Porous-Cladding Polydimethylsiloxane Optical Waveguide for Biomedical Pressure Sensing Applications
Abstract
1. Introduction
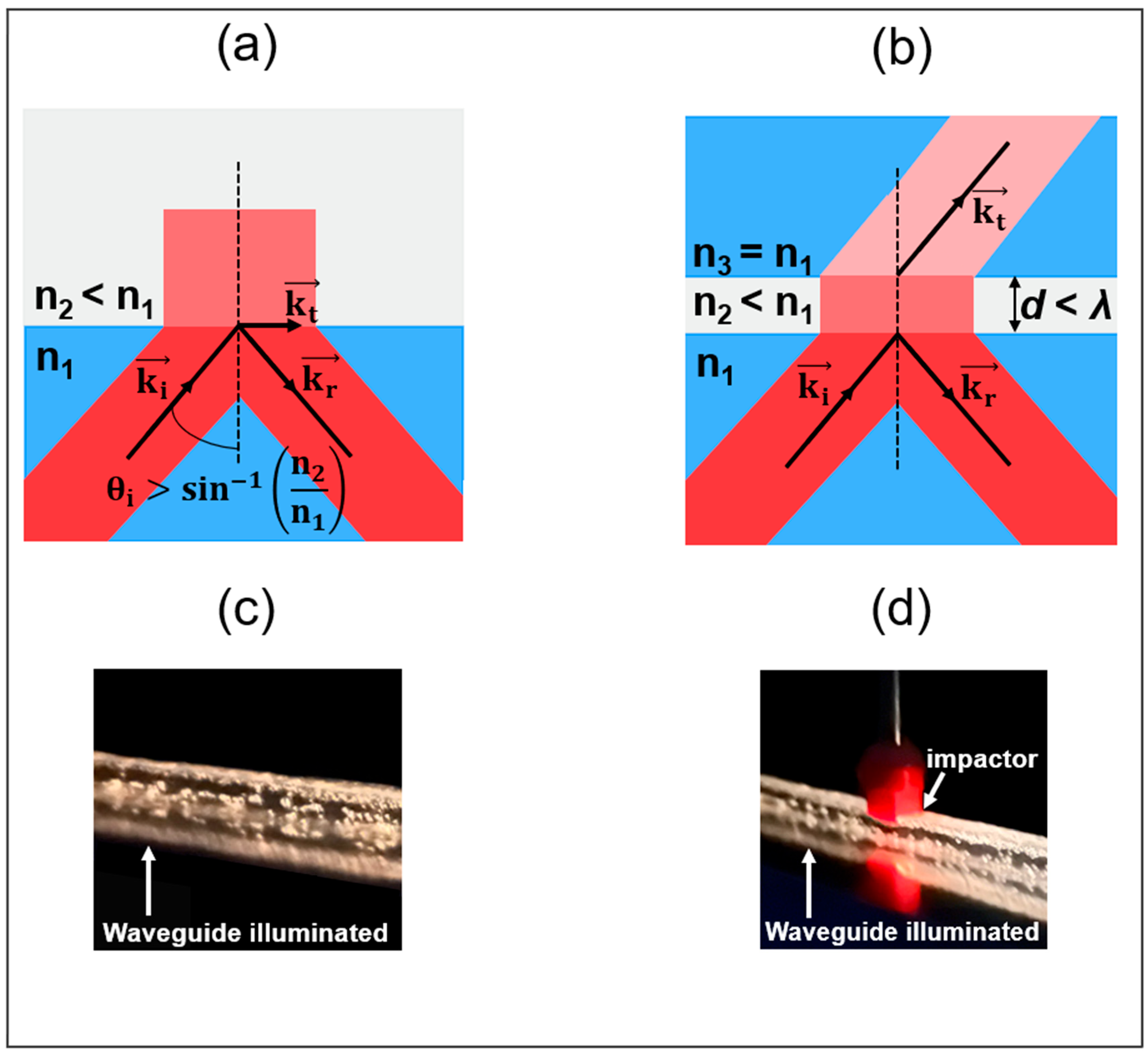
2. Sensor Structure and Optical Sensing Mechanism
3. Materials and Methods
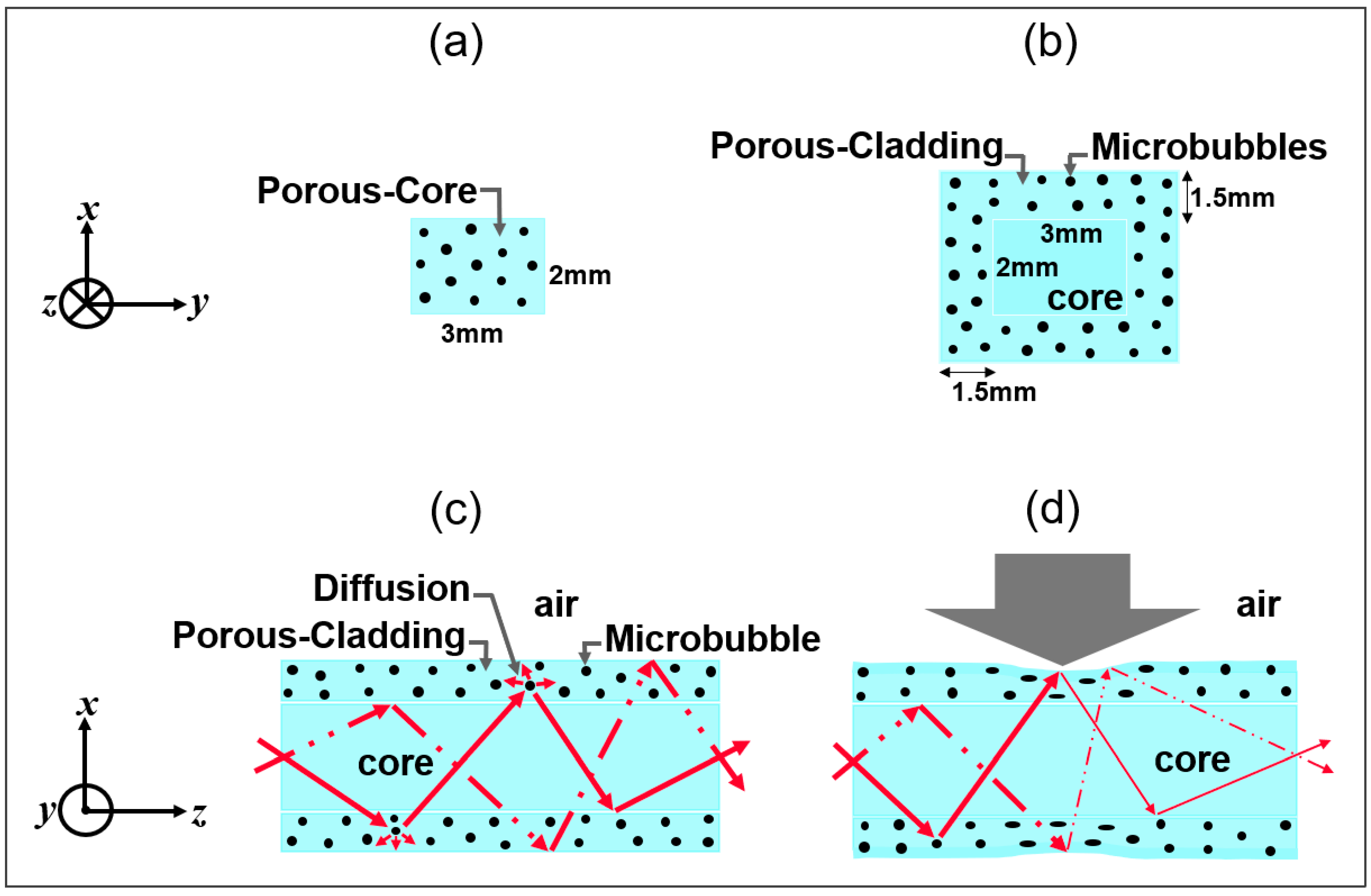
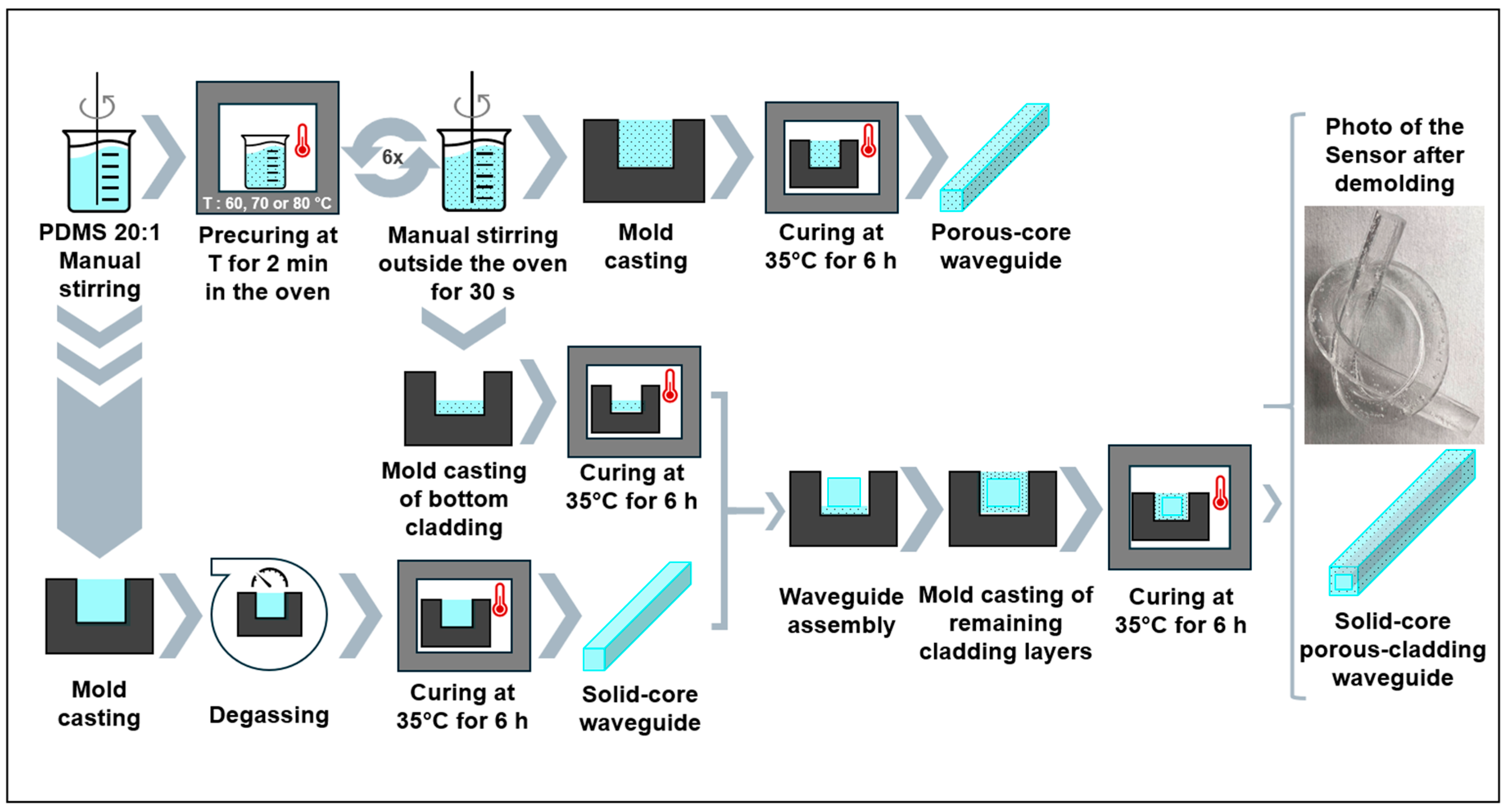
3.1. Waveguide Fabrications: Porous-Core/Air-Clad and Solid-Core/Porous Cladding
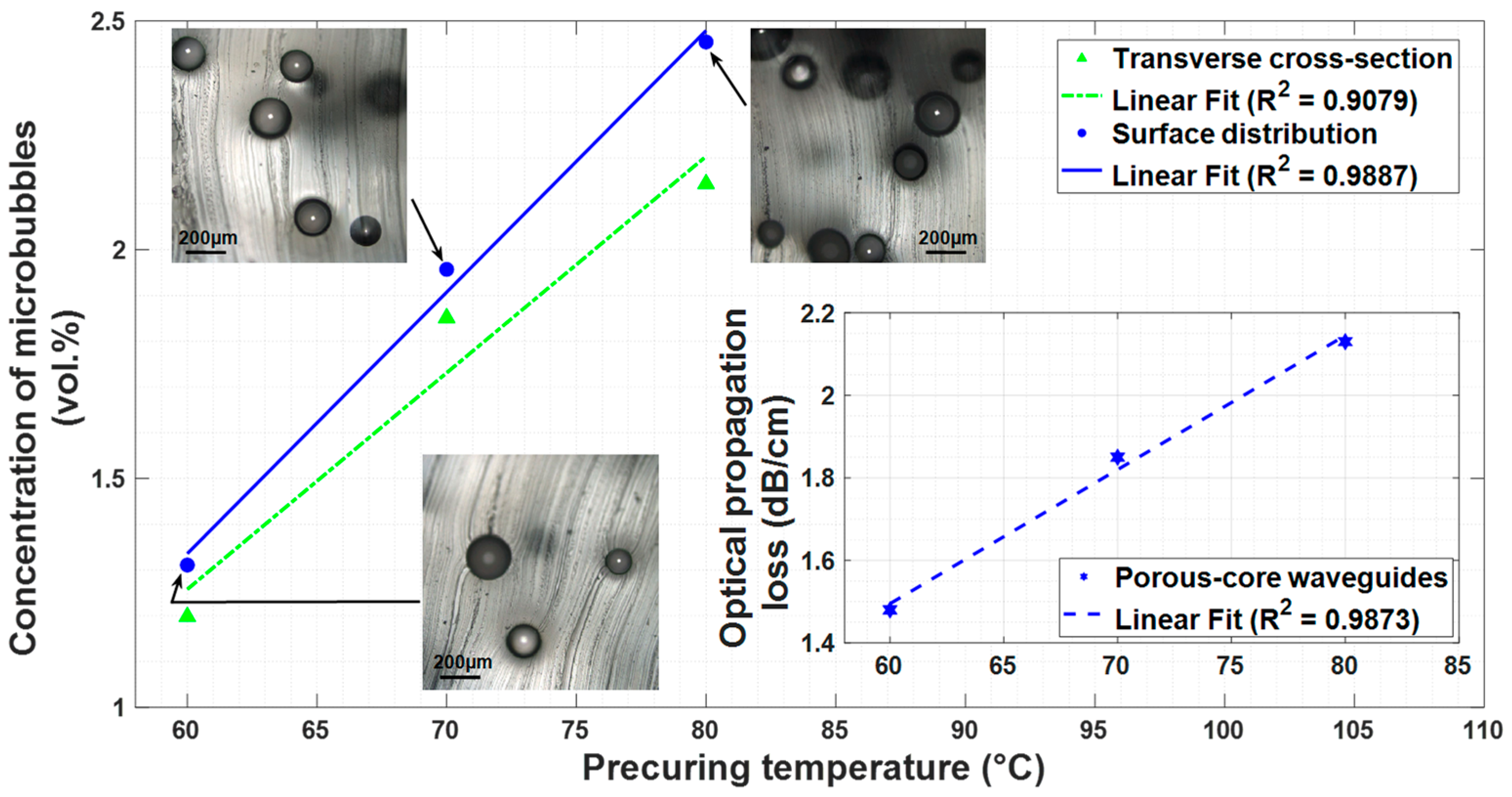
3.2. Quantitative Analysis of Microbubbles
3.2.1. Microbubble Effects on Waveguiding Optical Losses
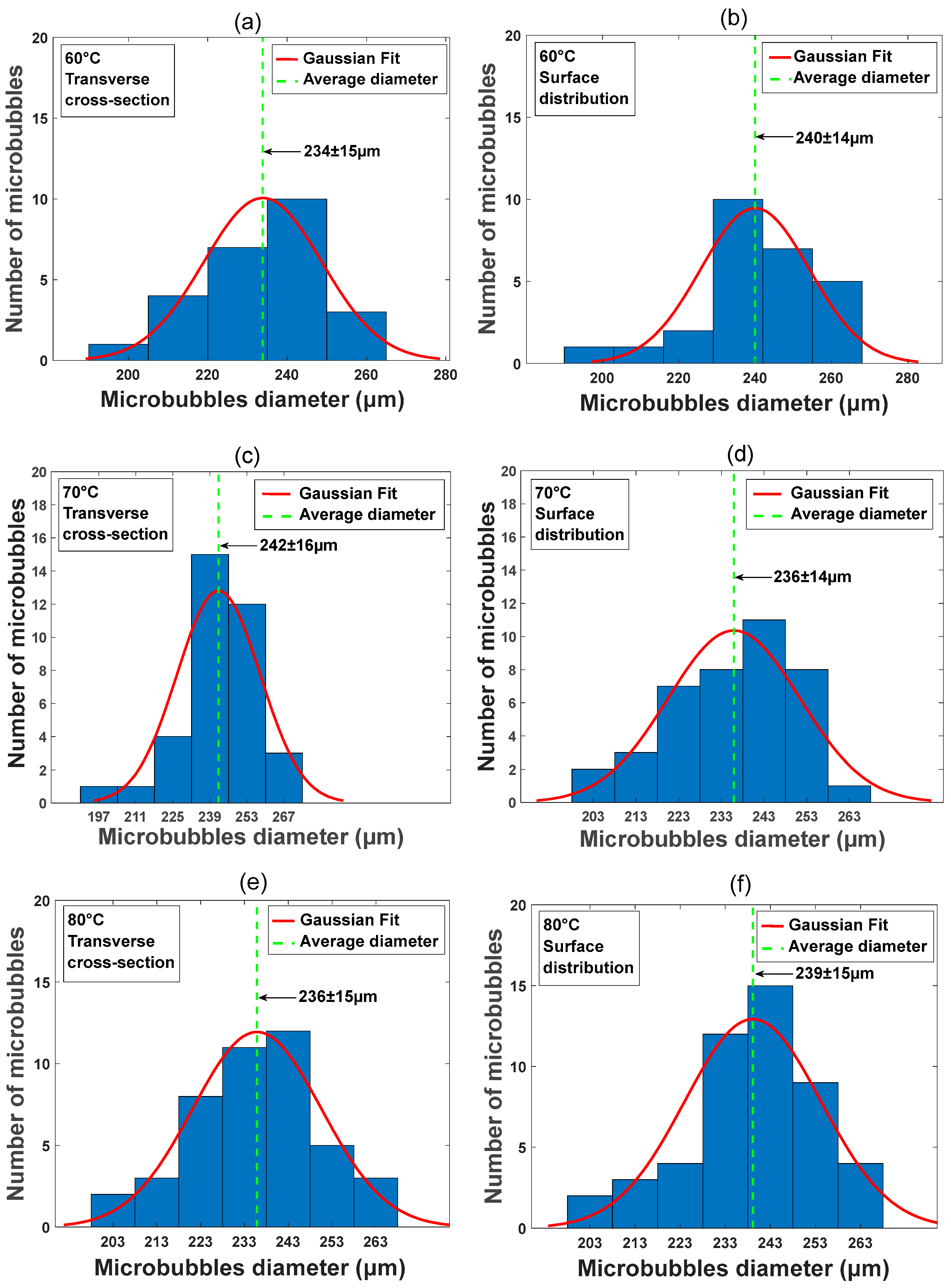
3.2.2. Average Microbubble Diameter Measurements
4. Characterization of Solid-Core/Porous-Cladding Waveguide
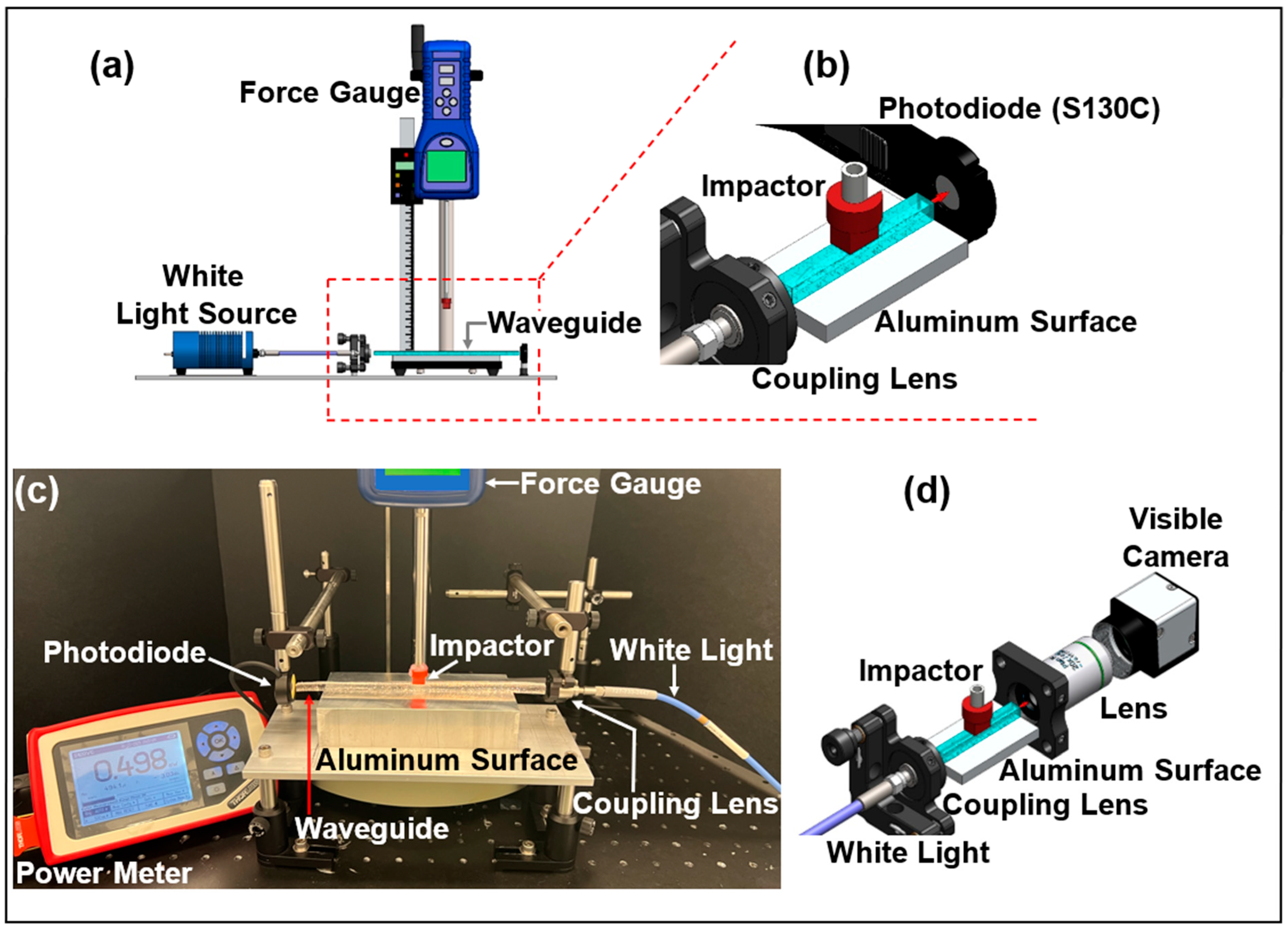
4.1. Transverse Compression Experimental Setup
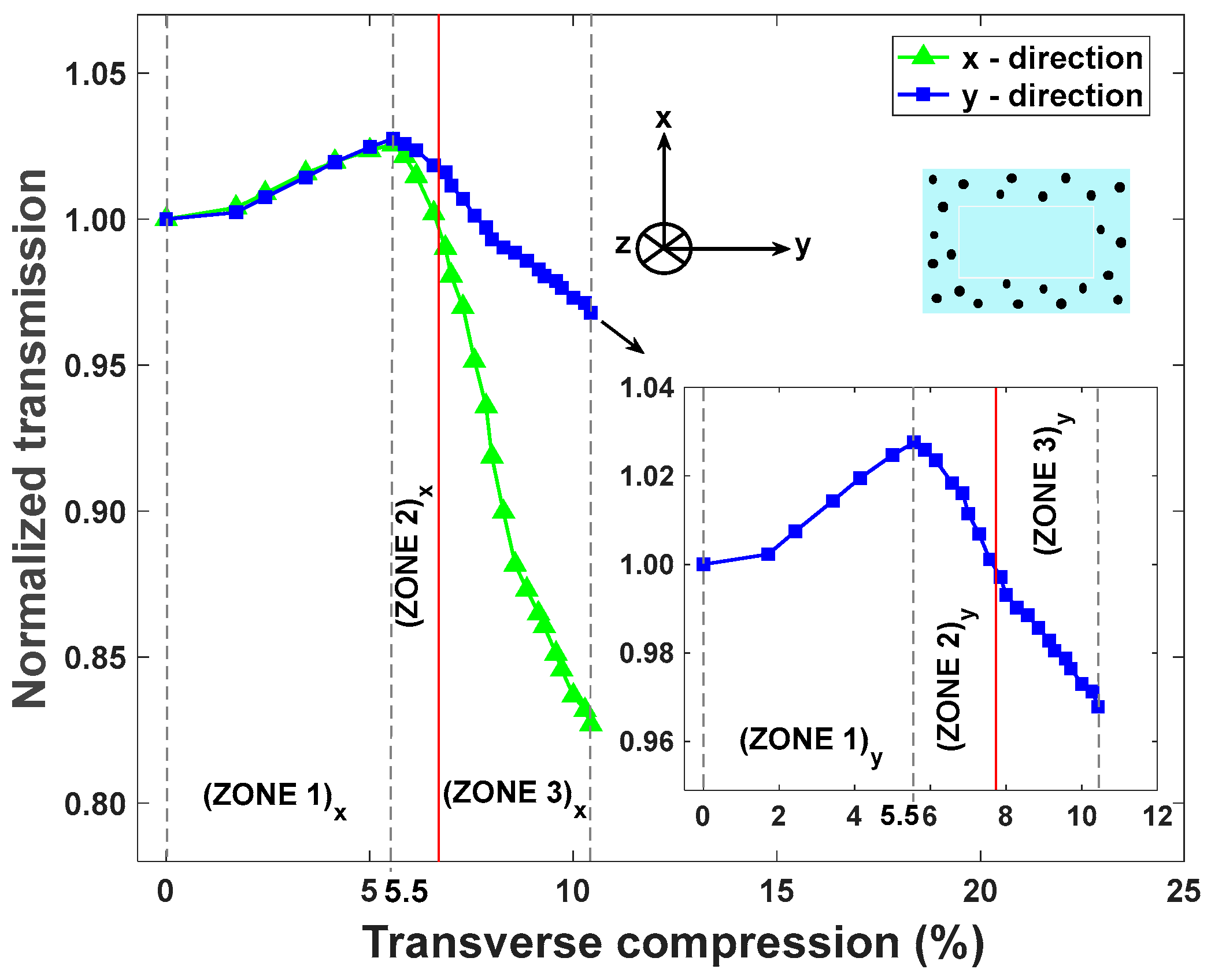
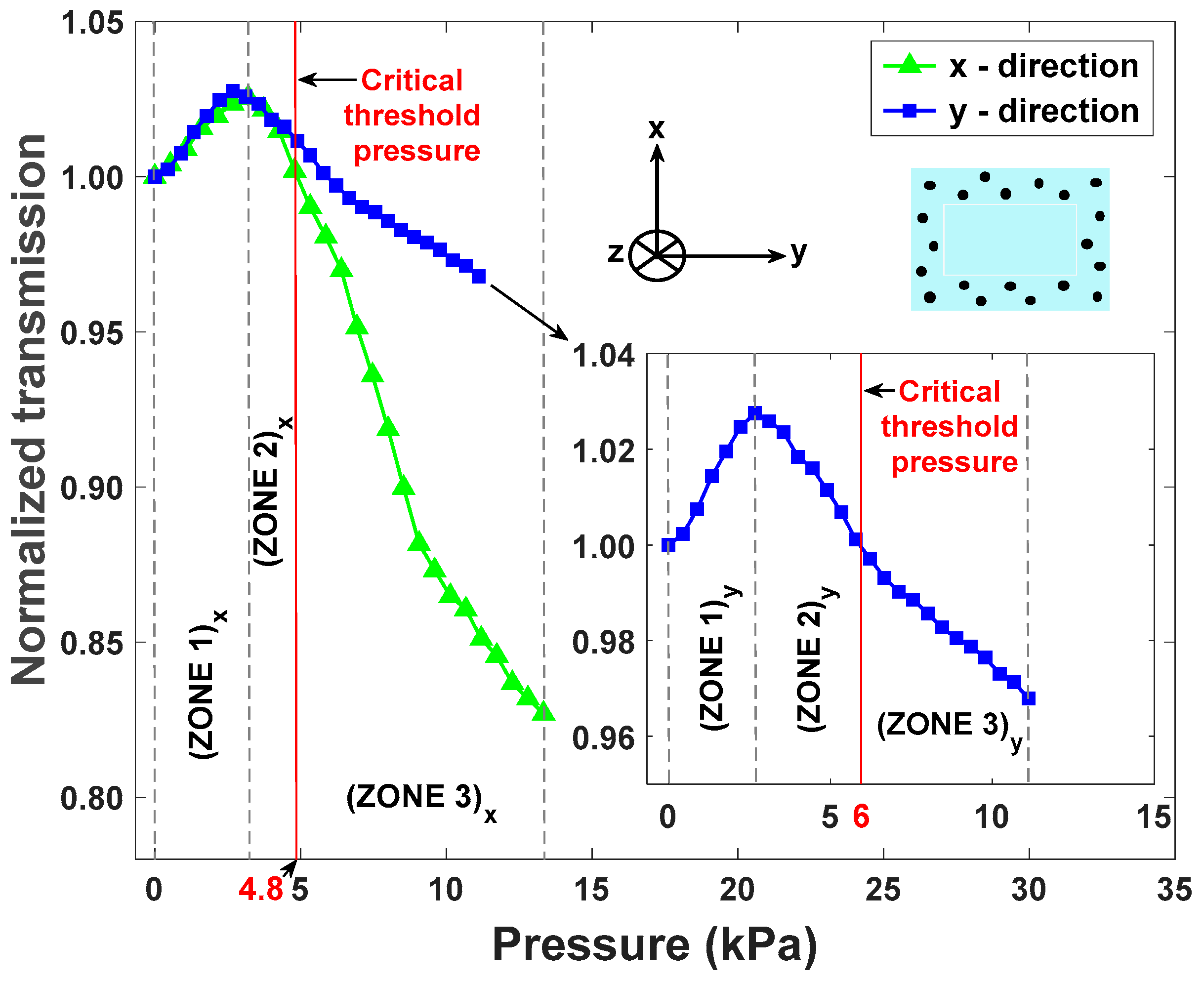
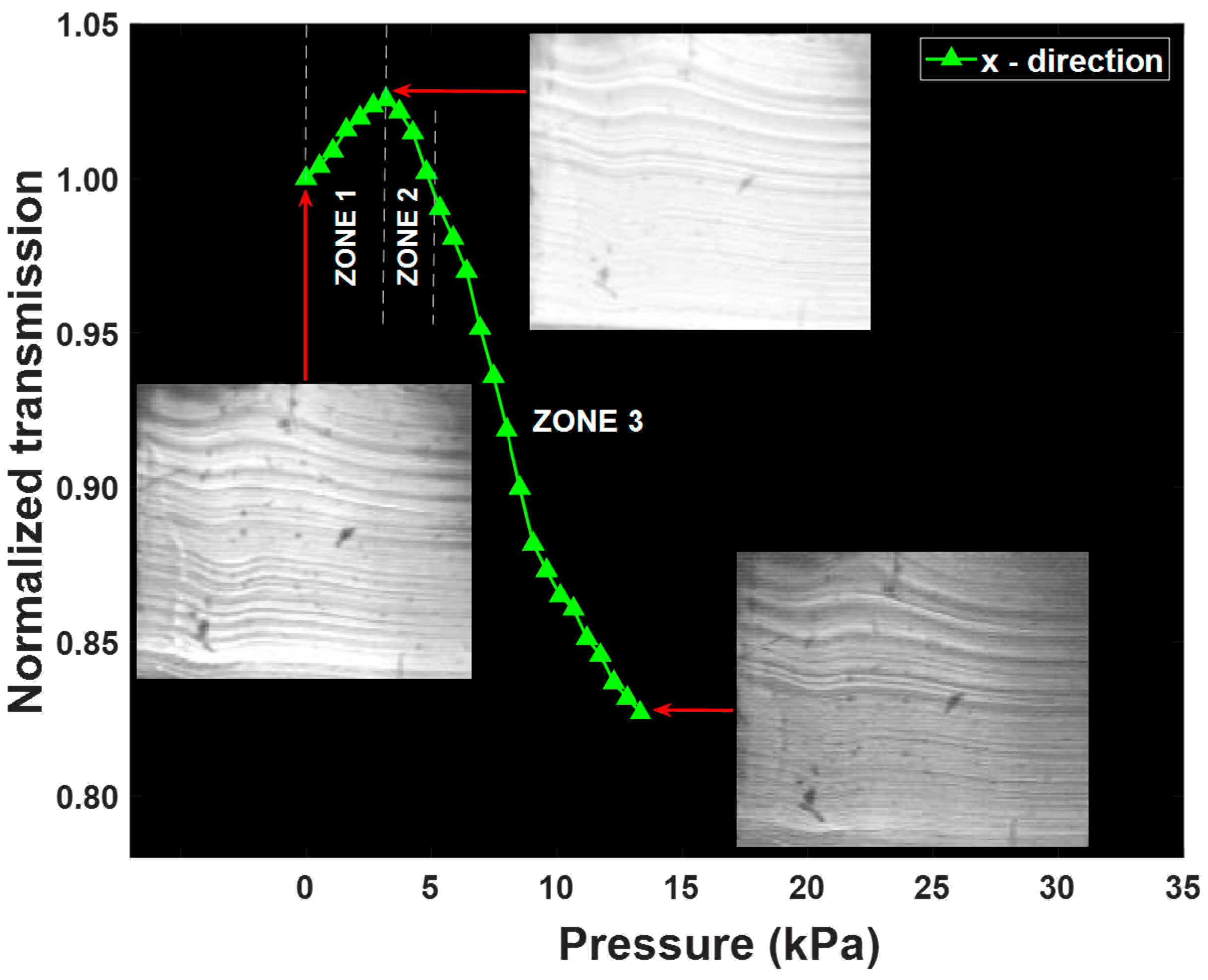
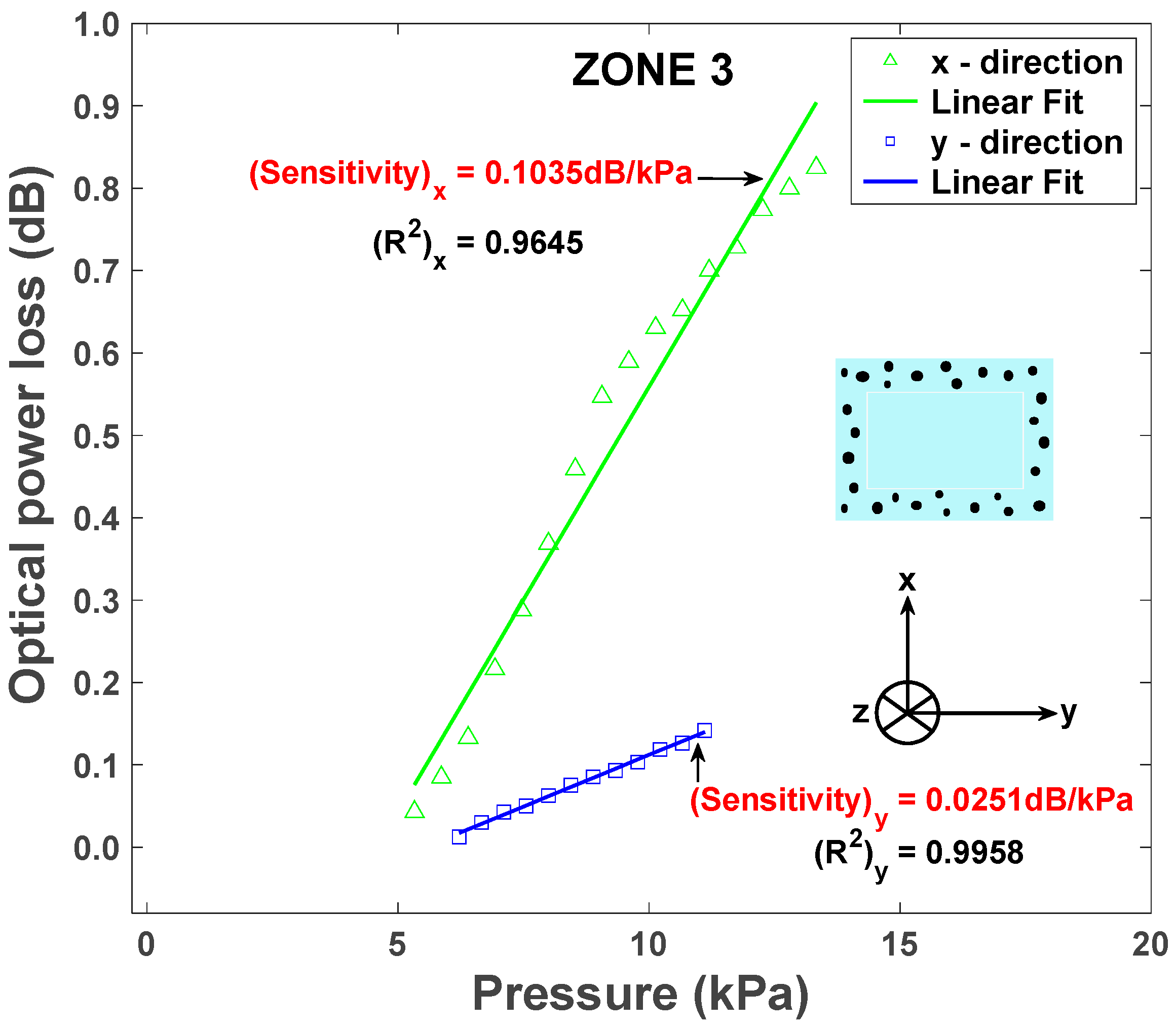
4.2. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Yang, C.; Dai, Q.; Kong, L. Soft and Stretchable Polymeric Optical Waveguide-Based Sensors for Wearable and Biomedical Applications. Sensors 2019, 19, 3771. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, A.; Avellar, L.; Biazi, V.; Soares, M.S.; Frizera, A.; Marques, C. Multifunctional flexible optical waveguide sensor: On the bioinspiration for ultrasensitive sensors development. Opto-Electron. Adv. 2022, 5, 210098. [Google Scholar] [CrossRef]
- Li, L.; Yang, C.; Wang, Z.; Xiao, K.; Min, R. Stretchable polymer optical fiber embedded in the mattress for respiratory and heart rate monitoring. Opt. Laser Technol. 2024, 171, 110356. [Google Scholar] [CrossRef]
