Electrocatalytical Nitrite Oxidation via Manganese and Copper Oxides on Carbon Screen-Printed Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Working Electrode Functionalization
2.3. Electrode Performance Analysis
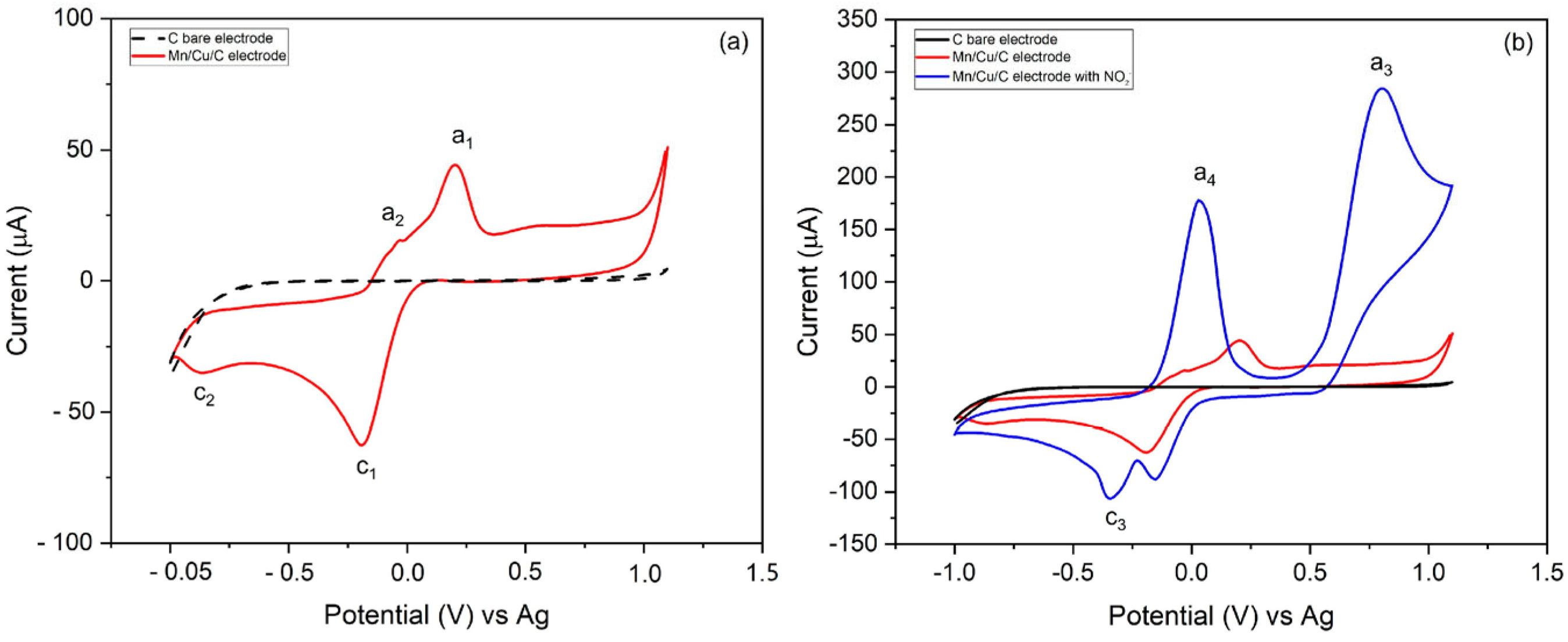
3. Results
3.1. Structural Characterization
3.2. Electrochemical Characterization
3.3. Sensor Performance for Nitrite Ion Detection
3.4. Reproducibility and Repeatability of the Sensor
3.5. Interference Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhang, G.; Chen, C.; Jiang, S.; Tang, H.; Tan, L.; Ma, M. A Metal–Organic Gel-Carbon Nanotube Nanocomposite for Electrochemical Detection of Nitrite. ACS Appl. Electron. Mater. 2021, 3, 761–768. [Google Scholar] [CrossRef]
- Rajab, N.; Ibrahim, H.; Hassan, R.Y.A.; Youssef, A.F.A. Selective Determination of Nitrite in Water and Food Samples Using Zirconium Oxide (ZrO2)@MWCNTs Modified Screen Printed Electrode. RSC Adv. 2023, 13, 21259–21270. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, Y.; Zhang, Y.; Duan, J.; Guan, F.; Hou, B. Effects of marine eutrophication environment on microbial corrosion: A review. Mar. Pollut. Bull. 2024, 205, 116637. [Google Scholar] [CrossRef] [PubMed]
- Preussmann, R. Carcinogenic N-nitroso compounds and their environmental significance. Naturwissenschaften 1984, 71, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Isaacson, R.L. Pretreatment effects on nitrite-induced methemoglobinemia: Saline and calcium channel antagonists. Pharmacol. Biochem. Behav. 1990, 37, 457–459. [Google Scholar] [CrossRef]
- Piazza, J.; Douin, C.; Bodson, L.; Ghuysen, A.; D’Orio, V. Le cas clinique du mois. Syndrome du bébé bleu: La vérité cachée au fond du puits [Blue baby syndrome: The source of the truth]. Rev. Médicale Liège 2014, 69, 175–179. (In French) [Google Scholar] [PubMed]
- Kross, B.C.; Ayebo, A.D.; Fuortes, L.J. Methemoglobinemia: Nitrate toxicity in rural America. Am. Fam. Physician 1992, 46, 183–188. [Google Scholar] [PubMed]
- Yang, Y.; Lei, Q.; Li, J.; Hong, C.; Zhao, Z.; Xue, H.; Hu, J. Ultrasensitive Electrochemical Detection of Nitrite Using Hierarchical Nanocomposites. Microchem. J. 2022, 172, 106904. [Google Scholar] [CrossRef]
- Shi, H.; Fu, L.; Chen, F.; Zhao, S.; Lai, G. Environmental Risk Assessment of Nitrite Contamination in Groundwater. Environ. Res. 2022, 209, 112747. [Google Scholar]
- Fan, A.M.; Steinberg, V.E. Health implications of nitrate and nitrite in drinking water: An update on methemoglobinemia occurrence and reproductive and developmental toxicity. Regul. Toxicol. Pharmacol. 1996, 23 Pt 1, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Capuano, G.E.; Farina, R.A.; Screpis, G.A.; Corso, D.; Coniglio, M.A.; Libertino, S. In-Situ Contaminant Detection by Portable and Potentially Real-Time Sensing Systems. In Sustaining Water Quality; Massarelli, C., Ed.; IntechOpen: Rijeka, Croatia, 2024; Chapter 4. [Google Scholar] [CrossRef]
