Optical Spectroscopic Detection of Mitochondrial Biomarkers (FMN and NADH) for Hypothermic Oxygenated Machine Perfusion: A Comparative Study in Different Perfusion Media
Abstract
Highlights
- First systematic optical characterization of FMN/NADH in clinically relevant perfusion media, revealing medium-specific fluorescence quenching effects.
- Identification of critical sensor design parameters: optimal excitation wavelengths (FMN: 455 nm; NADH: 360 nm), concentration-dependent NADH nonlinearity (>100 µg mL−1), and FMN-NADH spectral interference.
- Providing standardized calibration protocols enabling direct translation to portable optical sensors for real-time perfusion monitoring.
- Providing the theoretical framework to develop portable optical sensors for clinical perfusion monitoring, with specific guidelines for excitation wavelengths, interference mitigation, and medium-specific calibration.
Abstract
1. Introduction
- Incomplete calibration details: Many works do not report the calibration curves or experimental parameters (e.g., integration times, instrument settings) necessary to reproduce and compare their results.
- Limited detection methods: Most studies rely solely on fluorescence measurements—even though only FMN typically exhibits a robust fluorescence response—while neglecting absorbance techniques, which may be more suitable for quantifying NADH, particularly at high concentrations where nonlinear effects and signal saturation occur.
- Mismatch between calibration and measurement conditions: In several instances, the calibration medium differs from the perfusion medium used in actual settings, which can compromise the reliability of the optical readout.
