Exposure Time Dependence of Operators’ Head Entrance Air Kerma in Interventional Radiology Measured by TLD-100H Chips
Abstract
1. Introduction
2. Materials and Methods
2.1. TLD Analysis System
2.2. TLD Calibration Factor ()
2.3. Dosimeter Sensitivity Characterization
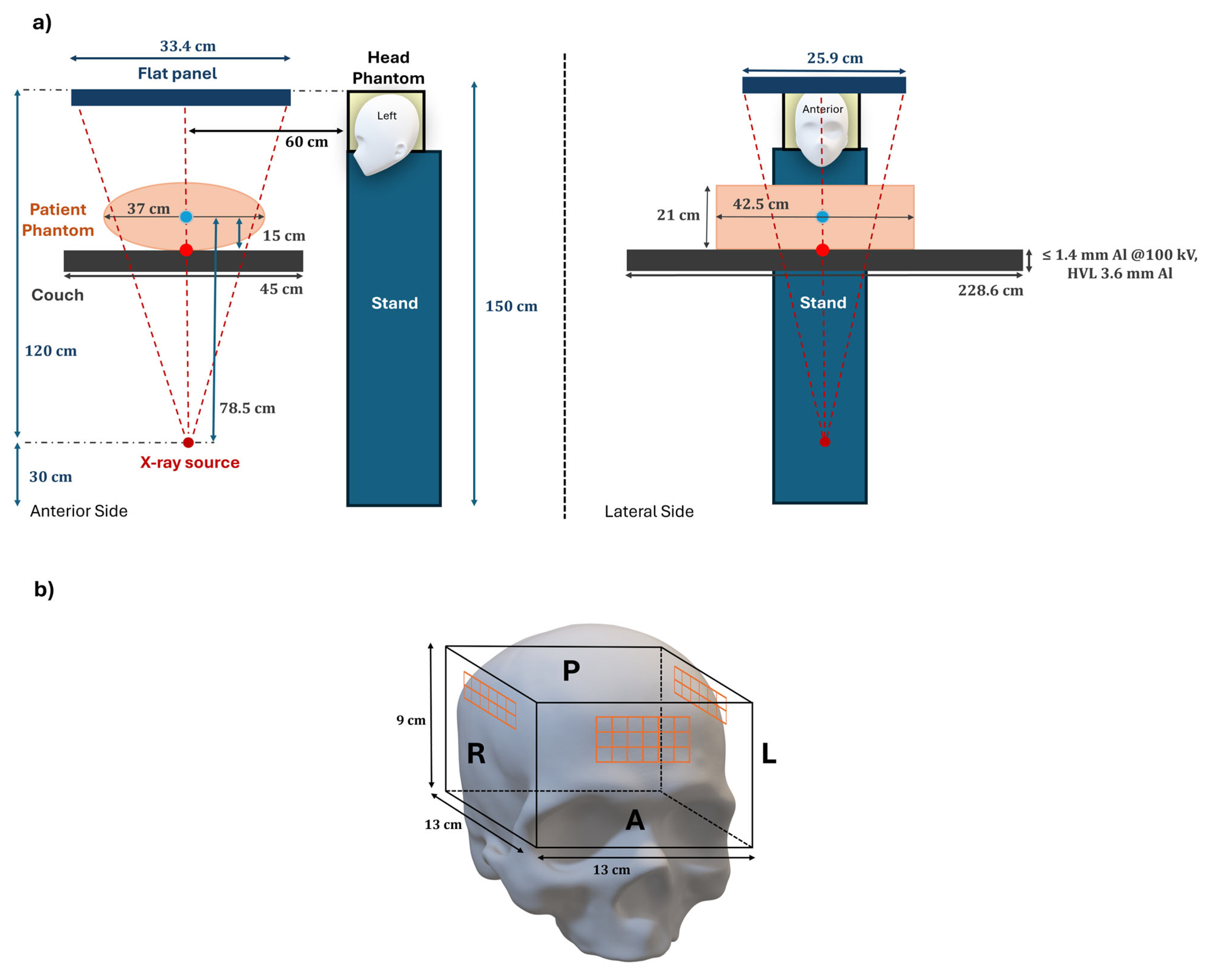
2.4. Setup and Irradiation
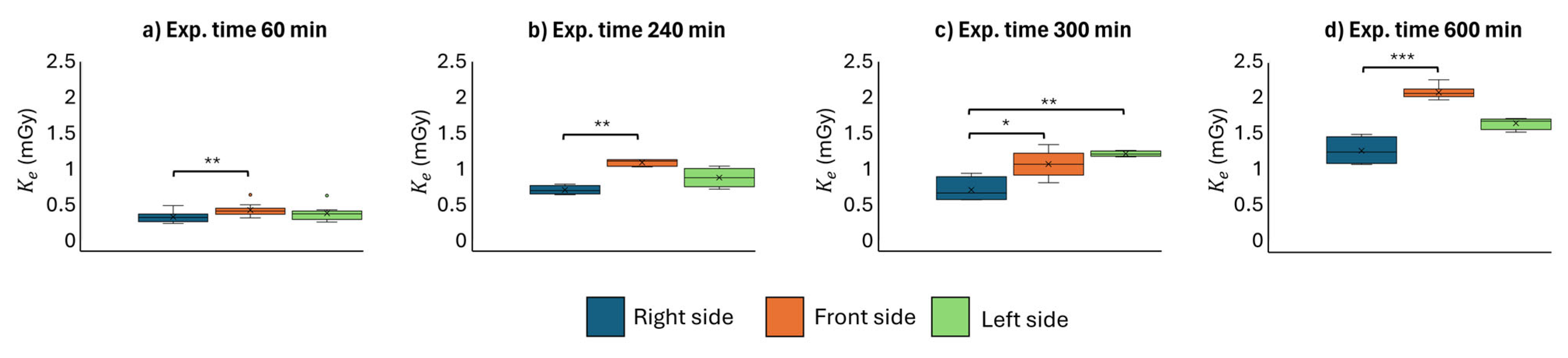
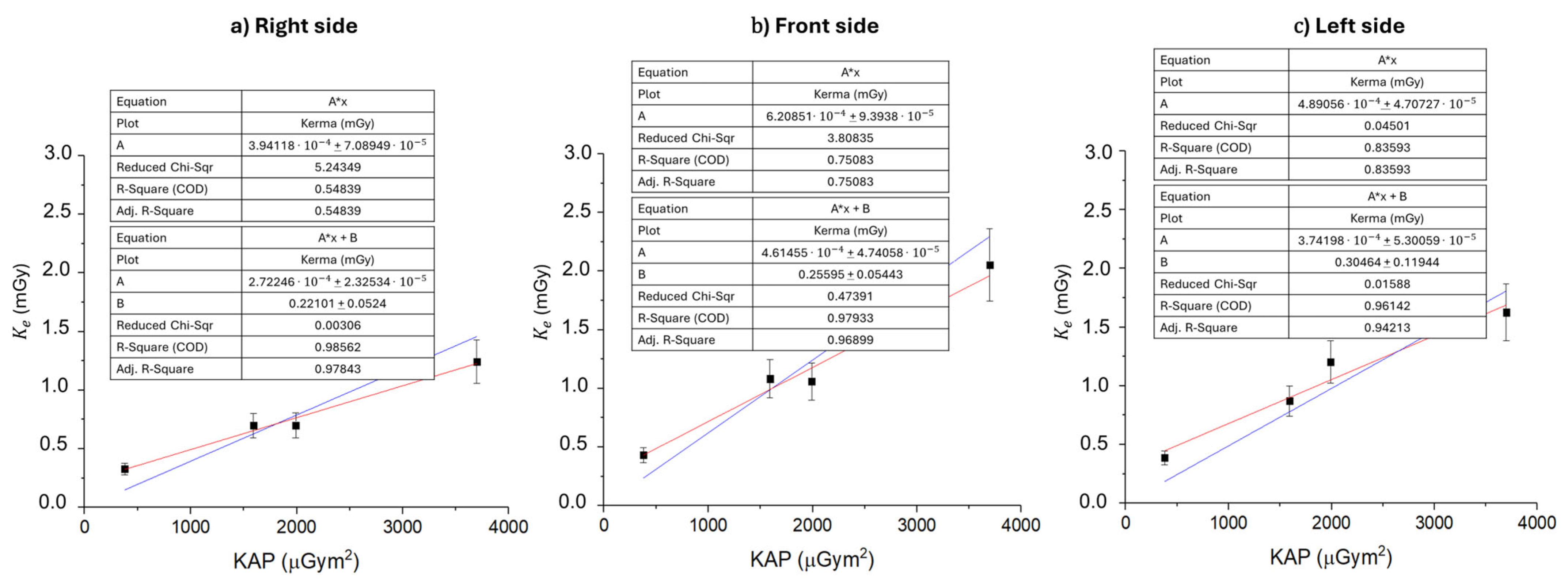
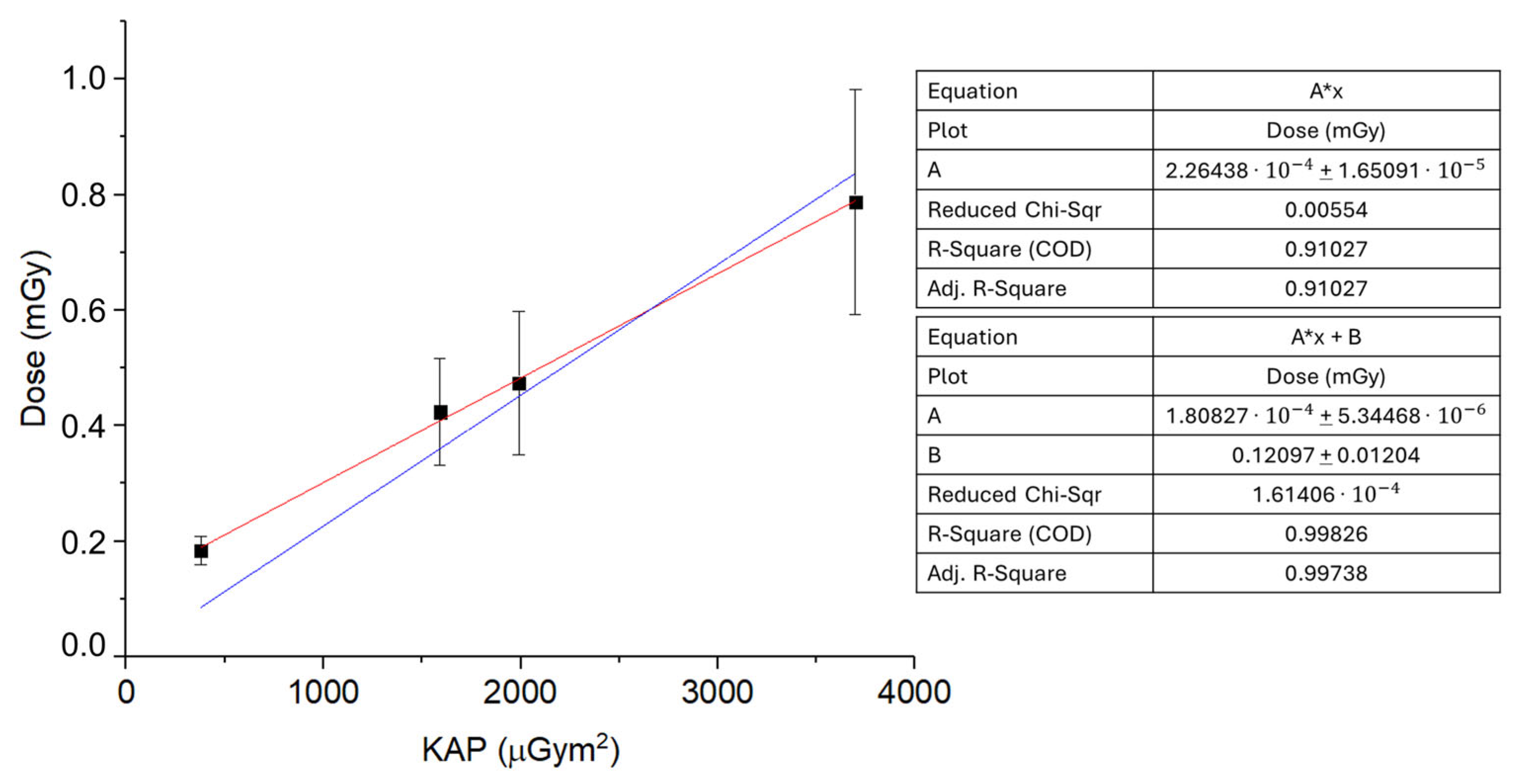
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doherty, M. Value of Interventional Radiology: Past, Present, and Future. Semin. Intervent. Radiol. 2019, 36, 026–028. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.P.; Soares, G.M. The Evolution of Interventional Radiology. Semin. Intervent. Radiol. 2005, 22, 6–9. [Google Scholar] [CrossRef]
- Rösch, J.; Keller, F.S.; Kaufman, J.A. The Birth, Early Years, and Future of Interventional Radiology. J. Vasc. Interv. Radiol. 2003, 14, 841–853. [Google Scholar] [CrossRef]
- Dotter, C.T.; Judkins, M.P. Transluminal Treatment of Arteriosclerotic Obstruction: Description of a New Technic and a Preliminary Report of Its Application. Circulation 1964, 30, 654–670. