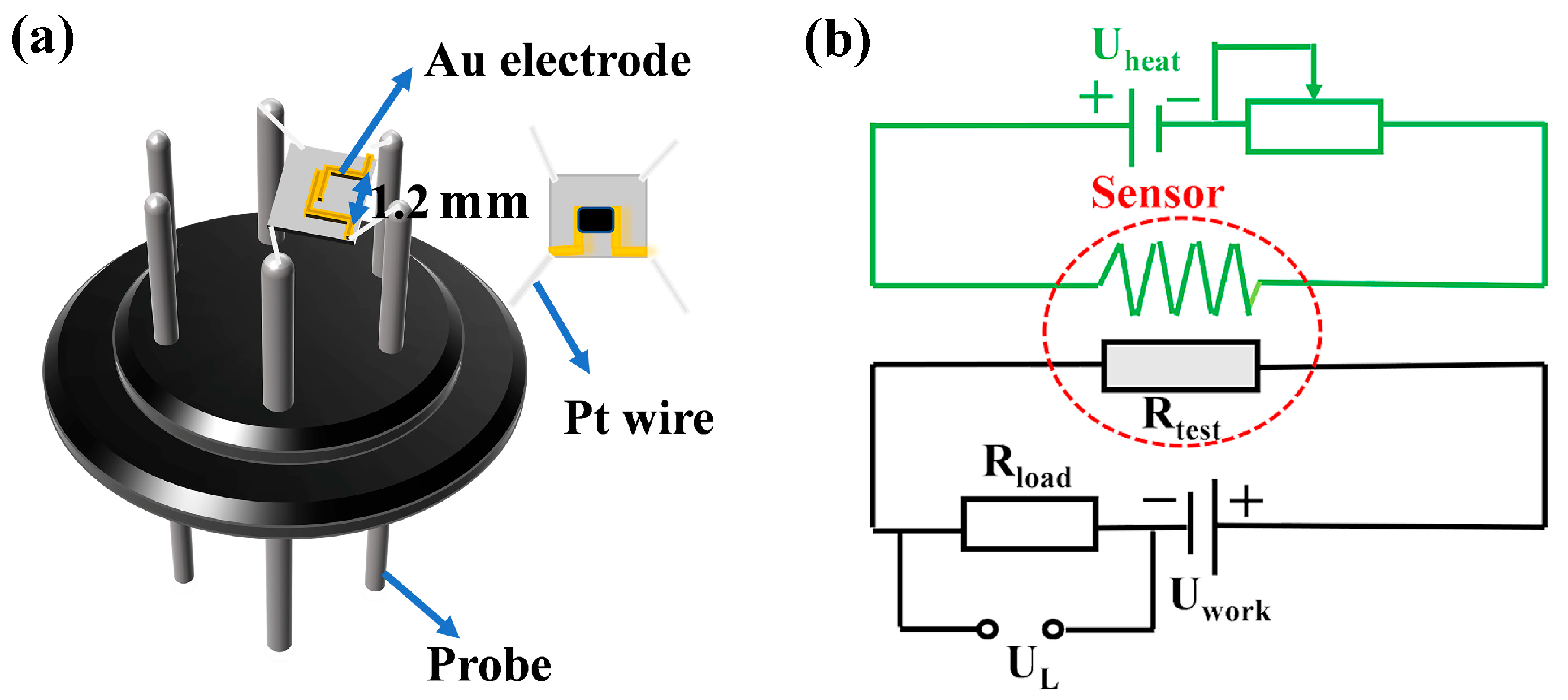
2.4. Fabrication and Performance Testing of Gas Sensors
3. Results and Discussion
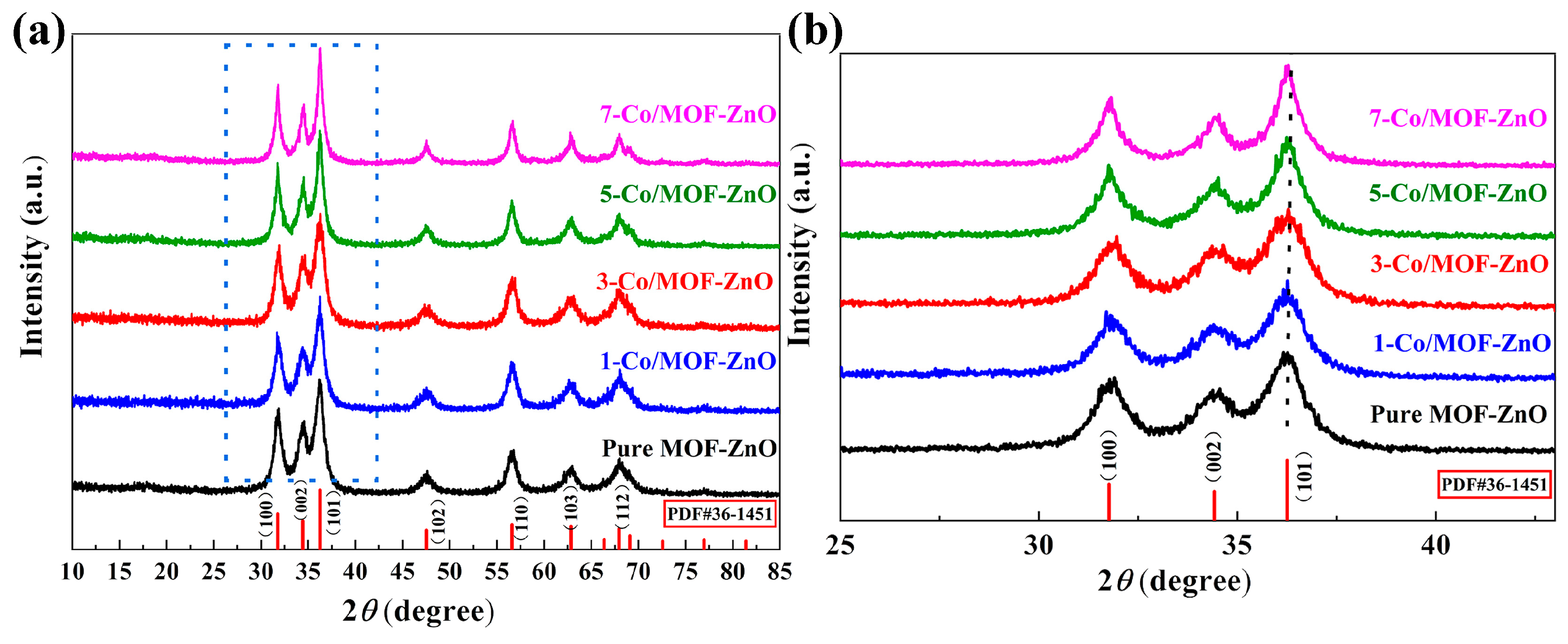
3.1. Structure and Morphology Analysis
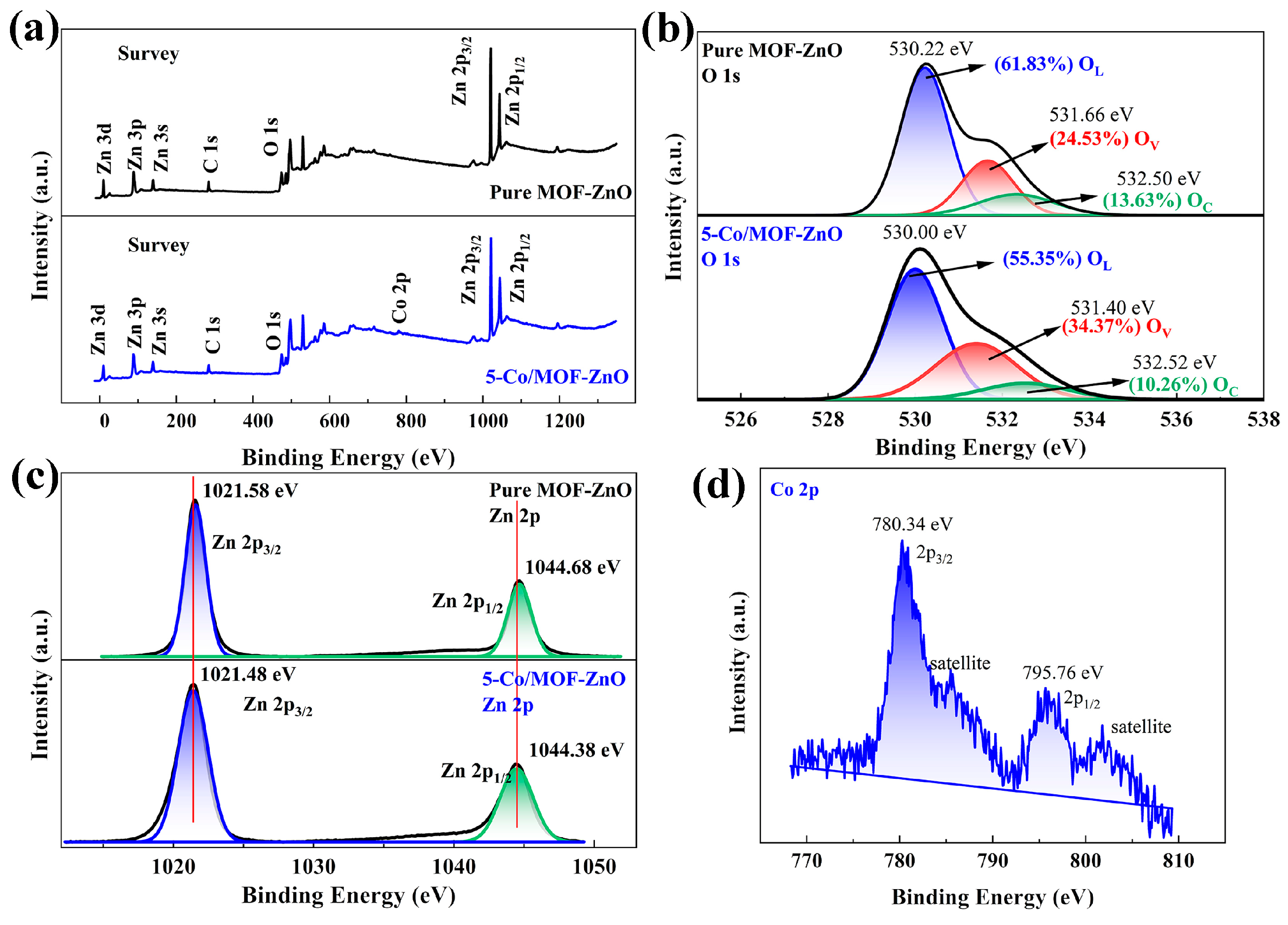
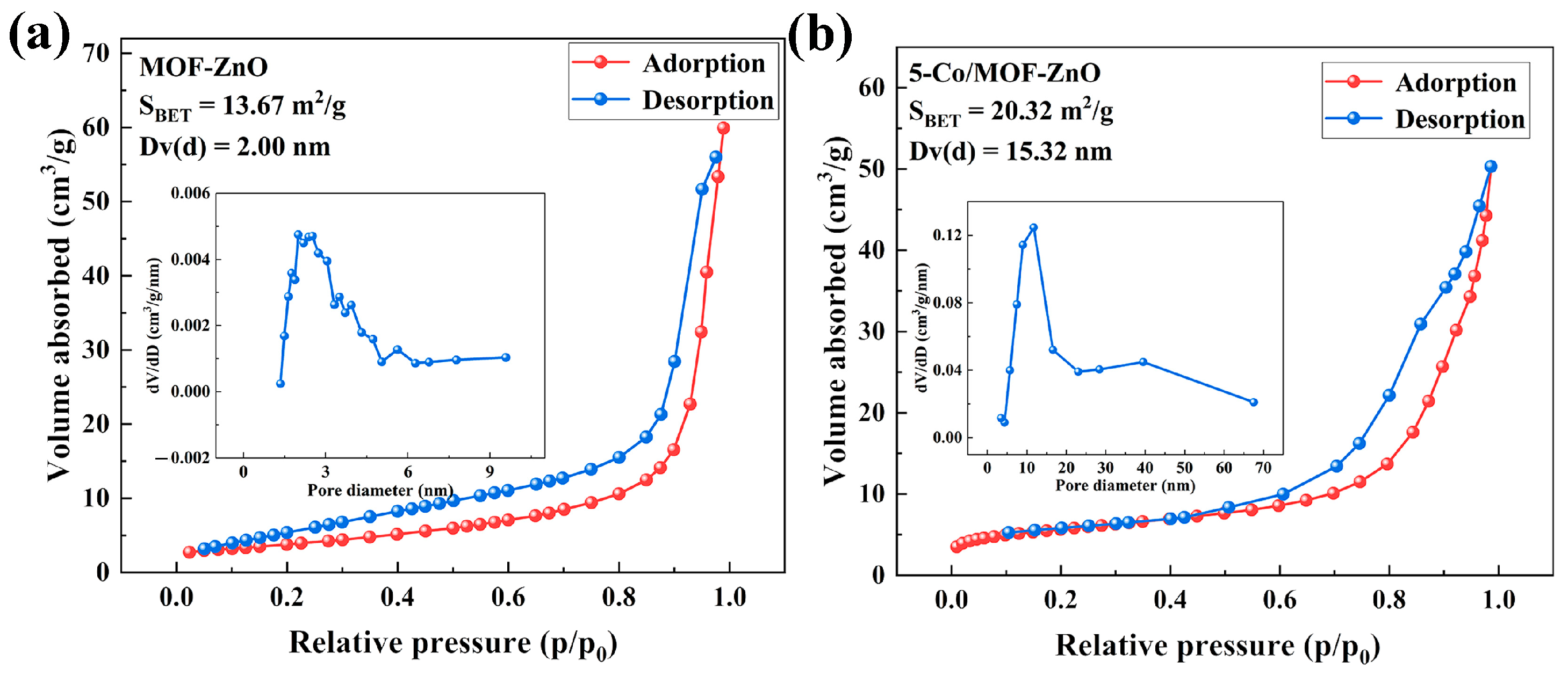
3.2. Surface and Bandwidth Analysis
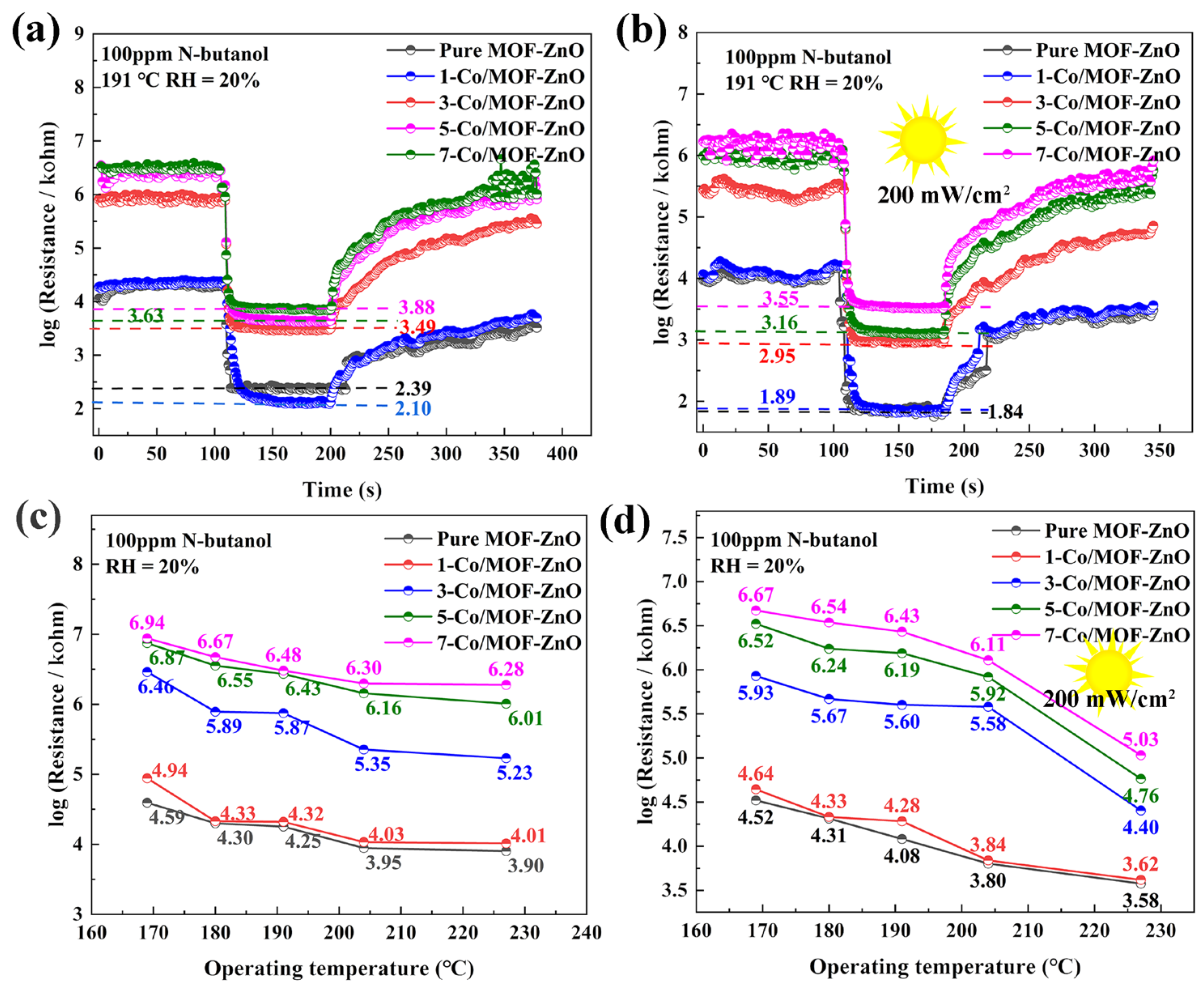
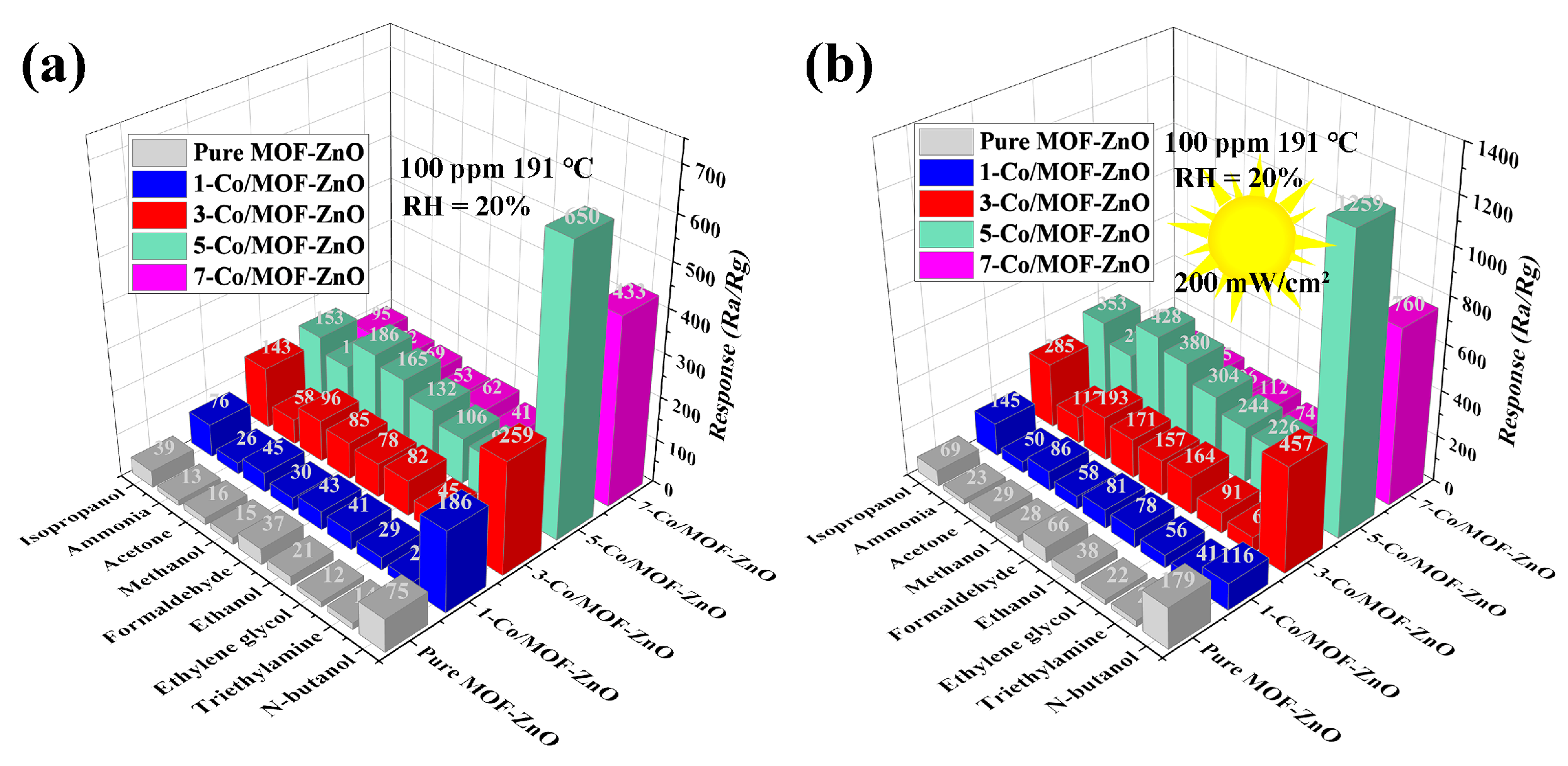
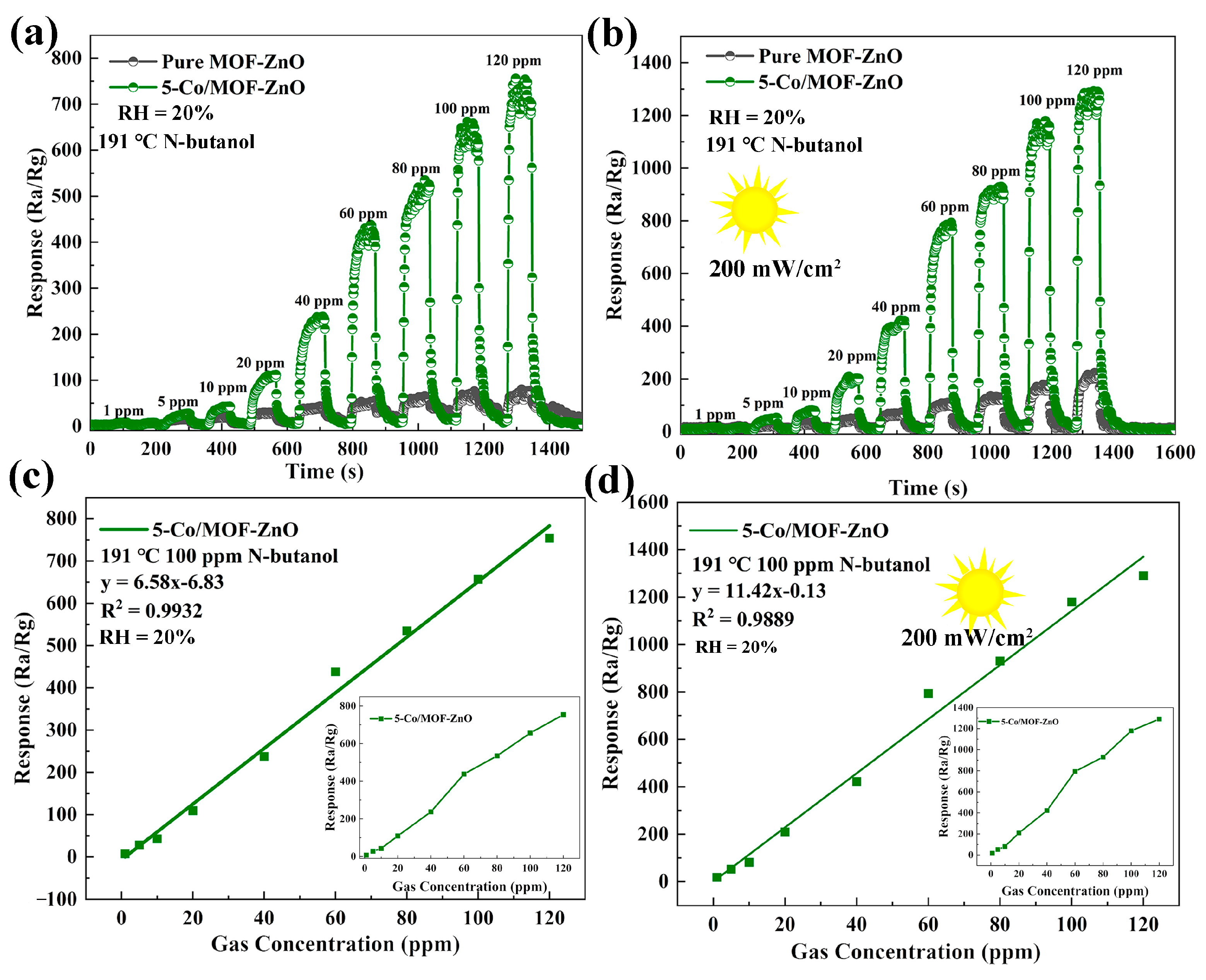
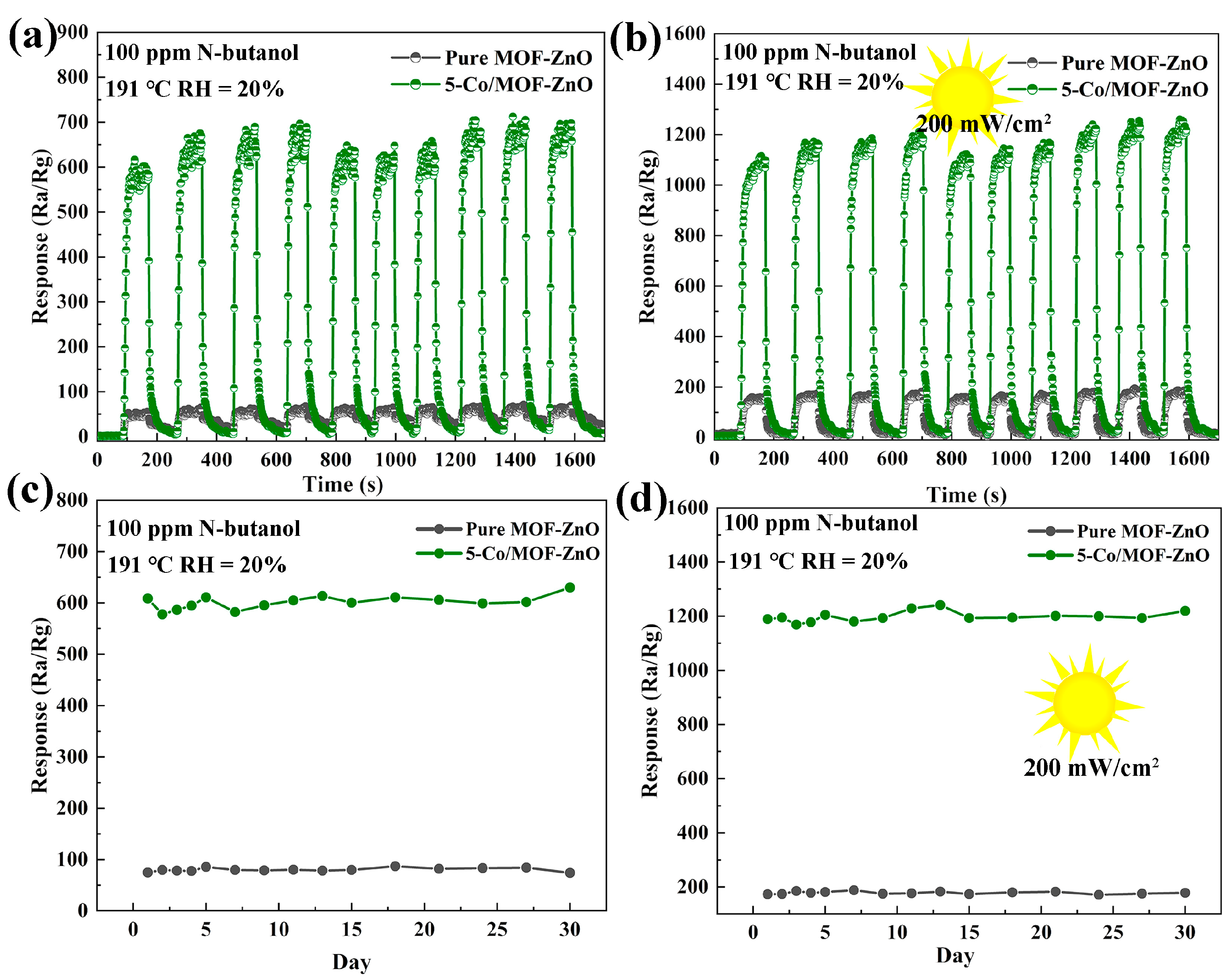
3.3. Gas-Sensing Performances
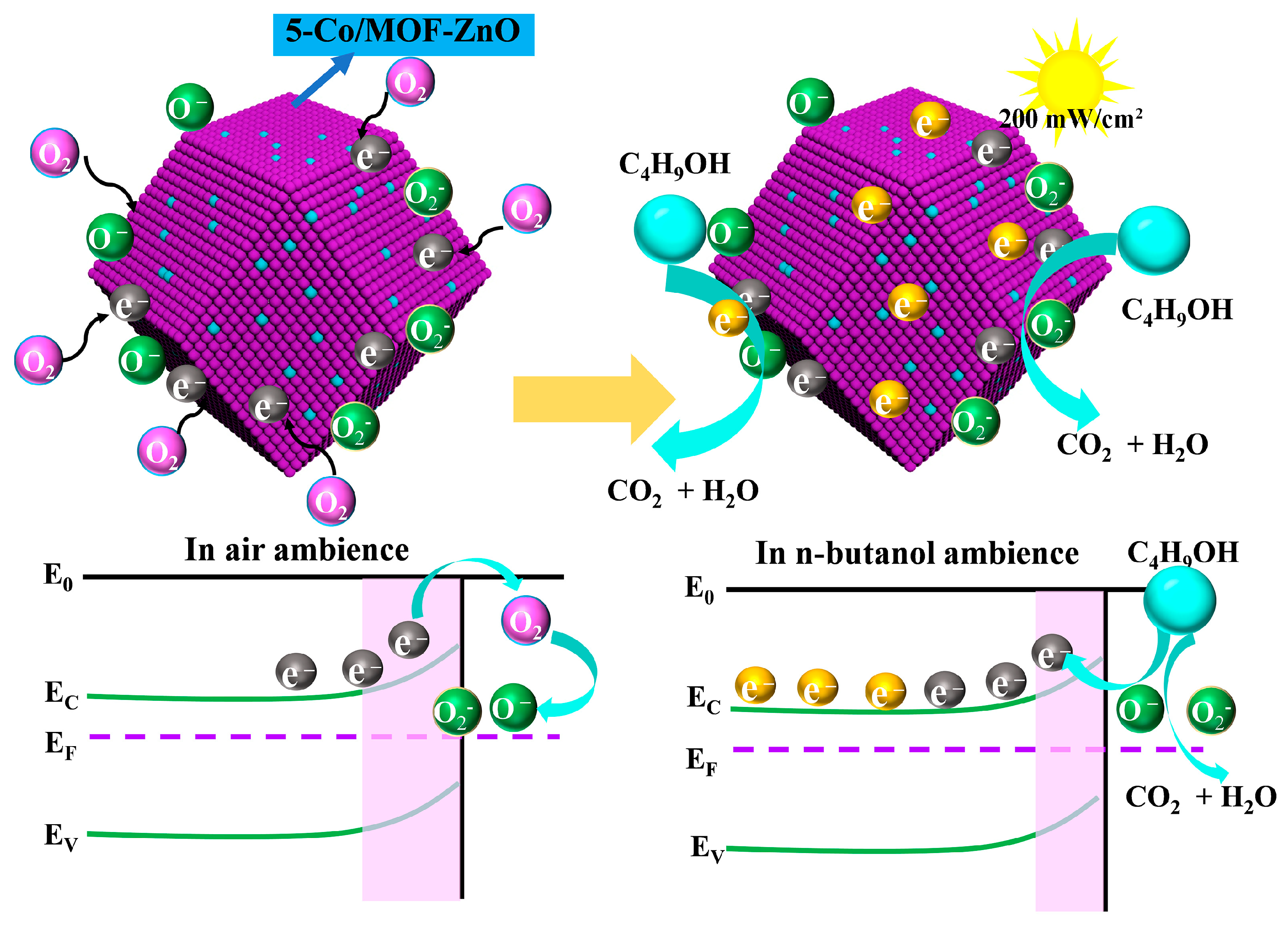
3.4. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Wang, Y.-Q.; Liu, X.-G.; Fu, H.-T.; An, X.-Z.; Yu, J.-K. Enhanced n-butanol sensitivity and selectivity of Sb-doped ZnO–Co3O4 nanoparticles synthesized by solvothermal method. Ceram. Int. 2021, 47, 34979–34986. [Google Scholar] [CrossRef]
- Zhao, R.; Li, K.; Wang, Z.; Xing, X.; Wang, Y. Gas-sensing performances of Cd-doped ZnO nanoparticles synthesized by a surfactant-mediated method for n-butanol gas. J. Phys. Chem. Solids 2018, 112, 43–49. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Yang, F.; Zhu, H.-M.; Meng, F.-L.; Ibrahim, M. High-response n-butanol gas sensor based on ZnO/In2O3 heterostructure. Rare Met. 2022, 42, 198–209. [Google Scholar] [CrossRef]
- Acharyya, S.; Bhowmick, P.K.; Guha, P.K. Selective identification and quantification of VOCs using metal nanoparticles decorated SnO2 hollow-spheres based sensor array and machine learning. J. Alloys Compd. 2023, 968, 171891. [Google Scholar] [CrossRef]