- Li, L.; Zheng, J.; Chen, J.; Luo, Z.; Su, Y.; Tang, W.; Gao, X.; Li, Y.; Cao, C.; Liu, Q.; et al. Flexible Pressure Sensors for Biomedical Applications: From Ex Vivo to In Vivo. Adv. Mater. Interfaces 2020, 7, 2000743. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Shen, G. Recent advances of flexible sensors for biomedical applications. Prog. Nat. Sci. Mater. Int. 2021, 31, 872–882. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Su, L. Soft Optical Waveguides for Biomedical Applications, Wearable Devices, and Soft Robotics: A Review. Adv. Intell. Syst. 2024, 6, 2300482. [Google Scholar] [CrossRef]
- Yan, J.; Chen, A.; Liu, S. Flexible sensing platform based on polymer materials for health and exercise monitoring. Alex. Eng. J. 2024, 86, 405–414. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Gao, C. Research on the Fabrication and Parameters of a Flexible Fiber Optic Pressure Sensor with High Sensitivity. Photonics 2024, 11, 919. [Google Scholar] [CrossRef]
- Wang, X.; Kang, S.; Dai, Z.; Li, H.; Guo, J.; Zeng, Q.; Tang, D.; Wang, Z.; Wang, G. Flexible electrospinning pressure sensing film with wide pressure detection range and high sensitivity. J. Text. Inst. 2025, 116, 71–79. [Google Scholar] [CrossRef]
- Mahato, R.; Masiul Islam, S.; Maurya, R.K.; Kumar, S.; Purohit, G.; Singh, S. Flexible piezo-resistive strain sensors using all-polydimethylsiloxane based hybrid nanocomposites for wearable electronics. Phys. Chem. Chem. Phys. 2024, 26, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tanusha, D.; Badhulika, S. Comparative analysis of micro patterned PDMS-based piezoresistive pressure sensors with multifunctional strain and health monitoring applications. Sens. Actuators Phys. 2024, 369, 115139. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Song, P.; Manshaii, F.; Bell, J.; Chen, J.; Dinh, T. Advances in Soft Strain and Pressure Sensors. ACS Nano 2025, 19, 6663–6704. [Google Scholar] [CrossRef]