- Inconsistent methodology across studies: Varied excitation/emission wavelengths and different spectrometers are currently used, making cross-comparisons challenging. For instance, while some studies use 450 nm excitation for FMN detection, others deploy suboptimal wavelengths (e.g., 405 nm or 485 nm) without justifying their choice, leading to potentially longer integration times and added complexity in sensor design.
- The linearity and detection limits of calibration curves,
- The influence of excitation wavelength on signal strength and specificity,
- The impact of media composition on spectral profiles, and
- The reproducibility and robustness of the optical measurements.
2. Materials and Methods
3. Results and Discussion
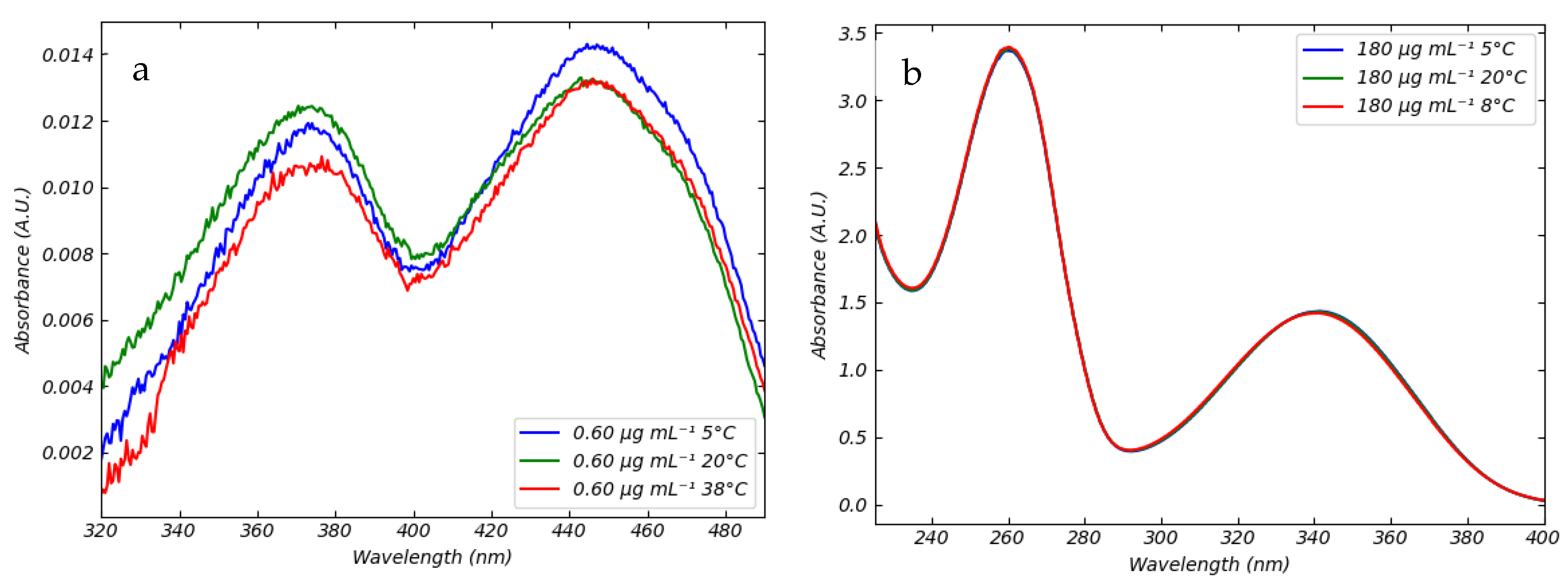
3.1. Absorption Spectra of FMN and NADH in Saline Solution in Different Conditions of Temperatures and pH
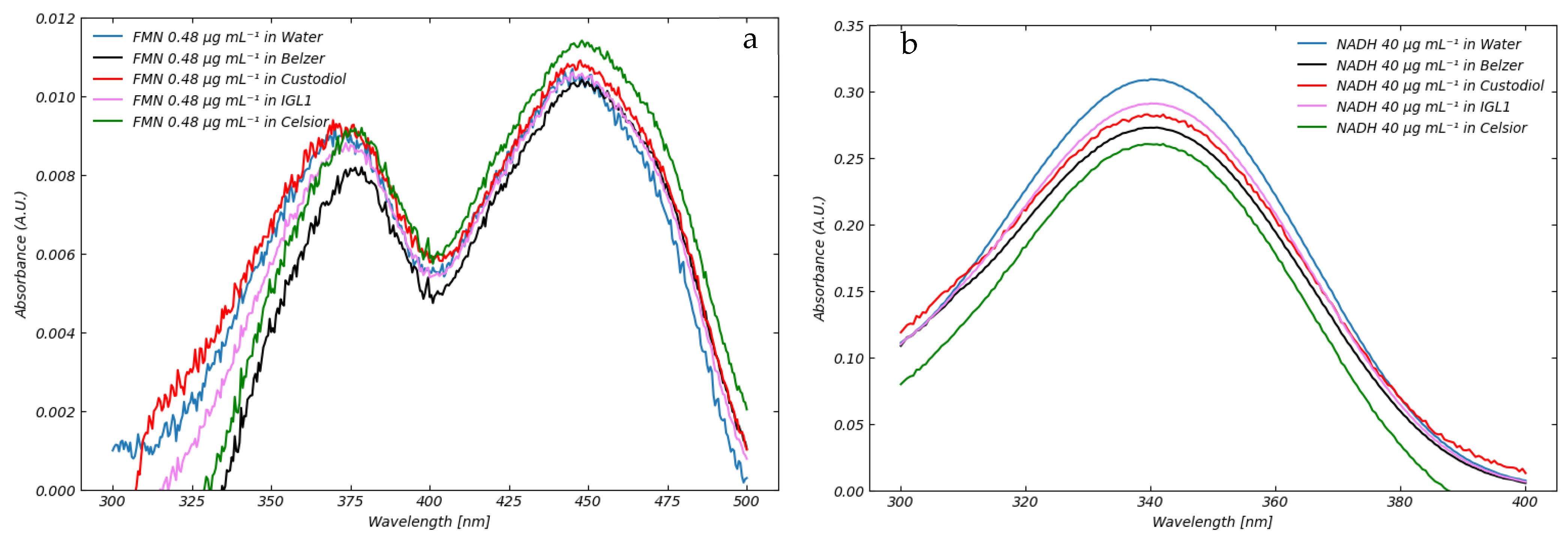
3.2. Absorption of Standard Perfusion Solutions
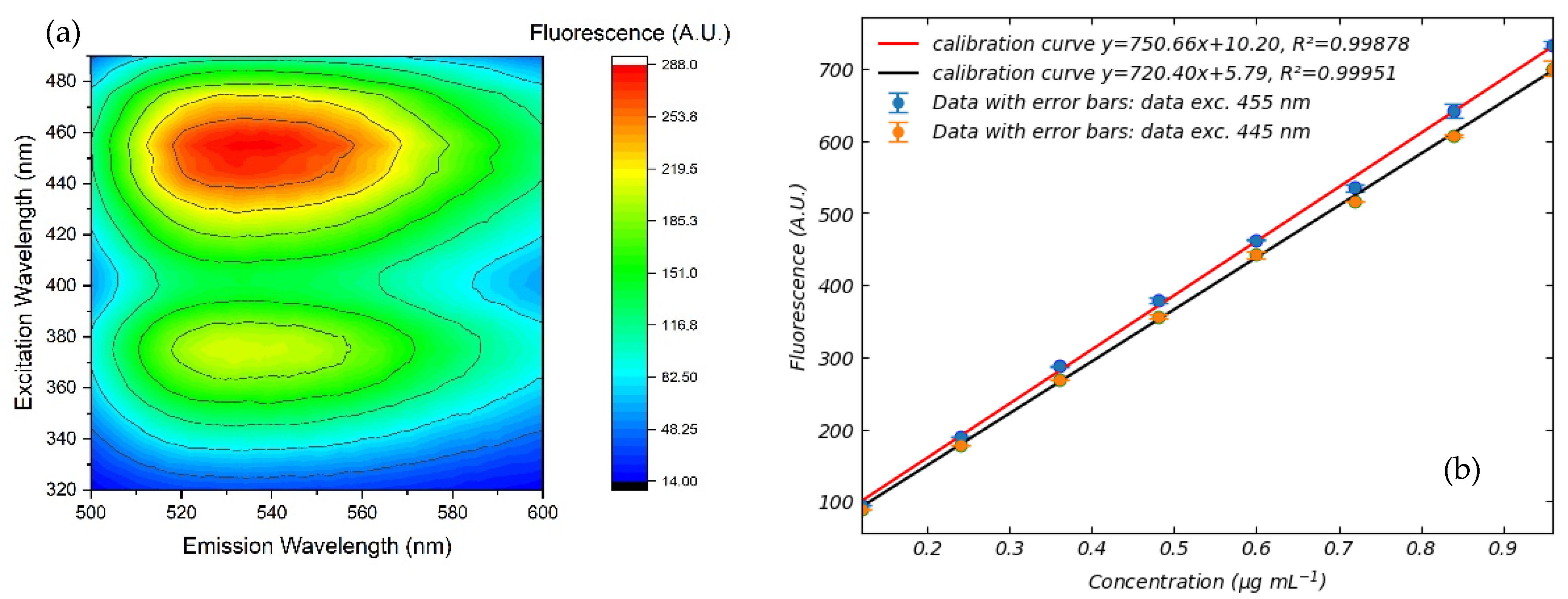
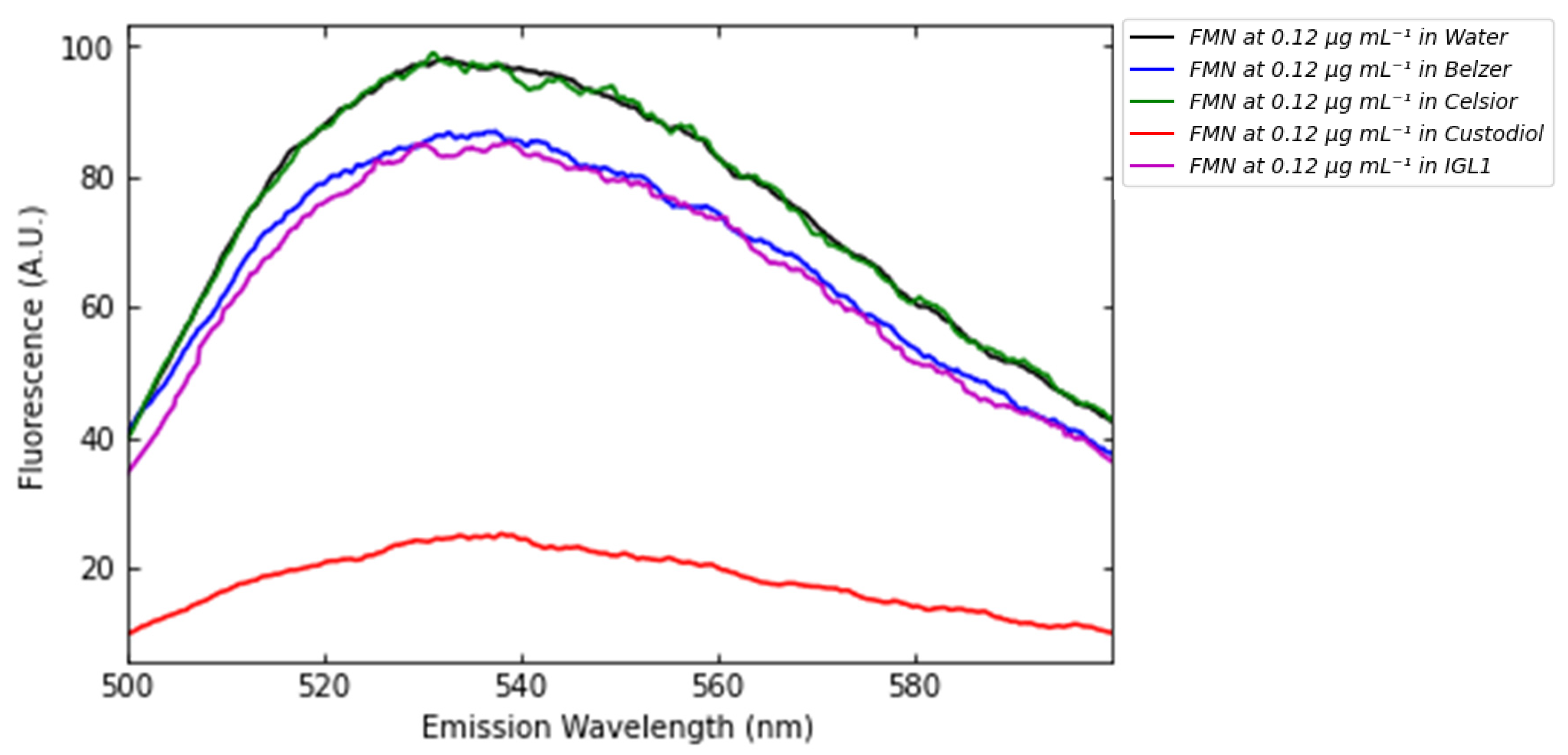
3.3. Fluorescence of FMN and NADH in Standard Perfusion Solutions
4. Contribution to the Development of Sensing Technologies
- FMN–NADH fluorescence interaction (Figure 13) demands embedded spectral deconvolution algorithms to prevent crosstalk in multi-analyte detection.
- Acknowledging that the detection by fluorescence of NADH has a limitation due to signal saturation effect (Figure 11).
- The implementation of machine learning algorithms capable of recognizing the coenzyme signal from other signals and consequently calculating the corresponding concentration.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.T.P.R.; Isaac, J.R.; Clavien, P.-A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef]
- Quintini, C.; Del Prete, L.; Simioni, A.; Del Angel, L.; Uso, T.D.; D’Amico, G.; Hashimoto, K.; Aucejo, F.; Fujiki, M.; Eghtesad, B.; et al. Transplantation of declined livers after normothermic perfusion. Surgery 2022, 171, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, F.; Knight, S.R.; Ploeg, R.J.; Hunter, J.P. A