Patient Health Record Smart Network Challenges and Trends for a Smarter World
Abstract
1. Introduction
2. Electronic Health Record Systems: Current Trends
3. Personal Health Records Benefits and Challenges
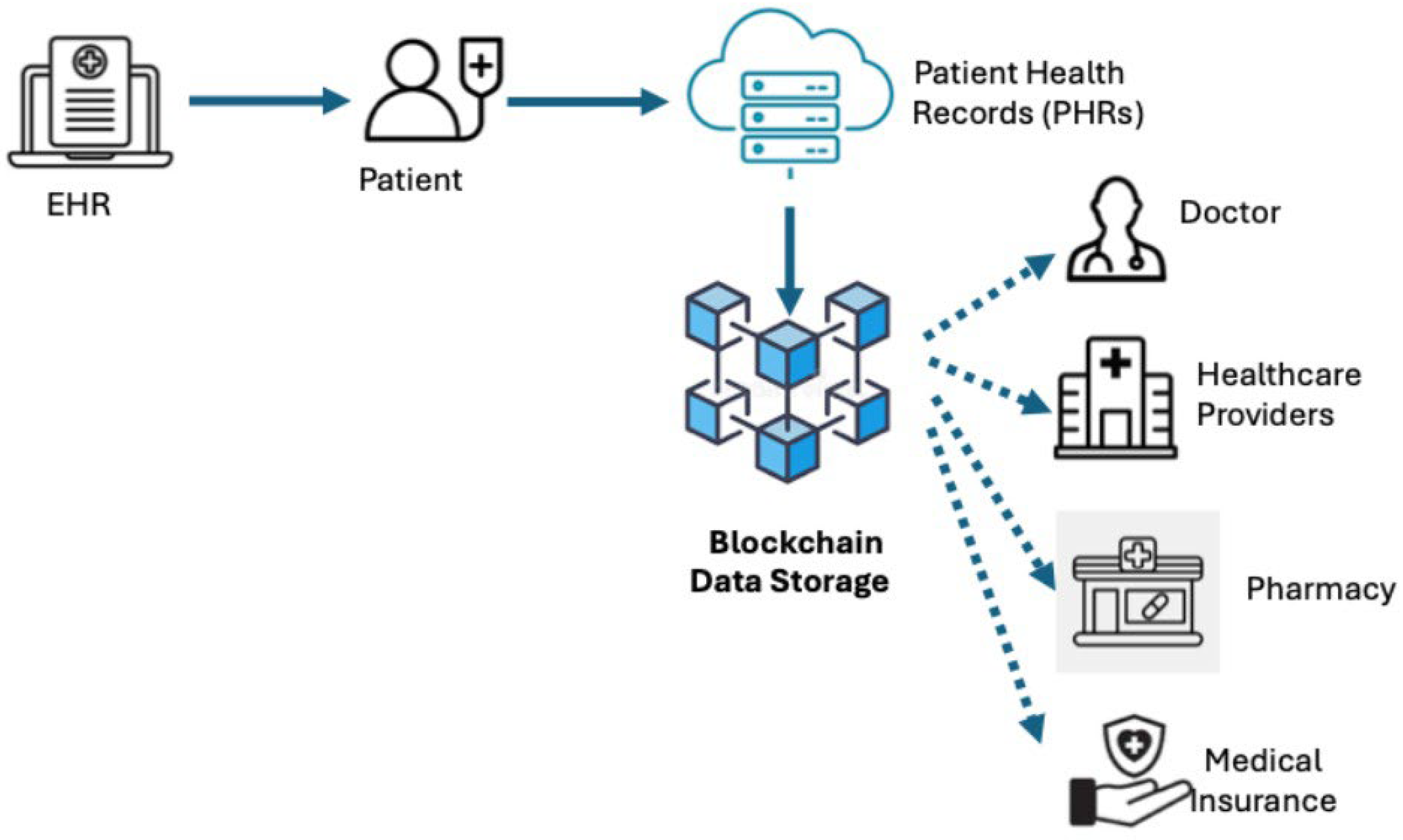
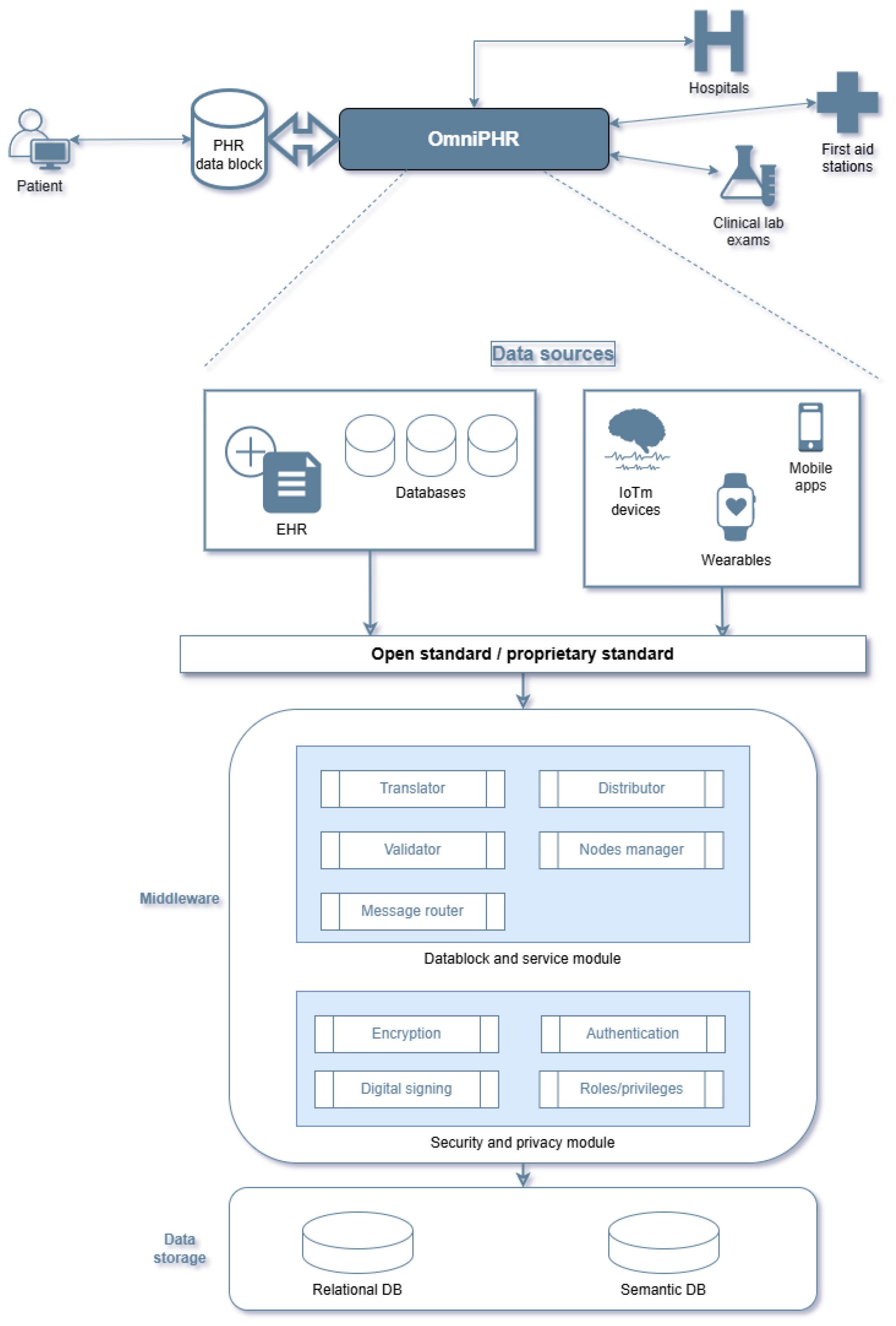
4. PHR Data Sources and System Architectures
4.1. Health Record Applications
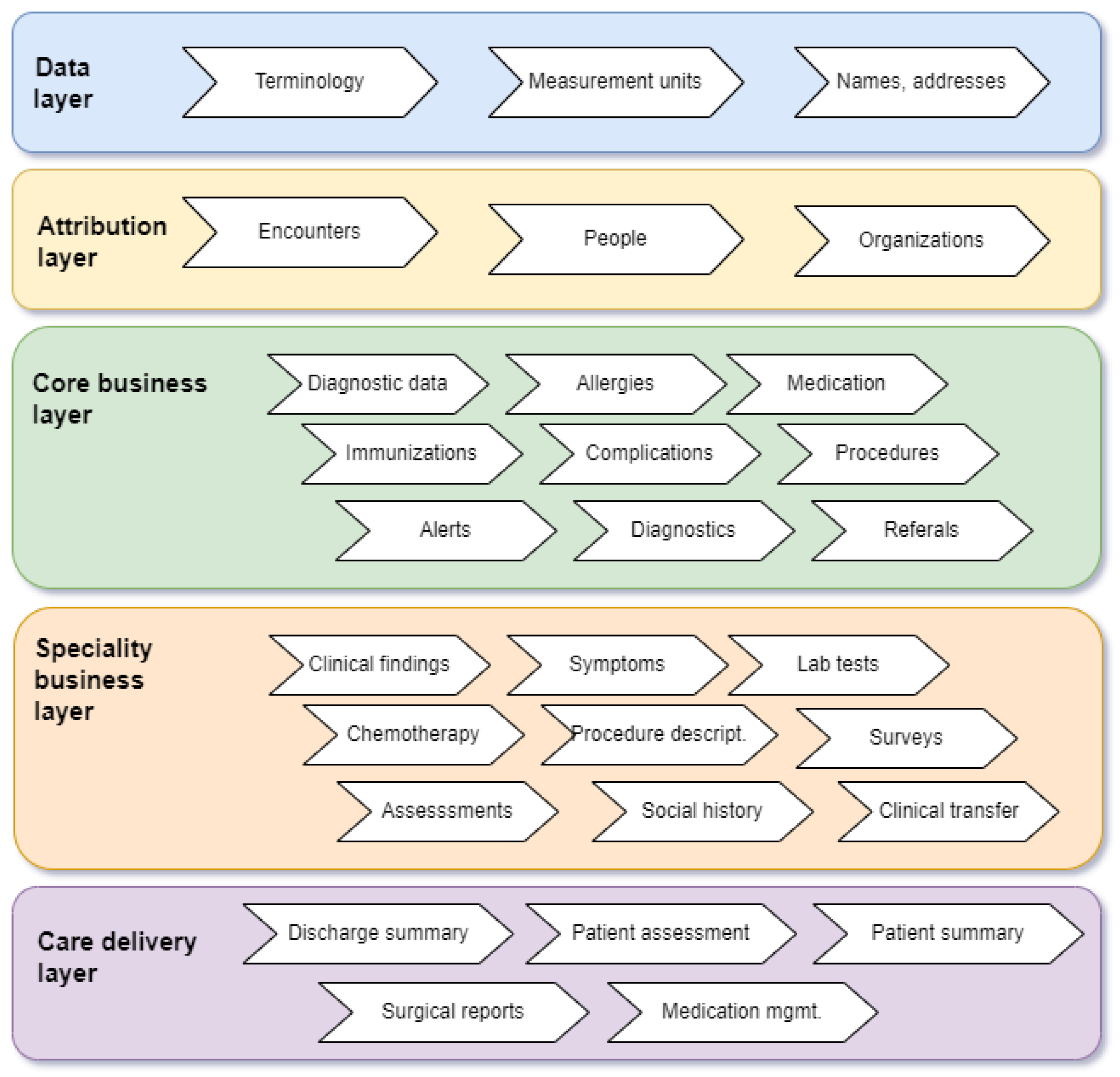
4.2. Standards for Personal Health Records
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vayena, E.; Dzenowagis, J.; Brownstein, J.S.; Sheikh, A. Policy implications of big data in the health sector. Bull. World Health Organ. 2018, 96, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.; North, F.; Raghu, T.S. Personal Health Records (PHR) and the future of the physician-patient relationship. In Proceedings of the 2011 iConference; ACM: Seattle, WA, USA, 2011; pp. 281–288. [Google Scholar] [CrossRef]
- Alanazi, A.; Anazi, Y.A. The Challenges in Personal Health Record Adoption. J. Healthc. Manag. 2019, 64, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, Y.A.; Gay, V. An overview of electronic personal health records. Health Policy Technol. 2018, 7, 427–432. [Google Scholar] [CrossRef]
- Manias, E.; Gray, K.; Wickramasinghe, N.; Manojlovich, M. Using electronic medical records to create big data and to communicate with patients—Is there room for both? Collegian 2018, 25, 467–469. [Google Scholar] [CrossRef]
- Bouayad, L.; Ialynytchev, A.; Padmanabhan, B. Patient Health Record Systems Scope and Functionalities: Literature Review and Future Directions. J. Med. Internet Res. 2017, 19, e388. [Google Scholar] [CrossRef]
- Paydar, S.; Emami, H.; Asadi, F.; Moghaddasi, H.; Hosseini, A. Functions and Outcomes of Personal Health Records for Patients with Chronic Diseases: A Systematic Review. Perspect. Health Inf. Manag. 2021, 18, 1l. [Google Scholar]
- Price, M.; Bellwood, P.; Kitson, N.; Davies, I.; Weber, J.; Lau, F. Conditions potentially sensitive to a Personal Health Record (PHR) intervention, a systematic review. BMC Med. Inform. Decis. Mak. 2015, 15, 32. [Google Scholar] [CrossRef]