- Zhang, C.; Lang, S.; Tao, M.; Li, P.; Liang, T.; Zhao, X.; Gou, X.; Zhao, X.; Xiong, S.; Zheng, L.; et al. Deep learning-assisted piezoresistive pressure sensors with broad-range ultrasensitivity for wearable motion monitoring. Nano Energy 2025, 140, 111035. [Google Scholar] [CrossRef]
- Jin, Y.; Xue, S.; He, Y. Flexible Pressure Sensors Enhanced by 3D-Printed Microstructures. Adv. Mater. 2025, 2500076. [Google Scholar] [CrossRef]
- Chen, W.; Yan, X. Progress in achieving high-performance piezoresistive and capacitive flexible pressure sensors: A review. J. Mater. Sci. Technol. 2020, 43, 175–188. [Google Scholar] [CrossRef]
- Cheng, A.J.; Wu, L.; Sha, Z.; Chang, W.; Chu, D.; Wang, C.H.; Peng, S. Recent Advances of Capacitive Sensors: Materials, Microstructure Designs, Applications, and Opportunities. Adv. Mater. Technol. 2023, 8, 2201959. [Google Scholar] [CrossRef]
- Mishra, R.B.; El-Atab, N.; Hussain, A.M.; Hussain, M.M. Recent Progress on Flexible Capacitive Pressure Sensors: From Design and Materials to Applications. Adv. Mater. Technol. 2021, 6, 2001023. [Google Scholar] [CrossRef]
- Shang, E.; Zhao, Z.; Peng, S.; Zhang, N.; Fan, D.; Liu, Y. Flexible Linkage Design of Composite Dielectric Layer for High-Performance Capacitive Pressure Sensor. ACS Appl. Electron. Mater. 2025, 7, 5050–5060. [Google Scholar] [CrossRef]
- Mathew, S.; Chintagumpala, K. A review of recent progress in flexible capacitance pressure sensors: Materials design, printing methods, and applications. Adv. Compos. Hybrid Mater. 2025, 8, 236. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G. Development of flexible capacitive pressure sensor with improved sensitivity using microdome and micropyramid polydimethylsiloxane dielectric layers. AIP Conf. Proc. 2025, 3253, 020008. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Khademolqorani, S.; Jadhav, V.V.; Chamanehpour, E.; Mishra, Y.K.; Mostafavi, E.; Kaushik, A. Recent progress of bio-based smart wearable sensors for healthcare applications. Mater. Today Electron. 2023, 5, 100055. [Google Scholar] [CrossRef]
