A Highly Sensitive Formaldehyde Gas Sensor Based on Ag2O and PtO2 Co-Decorated LaFeO3 Nanofibers Prepared by Electrospinning
Abstract
Highlights
- High-stability gas-sensing material for industrial potential.
- Sensor for detecting low-concentration formaldehyde with significant application potential.
- Ag2O and PtO2 bi-metal oxide nanoparticles co-decorated on LaFeO3 nanofibers exhibit remarkable gas-sensing performance for formaldehyde detection.
- The sensor based on Ag2O and PtO2 co-decorated LaFeO3 shows ultra-low detection limits (10 ppb) and high response values.
- The simple and controllable synthesis process is beneficial for industrial production.
- The ultra-low detection limit is crucial for environmental health monitoring.
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Ag2O and PtO2 Co-Decorated LaFeO3 Nanofibers
2.2. Fabrication of Gas Sensor
2.3. Materials Characterization
2.4. Gas-Sensing Performance Test
3. Results and Discussion
3.1. Characterizations of Sensing Materials
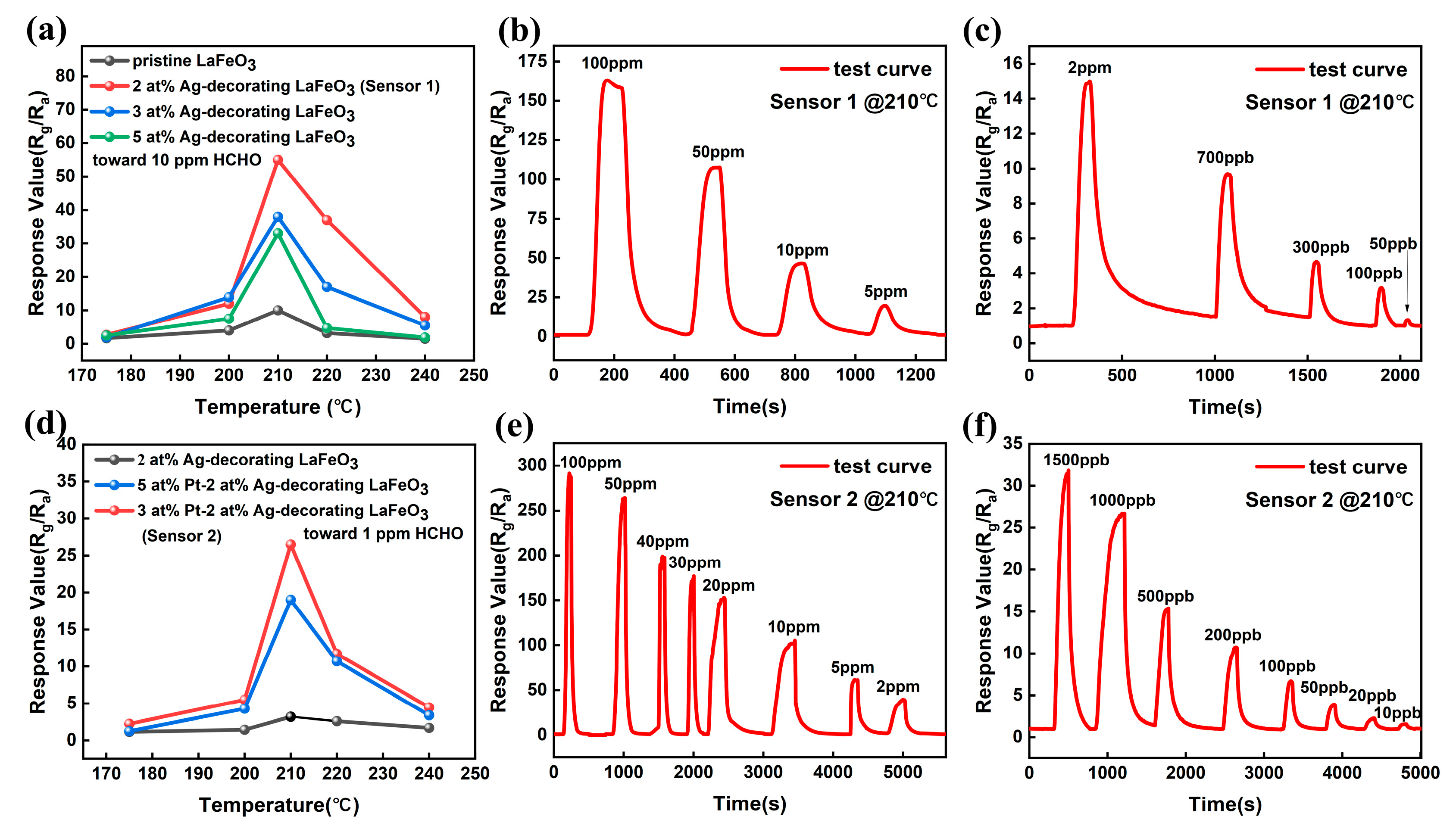
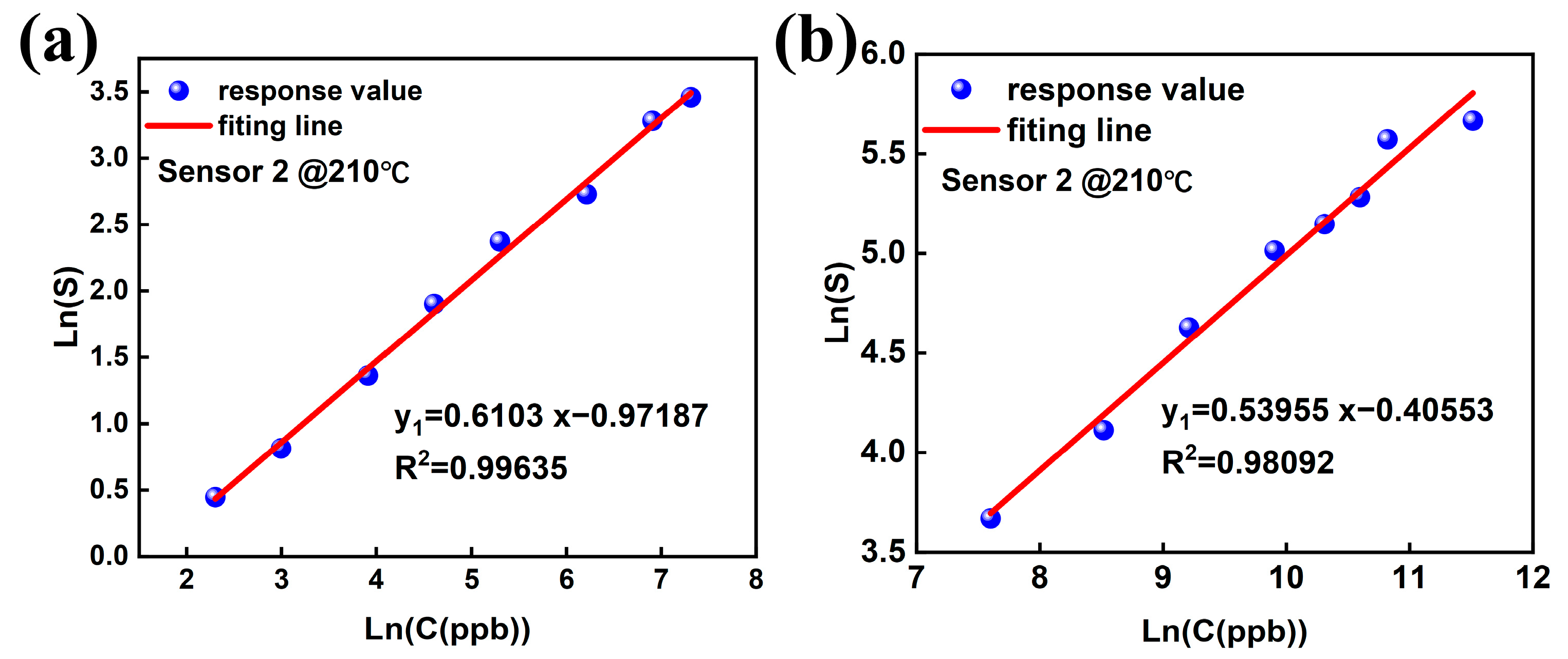
3.2. Gas-Sensing Performance
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| XRD | X-ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
| SEM | Scanning Electron Microscopy |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| VOCs | Volatile Organic Compounds |
| IARC | International Agency for Research on Cancer |
| WHO | World Health Organization |
| MOS | Metal Oxide Semiconductor |
| DMF | N, N-Dimethylformamide |
| PVP | Polyvinylpyrrolidone |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| HAL | Hole Accumulation Layer |
References
- Leso, V.; Macrini, M.C.; Russo, F.; Iavicoli, I. Formaldehyde Exposure and Epigenetic Effects: A Systematic Review. Appl. Sci. 2020, 10, 2319. [Google Scholar] [CrossRef]
- Rovira, J.; Roig, N.; Nadal, M.; Schuhmacher, M.; Domingo, J. Human health risks of formaldehyde indoor levels: An issue of concern. J. Environ. Sci. Health Part A 2016, 51, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, H.; Hu, H.; Niu, Y.; Hao, R.; Umar, A.; Al-Assiri, M.S.; Alsaiari, M.A.; Wang, Y. An insight into improvement of room temperature formaldehyde sensitivity for graphene-based gas sensors. Microchem. J. 2021, 160, 105607. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Duan, Y. Diagnosis of breast cancer based on breath analysis: An emerging method. Crit. Rev. Oncol. 2013, 87, 28–40. [Google Scholar] [CrossRef]