- Paccoud, I.; Baumann, M.; Le Bihan, E.; Pétré, B.; Breinbauer, M.; Böhme, P.; Chauvel, L.; Leist, A.K. Socioeconomic and behavioural factors associated with access to and use of Personal Health Records. BMC Med. Inform. Decis. Mak. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.-T.; Park, Y.R.; Lee, J.-H. Review of National-Level Personal Health Records in Advanced Countries. Healthc. Inform. Res. 2021, 27, 102–109. [Google Scholar] [CrossRef]
- European Commission. Directorate General for Communications Networks, Content and Technology, RAND Europe, Open Evidence, and BDI Research. In Benchmarking Deployment of eHealth Among General Practitioners (2018): Final Report; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Australian Digital Health Agency. View Your Record Using an App Sydney; Australian Digital Health Agency: Sydney, Australia, 2021. [Google Scholar]
- Armstrong, S. Patient access to health records: Striving for the Swedish ideal. BMJ 2017, 357, j2069. [Google Scholar] [CrossRef]
- Moll, J.; Grünloh, C.; Hägglund, M.; Cajander, Å.; Huvila, I.; Rexhepi, H.; Scandurra, I.; Åhlfeldt, R.-M.; Myreteg, G. Patients’ Experiences of Accessing Their Electronic Health Records: National Patient Survey in Sweden. J. Med. Internet Res. 2018, 20, e278. [Google Scholar] [CrossRef] [PubMed]
- Birinci, Ş. A Digital Opportunity for Patients to Manage Their Health: Turkey National Personal Health Record System (The e-Nabız). Balkan Med. J. 2023, 40, 215–221. [Google Scholar] [CrossRef] [PubMed]
- UK Interested in Turkey’s Award-Winning e-Nabız Healthcare App|Daily Sabah. Available online: https://www.dailysabah.com/business/tech/uk-interested-in-turkeys-award-winning-e-nabiz-healthcare-app (accessed on 28 November 2024).
- Grad, D.A.; Mureșanu, D. Electronic health records in Romania—Window of opportunity in improving population’s health? J. Med. Life 2022, 15, 1327–1329. [Google Scholar] [CrossRef]