- Xiong, X.; Liang, J.; Wu, W. Principle and recent progress of triboelectric pressure sensors for wearable applications. Nano Energy 2023, 113, 108542. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, Y.; Wang, P. Textile-Based Flexible Pressure Sensors: A Review. Polym. Rev. 2022, 62, 65–94. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, R.; Zhao, Q.; He, H.; Huang, H.; Yang, L.; Xu, Y. High-linearity flexible sensor for real-time pressure monitoring across wide frequency range by integrating piezoelectric and piezoresistive effects. Chem. Eng. J. 2025, 506, 159919. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Wang, B.; Zhou, B.; Lin, H.-J.; Liu, Y.-X.; Yang, F.-M.; Sun, S.-K.; Song, Q.-H.; Wu, Q. Recent advances in MXene-based flexible pressure sensors for medical monitoring. Rare Met. 2025, 44, 3653–3685. [Google Scholar] [CrossRef]
- Li, M.; Zang, H.; Long, J.; Sun, S.; Zhang, Y. Flexible Pressure Sensors Based on Polyvinylidene Fluoride: A Critical Review. Materials 2025, 18, 615. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Z.; Huang, Z.; Yu, H.; Wang, C.; Zhang, H.; Li, T.; Huang, Q.; Yuan, Z.; Jiang, Y.; et al. Constructing a high-power self-powered electrochemical pressure sensor for multimode pressure detections. Nano Energy 2025, 136, 110747. [Google Scholar] [CrossRef]
- Lu, H.; He, B.; Gao, B. Emerging electrochemical sensors for life healthcare. Eng. Regen. 2021, 2, 175–181. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, Y.; Duan, Z.; Wu, Y.; Yuan, Z.; Zhang, M.; Tai, H. Ion gradient induced self-powered flexible pressure sensor. Chem. Eng. J. 2024, 490, 151660. [Google Scholar] [CrossRef]