- Economou, A. Screen-Printed Electrodes Modified with “Green” Metals for Electrochemical Stripping Analysis of Toxic Elements. Sensors 2018, 18, 1032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Screen-Printed Electrodes: Promising Paper and Wearable Transducers for (Bio)Sensing. Biosensors 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamalasekaran, K.; Sundramoorthy, A.K. Applications of chemically modified screen-printed electrodes in food analysis and quality monitoring: A review. RSC Adv. 2024, 14, 27957–27971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farina, R.; Scalese, S.; Corso, D.; Capuano, G.E.; Screpis, G.A.; Coniglio, M.A.; Condorelli, G.G.; Libertino, S. Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles. Molecules 2024, 29, 3028. [Google Scholar] [CrossRef]
- Costa-Rama, E.; Fernández-Abedul, M.T. Paper-Based Screen-Printed Electrodes: A New Generation of Low-Cost Electroanalytical Platforms. Biosensors 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farina, R.; D’Arrigo, G.; Alberti, A.; Scalese, S.; Capuano, G.E.; Corso, D.; Screpis, G.A.; Coniglio, M.A.; Condorelli, G.G.; Libertino, S. Copper Micro-Flowers for Electrocatalytic Sensing of Nitrate Ions in Water. Sensors 2024, 24, 4501. [Google Scholar] [CrossRef]
- Hassan, M.H.; Khan, R.; Andreescu, S. Advances in electrochemical detection methods for measuring contaminants of emerging concerns. Electrochem. Sci. Adv. 2022, 2, e2100184. [Google Scholar] [CrossRef]
- Ghezal, A.; Al-Hamry, A.; Bouhamed, A.; Azzouzi, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Electrochemical detection of nitrite using screen printed graphite electrode modified by ION/rGO. In Proceedings of the 2019 16th International Multi-Conference on Systems, Signals & Devices (SSD), Istanbul, Turkey, 21–24 March 2019; pp. 611–614. [Google Scholar] [CrossRef]
- Talbi, M.; Al-Hamry, A.; Teixeira, P.R.; Bouhamed, A.; Azzouzi, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Graphite Screen Printed Electrodes Functionalized with AuNPs-PEI for Nitrite Detection. In Proceedings of the 2019 16th International Multi-Conference on Systems, Signals & Devices (SSD), Istanbul, Turkey, 21–24 March 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Zainal, Z.; Ahmad, S.A.A. A copper-based metal-organic framework decorated with electrodeposited Fe2O3 nanoparticles for electrochemical nitrite sensing. Microchim. Acta 2022, 189, 356. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, A.D.; Muchindu, M.; Jijana, A.; Mishra, S.; Nxumalo, E. Screen-printed electrode system based on carbon black/copper-organic framework hybrid nanocomposites for the electrochemical detection of nitrite. Mater. Today Commun. 2023, 35, 105567. [Google Scholar] [CrossRef]