- ISO 18308:2011: Health Informatics—Requirements for an Electronic Health Record Architecture. 2011. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:18308:ed-1:v1:en (accessed on 28 November 2024).
- ISO, ISO/TR 14292: Health Informatics—Personal Health Records—Definition, Scope and Context. 2012. Available online: https://www.iso.org/obp/ui/#iso:std:iso:tr:14292:ed-1:v1:en (accessed on 28 November 2024).
- Shortliffe, E.H.; Cimino, J.J.; Chiang, M.F. (Eds.) Biomedical Informatics: Computer Applications in Health Care and Biomedicine, 5th ed.; Springer: Cham, Switzerland, 2021; ISBN 978-3-030-58721-5. [Google Scholar]
- Roehrs, A.; Da Costa, C.A.; Da Rosa Righi, R.; Rigo, S.J.; Wichman, M.H. Toward a Model for Personal Health Record Interoperability. IEEE J. Biomed. Health Inform. 2019, 23, 867–873. [Google Scholar] [CrossRef] [PubMed]
- ISO/HL7 10781:2009 (en) Electronic Health Record-System Functional Model. 2009. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso-hl7:10781:ed-1:v2:en (accessed on 28 November 2024).
- Heart, T.; Ben-Assuli, O.; Shabtai, I. A review of PHR, EMR and EHR integration: A more personalized healthcare and public health policy. Health Policy Technol. 2017, 6, 20–25. [Google Scholar] [CrossRef]
- Hayrinen, K.; Saranto, K.; Nykanen, P. Definition, structure, content, use and impacts of electronic health records: A review of the research literature. Int. J. Med. Inform. 2008, 77, 291–304. [Google Scholar] [CrossRef]
- Melton, G.B.; McDonald, C.J.; Tang, P.C.; Hripcsak, G. Electronic Health Records. In Biomedical Informatics; Shortliffe, E.H., Cimino, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 467–509. ISBN 978-3-030-58720-8. [Google Scholar] [CrossRef]
- Palojoki, S.; Lehtonen, L.; Vuokko, R. Semantic Interoperability of Electronic Health Records: Systematic Review of Alternative Approaches for Enhancing Patient Information Availability. JMIR Med. Inform. 2024, 12, e53535. [Google Scholar] [CrossRef]
- ISO/IEEE 11073-10701:2024. Available online: https://www.iso.org/standard/88168.html (accessed on 11 May 2025).