- Mahato, K.; Wang, J. Electrochemical sensors: From the bench to the skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- Zhao, Y.; Miao, L.; Xiao, Y.; Sun, P. Research Progress of Flexible Piezoresistive Pressure Sensor: A Review. IEEE Sens. J. 2024, 24, 31624–31644. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.; Jiang, L.; Li, L.; Li, X.; Zhao, J. Flexible Optical Fiber Sensor for Non-Invasive Continuous Monitoring of Human Physiological Signals. Small Methods 2025, 9, 2401368. [Google Scholar] [CrossRef]
- Ballarini, R.; Taccheo, S. (INVITED) Flexible photonics for biomedical applications: A review. Opt. Mater. X 2023, 20, 100265. [Google Scholar] [CrossRef]
- Seo, H.; Hong, Y.-M.; Chung, W.G.; Park, W.; Lee, J.; Kim, H.K.; Byeon, S.H.; Kim, D.W.; Park, J.-U. Real-time in vivo monitoring of intraocular pressure distribution in the anterior chamber and vitreous chamber for diagnosis of glaucoma. Sci. Adv. 2024, 10, eadk7805. [Google Scholar] [CrossRef]
- Sharma, K.; Morlec, E.; Valet, S.; Camenzind, M.; Weisse, B.; Rossi, R.M.; Sorin, F.; Boesel, L.F. Polydimethylsiloxane based soft polymer optical fibers: From the processing-property relationship to pressure sensing applications. Mater. Des. 2023, 232, 112115. [Google Scholar] [CrossRef]
- Brunchi, C.E.; Filimon, A.; Cazacu, M.; Ioan, S. Properties of Some Poly(siloxane)s for Optical Applications. High Perform. Polym. 2009, 21, 31–47. [Google Scholar] [CrossRef]
- Lötters, J.C.; Olthuis, W.; Veltink, P.H.; Bergveld, P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J. Micromech. Microeng. 1997, 7, 145. [Google Scholar] [CrossRef]
- Roychowdhury, T. Polydimethylsiloxane: Optical properties from 191 to 1688 nm (0.735–6.491 eV) of the liquid material by spectroscopic ellipsometry. Surf. Sci. Spectra 2018, 25, 026001. [Google Scholar] [CrossRef]