systematic review to identify whether perfusate biomarkers produced during hypothermic machine perfusion can predict graft outcomes in kidney transplantation. Transpl. Int. 2020, 33, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, R.H.; Mirza, D.F.; Afford, S.C.; Mergental, H. Biomarkers of liver injury during transplantation in an era of machine perfusion. Int. J. Mol. Sci. 2020, 21, 1578. [Google Scholar] [CrossRef] [PubMed]
- Müller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.-A.; Dutkowski, P. Novel real-time prediction of liver graft function during hypothermic oxygenated machine perfusion before liver transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Schlegel, A.; Müller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.-A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. eBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef]
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.E.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin mononucleotide as a biomarker of organ quality—A pilot study. Transplant. Direct 2020, 6, e600. [Google Scholar] [CrossRef]
- Panconesi, R.; Carvalho, M.F.; Eden, J.; Fazi, M.; Ansari, F.; Mancina, L.; Navari, N.; Da Silva, R.X.; Dondossola, D.; Borrego, L.B.; et al. Mitochondrial injury during normothermic regional perfusion (NRP) and hypothermic oxygenated perfusion (HOPE) in a rodent model of DCD liver transplantation. eBioMedicine 2023, 98, 104861. [Google Scholar] [CrossRef]
- Sousa Da Silva, R.X.; Darius, T.; Mancina, L.; Eden, J.; Wernlé, K.; Ghoneima, A.S.; Barlow, A.D.; Clavien, P.-A.; Dutkowski, P.; Kron, P. Real-time assessment of kidney allografts during HOPE using flavin mononucleotide (FMN)—A preclinical study. Front. Transplant. 2023, 2, 1132673. [Google Scholar] [CrossRef]