- Yang, X.; Chen, A.; PourNejatian, N.; Shin, H.C.; Smith, K.E.; Parisien, C.; Compas, C.; Martin, C.; Costa, A.B.; Flores, M.G.; et al. A large language model for electronic health records. npj Digit. Med. 2022, 5, 194. [Google Scholar] [CrossRef]
- Acosta, J.N.; Falcone, G.J.; Rajpurkar, P.; Topol, E.J. Multimodal biomedical AI. Nat. Med. 2022, 28, 1773–1784. [Google Scholar] [CrossRef]
- Juhn, Y.; Liu, H. Artificial intelligence approaches using natural language processing to advance EHR-based clinical research. J. Allergy Clin. Immunol. 2020, 145, 463–469. [Google Scholar] [CrossRef]
- Biro, J.M.; Visconti, A.; Trafton, J.G.; Ratwani, R.M.; McCurry, J.M.; Handley, J.L.; Weinfeld, J. Opportunities and risks of artificial intelligence in patient portal messaging in primary care. npj Digit. Med. 2025, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, S.; Gandhi, P.; Shinde, G.; Subramanian, V. Deep Learning and Explainable AI in Healthcare Using EHR. In Deep Learning Techniques for Biomedical and Health Informatics; Dash, S., Acharya, B.R., Mittal, M., Abraham, A., Kelemen, A., Eds.; Studies in Big Data; Springer International Publishing: Cham, Switzerland, 2020; Volume 68, pp. 129–148. [Google Scholar] [CrossRef]
- Significance of Using AI Technology in EHR. Available online: https://www.managedoutsource.com/blog/ai-ehrs-usage-ai-helps-improve-electronic-health-records/ (accessed on 28 November 2024).
- Tran, D.T.; Zhang, X.; Stolyar, A.; Lober, W.B. Patient-centered design for a personal health record system. AMIA Annu. Symp. Proc. 2005, 1140, 2005. [Google Scholar]
- Sarwal, D.; Gupta, V. Personal Health Record. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Haddad, A.; Habaebi, M.H.; Islam, M.R.; Hasbullah, N.F.; Zabidi, S.A. Systematic Review on AI-Blockchain Based E-Healthcare Records Management Systems. IEEE Access 2022, 10, 94583–94615. [Google Scholar] [CrossRef]
- Tagde, P.; Kaushik, D.; Chopra, H.; Bhattacharya, T.; Akter, R.; Tagde, P.; Tagde, S.; Rahman, H. Blockchain and artificial intelligence technology in e-Health. Environ. Sci. Pollut. Res. 2021, 28, 52810–52831. [Google Scholar] [CrossRef]
- Chang, H.; Choi, J.-Y.; Shim, J.; Kim, M.; Choi, M. Benefits of Information Technology in Healthcare: Artificial Intelligence, Internet of Things, and Personal Health Records. Healthc. Inform. Res. 2023, 29, 323–333. [Google Scholar] [CrossRef]
- Flaumenhaft, Y.; Ben-Assuli, O. Personal health records, global policy and regulation review. Health Policy 2018, 122, 815–826. [Google Scholar] [CrossRef]
- Spriggs, M.; Arnold, M.V.; Pearce, C.M.; Fry, C. Ethical questions must be considered for electronic health records. J. Med. Ethics 2012, 38, 535–539. [Google Scholar] [CrossRef]
- Laranjo, L.; Rodolfo, I.; Pereira, A.M.; De Sá, A.B. Characteristics of Innovators Adopting a National Personal Health Record in Portugal: Cross-Sectional Study. JMIR Med. Inform. 2017, 5, e37. [Google Scholar] [CrossRef]
- Abd-Alrazaq, A.; Alalwan, A.A.; McMillan, B.; Bewick, B.M.; Househ, M.; AL-Zyadat, A.T. Patients’ Adoption of Electronic Personal Health Records in England: Secondary Data Analysis. J. Med. Internet Res. 2020, 22, e17499. [Google Scholar] [CrossRef]
- Niazkhani, Z.; Toni, E.; Cheshmekaboodi, M.; Georgiou, A.; Pirnejad, H. Barriers to patient, provider, and caregiver adoption and use of electronic personal health records in chronic care: A systematic review. BMC Med. Inform. Decis. Mak. 2020, 20, 153. [Google Scholar] [CrossRef]
- Patients to Get Full Access to Record on NHS App. Available online: https://www.bbc.com/news/articles/cz7j73vx9v3o (accessed on 19 May 2025).
- Iakovidis, I. From electronic medical record to personal health records: Present situation and trends in European Union in the area of electronic healthcare records. Stud. Health Technol. Inform. 1998, 52 Pt 1, suppl 18–22. [Google Scholar]
- Feather, J.; Misselbrook, C.A.; Zipchen, P.; Matthews, V.L. Evaluation of a personal health record given to newborns in Saskatoon. Can. J. Public. Health 1987, 78, 350–351. [Google Scholar]
- Toni, E.; Pirnejad, H.; Makhdoomi, K.; Mivefroshan, A.; Niazkhani, Z. Patient empowerment through a user-centered design of an electronic personal health record: A qualitative study of user requirements in chronic kidney disease. BMC Med. Inform. Decis. Mak. 2021, 21, 329. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.; Conway, N.T.; Allardice, B.; Wake, D.J.; Cunningham, S.G. Continuing the quality improvement of an electronic personal health record and interactive website for people with diabetes in Scotland (My Diabetes My Way). Diabet. Med. 2023, 40, e15085. [Google Scholar] [CrossRef]
- Kaelber, D.; Pan, E.C. The value of personal health record (PHR) systems. AMIA Annu. Symp. Proc. 2008, 2008, 343–347. [Google Scholar]
- Archer, N.; Fevrier-Thomas, U.; Lokker, C.; McKibbon, K.A.; Straus, S.E. Personal health records: A scoping review. J. Am. Med. Inform. Assoc. 2011, 18, 515–522. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; A Hidalgo, J.; López, D.M.; Quintero, V.; Rendón, A.; Cajiao, A.; Cerón, J.D. A vital signs telemonitoring system—Interoperability supported by a personal health record systema and a cloud service. Stud. Health Technol. Inform. 2014, 200, 124–130. [Google Scholar]