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, T.; Fotiadis, N.; Adam, A. Modern Trends in Interventional Radiology. Br. Med. Bull. 2007, 81–82, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.K.; Bender, C.E.; Classic, K.L.; Zink, F.E.; Quam, J.P.; Ward, E.M.; Oberg, A.L. Benefits and Safety of CT Fluoroscopy in Interventional Radiologic Procedures. Radiology 2001, 219, 515–520. [Google Scholar] [CrossRef]
- O’Brien, B.; Van Der Putten, W. Quantification of Risk-Benefit in Interventional Radiology. Radiat. Prot. Dosim. 2008, 129, 59–62. [Google Scholar] [CrossRef]
- Gerić, M.; Popić, J.; Gajski, G.; Garaj-Vrhovac, V. Cytogenetic Status of Interventional Radiology Unit Workers Occupationally Exposed to Low-Dose Ionising Radiation: A Pilot Study. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 843, 46–51. [Google Scholar] [CrossRef]
- Martin, C.J.; Magee, J.S. Assessment of Eye and Body Dose for Interventional Radiologists, Cardiologists, and Other Interventional Staff. J. Radiol. Prot. 2013, 33, 445–460. [Google Scholar] [CrossRef]
- Zakeri, F.; Hirobe, T.; Akbari Noghabi, K. Biological Effects of Low-Dose Ionizing Radiation Exposure on Interventional Cardiologists. Occup. Med. 2010, 60, 464–469. [Google Scholar] [CrossRef]
- Reeves, R.R.; Ang, L.; Bahadorani, J.; Naghi, J.; Dominguez, A.; Palakodeti, V.; Tsimikas, S.; Patel, M.P.; Mahmud, E. Invasive Cardiologists Are Exposed to Greater Left Sided Cranial Radiation. JACC Cardiovasc. Interv. 