- Dadkhah, M.; Tulliani, J.-M. Green Synthesis of Metal Oxides Semiconductors for Gas Sensing Applications. Sensors 2022, 22, 4669. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, X.; Huang, S.; Hu, Y.; Yang, Z.; Li, T.T.; Li, Q.; Qian, J. MOF-on-MOF-Derived Hollow Co3O4/In2O3 Nanostructure for Efficient Photocatalytic CO2 Reduction. Adv. Sci. 2023, 10, 2300797. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Feng, Z.; Liu, S.-S.; Wang, P.; Zhao, C.; Wang, C.-C. Enhanced ethanol sensing performance of N-doped ZnO derived from ZIF-8. Chin. Chem. Lett. 2023, 34, 107425. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Ling, W.Y.; Zhao, T.Y.; Pu, Y.; Cao, S.X.; Zhu, D.C. High response ZnO gas sensor derived from Tb@Zn-MOFs to acetic acid under UV excitation. Sens. Actuators A Phys. 2024, 365, 114862. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, L.; Wang, X.; Chai, L.; Wang, Y.; Jiang, M.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. General approach to MOF-derived core-shell bimetallic oxide nanowires for fast response to glucose oxidation. Sens. Actuators B Chem. 2020, 306, 127551. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, D.; Huang, Q.; Huang, S.; Qian, J. Carbon nanotubes anchored onto hollow carbon for efficient oxygen reduction. Sci. China Mater. 2022, 66, 641–650. [Google Scholar] [CrossRef]
- Shi, T.; Hussain, S.; Ge, C.; Liu, G.; Wang, M.; Qiao, G. ZIF-X (8, 67) based nanostructures for gas-sensing applications. Rev. Chem. Eng. 2023, 39, 911–939. [Google Scholar] [CrossRef]
- Kukkar, P.; Kim, K.-H.; Kukkar, D.; Singh, P. Recent advances in the synthesis techniques for zeolitic imidazolate frameworks and their sensing applications. Coord. Chem. Rev. 2021, 446, 214109. [Google Scholar] [CrossRef]