- Adiraju, A.; Munjal, R.; Viehweger, C.; Al-Hamry, A.; Brahem, A.; Hussain, J.; Kommisetty, S.; Jalasutram, A.; Tegenkamp, C.; Kanoun, O. Towards Embedded Electrochemical Sensors for On-Site Nitrite Detection by Gold Nanoparticles Modified Screen Printed Carbon Electrodes. Sensors 2023, 23, 2961. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Liu, Y.; Song, P.; Chen, P.; Liu, X.; Cai, L.; Zhang, L. In situ growth of copper/reduced graphene oxide on graphite surfaces for the electrocatalytic reduction of nitrate. Electrochim. Acta 2019, 324, 134846. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim. Acta 2008, 53, 5977–5984. [Google Scholar] [CrossRef]
- Farina, R.; D’Arrigo, G.; Alberti, A.; Capuano, G.E.; Corso, D.; Screpis, G.A.; Coniglio, M.A.; Condorelli, G.G.; Libertino, S. Electrochemical Growth of Copper Crystals on SPCE for Electrocatalysis Nitrate Reduction. Nanomaterials 2024, 14, 1704. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Tranchida, G.; Milazzo, R.G.; Leonardi, M.; Scalese, S.; Farina, R.A.; Lombardo, S.; Privitera, S.M.S. Ultra-Low Loading of Gold on Nickel Foam for Nitrogen Electrochemistry. Nanomaterials 2023, 13, 2850. [Google Scholar] [CrossRef]
- Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramakrishnappa, T.; Sureshkumar, K.; Pandurangappa, M. Copper oxide impregnated glassy carbon spheres based electrochemical interface for nitrite/nitrate sensing. Mater. Chem. Phys. 2020, 245, 122744. [Google Scholar] [CrossRef]
- Šljukić, B.; Banks, C.E.; Crossley, A.; Compton, R.G. Copper Oxide—Graphite Composite Electrodes: Application to Nitrite Sensing. Electroanalysis 2007, 19, 79–84. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, F.; Zhang, X.; Yang, L. Facile synthesis of flower like copper oxide their application to hydrogen peroxide nitrite sensing. Chem. Cent. J. 2011, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Nozari-Asbemarz, M.; Amiri, M.; Imanzadeh, H.; Bezaatpour, A.; Nouhi, S.; Hosseini, P.; Wark, M.; Seifzadeh, D. Mixed metal oxides as efficient electrocatalysts for water oxidation. Int. J. Hydrog. Energy 2022, 47, 5250–5259. [Google Scholar] [CrossRef]
- Sannasi, V.; Subbian, K. Influence of Moringa oleifera gum on two polymorphs synthesis of MnO2 and evaluation of the pseudo-capacitance activity. J. Mater. Sci. Mater. Electron. 2020, 31, 17120–17132. [Google Scholar] [CrossRef]
- Srivastava, G.; Dalela, S.; Kumar, S.; Choudhary, B.; Alvi, P. Structural and Raman studies of MnO2 and Mn2O3 nano-particles. Mater. Today Proc. 2023, 79, 169–171. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Lee, J.; Kim, Y.; Shin, D.; Park, J.; Kim, S.; Byon, H.R.; Kim, H.; Song, H. Switching Methane Selectivity in Carbon Dioxide Electroreduction via Confining Copper(I) Oxide Nanocubes by Polyimine Shells. ACS Catal. 2025, 15, 2642–2653. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’MUllane, A.P.; Kalantar-Zadeh, K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef]