2015, 8, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Bohari, A.; Hashim, S.; Mohd Mustafa, S.N. Scatter Radiation in the Fluoroscopy-Guided Interventional Room. Radiat. Prot. Dosim. 2020, 188, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Roguin, A.; Nolan, J. Radiation Protection in the Cardiac Catheterisation Lab: Best Practice. Heart 2021, 107, 76–82. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.A.; Chopra, R.; Decker, J.M.; McNamara, M.W.; VanOosterhout, S.M.; Berkompas, D.C.; Dahu, M.I.; Kenaan, M.A.; Jawad, W.I.; Merhi, W.M.; et al. Comparison of Radiation Exposure Among Interventional Echocardiographers, Interventional Cardiologists, and Sonographers During Percutaneous Structural Heart Interventions. JAMA Netw. Open 2022, 5, e2220597. [Google Scholar] [CrossRef]
- Braganza, M.Z.; Kitahara, C.M.; Berrington De Gonzalez, A.; Inskip, P.D.; Johnson, K.J.; Rajaraman, P. Ionizing Radiation and the Risk of Brain and Central Nervous System Tumors: A Systematic Review. Neuro-Oncol. 2012, 14, 1316–1324. [Google Scholar] [CrossRef]
- Hattori, S.; Monzen, H.; Tamura, M.; Kosaka, H.; Nakamura, Y.; Nishimura, Y. Estimating Radiation Exposure of the Brain of a Physician with a Protective Flap in Interventional Radiology: A Phantom Study. J. Appl. Clin. Med. Phys. 2022, 23, e13532. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Linet, M.S.; Balter, S.; Miller, D.L.; Rajaraman, P.; Cahoon, E.K.; Velazquez-Kronen, R.; Simon, S.L.; Little, M.P.; Doody, M.M.; et al. Occupational Radiation Exposure and Deaths From Malignant Intracranial Neoplasms of the Brain and CNS in U.S. Radiologic Technologists, 1983–2012. Am. J. Roentgenol. 2017, 208, 1278–1284. [Google Scholar] [CrossRef]
- Picano, E.; Vano, E.; Domenici, L.; Bottai, M.; Thierry-Chef, I. Cancer and Non-Cancer Brain and Eye Effects of Chronic Low-Dose Ionizing Radiation Exposure. BMC Cancer 2012, 12, 157. [Google Scholar] [CrossRef]
- Wenzl, T.B. Increased Brain Cancer Risk in Physicians with High Radiation Exposure. Radiology 2005, 235, 709–711. [Google Scholar] [CrossRef]
- Pugliese, M.; Amatiello, A.; Correra, M.; Stoia, V.; Cerciello, V.; Roca, V.; Loffredo, F.; Fiore, F.; La Verde, G. Evaluation of the Current Status of the Eye Lens Radiation Exposure in an Interventional Radiology Department. La Med. del Lav. 2018, 109, 471–477. [Google Scholar] [CrossRef]
- Liverani, A.; Loffredo, F.; Fiore, F.; Correra, M.; La Verde, G.; Pugliese, M. Evaluation of the Dose Dependence on the Eye Lens from the Position of the Dosimeter for the Operators Exposed in Interventional Radiology. Il Nuovo Cimento C 2018, 41, 213–217. [Google Scholar] [CrossRef]