- Bai, H.; Li, S.; Barreiros, J.; Tu, Y.; Pollock, C.R.; Shepherd, R.F. Stretchable distributed fiber-optic sensors. Science 2020, 370, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Choi, Y. Soft Optical Waveguide Sensors Tuned by Reflective Pigmentation for Robotic Applications. J. Korea Robot. Soc. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Kim, T.A.; Robb, M.J.; Moore, J.S.; White, S.R.; Sottos, N.R. Mechanical Reactivity of Two Different Spiropyran Mechanophores in Polydimethylsiloxane. Macromolecules 2018, 51, 9177–9183. [Google Scholar] [CrossRef]
- Samy, R.; Glawdel, T.; Ren, C.L. Method for Microfluidic Whole-Chip Temperature Measurement Using Thin-Film Poly(dimethylsiloxane)/Rhodamine B. Anal. Chem. 2008, 80, 369–375. [Google Scholar] [CrossRef]
- Bu, Y.; Wu, J.; Zhang, Z.; Wei, Q.; Su, B.; Wang, Y. Design and Analysis of Porous Elastomeric Polymer Based on Electro-Mechanical Coupling Characteristics for Flexible Pressure Sensor. Polymers 2024, 16, 701. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Lv, R.-Y.; Zou, Y.-L.; Peng, H.-L. Flexible pressure sensor with wide pressure range based on 3D microporous PDMS/MWCNTs for human motion detection. Microelectron. Eng. 2024, 283, 112105. [Google Scholar] [CrossRef]
- Lee, S.-J.; Sung, Y.-G.; Kesavan, S.; Kim, C.-L. Development of highly sensitive/durable porous carbon nanotube–polydimethylsiloxane sponge electrode for wearable human motion monitoring sensor. New J. Chem. 2024, 48, 2146–2154. [Google Scholar] [CrossRef]
- Amouzou, K.N.; Romero, A.A.; Sengupta, D.; Mishra, S.K.; Richard-Denis, A.; Mac-Thiong, J.-M.; Petit, Y.; Lina, J.-M.; Ung, B. Development of High Refractive Index Polydimethylsiloxane Waveguides Doped with Benzophenone via Solvent-Free Fabrication for Biomedical Pressure Sensing. Photonics 2022, 9, 557. [Google Scholar] [CrossRef]
- Correia, R.; James, S.; Lee, S.-W.; Morgan, S.P.; Korposh, S. Biomedical application of optical fibre sensors. J. Opt. 2018, 20, 073003. [Google Scholar] [CrossRef]