- Sivkov, D.V.; Petrova, O.V.; Nekipelov, S.V.; Vinogradov, A.S.; Skandakov, R.N.; Isaenko, S.I.; Ob’edkov, A.M.; Kaverin, B.S.; Vilkov, I.V.; Korolev, R.I.; et al. The Identification of Cu–O–C Bond in Cu/MWCNTs Hybrid Nanocomposite by XPS and NEXAFS Spectroscopy. Nanomaterials 2021, 11, 2993. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Di Castro, V.; Polzonetti, G. XPS study of MnO oxidation. J. Electron Spectrosc. Relat. Phenom. 1989, 48, 117–123. [Google Scholar] [CrossRef]
- Su, X.; Feng, G.; Yu, L.; Li, Q.; Zhang, H.; Song, W.; Hu, G. In-situ green synthesis of CuO on 3D submicron-porous/solid copper current collectors as excellent supercapacitor electrode material. J. Mater. Sci. Mater. Electron. 2019, 30, 3545–3551. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Nie, F.; Zhang, R.; Fang, X.; Wang, Y. The role of MnO2 crystal morphological scale and crystal structure in selective catalytic degradation of azo dye. Environ. Sci. Pollut. Res. 2023, 30, 15377–15391. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Farina, R.A.; Malannata, E.M.; Lo Presti, F.; Balsamo, S.A. VOCs Photothermo-Catalytic Removal on MnOx-ZrO2 Catalysts. Catalysts 2022, 12, 85. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, S.; Qu, S.; Zhu, Y.; Zhang, Z. Surface decoration of multi-walled carbon nanotubes modified carbon paste electrode with CuO nanoparticles for electrocatalytic oxidation of nitrite. Indian J. Chem. 2020, 59, 625–632. [Google Scholar] [CrossRef]
- Xu, M.-W.; Bao, S.-J. Nanostructured MnO2 for Electrochemical Capacitor. In Energy Storage in the Emerging Era of Smart Grids; InTech: Vienna, Austria, 2011. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Xu, Y. Nucleation and Growth of Porous MnO2 Coatings Prepared on Nickel Foam and Evaluation of Their Electrochemical Performance. Materials 2018, 11, 716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desai, M.A.; Vedpathak, A.S.; Bhapkar, A.R.; Saratale, G.D.; Sartale, S.D. An investigation of chemical and electrochemical conversion of SILAR grown Mn3O4 into MnO2 thin films. J Environ. Manag. 2021, 299, 113564. [Google Scholar] [CrossRef] [PubMed]
- Malha, S.I.R.; Mandli, J.; Ourari, A.; Amine, A. Carbon black-modified electrodes as sensitive tools for the electrochemicaldetection of nitrite and nitrate. Electroanalysis 2013, 25, 2289–2297. [Google Scholar] [CrossRef]
- Abdi, Z.; Vandichel, M.; Sologubenko, A.S.; Willinger, M.-G.; Shen, J.-R.; Allakhverdiev, S.I.; Najafpour, M.M. The importance of identifying the true catalyst when using Randles-Sevcik equation to calculate turnover frequency. Int. J. Hydrog. Energy 2021, 46, 37774–37781. [Google Scholar] [CrossRef]