- Kim, J.; Hong, U.G.; Choi, Y.; Hong, S. Enhancing the evanescent field in TiO2/Au hybrid thin films creates a highly sensitive room-temperature formaldehyde gas biosensor. Colloids Surf. B Biointerfaces 2019, 182, 110303. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Qi, Y.; Yang, Z.; Han, H.; Jiang, Y.; Du, W.; Zhang, X.; Zhang, J.; Dai, Z.; Wu, L.; et al. Recent advances and perspectives on constructing metal oxide semiconductor gas sensing materials for efficient formaldehyde detection. J. Mater. Chem. C. 2020, 8, 13169–13188. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-890-0213-4.
- Liu, C.; Hu, J.; Wu, G.; Cao, J.; Zhang, Z.; Zhang, Y. Carbon nanotube-based field-effect transistor-type sensor with a sensing gate for ppb-level formaldehyde detection. ACS Appl. Mater. Interfaces 2021, 13, 56309–56319. [Google Scholar] [CrossRef]
- Roy, S.; Pan, S.; Sivaram, S.; De, P. Naphthalimide-based fluorescent polymeric probe: A dual-phase sensor for formaldehyde detection. Sci. Technol. Adv. Mater. 2025, 26, 2469493. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; Ren, J.; Zhang, G.; Jia, Y. Enhanced N-Butanol Sensing Performance of Cr-Doped CeO2 Nanomaterials. Sensors 2025, 25, 1208. [Google Scholar] [CrossRef]
- Flores, S.Y.; Pacheco, E.; Malca, C.; Peng, X.; Chen, Y.; Zhou, B.; Pinero, D.M.; Diaz-Vazquez, L.M.; Zhou, A.F.; Feng, P.X. Highly Selective Room-Temperature Blue LED-Enhanced NO2 Gas Sensors Based on ZnO-MoS2-TiO2 Heterostructures. Sensors 2025, 25, 1781. [Google Scholar] [CrossRef]
- Fu, B.; Bi, L.; Lin, J.; Fu, J.; Wen, J.; Zou, B.; Wang, C.; Wang, Y. Low-temperature detection and excellent selectivity of ethanol using LaFeO3 gas sensors with dual regulation of doping and non-stoichiometry. J. Alloys Compd. 2025, 1013, 178590. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.-F.; Chen, G.-X.; Wang, Z.-L.; Cui, Y.; Li, S.; Qu, W.-L.; Qiu, J. Enhancing the responsiveness of porous LaFeO3 microspheres to ethanol under high humidity. J. Mater. Sci. 2025, 60, 662–673. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Tang, B.; Zheng, C. Pt/Ru-Modified ZnO Sensor Grown In Situ for Detection of ppb H2S. Sensors 2025, 25, 1995. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yuan, T.; Zhang, W.; Tan, X.; Chen, Y.; Xu, J. Industrial Potential of Formaldehyde Gas Sensor Based on PdPt Bimetallic Loaded SnO2 Nanoparticles. Sensors 2025, 25, 1627. [Google Scholar] [CrossRef]
- Xia, Z.; Zheng, C.; Hu, J.; Yuan, Q.; Zhang, C.; Zhang, J.; He, L.; Gao, H.; Jin, L.; Chu, X.; et al. Synthesis of SnO2 quantum dot sensitized LaFeO3 for conductometric formic acid gas sensors. Sens. Actuators B Chem. 2023, 379, 133198. [Google Scholar] [CrossRef]