- Van de Leemkolk, F.E.; Lo Faro, M.L.; Shaheed, S.; Mulvey, J.F.; Huurman, V.A.L.; Alwayn, I.P.J.; Putter, H.; Jochmans, I.; Lindeman, J.H.N.; Ploeg, R.J.; et al. The role of flavin mononucleotide (FMN) as a potentially clinically relevant biomarker to predict the quality of kidney grafts during hypothermic (oxygenated) machine perfusion. PLoS ONE 2023, 18, e0287713. [Google Scholar] [CrossRef]
- Huwyler, F.; Eden, J.; Binz, J.; Cunningham, L.; Sousa Da Silva, R.X.; Clavien, P.A.; Dutkowski, P.; Tibbitt, M.W.; Hefti, M. A Spectrofluorometric Method for Real-Time Graft Assessment and Patient Monitoring. Adv. Sci. 2023, 10, 2301537. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Vergauwen, M.; Maistriaux, L.; Evrard, R.; Schlegel, A.; Mueller, M.; O’Neil, D.; Southam, A.; Aydin, S.; Devresse, A.; et al. Intermittent Surface Oxygenation Results in Similar Mitochondrial Protection and Maintenance of Aerobic Metabolism as Compared to Continuous Oxygenation during Hypothermic Machine Kidney Machine Perfusion. J. Clin. Med. 2023, 12, 3731. [Google Scholar] [CrossRef] [PubMed]
- Dingfelder, J.; Kollmann, D.; Rauter, L.; Pereyra, D.; Kacar, S.; Weijler, A.M.; Zadeh, T.S.; Tortopis, C.; Silberhumer, G.; Salat, A.; et al. Validation of Mitochondrial FMN as a Predictor for Early Allograft Dysfunction and Patient Survival Measured During Hypothermic Oxygenated Perfusion. Liver Transpl. 2025, 31, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jiao, C.; Panconesi, R.; Satish, S.; Karakaya, O.F.; De Goeij, F.H.; Diwan, T.; Ali, K.; Cadinu, L.A.; Cazzaniga, B.; et al. Quantifying Flavin Mononucleotide: An Internationally Validated Methodological Approach for Enhanced Decision Making in Organ Transplantation. EBioMedicine 2025, 116, 105761. [Google Scholar] [CrossRef]
- Antunović, M.; Aleksić, D. Preparation and testing of solutions for organ perfusion and preservation in transplantation. Vojnosanit. Pregl. 2008, 65, 596–600. [Google Scholar] [CrossRef]