- Groenen, C.J.M.; Kremer, J.A.M.; IntHout, J.; Meinders, M.J.; Van Duijnhoven, N.T.L.; Vandenbussche, F.P.H.A. Effects of a Personal Health Record in Maternity Care: A Stepped-Wedge Trial. Int. J. Environ. Res. Public Health 2021, 18, 10343. [Google Scholar] [CrossRef]
- Fisher-Grace, K.; Turk, M.T.; Anthony, M.K.; Chia, L.R. Use of Personal Health Records to Support Diabetes Self-management: An Integrative Review. CIN Comput. Inform. Nurs. 2021, 39, 298–305. [Google Scholar] [CrossRef]
- Kumar, S.; Wajeed, M.A.; Singhal, P.; Dwivedi, N.; Jamal, S.S.; Kunabeva, R.; Akwafo, R. Novel Method for Safeguarding Personal Health Record in Cloud Connection Using Deep Learning Models. Comput. Intell. Neurosci. 2022, 2022, 3564436. [Google Scholar] [CrossRef]
- Jacob, P.D. Management of patient healthcare information. In Fundamentals of Telemedicine and Telehealth; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–57. ISBN 978-0-12-814309-4. [Google Scholar] [CrossRef]
- Salehi, F.; Bastani, P.; Ahmadian, L.; Samadi, K.; Yazdani, A.; Sharifian, R. Prerequisites of Personal Health Record for Chronic Kidney Disease: A Scoping Review and Evaluation of the Content Validity. In Studies in Health Technology and Informatics; Blobel, B., Giacomini, M., Eds.; IOS Press: Amsterdam, The Netherlands, 2021; ISBN 978-1-64368-226-6. [Google Scholar] [CrossRef]
- Nekar, D.M.; Kang, H.; Alao, H.; Yu, J. Feasibility of Using Multiplayer Game-Based Dual-Task Training with Augmented Reality and Personal Health Record on Social Skills and Cognitive Function in Children with Autism. Children 2022, 9, 1398. [Google Scholar] [CrossRef]
- Roehrs, A.; Da Costa, C.A.; Righi, R.D.R.; De Oliveira, K.S.F. Personal Health Records: A Systematic Literature Review. J. Med. Internet Res. 2017, 19, e13. [Google Scholar] [CrossRef] [PubMed]
- Walle, A.D.; Walle, A.D.; Ferede, T.A.; Baykemagn, N.D.; Shimie, A.W.; Kebede, S.D.; Tegegne, M.D.; Wubante, S.M.; Yehula, C.M.; Demsash, A.W.; et al. Predicting healthcare professionals’ acceptance towards electronic personal health record systems in a resource-limited setting: Using modified technology acceptance model. BMJ Health Care Inform. 2023, 30, e100707. [Google Scholar] [CrossRef] [PubMed]
- Cronin, R.M.; Jimison, H.; Johnson, K.B. Personal Health Informatics. In Biomedical Informatics; Shortliffe, E.H., Cimino, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 363–389. ISBN 978-3-030-58720-8. [Google Scholar] [CrossRef]
- Katz, R.; Mesfin, T.; Barr, K. Lessons From a Community-Based mHealth Diabetes Self-Management Program: ‘It’s Not Just About the Cell Phone’. J. Health Commun. 2012, 17, 67–72. [Google Scholar] [CrossRef]
- Jeddi, F.R.; Nabovati, E.; Rezayi, S.; Saeedi, S.; Amirazodi, S. Identifying a minimum data set as a necessity to design a web-based personal health record for patients under chronic dialysis. J. Fam. Med. Prim. Care 2022, 11, 969–975. [Google Scholar] [CrossRef]
- Galvin, H.K.; DeMuro, P.R. Developments in Privacy and Data Ownership in Mobile Health Technologies, 2016–2019. Yearb Med. Inform. 2020, 29, 032–043. [Google Scholar] [CrossRef]
- Frutos, E.; Descalzo, J.; Colussi, G.; Rapisarda, R.; Plazzotta, F.; Luna, D. Exploring the Digital Divide as a Barrier to Use of a Personal Health Record in the Elderly. In Studies in Health Technology and Informatics; Séroussi, B., Weber, P., Dhombres, F., Grouin, C., Liebe, J.-D., Pelayo, S., Pinna, A., Rance, B., Sacchi, L., Ugon, A., et al., Eds.; IOS Press: Amsterdam, The Netherlands, 2022; ISBN 978-1-64368-284-6. [Google Scholar] [CrossRef]
- Fosser, S.M.; Sommer, J.; Simón, M.; Giraldo, L.; Plazzotta, F.; Luna, D. User-Centered Design of a Patient Medication Reconciliation Module in an Integrated Personal Health Record. Stud. Health Technol. Inform. 2019, 264, 1278–1282. [Google Scholar] [CrossRef]
- Farinango, C.; Benavides, J.; Cerón, J.; López, D.; Álvarez, R. Human-centered design of a personal health record system for metabolic syndrome management based on the ISO 9241-210:2010 standard. J. Multidiscip. Healthc. 2018, 11, 21–37. [Google Scholar] [CrossRef]
- Diffin, J.; McLaughlin, D.; Price, J.; Abbott, A.; O’Halloran, P.; Byrne, B.; Kerr, H. The usefulness and acceptability of a personal health record to children and young people living with a complex health condition: A realist review of the literature. Child 2019, 45, 313–332. [Google Scholar] [CrossRef]
- Druss, B.G.; Li, J.; Tapscott, S.; Lally, C.A. Randomized Trial of a Mobile Personal Health Record for Behavioral Health Homes. Psychiatr. Serv. 2020, 71, 803–809. [Google Scholar] [CrossRef]
- Evans, B.A.; Beverly, C.J.; Tsai, P.-F.; Rettiganti, M.; Lefler, L.L.; Parks, R.F. Older Adults’ Live Demonstration of Electronic Personal Health Record Use: Factors Mediating Initial Proficiency. CIN Comput. Inform. Nurs. 2018, 36, 603–609. [Google Scholar] [CrossRef] [PubMed]
- ISO 13606 Standard—EHR Interoperability. Available online: http://www.en13606.org/information.html (accessed on 11 May 2025).
- The World’s Leading Open-Source Medical Record Software. Available online: https://www.open-emr.org/ (accessed on 28 November 2024).
- IEEE. IEEE Recommended Practice for Software Requirements Specifications. Available online: https://ieeexplore.ieee.org/servlet/opac?punumber=5841 (accessed on 11 April 2024).