- ICRP. ICRP Statement on Tissue Reactions/Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context. ICRP Publication 118. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Radiation Volume 100D, a Review of Human Carcinogens. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012. [Google Scholar]
- Council Directive 2013/59/Euratom of 5 December 2013 Laying down Basic Safety Standards for Protection Against the Dangers Arising from Exposure to Ionising Radiation, and Repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom; Official Journal of the European Union. 2014, pp. 1–73. Available online: https://eur-lex.europa.eu/eli/dir/2013/59/oj (accessed on 2 June 2025).
- Decreto Legislativo 31 Luglio 2020, n. 101 Attuazione Della Direttiva 2013/59/Euratom, Che Stabilisce Norme Fondamentali Di Sicurezza Relative Alla Protezione Contro i Pericoli Derivanti Dall’Esposizione Alle Radiazioni Ionizzanti, e Che Abroga le Direttive 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom e 2003/122/Euratom e Riordino Della Normativa Di Settore in Attuazione Dell’Articolo 20, Comma 1, Lettera a), Della Legge 4 Ottobre 2019, n. 117; Gazzetta Ufficiale della Repubblica Italiana, Serie Generale n.201 del 12-08-2020—Suppl. Ordinario n. 29. 2020. Available online: https://www.gazzettaufficiale.it/eli/id/2020/08/12/20G00121/sg (accessed on 2 June 2025).