- Panayotova, G.; Paterno, F.; Galan, M.; Simonishvili, S.; Qin, Y.; McCarty, M.; Brown, L.; Amin, A.; Lunsford, K.E.; Guarrera, J.V. Hypothermic oxygenated machine perfusion preserves cholangiocyte homeostasis and protects against biliary complications post liver transplantation: Preliminary results from a single center. J. Am. Coll. Surg. 2021, 233, S267–S268. [Google Scholar] [CrossRef]
- Edwards, A.M. Structure and General Properties of Flavins. In Flavins and Flavoproteins. Methods in Molecular Biology; Weber, S., Schleicher, E., Eds.; Humana Press: New York, NY, USA, 2014; pp. 3–13. [Google Scholar] [CrossRef]
- Hull, R.; Conger, P.; Hoobler, R. Conformation of NADH studied by fluorescence excitation transfer spectroscopy. Biophys. Chem. 2001, 90, 9–16. [Google Scholar] [CrossRef]
- De Vries, Y.; Berendsen, T.A.; Fujiyoshi, M.; van den Berg, A.P.; Blokzijl, H.; de Boer, M.T.; van der Heide, F.; de Kleine, R.H.; van Leeuwen, O.B.; Matton, A.P.; et al. Transplantation of high-risk donor livers after resuscitation and viability assessment using a combined protocol of oxygenated hypothermic, rewarming and normothermic machine perfusion: Study protocol for a prospective, single-arm study (DHOPE-COR-NMP). BMJ Open 2019, 9, e028596. [Google Scholar] [CrossRef]
- Van Beekum, C.J.; Vilz, T.O.; Glowka, T.R.; von Websky, M.W.; Kalff, J.C.; Manekeller, S. Normothermic machine perfusion (NMP) of the liver—Current status and future perspectives. Ann. Transplant. 2021, 26, e931664. [Google Scholar] [CrossRef]
- Ghisla, S.; Massey, V.; Lhoste, J.M.; Mayhew, S.G. Fluorescence and optical characteristics of reduced flavines and flavoproteins. Biochemistry 1974, 13, 589–597. [Google Scholar] [CrossRef]