- Arbia, M.B.; Kim, S.-H.; Yoon, J.-B.; Comini, E. Preparation of NiO NWs by Thermal Oxidation for Highly Selective Gas-Sensing Applications. Sensors 2025, 25, 2075. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, C.; Shen, J.; Liu, S.; Ma, Q.; Zheng, Y. Fabrication of Oxygen Vacancy-Rich WO3 Porous Thin Film by Sputter Deposition for Ultrasensitive Mustard-Gas Simulants Sensor. Sensors 2025, 25, 3049. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Lin, Y.T.; Chen, J.L.; Zhang, J.; Zhu, Z.Q.; Liu, Q.J. A high sensitivity gas sensor for formaldehyde based on silver doped lanthanum ferrite. Sens. Actuators B Chem. 2014, 190, 171–176. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, S.; Yang, G.; Gao, L.; Wu, Y.; Zhang, X. Oxygen vacancies assisted LaFeO3 derived from metal organic frameworks endows a practical HCHO sensor with excellent sensing characteristics. J. Ind. Eng. Chem. 2023, 126, 501–509. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Wu, Z.; Xiong, X.; Li, J.; Zhou, J.; Gao, X. Excellent ethanol sensor based on LaFeO3 modified with gold nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 27587–27595. [Google Scholar] [CrossRef]
- Sun, B.; Chen, R.; Liu, M.; Song, P. Highly sensitive formaldehyde gas sensor based on Au nanoparticle functionalized 1D LaFeO3 nanofibers. Microchem. J. 2025, 211, 113140. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Yuan, Q.; Yu, X.; Wang, D.; Chen, Y. Ag-Modified Porous Perovskite-Type LaFeO3 for Efficient Ethanol Detection. Nanomaterials 2022, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhu, G.; Rao, F.; Liu, Z.; Zhang, W.; Zhu, L.; Shi, X.; Huang, Y.; Jia, Y.; Hojamberdiev, M. Platinum Nanoclusters-Modified Porous In2O3 Nanocubes for Highly Sensitive and Selective Formaldehyde Gas Sensing at Room Temperature. Sens. Actuators B Chem. 2024, 399, 134805. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Chen, J.; Wang, Y.; Zhang, L.; Yue, S.; Li, S.; Huang, T.; Sun, D. Pd-modified LaFeO3 as a high-efficiency gas-sensing material for H2S gas detection. Nanomaterials 2022, 12, 2460. [Google Scholar] [CrossRef]
- Chumakova, V.; Marikutsa, A.; Platonov, V.; Khmelevsky, N.; Rumyantseva, M. Distinct Roles of Additives in the Improved Sensitivity to CO of Ag-and Pd-Modified Nanosized LaFeO3. Chemosensors 2023, 11, 60. [Google Scholar] [CrossRef]
- Fan, H.T.; Xu, X.J.; Ma, X.K.; Zhang, T. Preparation of LaFeO3 nanofibers by electrospinning for gas sensors with fast response and recovery. Nanotechnology 2011, 22, 115502. [Google Scholar] [CrossRef]
