Mesoporous Bi2S3/Bi2O3 Heterostructure-Based Sensors for Sub-ppm NO2 Detection at Room Temperature
Abstract
1. Introduction
2. Experimental Section
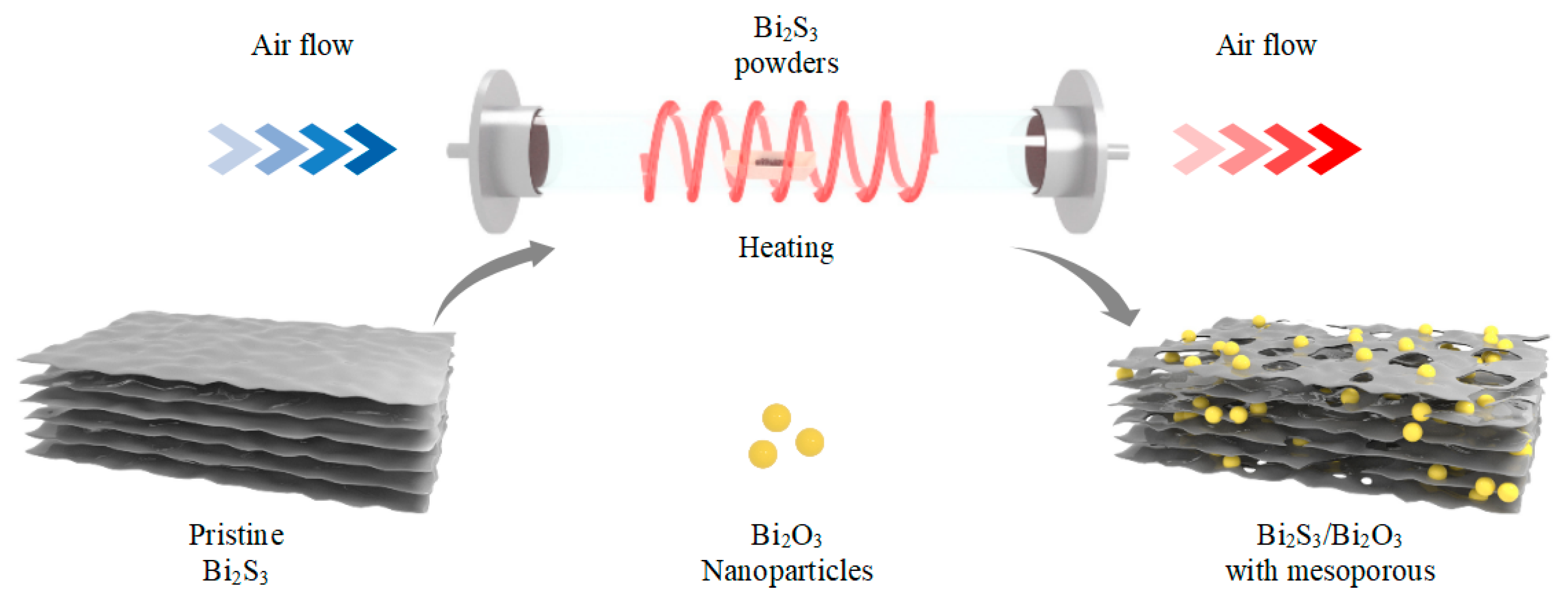
2.1. Fabrication of Mesoporous Bi2S3/Bi2O3 Hybrid Materials