- Gorbunova, I.A.; Sasin, M.E.; Yachkov, D.V.; Volkov, D.A.; Vedyaykin, A.D.; Nikiforov, A.A.; Vasyutinskii, O.S. Two-photon excited fluorescence of NADH-alcohol dehydrogenase complex in a mixture with bacterial enzymes. Biomolecules 2023, 13, 256. [Google Scholar] [CrossRef]
- Kotaki, A.; Yagi, K. Fluorescence properties of flavins in various solvents. J. Biochem. 1970, 68, 509–516. [Google Scholar] [CrossRef]




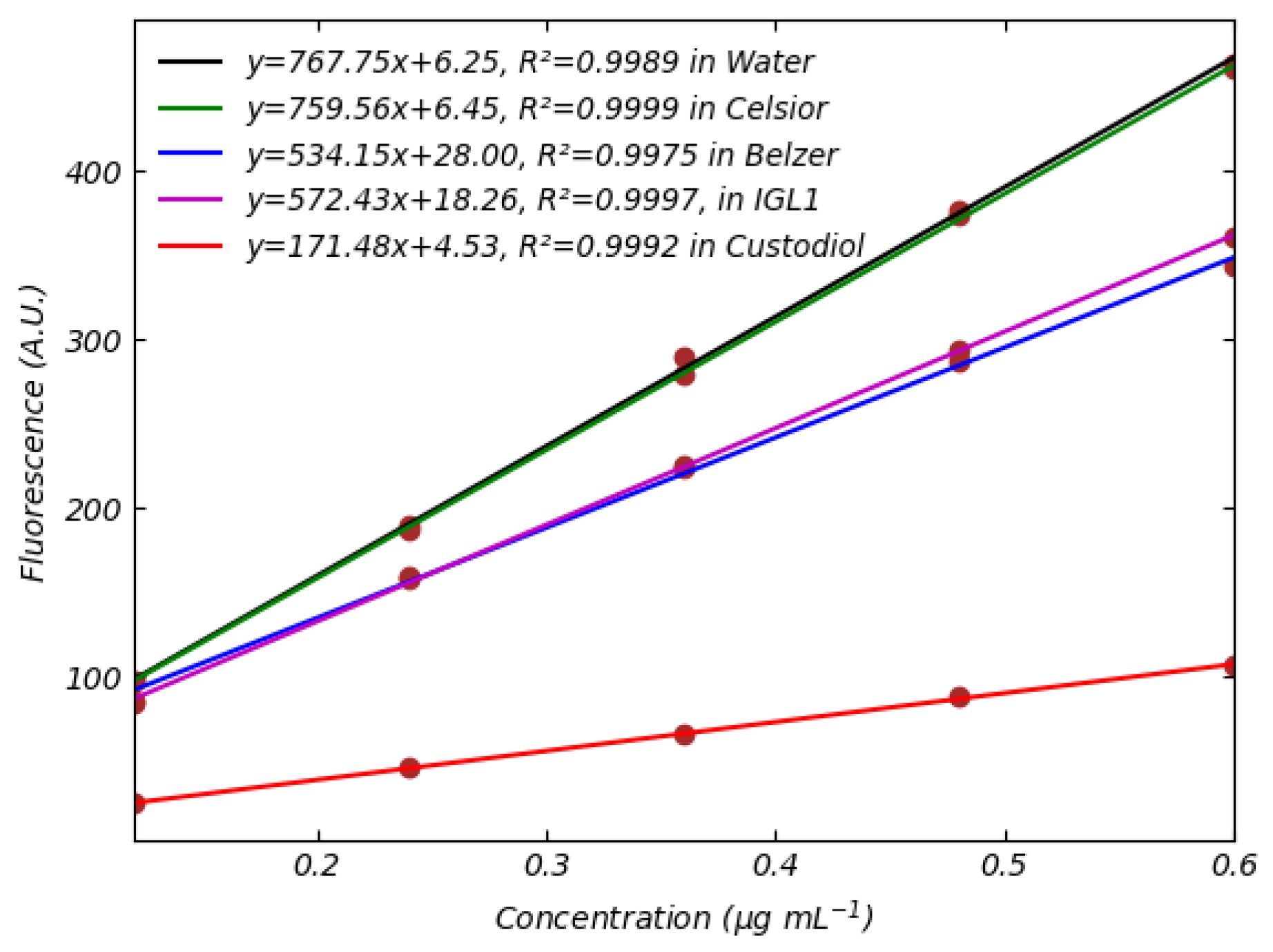
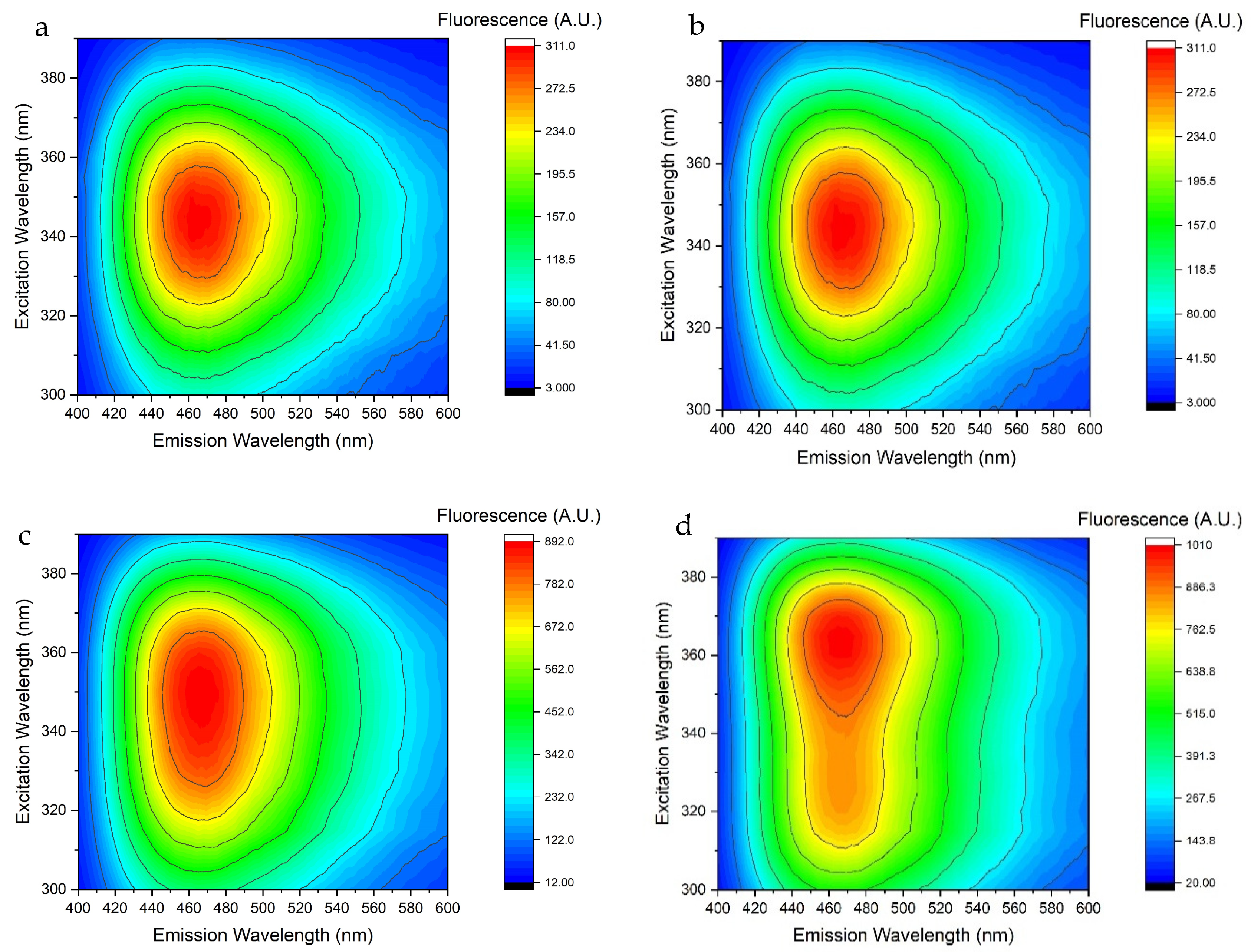
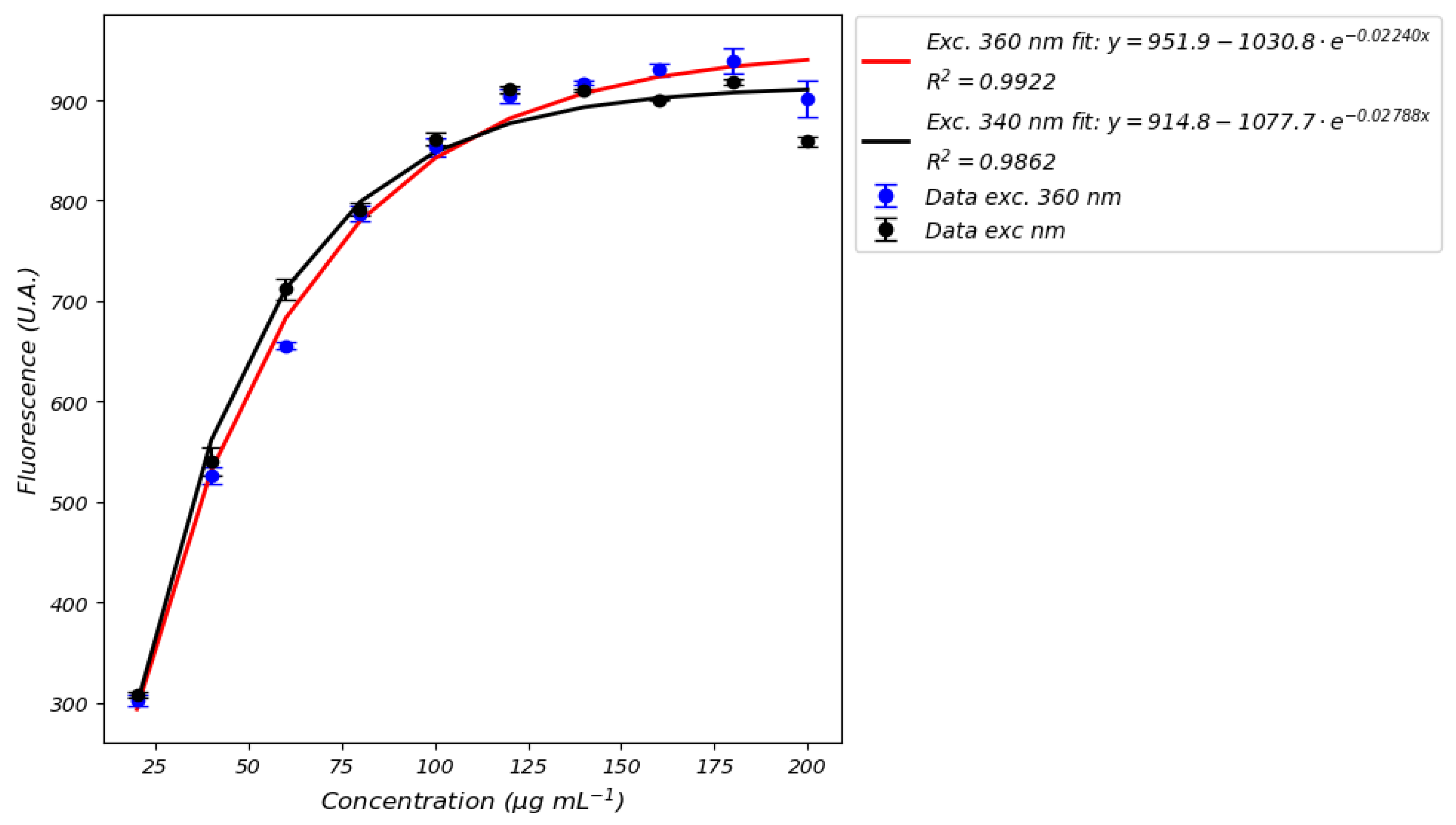


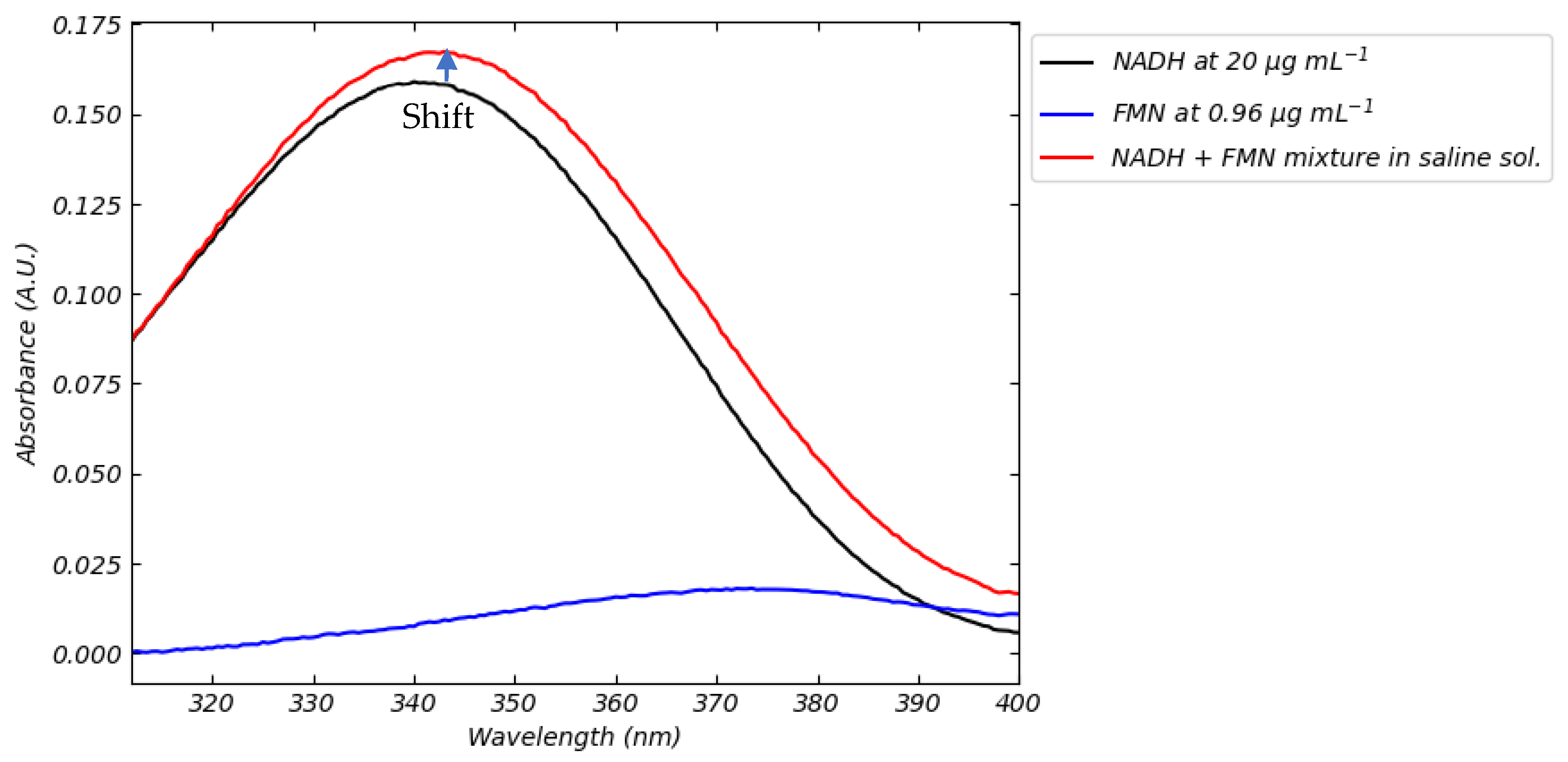
| Ref. | Type of Analyte | Type of Method | Detection Method | Effects of Media Composition | Calibration Medium/Perfusion Medium | Calibration Curve | Reference Excitation/Emission Wavelengths | Spectrometer Used | Type of Study | LOQ and LOD |
|---|---|---|---|---|---|---|---|---|---|---|
| [5] | FMN | Fluorescence | Direct on perfusion line | Not reported | Not reported/Belzer | Not reported | Exc: 450 nm; Em: 500–600 nm | Portable | Clinical | Not reported in clinical context |
| [6] | FMN/NADH | Fluorescence | Direct on perfusion line/Perfusate Sampling | Not reported | Not reported/Belzer | Not reported | FMN-Exc: 450 nm; Em: 500–600 nm NADH-Exc: 360 nm Em: 465 nm | Portable | Preclinical | Not reported in pre-clinical context |
| [7] | FMN | Fluorescence | Perfusate Sampling | Not reported | Saline/Belzer | Not reported | Exc: 450 nm; Em: 525 nm | Microplate reader | Clinical | Not reported in clinical context |
| [9] | FMN | Fluorescence | Perfusate Sampling | Not reported | Not reported/Belzer | Yes | Exc: 485 nm; Em: 528 nm | Microplate reader | Preclinical | Not reported in preclinical context |
| [10] | FMN | Fluorescence | Perfusate Sampling | Not reported | Belzer/Belzer | Yes | Exc: 450 nm; Em: 500–600 nm | Microplate reader | Clinical | Reported for calibration: 0.00356 ng mL−1 |