- Kruger, E.A.; Pires, M.; Ngann, Y.; Sterling, M.; Rubayi, S. Comprehensive management of pressure ulcers in spinal cord injury: Current concepts and future trends. J. Spinal Cord Med. 2013, 36, 572–585. [Google Scholar] [CrossRef]
- Mervis, J.S.; Phillips, T.J. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J. Am. Acad. Dermatol. 2019, 81, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Cluzel, B.; De Fornel, F. Frustrated total internal reflection: The Newton experiment revisited. Photoniques 2022, 116, 32–37. [Google Scholar] [CrossRef]
- You, Y.; Wang, X.; Wang, S.; Pan, Y.; Zhou, J. A new method to demonstrate frustrated total internal reflection in the visible band. Am. J. Phys. 2008, 76, 224–228. [Google Scholar] [CrossRef]
- Pérez-Calixto, D.; Zamarrón-Hernández, D.; Cruz-Ramírez, A.; Hautefeuille, M.; Hérnandez-Cordero, J.; Velázquez, V.; Grether, M. Fabrication of large all-PDMS micropatterned waveguides for lab on chip integration using a rapid prototyping technique. Opt. Mater. Express 2017, 7, 1343–1350. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, B.; Yang, C.; Dai, Q.; Kong, L. Stretchable and Temperature-Sensitive Polymer Optical Fibers for Wearable Health Monitoring. Adv. Funct. Mater. 2019, 29, 1902898. [Google Scholar] [CrossRef]
- Bardelli, T.; Marano, C.; Briatico Vangosa, F. Polydimethylsiloxane crosslinking kinetics: A systematic study on Sylgard184 comparing rheological and thermal approaches. J. Appl. Polym. Sci. 2021, 138, 51013. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Wang, X.; Zhang, X.; Liu, S.; Yuan, H.; Yang, X.; Li, D.; Jin, Z.; Zhang, Y.; et al. Flexible Piezoresistive Sensor with High Stability Based on GO@PDMS-PU Porous Structure. Symmetry 2025, 17, 773. [Google Scholar] [CrossRef]