- Disposizioni Integrative e Correttive al Decreto Legislativo 31 Luglio 2020, n. 101, di Attuazione della Direttiva 2013/59/Euratom, che Stabilisce Norme Fondamentali di Sicurezza Relative alla Protezione Contro i Pericoli Derivanti dall’Esposizione alle Radiazioni Ionizzanti, e che Abroga le Direttive 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom e 2003/122/Euratom e Riordino della Normativa di Settore in Attuazione dell’Articolo 20, Comma 1, Lettera a), della Legge 4 Ottobre 2019, n. 117; Gazzetta Ufficiale della Repubblica Italiana, Serie Generale n.2 del 03-01-2023. Available online: https://www.gazzettaufficiale.it/eli/id/2023/01/03/22G00207/sg (accessed on 2 June 2025).
- International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Moriña, D.; Grellier, J.; Carnicer, A.; Pernot, E.; Ryckx, N.; Cardis, E. InterCardioRisk: A Novel Online Tool for Estimating Doses of Ionising Radiation to Occupationally-Exposed Medical Staff and Their Associated Health Risks. J. Radiol. Prot. 2016, 36, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Kunert, P.; Matyja, E.; Prokopienko, M.; Marchel, A. Radiation-Induced Tumours of Meninges. Report on Eight Cases and Review of the Literature. Neurol. Neurochir. Pol. 2012, 46, 542–552. [Google Scholar] [CrossRef][Green Version]
- Roguin, A.; Goldstein, J.; Bar, O.; Goldstein, J.A. Brain and Neck Tumors Among Physicians Performing Interventional Procedures. Am. J. Cardiol. 2013, 111, 1368–1372. [Google Scholar] [CrossRef]
- Ferrari, P.; Jovanovic, Z.; Bakhanova, E.; Becker, F.; Krstic, D.; Jansen, J.; Principi, S.; Teles, P.; Clairand, I.; Knezevic, Ž. Absorbed Dose in the Operator’s Brain in Interventional Radiology Practices: Evaluation through KAP Value Conversion Factors. Phys. Medica 2020, 76, 177–181. [Google Scholar] [CrossRef]
- Roguin, A.; Goldstein, J.; Bar, O. Brain Tumours among Interventional Cardiologists: A Cause for Alarm? Report of Four New Cases from Two Cities and a Review of the Literature. EuroIntervention 2012, 7, 1081–1086. [Google Scholar] [CrossRef]
- D’Avino, V.; Scarica, M.; Ametrano, G.; La Verde, G.; Manti, L.; Muto, P.; Pugliese, M.; Arrichiello, C. Preliminary Investigation of Performance of Thermoluminescent Dosimeters for Dose Verification in Brachytherapy. Il Nuovo Cimento C 2020, 43, 149. [Google Scholar] [CrossRef]
- Liuzzi, R.; Piccolo, C.; D’Avino, V.; Clemente, S.; Oliviero, C.; Cella, L.; Pugliese, M. Dose–Response of TLD-100 in the Dose Range Useful for Hypofractionated Radiotherapy. Dose-Response 2020, 18, 155932581989408. [Google Scholar] [CrossRef]
- Manna, F.; De Nardellis, G.; Carmosino, P.A.; Ambrosino, F.; Caruso, U.; Correra, M.; Fiore, F.; La Verde, G.; Tarotto, L.; Pugliese, M. Hp(3) vs TLD-100 for Eye Lens Dosimetry in Interventional Radiology Procedures: A Preliminary Study. Eur. Phys. J. Plus 2023, 138, 811. [Google Scholar] [CrossRef]
- Davis, S.D.; Ross, C.K.; Mobit, P.N.; Van der Zwan, L.; Chase, W.J.; Shortt, K.R. The Response of LiF Thermoluminescence Dosemeters to Photon Beams in the Energy Range from 30 kV X Rays to 60Co Gamma Rays. Radiat. Prot. Dosim. 2003, 106, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Guni, E.; Hellmann, I.; Wucherer, M.; Knappe-Kagan, P.; Hartmann, J.; Lell, M.; Adamus, R. Effectiveness of Radiation Protection Caps for Lowering Dose to the Brain and the Eye Lenses. Cardiovasc. Intervent. Radiol. 2021, 44, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Indicazioni Operative per gli Utenti del Servizio di Irraggiamento dell’Istituto Superiore di Sanità con Radiazioni Gamma da Sorgenti di Cs-137; Rapporti ISTISAN 22/11; Istituto Superiore di Sanità, Rome, Italy. 2022. Available online: https://www.iss.it/documents/20126/6682486/22-15+web.pdf/a4ce8be2-ecef-4843-4a52-21ca99a91728?t=1656923451825 (accessed on 2 June 2025).