- ISO 9241-210:2019—Ergonomics of Human-System Interaction—Part 210: Human-Centred Design for Interactive Systems. Available online: https://www.iso.org/standard/77520.html (accessed on 11 April 2024).
- Braunstein, M.L. Health care in the age of interoperability part 5: The personal health record. IEEE Pulse 2019, 10, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Featured Apps. Available online: https://apps.smarthealthit.org/apps/featured (accessed on 11 March 2024).
- Lee, J.; Yoo, B.; Lim, H.-S.; Shin, S.-A.; Cho, K.-H.; Kim, D.-W.; Kim, J. The development and implementation of stroke risk prediction model in National Health Insurance Service’s personal health record. Comput. Methods Programs Biomed. 2018, 153, 253–257. [Google Scholar] [CrossRef]
- Roehrs, A.; Da Costa, C.A.; Da Rosa Righi, R. OmniPHR: A distributed architecture model to integrate personal health records. J. Biomed. Inform. 2017, 71, 70–81. [Google Scholar] [CrossRef]
- Veazie, S.; Helfand, M.; Eden, K.B.; Guise, J.-M.; Gilbert, J.; Winchell, K.; Nussbaum, K.; Ivlev, I.; Paynter, R.; Weiskopf, N. Rapid Evidence Review of Mobile Applications for Self-management of Diabetes. J. Gen. Intern. Med. 2018, 33, 1167–1176. [Google Scholar] [CrossRef]
- Park, Y.-T.; Park, H.-A.; Lee, J.M.; Choi, B.K. Hospitals’ Adoption of Mobile-Based Personal Health Record Systems and Patients’ Characteristics: A Cross-Sectional Study Analyzing National Healthcare Big Data. Inquiry 2023, 60, 00469580231160892. [Google Scholar] [CrossRef]
- Pérez, Y.I.V.; Medlow, S.; Ho, J.; Steinbeck, K. Mobile and Web-Based Apps That Support Self-Management and Transition in Young People With Chronic Illness: Systematic Review. J. Med. Internet Res. 2019, 21, e13579. [Google Scholar] [CrossRef]
- Costa, T.B.D.S.; Shinoda, L.; Moreno, R.A.; Krieger, J.E.; Gutierrez, M. Blockchain-Based Architecture Design for Personal Health Record: Development and Usability Study. J. Med. Internet Res. 2022, 24, e35013. [Google Scholar] [CrossRef]
- Roehrs, A.; Schmidt, D.C.; Goldim, J.R.; Righi, R.R.; Mayer, A.H.; da Silva, V.F.; A da Costa, C. Integrating multiple blockchains to support distributed personal health records. Health Inform. J. 2021, 27, 14604582211007546. [Google Scholar] [CrossRef]
- Roehrs, A.; Da Costa, C.A.; Da Rosa Righi, R.; Da Silva, V.F.; Goldim, J.R.; Schmidt, D.C. Analyzing the performance of a blockchain-based personal health record implementation. J. Biomed. Inform. 2019, 92, 103140. [Google Scholar] [CrossRef]
- Yoon, H.-J. Blockchain Technology and Healthcare. Healthc. Inform. Res. 2019, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.S.A.; Tan, T.H.; Tan, Y.F.C.; Tan, C.J.M. Blockchain Personal Health Records: Systematic Review. J. Med. Internet Res. 2021, 23, e25094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Diro, A.; Saini, A.; Kaisar, S.; Hiep, P.C. Leveraging zero knowledge proofs for blockchain-based identity sharing: A survey of advancements, challenges and opportunities. J. Inf. Secur. Appl. 2024, 80, 103678. [Google Scholar] [CrossRef]
- Kumar, J.; Zakria; Zeng, S.; Khan, A.A.; Kumar, R.; Khan, R.U.; Ali, H.; Bernard, C.M. Blockchain and homomorphic encryption based privacy-preserving model aggregation for medical images. Comput. Med. Imaging Graph. 2022, 102, 102139. [Google Scholar] [CrossRef]
- Van Gorp, P.; Comuzzi, M. MyPHRMachines: Lifelong Personal Health Records in the cloud. In Proceedings of the 2012 25th IEEE International Symposium on Computer-Based Medical Systems (CBMS), Rome, Italy, 20–22 June 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1–6. [Google Scholar] [CrossRef]
- Van Gorp, P.; Comuzzi, M.; Jahnen, A.; Kaymak, U.; Middleton, B. An open platform for personal health record apps with platform-level privacy protection. Comput. Biol. Med. 2014, 51, 14–23. [Google Scholar] [CrossRef]
- Celi, L.A.; Majumder, M.S.; Ordóñez, P.; Osorio, J.S.; Paik, K.E.; Somai, M. (Eds.) Leveraging Data Science for Global Health; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-47993-0. [Google Scholar] [CrossRef]
- OmniPHR: A Blockchain Based Interoperable Architecture for Personal Health Records. Available online: https://repositorio.jesuita.org.br/handle/UNISINOS/8867 (accessed on 29 November 2024).
- Home—My HealtheVet—My HealtheVet. Available online: https://www.myhealth.va.gov/mhv-portal-web/home (accessed on 28 May 2025).
- Cernian, A.; Tiganoaia, B.; Sacala, I.; Pavel, A.; Iftemi, A. PatientDataChain: A Blockchain-Based Approach to Integrate Personal Health Records. Sensors 2020, 20, 6538. [Google Scholar] [CrossRef]
- Benson, T.; Grieve, G. Health Information Technology Standards. In Principles of Health Interoperability: SNOMED CT, HL7 and FHIR.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-30368-0. [Google Scholar] [CrossRef]
- openEHR Home. Available online: https://openehr.org/ (accessed on 29 November 2024).
- Healthcare Integration Engine|Mirth® Connect by NextGen Healthcare. Available online: https://www.nextgen.com/solutions/interoperability/mirth-integration-engine (accessed on 28 November 2024).
- iNTERFACEWARE—Iguana Healthcare Integration Engine. Available online: https://www.interfaceware.com/iguana-integration-engine (accessed on 28 November 2024).
- USCDI—US Core Implementation Guide v8.0.0. Available online: https://build.fhir.org/ig/HL7/US-Core/uscdi.html (accessed on 22 May 2025).