- Luo, Y.; Ly, A.; Lahem, D.; Martin, J.D.M.; Romain, A.-C.; Zhang, C.; Debliquy, M. Role of cobalt in Co-ZnO nanoflower gas sensors for the detection of low concentration of VOCs. Sens. Actuators B Chem. 2022, 360, 131674. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Zhu, H.; Wang, H.; Meng, F. High-Performance Conductometric Acetone Gas Sensor Based on Co3O4/ZnO Nanorods with Abundant Oxygen Vacancies. IEEE Trans. Instrum. Meas. 2024, 73, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.; Meng, F. Low Detection Limit and High Sensitivity 2-Butanone Gas Sensor Based on ZnO Nanosheets Decorated by Co Nanoparticles Derived from ZIF-67. Nanomaterials 2023, 13, 2398. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors Under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, X.; Sun, J. UV-enhanced NO2 sensor using ZnO quantum dots sensitized SnO2 porous nanowires. Nanotechnology 2022, 33, 185501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hong, J.S.; Zhang, Z.L.; Li, J.D.; Cao, X.D.; Tang, J.H.; Geng, Y.F.; Wang, J.Q.; Li, X.J.; Pei, K.; et al. Visible light-activated ethanol sensor based on flower-like N3-loaded ZnO composites. Sens. Actuators B Chem. 2022, 370, 132399. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Y.; Tang, Y.; Yu, F. UV-Activated ZnO–NiO heterojunction sensor for ethanol gas detection at low working temperature. Mater. Sci. Semicond. Process. 2024, 169, 107925. [Google Scholar] [CrossRef]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling zeolitic imidazolate framework nano-and microcrystal formation: Insight into crystal growth by time-resolved in situ static light scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Xiao, S.; Jiao, Z.; Yang, X.C. Ultra-Sensitive Gas Sensor Based on CDs@ZnO. Sensors 2025, 25, 905. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Ding, Z.W.; Yuan, X.T.; Zhang, F.Y.; Xu, K.; Lu, X.; Liu, S.; Li, S.Q.; Ji, W. Highly responsive n-butanol gas sensor based on double-shell ZnO hollow microspheres. Microchem. J. 2024, 200, 110242. [Google Scholar] [CrossRef]
- Naik, E.I.; Naik, H.B.; Sarvajith, M.; Pradeepa, E. Co-precipitation synthesis of cobalt doped ZnO nanoparticles: Characterization and their applications for biosensing and antibacterial studies. Inorg. Chem. Commun. 2021, 130, 108678. [Google Scholar] [CrossRef]
- Hu, J.; Gao, F.; Zhao, Z.; Sang, S.; Li, P.; Zhang, W.; Zhou, X.; Chen, Y. Synthesis and characterization of Cobalt-doped ZnO microstructures for methane gas sensing. Appl. Surf. Sci. 2016, 363, 181–188. [Google Scholar] [CrossRef]
- Nair, M.G.; Nirmala, M.; Rekha, K.; Anukaliani, A. Structural, optical, photo catalytic and antibacterial activity of ZnO and Co doped ZnO nanoparticles. Mater. Lett. 2011, 65, 1797–1800. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Z.; Liu, D.; Li, Y.; Zhang, Z.; Tang, Z. Conductometric NO2 gas sensors based on MOF-derived porous ZnO nanoparticles. Sens. Actuators B Chem. 2022, 357, 131384. [Google Scholar] [CrossRef]
- Wongrat, E.; Ta-om, T.; Khamprakaysit, S.; Chanlek, N.; Choopun, S. Effect of Cu or Ni addition to ZnO nanostructures on their n-butanol sensing performance. Thin Solid Film. 2023, 774, 139839. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Li, Z.; Guo, L.; Feng, Z.; Gao, S.; Zhang, H.; Yang, X.; Liu, H.; Shao, J.; Sun, C.; Cheng, Y.; et al. Metal-organic framework-derived ZnO decorated with CuO for ultra-high response and selectivity H2S gas sensor. Sens. Actuators B Chem. 2022, 366, 131995. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Liu, Z.; Xia, P.; Xie, Y.; Xiong, D. Synthesis of the 0D/3D CuO/ZnO Heterojunction with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2018, 122, 9531–9539. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, W.; Qiao, X.; Song, X.; Wang, S.; Zhang, X.; Wang, X.; Tian, J. Confined synthesis of 2D ultrathin ZnO/Co3O4 nanomeshes heterostructure for superior triethylamine detection at low temperature. Sens. Actuators B Chem. 2021, 346, 130486. [Google Scholar] [CrossRef]
- Singh, K.; Nancy; Kaur, H.; Sharma, P.K.; Singh, G.; Singh, J. ZnO and cobalt decorated ZnO NPs: Synthesis, photocatalysis and antimicrobial applications. Chemosphere 2023, 313, 137322. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xie, Z.; Zheng, M. Room temperature phosphorescent carbon dots for latent fingerprints detection and in vivo phosphorescence bioimaging. Sens. Actuators B Chem. 2022, 351, 130976. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, L.; Yang, S.; Zhou, X.; Chen, X.; Dong, B.; Bai, X.; Lu, G.; Song, H. Cobalt-doped ZnO nanoparticles derived from zeolite imidazole frameworks: Synthesis, characterization, and application for the detection of an exhaled diabetes biomarker. J. Colloid Interface Sci. 2020, 569, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Ahemad, M.J.; Kumar, S.; Van Dung, D.; Rai, P.; Kumar, M.; Awasthi, K.; Yu, Y.-T. Enhanced hydrogen sensing performances of PdO nanoparticles-decorated ZnO flower-like nanostructures. J. Alloys Compd. 2022, 900, 163545. [Google Scholar] [CrossRef]