- Nunn, A.A.; Davis, S.D.; Micka, J.A.; DeWerd, L.A. LiF:Mg,Ti TLD Response as a Function of Photon Energy for Moderately Filtered X-ray Spectra in the Range of 20–250 kVp Relative to Co-60. Med. Phys. 2008, 35, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.M.; Boone, J.M. Tungsten Anode Spectral Model Using Interpolating cubic Splines: Unfiltered X-ray Spectra from 20 kV to 640 kV. Med. Phys. 2014, 41, 042101. [Google Scholar] [CrossRef]
- Plato, P.; Miklos, J. Production of Element Correction Factors for Thermoluminescent Dosimeters. Health Phys. 1985, 49, 873–881. [Google Scholar] [CrossRef]
- Ohno, S.; Shindo, R.; Konta, S.; Yamamoto, K.; Inaba, Y.; Chida, K. Radiation Exposure to the Brains of Interventional Radiology Staff: A Phantom Study. Bioengineering 2024, 11, 1083. [Google Scholar] [CrossRef]
- Hulthén, M.; Tsapaki, V.; Karambatsakidou, A. Estimating Brain and Eye Lens Dose for the Cardiologist in Interventional Cardiology—Are the Dose Levels of Concern? Br. J. Radiol. 2024, 97, 1191–1201. [Google Scholar] [CrossRef]


| Tube Voltage (kV) | Tube Current (mA) | Inherent Filtration (mm Al) | Added Filtration (mm Cu) | Focal Spot (mm2) |
|---|---|---|---|---|
| 68 | 21 | 2.5 | 0.6 | 1.0 × 1.0 |
| (mGy) | ||
|---|---|---|
| Right side 24.16 | Front side 31.43 | Left side 28.09 |
| (mGy) | ||
| Right side 9.32 | Front side 15.41 | Left side 12.19 |
| (mGy) [y = A*x + B] | ||
| Right side 7.72 | Front side 13.59 | Left side 9.66 |
| (mGy) [y = A*x] | ||
| Right side 10.08 | Front side 16.16 | Left side 13.82 |
| 4.2% | 1.9% | 1.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottareale, R.; Manna, F.; Carmosino, P.A.; Fiore, F.; Correra, M.; Stilo, S.; Tarotto, L.; Pugliese, M. Exposure Time Dependence of Operators’ Head Entrance Air Kerma in Interventional Radiology Measured by TLD-100H Chips. Sensors 2025, 25, 3666. https://doi.org/10.3390/s25123666
Mottareale R, Manna F, Carmosino PA, Fiore F, Correra M, Stilo S, Tarotto L, Pugliese M. Exposure Time Dependence of Operators’ Head Entrance Air Kerma in Interventional Radiology Measured by TLD-100H Chips. Sensors. 2025; 25(12):3666. https://doi.org/10.3390/s25123666
Chicago/Turabian StyleMottareale, Rocco, Francesco Manna, Patrizio Antonio Carmosino, Francesco Fiore, Marco Correra, Salvatore Stilo, Luca Tarotto, and Mariagabriella Pugliese. 2025. "Exposure Time Dependence of Operators’ Head Entrance Air Kerma in Interventional Radiology Measured by TLD-100H Chips" Sensors 25, no. 12: 3666. https://doi.org/10.3390/s25123666
APA StyleMottareale, R., Manna, F., Carmosino, P. A., Fiore, F., Correra, M., Stilo, S., Tarotto, L., & Pugliese, M. (2025). Exposure Time Dependence of Operators’ Head Entrance Air Kerma in Interventional Radiology Measured by TLD-100H Chips. Sensors, 25(12), 3666. https://doi.org/10.3390/s25123666