- European Health Data Space Regulation (EHDS)—European Commission. Available online: https://health.ec.europa.eu/ehealth-digital-health-and-care/european-health-data-space-regulation-ehds_en (accessed on 22 May 2025).
- Marelli, L.; Stevens, M.; Sharon, T.; Van Hoyweghen, I.; Boeckhout, M.; Colussi, I.; Degelsegger-Márquez, A.; El-Sayed, S.; Hoeyer, K.; van Kessel, R. The European health data space: Too big to succeed? Health Policy 2023, 135, 104861. [Google Scholar] [CrossRef]
- Rinaldi, E.; Thun, S. Mapping from openEHR to FHIR and OMOP CDM to support interoperability for infection control. GMS Med Inf. Biom Epidemiol. 2021, 17, Doc07. [Google Scholar] [CrossRef]
- Veratech for Health. Available online: https://www.veratech.es/en/2023/08/25/compatibilidad-de-estandares-sanitarios-openehr-hl7-fhir-y-omop/ (accessed on 22 May 2025).
- X-eHealth: Exchanging Electronic Health Records in a Commom Framework|X-eHealth|Projekt|Fact Sheet|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/951938 (accessed on 22 May 2025).
- Hospitals on FHIR-HL7 Europe-Confluence. Available online: https://confluence.hl7.org/spaces/HEU/pages/218829591/Hospitals+on+FHIR (accessed on 22 May 2025).
- Piesche, K. Assessing SNOMED CT for Large Scale eHealth Deployments in the EU. Final Report. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://research.tue.nl/files/300928883/assessct_final_brochure.pdf&ved=2ahUKEwj3hvaEt-2NAxV50jQHHVkBCokQFnoECBgQAQ&usg=AOvVaw1QCEtuorkM97tU2A0Viul3 (accessed on 22 May 2025).
- Chatterjee, A.; Pahari, N.; Prinz, A. HL7 FHIR with SNOMED-CT to Achieve Semantic and Structural Interoperability in Personal Health Data: A Proof-of-Concept Study. Sensors 2022, 22, 3756. [Google Scholar] [CrossRef]
- Pedrera-Jiménez, M.; García-Barrio, N.; Frid, S.; Moner, D.; Boscá-Tomás, D.; Lozano-Rubí, R.; Kalra, D.; Beale, T.; Muñoz-Carrero, A.; Serrano-Balazote, P. Can OpenEHR, ISO 13606, and HL7 FHIR Work Together? An Agnostic Approach for the Selection and Application of Electronic Health Record Standards to the Next-Generation Health Data Spaces. J. Med. Internet Res. 2023, 25, e48702. [Google Scholar] [CrossRef]
- Ouhbi, S.; Fernández-Alemán, J.L.; Carrillo-de-Gea, J.M.; Toval, A.; Idri, A. E-Health Internationalization Requirements for Audit Purposes. Comput. Methods Programs Biomed. 2017, 144, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Health Level Seven International—Homepage|HL7 International. Available online: https://www.hl7.org/index.cfm (accessed on 11 May 2025).
- Index—FHIR v5.0.0. Available online: https://hl7.org/fhir/ (accessed on 11 May 2025).
- ISO/HL7 27931:2009. Available online: https://www.iso.org/standard/44428.html (accessed on 11 May 2025).
- Current Edition. Available online: https://www.dicomstandard.org/current (accessed on 11 May 2025).
- ISO 21090:2011. Available online: https://www.iso.org/standard/35646.html (accessed on 11 May 2025).
- SIST-TP CEN/TR 15212:2006—Health Informatics—Vocabulary—Maintenance Procedure for a Web-Based Terms and Concepts Database. Available online: https://standards.iteh.ai/catalog/standards/sist/d82a892b-bc9c-4759-a402-55cd3db6aacb/sist-tp-cen-tr-15212-2006 (accessed on 11 May 2025).
- ISO/TR 20514:2005. Available online: https://www.iso.org/standard/39525.html (accessed on 11 May 2025).
- ISO 27799:2016. Available online: https://www.iso.org/standard/62777.html (accessed on 11 May 2025).
- ISO/IEC 27002:2022. Available online: https://www.iso.org/standard/75652.html (accessed on 11 May 2025).
- P3P: The Platform for Privacy Preferences. Available online: https://www.w3.org/P3P/ (accessed on 11 May 2025).
- ISO/TS 14265:2024. Available online: https://www.iso.org/standard/83447.html (accessed on 11 May 2025).
- ISO/IEC 25010:2023. Available online: https://www.iso.org/standard/78176.html (accessed on 11 May 2025).
- ISO 9241-151:2008. Available online: https://www.iso.org/standard/37031.html (accessed on 11 May 2025).
- IEC/TC 62—Electrical Equipment in Medical Practice. Available online: https://standards.iteh.ai/catalog/tc/iso/e872eb05-a7d3-4680-9a66-57d67d9a6156/iec-tc-62 (accessed on 11 May 2025).
- WHY INTEROPERABILITY IS ESSENTIAL IN HEALTH CARE—Procuring Interoperability—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK594855/ (accessed on 22 May 2025).
- Overview—FHIR v5.0.0. Available online: https://www.hl7.org/fhir/overview.html (accessed on 29 November 2024).