| [11] | FMN/NADH | Fluorescence | Direct on perfusion line | Not reported | Belzer/Belzer | Yes | FMN-Exc: 405 nm; Em: 530 nm NADH-Exc: 385 nm Em: 460 nm | Portable | Clinical | Reported for calibration: 2.01 ng mL−1 |
| [12] | FMN/NADH | Fluorescence | Perfusate Sampling | Not reported | Not reported | Not Reported | Not Reported | Not reported | Preclinical | Not reported in pre-clinical context |
| [13] | Only FMN | Fluorescence | Perfusate Sampling | Not reported | Belzer/Belzer | Yes | Exc: 450 nm; Em: 525 nm | Microplate reader | Clinical | Reported for calibration: 7.9 ng mL−1 |
| Our study | Yes | Fluorescence/Absorption | Preparation in laboratory of perfusion solution simulating perfusate | Yes | Fully described | Yes | Exc: 300–500 nm. Em: 400–600 nm | Benchtop | Methodologic | Reported for FMN calibration: 120 ng mL−1 |
| Concentration (μg∙mL−1) | Absorbance (Mean, A.U.) | Standard Error (A.U.) |
|---|---|---|
| 0.12 | 0.002777 | 1.70 × 10−5 |
| 0.24 | 0.005138 | 2.63 × 10−5 |
| 0.36 | 0.007682 | 2.69 × 10−4 |
| 0.48 | 0.010725 | 4.20 × 10−4 |
| 0.60 | 0.013587 | 2.67 × 10−4 |
| 0.72 | 0.016343 | 7.40 × 10−5 |
| 0.84 | 0.019524 | 2.52 × 10−4 |
| 0.96 | 0.021873 | 6.51 × 10−5 |
| 20 | 0.155279 | 4.08 × 10−4 |
| 40 | 0.307527 | 6.66 × 10−4 |
| 60 | 0.465341 | 9.87 × 10−4 |
| 80 | 0.624495 | 1.39 × 10−3 |
| 100 | 0.775295 | 1.63 × 10−3 |
| 120 | 0.934276 | 8.05 × 10−4 |
| 140 | 1.114413 | 6.18 × 10−4 |
| 160 | 1.262550 | 4.58 × 10−4 |
| 180 | 1.426020 | 3.07 × 10−3 |
| Exc. 445 nm | Exc. 455 nm | |||
|---|---|---|---|---|
| Concentration (µg∙mL−1) | Fluorescence (Mean A.U.) | Standard Error (A.U.) | Fluorescence (Mean A.U.) | Standard Error (A.U.) |
| 0.12 | 94.4921 | 0.4302 | 88.7749 | 0.2351 |
| 0.24 | 189.2913 | 0.6099 | 177.7753 | 0.1832 |
| 0.36 | 287.3993 | 1.5043 | 268.9927 | 0.5976 |
| 0.48 | 379.3207 | 3.5497 | 356.0967 | 3.0412 |
| 0.60 | 462.7110 | 1.0385 | 441.9740 | 4.2942 |