- Hu, J.; Chen, X.; Zhang, Y. Batch fabrication of formaldehyde sensors based on LaFeO3 thin film with ppb-level detection limit. Sens. Actuators B Chem. 2021, 349, 130738. [Google Scholar] [CrossRef]
- Sun, L.H.; Hu, J.F.; Feng, G.; Qin, H.W. Adsorption of formaldehyde on the Fe site of clean and M2+ (Ca2+, Sr2+ and Ba2+) doped LaFeO3 (0 1 0) surface. Appl. Surf. Sci. 2011, 257, 8692–8695. [Google Scholar] [CrossRef]
- Li, C.; You, X.; Zhao, X.; Yin, P. A highly sensitive ethylene gas sensor based on PtO2-decorated SnO2 used to monitor the ripening and spoilage of fruits and vegetables that are stored at room temperature. New J. Chem. 2024, 48, 10686–10696. [Google Scholar] [CrossRef]
- Wang, J.; An, C.; Zhang, M.; Qin, C.; Ming, X.; Zhang, Q. Photochemical conversion of AgCl nanocubes to hybrid AgCl–Ag nanoparticles with high activity and long-term stability towards photocatalytic degradation of organic dyes. Can. J. Chem. 2012, 90, 858–864. [Google Scholar] [CrossRef]
- Akel, S.; Dillert, R.; Balayeva, N.O.; Boughaled, R.; Koch, J.; El Azzouzi, M.; Bahnemann, D.W. Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts 2018, 8, 647. [Google Scholar] [CrossRef]
- Tjeng, L.H.; Meinders, M.B.J.; Van Elp, J.; Ghijsen, J.; Sawatzky, G.A.; Johnson, R.L. Electronic structure of Ag2O. Phys. Rev. B Condens. Matter Mater. Phys. 1990, 41, 3190–3199. Available online: https://link.aps.org/doi/10.1103/PhysRevB.41.3190 (accessed on 16 June 2025). [CrossRef] [PubMed]
- Ding, M.; Shan, B.; Bo, P.; Zhou, J.; Zhang, K. Dynamic Pt–OH−·H2O–Ag species mediate coupled electron and proton transfer for catalytic hydride reduction of 4-nitrophenol at the confined nanoscale interface. Phys. Chem. Chem. Phys. 2022, 24, 7923–7936. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cano, L.A.; Rosado-Ortiz, G.; Valenzuela-Muñiz, A.M.; Ordóñez, L.C.; Gauvin, R.; Verde Gómez, Y. Solvent effect in the synthesis of nanostructured Pt–Sn/CNT as electrocatalysts for the electrooxidation of ethanol. Int. J. Hydrogen Energy 2019, 44, 12430–12438. [Google Scholar] [CrossRef]
- Hu, S.; Xiong, L.; Ren, X.; Wang, C.; Luo, Y. Pt–Ir binary hydrophobic catalysts: Effects of Ir content and particle size on catalytic performance for liquid phase catalytic exchange. Int. J. Hydrogen Energy 2009, 34, 8723–8732. [Google Scholar] [CrossRef]
- Hagiwara, H.; Nagatomo, M.; Seto, C.; Ida, S.; Ishiahara, T. Dye modification effects on TaON for photocatalytic hydrogen production from water. Catalysts 2013, 3, 614–624. [Google Scholar] [CrossRef]