2.2. Material Characterizations
2.3. Sensor Preparation and Measurements
2.4. DFT Computation
3. Results and Discussion
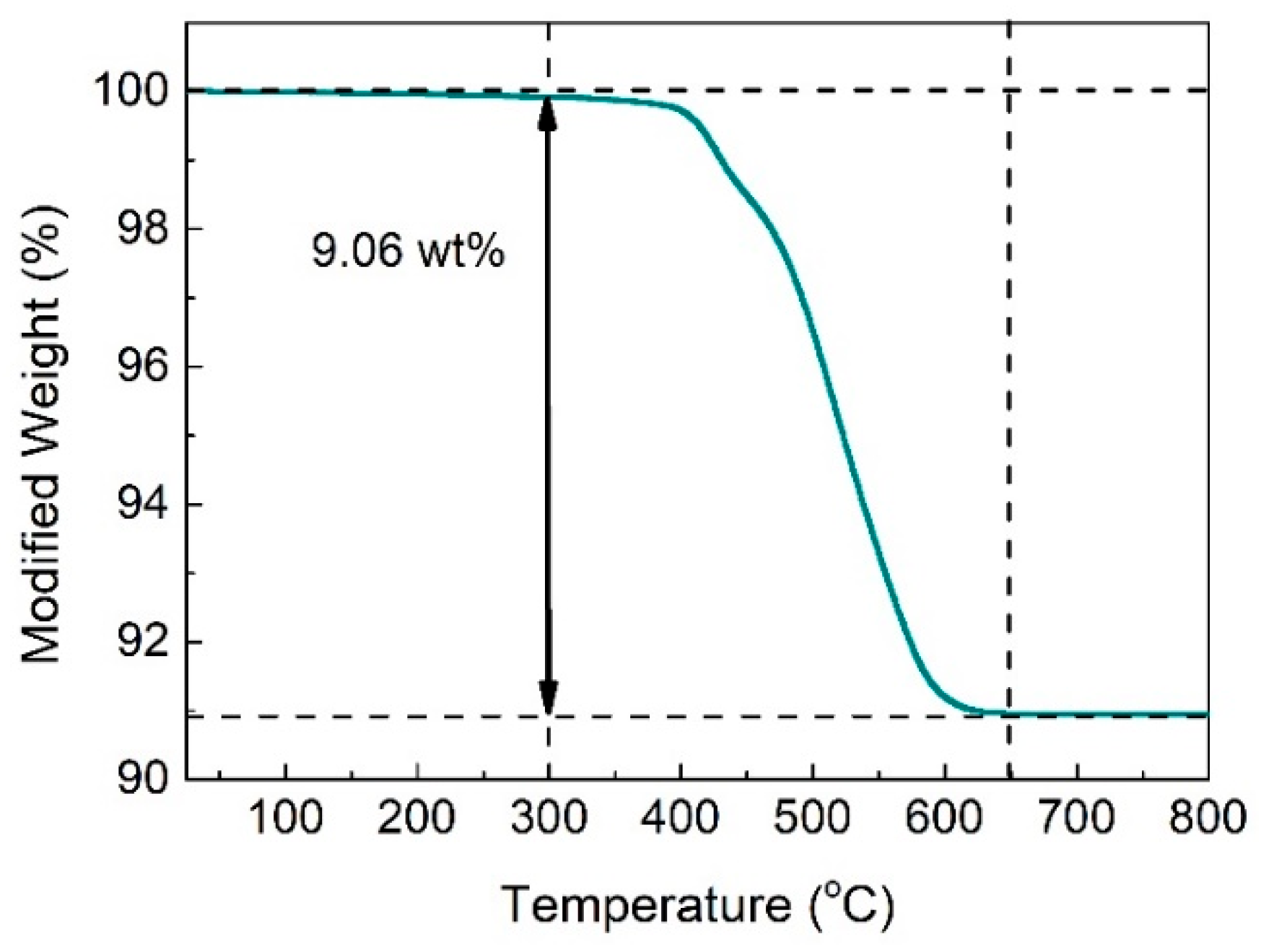
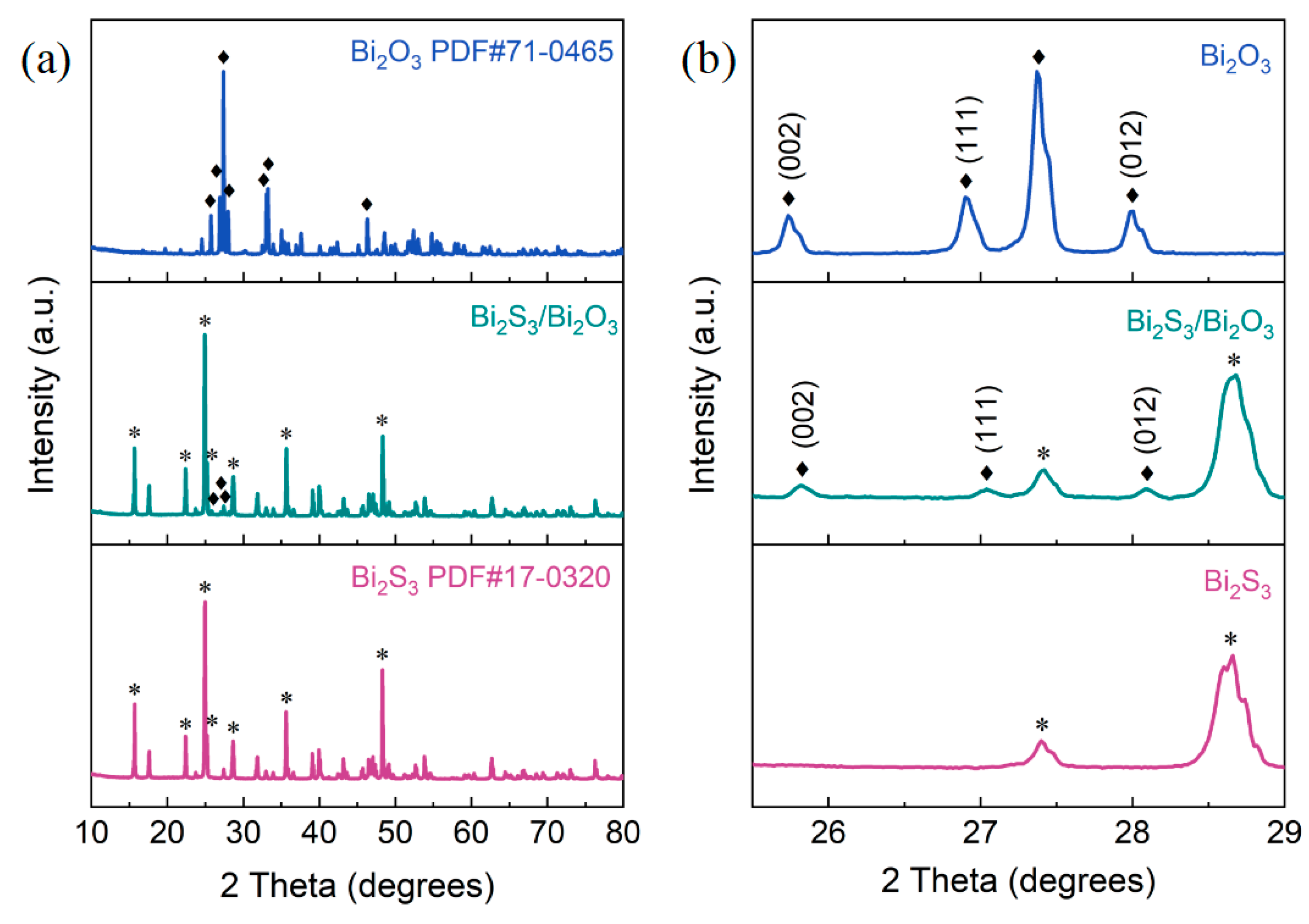
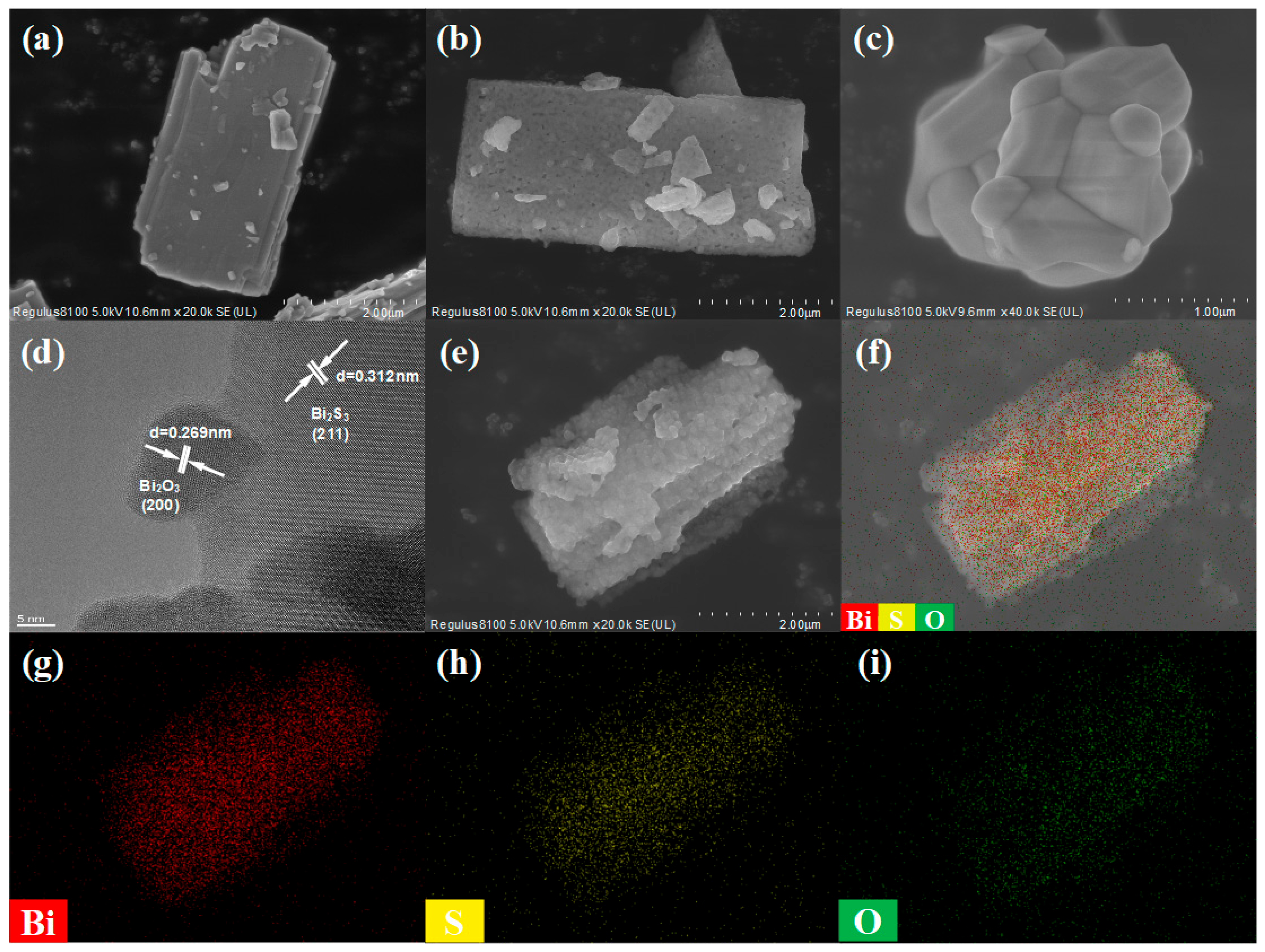