| 0.72 | 535.1927 | 4.4261 | 516.2127 | 0.2915 |
| 0.84 | 642.2623 | 10.0139 | 607.7856 | 1.9467 |
| 0.96 | 733.7770 | 4.5954 | 700.7743 | 10.7981 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadinu, L.A.; Sun, K.; Jiao, C.; Panconesi, R.; Satish, S.; Yildirim, F.S.; Karakaya, O.F.; Wehrle, C.J.; Crasta, G.S.; Fernandes, F.W.; et al. Optical Spectroscopic Detection of Mitochondrial Biomarkers (FMN and NADH) for Hypothermic Oxygenated Machine Perfusion: A Comparative Study in Different Perfusion Media. Sensors 2025, 25, 4031. https://doi.org/10.3390/s25134031
Cadinu LA, Sun K, Jiao C, Panconesi R, Satish S, Yildirim FS, Karakaya OF, Wehrle CJ, Crasta GS, Fernandes FW, et al. Optical Spectroscopic Detection of Mitochondrial Biomarkers (FMN and NADH) for Hypothermic Oxygenated Machine Perfusion: A Comparative Study in Different Perfusion Media. Sensors. 2025; 25(13):4031. https://doi.org/10.3390/s25134031
Chicago/Turabian StyleCadinu, Lorenzo Agostino, Keyue Sun, Chunbao Jiao, Rebecca Panconesi, Sangeeta Satish, Fatma Selin Yildirim, Omer Faruk Karakaya, Chase J. Wehrle, Geofia Shaina Crasta, Fernanda Walsh Fernandes, and et al. 2025. "Optical Spectroscopic Detection of Mitochondrial Biomarkers (FMN and NADH) for Hypothermic Oxygenated Machine Perfusion: A Comparative Study in Different Perfusion Media" Sensors 25, no. 13: 4031. https://doi.org/10.3390/s25134031
APA StyleCadinu, L. A., Sun, K., Jiao, C., Panconesi, R., Satish, S., Yildirim, F. S., Karakaya, O. F., Wehrle, C. J., Crasta, G. S., Fernandes, F. W., Eshraghi, N., Takase, K., Horie, H., Ricci, P. C., Bagnoli, D., Carvalho, M. F., Schlegel, A., & Barbaro, M. (2025). Optical Spectroscopic Detection of Mitochondrial Biomarkers (FMN and NADH) for Hypothermic Oxygenated Machine Perfusion: A Comparative Study in Different Perfusion Media. Sensors, 25(13), 4031. https://doi.org/10.3390/s25134031