- Kumar, K.P.; Prathap, B.R.; Thiruthuvanathan, M.M.; Murthy, H.; Pillai, V.J. Secure approach to sharing digitized medical data in a cloud environment. Data Sci. Manag. 2024, 7, 108–118. [Google Scholar] [CrossRef]
- Abdulnabi, M.; Al-Haiqi, A.; Kiah, M.L.M.; Zaidan, A.A.; Zaidan, B.B.; Hussain, M. A distributed framework for health information exchange using smartphone technologies. J. Biomed. Inform. 2017, 69, 230–250. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, W.S.; Song, J.Y.; Yoon, Y.K.; Kim, M.J.; Sohn, J.W. The role of smart monitoring digital health care system based on smartphone application and personal health record platform for patients diagnosed with coronavirus disease 2019. BMC Infect Dis. 2021, 21, 229. [Google Scholar] [CrossRef]
- Alsyouf, A.; Albugami, M.; Anshasi, R.J.; Lutfi, A.; Alhazmi, F.N.; Al-Mugheed, K.; Alsubahi, N.; Alharbi, N.I. The Use of a Technology Acceptance Model (TAM) to Predict Patients’ Usage of a Personal Health Record System: The Role of Security, Privacy, and Usability. Int. J. Environ. Res. Public Health 2023, 20, 1347. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, K.I.; Soh, J.Y.; Hyun, Y.H.; Jang, S.K.; Lee, S.; Hwang, G.Y.; Kim, H.S. Factors Influencing Acceptance of Personal Health Record Apps for Workplace Health Promotion: Cross-Sectional Questionnaire Study. JMIR mHealth uHealth 2020, 8, e16723. [Google Scholar] [CrossRef]
- Wubante, S.M.; Tegegne, M.D.; Melaku, M.S.; Mengiste, N.D.; Fentahun, A.; Zemene, W.; Fikadie, M.; Musie, B.; Keleb, D.; Bewoketu, H.; et al. Healthcare professionals’ knowledge, attitude and its associated factors toward electronic personal health record system in a resource-limited setting: A cross-sectional study. Front. Public Health 2023, 11, 1114456. [Google Scholar] [CrossRef]
- Cunningham, S.G.; Stoddart, A.; Wild, S.H.; Conway, N.J.; Gray, A.M.; Wake, D.J. Cost-Utility of an Online Education Platform and Diabetes Personal Health Record: Analysis Over Ten Years. J. Diabetes Sci. Technol. 2023, 17, 715–726. [Google Scholar] [CrossRef]




| Country | PHR System | Architecture Type | Challenges | Utilization Rate | Refs. |
|---|---|---|---|---|---|
| USA | Multiple (e.g., HealthVault, Dossia, PHR connected through patient portals) | Centralized (HealthVault), Open-source (Dossia-Indivo) | Reluctance from the medical profession, lack of interoperability between systems | Estimated adoption rate >75% in 2020 | [43] |
| UK | ePHR integrated with online general practice services (prescriptions, data access) | Centralized (tethered ePHR-connected to NHS systems) | Data protection concerns | 28% in 2019 | [41,44] |
| Sweden | Journalen–full access to EHR via patient portal | Centralized (integrated into the national EHR infrastructure) | Reluctance on the part of medical professionals (concerns about patient anxiety and loss of control) | Over 3.7 million users (37.9% of the population) by 2017; usage on the rise | [13] |
| Portugal | National web-based PHR | Centralized (opt-in, partially connected with EHR) | Lack of promotion and poor integration with EHR | 3 months after the official launch (May 2013), ~109,600 people, ~1% of the country’s population | [41] |
| Turkey | E-nabiz is a national PHR connected with all medical centres. | Centralized | Infrastructure development and integration of diverse systems | 82% of the population actively using e-Nabız in 2023 | [15] |
| Australia | My Health Record | Centralized | Privacy concerns citizens: engagement of providers and patients remains low despite high registration rates | 22.8 million records created (~90% of the population) by 2020; only ~10 million contain clinical data | [40] |
| Standard | Scope of Application | Objective |
|---|---|---|
| HL7 v2/v3 [109] | Standards for interoperability and data exchange | Exchange clinical data between information systems |
| FHIR [110] | Modern, RESTful API | |
| ISO/HL7 27931:2009 [111] | Data exchange | |
| DICOM [112] | Standard for medical imaging | |
| EN ISO 21090:2011 [113] | Standards for data modeling and semantics | Standardized data types for information exchange |
| CEN/TR 15212:2006 [114] | Standardized vocabulary procedures | |
| ISO/TR 20514:2005 [115] | EHR/PHR definition and context | |
| ISO 27799:2008 [116] | Privacy and security standards | Health information security management |
| ISO/IEC 27002 [117] | General guide to information security | |
| W3C P3P [118] | Privacy policies in web apps | |
| ISO/TS 14265:2011 [119] | Classification of purposes of processing personal health data | |
| ISO/IEC 25010 [120] | Standards for internationalization (i18n) and accessibility | Software quality, including internationalization |
| ISO 9241-151 [121] | Accessible and internationalized web interfaces | |
| ISO/IEEE 11073 [27] | Standards for medical devices and IoT integration | Communications between medical devices and IT systems |
| IEC/TC 62 [122] | Electrical equipment used in medical practice |
| Section | Recommendation |
|---|---|
| Design and functionalities | Improve the user interface Integrate predictive analytics features |
| Data quality | Standardize patient input to ensure consistency and validity (automatic validation and NLP for correction of patient-entered data) Consolidate data from multiple sources (devices, clinics, mobile apps) |
| Interoperability | Utilize standards (e.g., FHIR, HL7, and ISO 13606) SMART on FHIR for modular applications |
| Data protection | Implement advanced security measures (encryption, anonymization, access control) |
| Integration with AI and IoT | Use AI for analysis, but with rigorous validation Adapt PHR systems to integration with IoT devices for real-time monitoring |
| Institutional support | Actively involve service providers and insurance companies in promoting and supporting PHR |
| Validation | Assess the impact of PHR on clinical outcomes and healthcare costs Use multicenter randomized trials for clinical effectiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicoveanu, D.; Gherman, O.; Șoldănescu, I.; Lavric, A. Patient Health Record Smart Network Challenges and Trends for a Smarter World. Sensors 2025, 25, 3710. https://doi.org/10.3390/s25123710
Vicoveanu D, Gherman O, Șoldănescu I, Lavric A. Patient Health Record Smart Network Challenges and Trends for a Smarter World. Sensors. 2025; 25(12):3710. https://doi.org/10.3390/s25123710
Chicago/Turabian StyleVicoveanu, Dragoş, Ovidiu Gherman, Iuliana Șoldănescu, and Alexandru Lavric. 2025. "Patient Health Record Smart Network Challenges and Trends for a Smarter World" Sensors 25, no. 12: 3710. https://doi.org/10.3390/s25123710
APA StyleVicoveanu, D., Gherman, O., Șoldănescu, I., & Lavric, A. (2025). Patient Health Record Smart Network Challenges and Trends for a Smarter World. Sensors, 25(12), 3710. https://doi.org/10.3390/s25123710