- González-Meza, O.A.; Larios-Durán, E.R.; Gutiérrez-Becerra, A.; Casillas, N.; Escalante, J.I.; Bárcena-Soto, M. Development of a Randles-Ševčík-like equation to predict the peak current of cyclic voltammetry for solid metal hexacyanoferrates. J. Solid State Electrochem. 2019, 23, 3123–3133. [Google Scholar] [CrossRef]
- Algethami, F.K.; Rabti, A.; Mastouri, M.; Abdulkhair, B.Y.; Ben Aoun, S.; Raouafi, N. Highly sensitive capacitance-based nitrite sensing using polydopamine/AuNPs-modified screen-printed carbon electrode. RSC Adv. 2023, 13, 21336–21344. [Google Scholar] [CrossRef]
- Marino, N.; Milazzo, R.G.; Farina, R.; Bongiorno, C.; Libertino, S.; Lombardo, S.A.; Privitera, S.M.S. High Performance Fe-MoS2 Electrocatalyst for Efficient Nitrate Reduction to Ammonia: Synergistic Design for Sustainable Ammonia Production. ACS Sustain. Chem. Eng. J. Available online: https://ecs.confex.com/ecs/247/meetingapp.cgi/Paper/203301 (accessed on 11 May 2025).




| Electrode | LoD | Linear Range | Application | Reference |
|---|---|---|---|---|
| SPE modified with CuO and MnO2 (This work) | 71 nM | 0.2–60 µM | Drinking water | This work |
| SPCE modified with copper(II)-benzene-1,4-dicarboxylate (Cu-BDC) frameworks and Fe2O3 NPs | 74 nM | 1–2000 µM | Mineral water | [26] |
| AuNP-modified screen-printed carbon electrode (SPCE) | 0.38 µM | 5.0–100 µM | On-site detection in aqueous samples | [24] |
| SPE modified with ZrO2@MWCNTs | 0.94 µM | 5.0–100 µM | Food and water samples | [3] |
| Polydopamine/AuNPs-modified SPCE | 1.98 μM | 10 to 500 μM | Processed meat samples in water | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, R.; Scalese, S.; Alberti, A.; Privitera, S.M.S.; Capuano, G.E.; Corso, D.; Screpis, G.A.; Reina, S.C.R.; Condorelli, G.G.; Coniglio, M.A.; et al. Electrocatalytical Nitrite Oxidation via Manganese and Copper Oxides on Carbon Screen-Printed Electrode. Sensors 2025, 25, 3764. https://doi.org/10.3390/s25123764
Farina R, Scalese S, Alberti A, Privitera SMS, Capuano GE, Corso D, Screpis GA, Reina SCR, Condorelli GG, Coniglio MA, et al. Electrocatalytical Nitrite Oxidation via Manganese and Copper Oxides on Carbon Screen-Printed Electrode. Sensors. 2025; 25(12):3764. https://doi.org/10.3390/s25123764
Chicago/Turabian StyleFarina, Roberta, Silvia Scalese, Alessandra Alberti, Stefania Maria Serena Privitera, Giuseppe Emanuele Capuano, Domenico Corso, Giuseppe Andrea Screpis, Serena Concetta Rita Reina, Guglielmo Guido Condorelli, Maria Anna Coniglio, and et al. 2025. "Electrocatalytical Nitrite Oxidation via Manganese and Copper Oxides on Carbon Screen-Printed Electrode" Sensors 25, no. 12: 3764. https://doi.org/10.3390/s25123764
APA StyleFarina, R., Scalese, S., Alberti, A., Privitera, S. M. S., Capuano, G. E., Corso, D., Screpis, G. A., Reina, S. C. R., Condorelli, G. G., Coniglio, M. A., & Libertino, S. (2025). Electrocatalytical Nitrite Oxidation via Manganese and Copper Oxides on Carbon Screen-Printed Electrode. Sensors, 25(12), 3764. https://doi.org/10.3390/s25123764