- Cheng, R.; Huang, Y.; Zhang, X.; Sun, B.; Ye, F.; Nie, L.; Liao, G.; Shi, T. Flexible 3-D Pressure Sensor Based on PEI-Decorated Porous Sponge for Human Physiological Monitoring. IEEE Sens. J. 2025, 25, 22686–22695. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Wen, Y.; Zhao, J.; Chen, C.; Chen, Z.; Zhang, X.-S. Wearable, washable piezoresistive pressure sensor based on polyurethane sponge coated with composite CNT/CB/TPU. Mater. Today Phys. 2025, 52, 101681. [Google Scholar] [CrossRef]
- Patel, D.K.; Sakhaei, A.H.; Layani, M.; Zhang, B.; Ge, Q.; Magdassi, S. Highly Stretchable and UV Curable Elastomers for Digital Light Processing Based 3D Printing. Adv. Mater. 2017, 29, 1606000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amouzou, K.N.; Alonso Romero, A.; Sengupta, D.; Zimmermann, C.A.; Kumar, A.; Gravel, N.; Lina, J.-M.; Daxhelet, X.; Ung, B. Porous-Cladding Polydimethylsiloxane Optical Waveguide for Biomedical Pressure Sensing Applications. Sensors 2025, 25, 4311. https://doi.org/10.3390/s25144311
Amouzou KN, Alonso Romero A, Sengupta D, Zimmermann CA, Kumar A, Gravel N, Lina J-M, Daxhelet X, Ung B. Porous-Cladding Polydimethylsiloxane Optical Waveguide for Biomedical Pressure Sensing Applications. Sensors. 2025; 25(14):4311. https://doi.org/10.3390/s25144311
Chicago/Turabian StyleAmouzou, Koffi Novignon, Alberto Alonso Romero, Dipankar Sengupta, Camila Aparecida Zimmermann, Aashutosh Kumar, Normand Gravel, Jean-Marc Lina, Xavier Daxhelet, and Bora Ung. 2025. "Porous-Cladding Polydimethylsiloxane Optical Waveguide for Biomedical Pressure Sensing Applications" Sensors 25, no. 14: 4311. https://doi.org/10.3390/s25144311
APA StyleAmouzou, K. N., Alonso Romero, A., Sengupta, D., Zimmermann, C. A., Kumar, A., Gravel, N., Lina, J.-M., Daxhelet, X., & Ung, B. (2025). Porous-Cladding Polydimethylsiloxane Optical Waveguide for Biomedical Pressure Sensing Applications. Sensors, 25(14), 4311. https://doi.org/10.3390/s25144311