- Bai, M.; Li, C.; Zhao, X.; Wang, Q.; Pan, Q. Controllable Synthesis of Sheet-Flower ZnO for Low Temperature NO2 Sensor. Nanomaterials 2023, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Li, J.A.; He, D.W.; Wang, X.R.; Shi, Y.; Pan, L.J. Recent progress on performances and mechanisms of carbon dots for gas sensing. Luminescence 2022, 38, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Basak, D. Role of defects in the anomalous photoconductivity in ZnO nanowires. Appl. Phys. Lett. 2009, 94, 163119. [Google Scholar] [CrossRef]
- Cai, Z.; Park, J.; Park, S. Porous In2O3–ZnO nanofiber-based sensor for ultrasensitive room-temperature detection of toluene gas under UV illumination. J. Mater. Res. Technol. 2023, 24, 2482–2499. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Z.; Tian, M.; Miao, J.; Zhang, H.; Sun, J. UV excitation NO2 gas sensor sensitized by ZnO quantum dots at room temperature. Sens. Actuators B Chem. 2018, 259, 526–531. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wan, Y.; Wang, Y.; Gao, S.; Chen, Y.; Luo, Q.; Feng, C. Novel Bi-doped ZnFe2O4 nanofibers based gas sensor for enhanced n-butanol sensing. J. Taiwan Inst. Chem. Eng. 2024, 157, 105395. [Google Scholar] [CrossRef]
- Sun, X.Y.; Tang, M.X.; Yu, M.Q.; Fan, Y.Z.; Qin, C.; Cao, J.L.; Wang, Y. UV-activated CH4 gas sensor based on Pd@Ni/ZnO microspheres. Mater. Today Commun. 2024, 40, 109551. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yang, B.; Zhou, J.; Yi, G.; Wang, Y. Highly open skeleton In2O3/ZnO as a high-performance formaldehyde sensing material for gas sensors. Mater. Today Commun. 2025, 42, 111495. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Song, X.; Liu, T.; Gu, K.; Luo, Z.; Yang, X.; Zhang, M. UV-triggered and temperature-control carrier transport regulation of ZnO/Ti3C2Tx MXene heterostructures for dual selective HCHO and triethylamine detection. Sens. Actuators B Chem. 2024, 417, 136158. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Ling, W.; Zhu, D.; Pu, Y. UV excited gas sensing SnO2-ZnO aerogels to ppb-level ethanol detection. Sens. Actuators B Chem. 2021, 337, 129815. [Google Scholar] [CrossRef]
- Li, J.; Gu, D.; Yang, Y.; Du, H.; Li, X. UV Light Activated SnO2/ZnO Nanofibers for Gas Sensing at Room Temperature. Front. Mater. 2019, 6, 158. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.L.; Liu, Y.C.; Qu, Z.H.; Tian, S.Y.; Zhang, Y.H. UV light-activated Eu/ZnO flower-like microsphere for detecting NO2 gas with high response. Ceram. Int. 2024, 50, 39654–39665. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, A.-J.; Sun, Y.-R.; Li, J.; Ma, R.; Ding, Y. High selectivity and sensitivity gas sensors to n-butanol based on Ni-doped ultra-thin porous single-crystalline ZnO nanosheets. Sens. Actuators A Phys. 2024, 369, 115115. [Google Scholar] [CrossRef]



| Sample | Lattice Parameter a = b (Å) | Lattice Parameter c (Å) | Grain Size (nm) | FWHM (°) |
|---|---|---|---|---|
| pure MOF-ZnO | 3.2518 | 5.2406 | 7.06 | 1.17 |
| 1-Co/MOF-ZnO | 3.2475 | 5.2159 | 6.81 | 1.21 |
| 3-Co/MOF-ZnO | 3.2436 | 5.198 | 6.66 | 1.24 |
| 5-Co/MOF-ZnO | 3.2519 | 5.1796 | 8.09 | 1.02 |
| 7-Co/MOF-ZnO | 3.2532 | 5.1668 | 9.11 | 0.91 |
| Sample | Gas | Concentration (ppm) | Working Temperature (°C) | Response (Ra/Rg) | Light | Refs. |
|---|---|---|---|---|---|---|