- Long, B.M.; Cam, T.S.; Omarov, S.O.; Lebedev, L.A.; Seroglazova, A.S.; Stovpiaga, E.Y.; Gerasimov, E.Y.; Popkov, V.I. Structure and catalytic activity of ultra-high-entropy rare-earth orthoferrite (UHE REO) towards thermal hydrogen oxidation. Catal. Sci. Technol. 2024, 14, 5978–5988. [Google Scholar] [CrossRef]
- Sun, B.; Liu, M.; Wang, Q.; Song, P. Enhanced formaldehyde gas sensing properties of p-LaFeO3/n-Fe2O3 composite nanofibers synthesized by electrospinning method. Sens. Actuators B Chem. 2025, 426, 137010. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Y.; Zhang, Y.; Lin, Y.; Gui, Y.; Liu, L. Photocatalytic Degradation of Volatile Organic Compounds over WO2/SnS2 Nanofibers. ACS Appl. Nano Mater. 2023, 6, 22301–22310. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, G.; Liu, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Visible light responsive titania photocatalysts codoped by nitrogen and metal (Fe, Ni, Ag, or Pt) for remediation of aqueous pollutants. Chem. Eng. J. 2013, 231, 18–25. [Google Scholar] [CrossRef]
- Sun, N.; Tian, Q.; Bian, W.; Wang, X.; Dou, H.; Li, C.; Zhang, Y.; Gong, C.; You, X.; Du, X.; et al. Highly sensitive and lower detection-limit NO2 gas sensor based on Rh-doped ZnO nanofibers prepared by electrospinning. Appl. Surf. Sci. 2023, 614, 156213. [Google Scholar] [CrossRef]
- Capek, I. Dispersions based on noble metal nanoparticles-DNA conjugates. Adv. Colloid Interface Sci. 2011, 163, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, J.; Li, Z.; Yang, J.; Liu, H.; Wang, L.; Cui, Y.; Zhan, W.; Guo, Y.; Guo, Y. Nickel oxide regulating surface oxygen to promote formaldehyde oxidation on manganese oxide catalysts. Catal. Sci. Technol. 2021, 11, 7110–7124. [Google Scholar] [CrossRef]
- Xiao, C.; Ma, Z.; Gao, X.; Zou, P.; Jia, L. Fabrication of Pt functionalized LaFeO3 porous structures for highly sensitive detection of formaldehyde. Sens. Actuators B Chem. 2024, 410, 135644. [Google Scholar] [CrossRef]
- Goel, N.; Kunal, K.; Kushwaha, A.; Kumar, M. Metal oxide semiconductors for gas sensing. Eng. Rep. 2023, 5, e12604. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhang, J.; Chen, J.L.; Liu, Q.J. Improvement of response to formaldehyde at Ag–LaFeO3 based gas sensors through incorporation of SWCNTs. Sens. Actuators B Chem. 2014, 195, 509–514. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Wang, J.; Liu, J.; Zhao, W.; Yan, W. Efficient Formaldehyde Gas Sensing Performance via Promotion of Oxygen Vacancy on In-Doped LaFeO3 Nanofibers. Nanomaterials 2024, 14, 1595. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.; Xiao, C.; Jia, L. C-doped LaFeO3 Porous Nanostructures for Highly Selective Detection of Formaldehyde. Sens. Actuators B Chem. 2021, 347, 130550. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.H.; Yu, Q.C.; Kebeded, M.A.; Zhuang, Y.Y.; Huang, S.; Jiao, M.Z.; He, X.J. Advances in modification of metal and noble metal nanomaterials for metal oxide gas sensors: A review. Rare Met. 2025, 44, 1443–1496. [Google Scholar] [CrossRef]
- Al-Sarraj, A.; Salah, B.; Ayesh, A.I.; Saoud, K.M.; El Mel, A.A.; Rehman, A.; Bermak, A.; Haik, Y. Fabrication of Ag2O/WO3 based sensors for detection of hydrogen sulfide. Sens. Actuators A Phys. 2022, 333, 113256. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Q.; Xiao, Y.; Sun, P.; Wang, Z.; Gao, Y.; Ma, J.; Sun, Y.; Lu, G. A pulse-driven sensor based on ordered mesoporous Ag2O/SnO2 with improved H2S-sensing performance. Sens. Actuators B Chem. 2016, 228, 529–538. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Qin, H.; Liu, Q.; Jing, X.; Zhang, H.; Li, R.; Huang, G.; Wang, J. PtO2-nanoparticles functionalized CuO polyhedrons for n-butanol gas sensor application. Ceram. Int. 2018, 44, 10426–10432. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, X.; Ma, Z.; Yang, K.; Gao, X.; Wang, H.; Jia, L. Formal-dehyde gas sensor with 1 ppb detection limit based on In-doped LaFeO3 porous structure. Sens. Actuators B Chem. 2022, 371, 132558. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Song, F.; Fu, H.; Yu, S.; Zhang, K.; Tang, Z.; Meng, Q.; Jing, Q.; Liu, B. A Highly Sensitive Formaldehyde Gas Sensor Based on Ag2O and PtO2 Co-Decorated LaFeO3 Nanofibers Prepared by Electrospinning. Sensors 2025, 25, 3848. https://doi.org/10.3390/s25133848
Wang X, Song F, Fu H, Yu S, Zhang K, Tang Z, Meng Q, Jing Q, Liu B. A Highly Sensitive Formaldehyde Gas Sensor Based on Ag2O and PtO2 Co-Decorated LaFeO3 Nanofibers Prepared by Electrospinning. Sensors. 2025; 25(13):3848. https://doi.org/10.3390/s25133848
Chicago/Turabian StyleWang, Xin, Fei Song, Huai’an Fu, Shanshan Yu, Kai Zhang, Zhipeng Tang, Qingkuan Meng, Qiang Jing, and Bo Liu. 2025. "A Highly Sensitive Formaldehyde Gas Sensor Based on Ag2O and PtO2 Co-Decorated LaFeO3 Nanofibers Prepared by Electrospinning" Sensors 25, no. 13: 3848. https://doi.org/10.3390/s25133848
APA StyleWang, X., Song, F., Fu, H., Yu, S., Zhang, K., Tang, Z., Meng, Q., Jing, Q., & Liu, B. (2025). A Highly Sensitive Formaldehyde Gas Sensor Based on Ag2O and PtO2 Co-Decorated LaFeO3 Nanofibers Prepared by Electrospinning. Sensors, 25(13), 3848. https://doi.org/10.3390/s25133848