| In2O3/ZnO | formaldehyde | 100 | 300 | 53.20 | / | [44] |
| Pt-ZnO | triethylamine | 100 | 200 | 242.00 | / | [45] |
| ZnO/Ti3C2Tx MXene | triethylamine | 100 | 160 | 28.20 | / | [46] |
| N3-loaded ZnO nanocluster | ethyl alcohol | 200 | 225 | 75.10 | Green | [18] |
| SnO2-ZnO aerogels | ethyl alcohol | 100 | 300 | 15.50 | UV | [47] |
| ZnO-Ni nanotetrapods | n-butanol | 100 | 400 | 186.00 | / | [27] |
| 5-Co/MOF-ZnO | n-butanol | 100 | 191 | 1259.06 | UV | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wei, X.; Guo, Y.; Wang, L.; Guo, H.; Wang, Q.; Qiao, Y.; Zhu, X.; Yang, X.; Cheng, L.; et al. A Highly Sensitive Low-Temperature N-Butanol Gas Sensor Based on a Co-Doped MOF-ZnO Nanomaterial Under UV Excitation. Sensors 2025, 25, 4480. https://doi.org/10.3390/s25144480
Liu Y, Wei X, Guo Y, Wang L, Guo H, Wang Q, Qiao Y, Zhu X, Yang X, Cheng L, et al. A Highly Sensitive Low-Temperature N-Butanol Gas Sensor Based on a Co-Doped MOF-ZnO Nanomaterial Under UV Excitation. Sensors. 2025; 25(14):4480. https://doi.org/10.3390/s25144480
Chicago/Turabian StyleLiu, Yinzhong, Xiaoshun Wei, Yun Guo, Lingchao Wang, Hui Guo, Qingjie Wang, Yiyu Qiao, Xiaotao Zhu, Xuechun Yang, Lingli Cheng, and et al. 2025. "A Highly Sensitive Low-Temperature N-Butanol Gas Sensor Based on a Co-Doped MOF-ZnO Nanomaterial Under UV Excitation" Sensors 25, no. 14: 4480. https://doi.org/10.3390/s25144480
APA StyleLiu, Y., Wei, X., Guo, Y., Wang, L., Guo, H., Wang, Q., Qiao, Y., Zhu, X., Yang, X., Cheng, L., & Jiao, Z. (2025). A Highly Sensitive Low-Temperature N-Butanol Gas Sensor Based on a Co-Doped MOF-ZnO Nanomaterial Under UV Excitation. Sensors, 25(14), 4480. https://doi.org/10.3390/s25144480