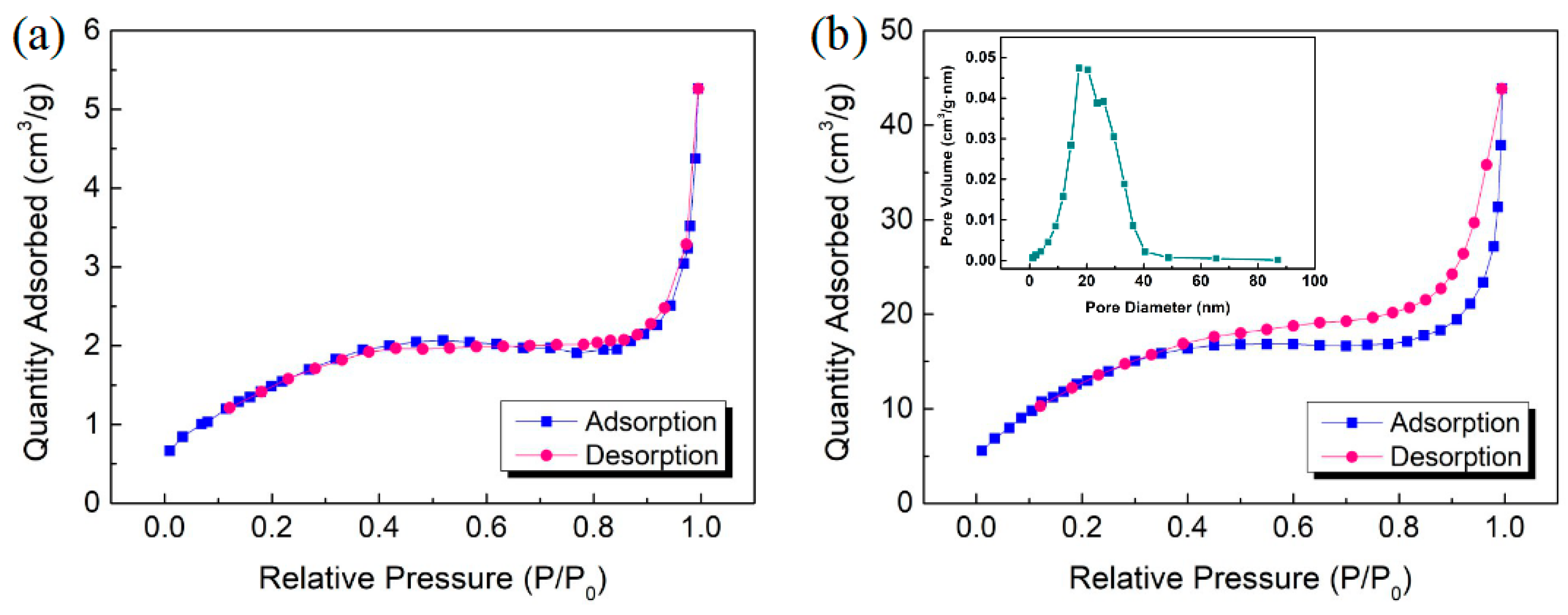
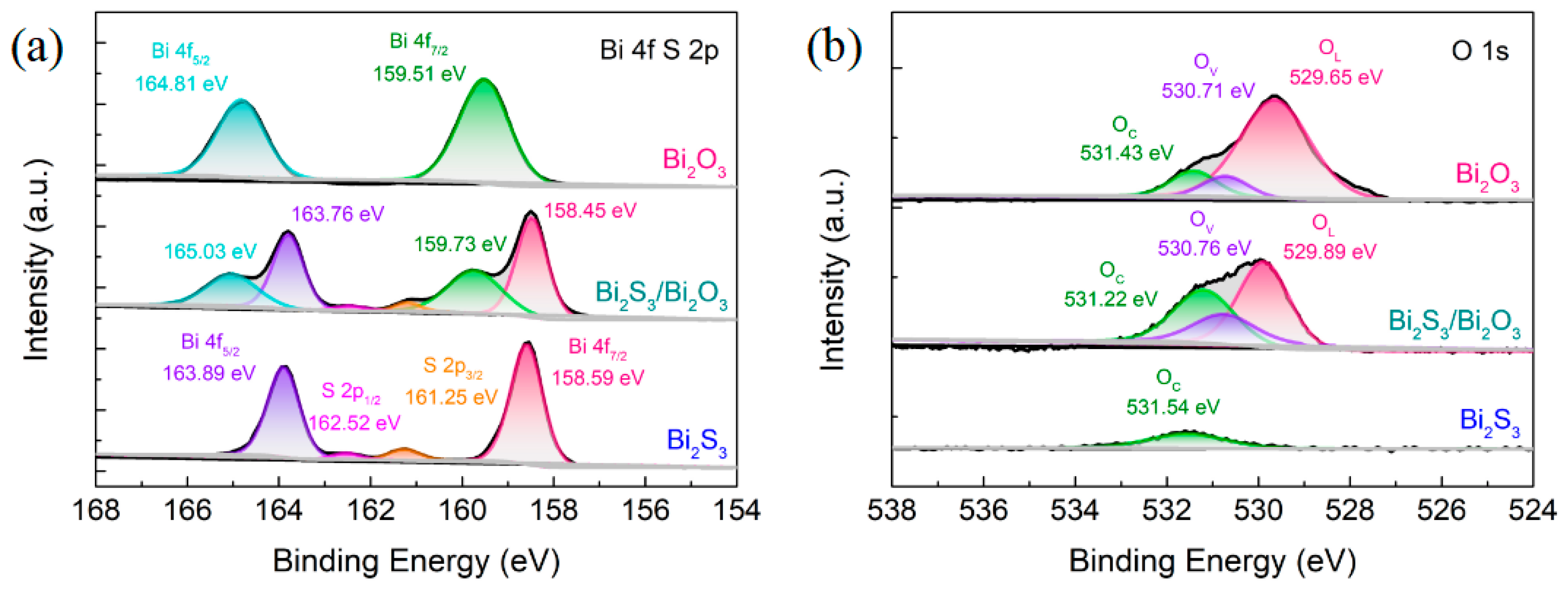
3.1. Microstructure of Bi2S3/Bi2O3 Hybrid Materials
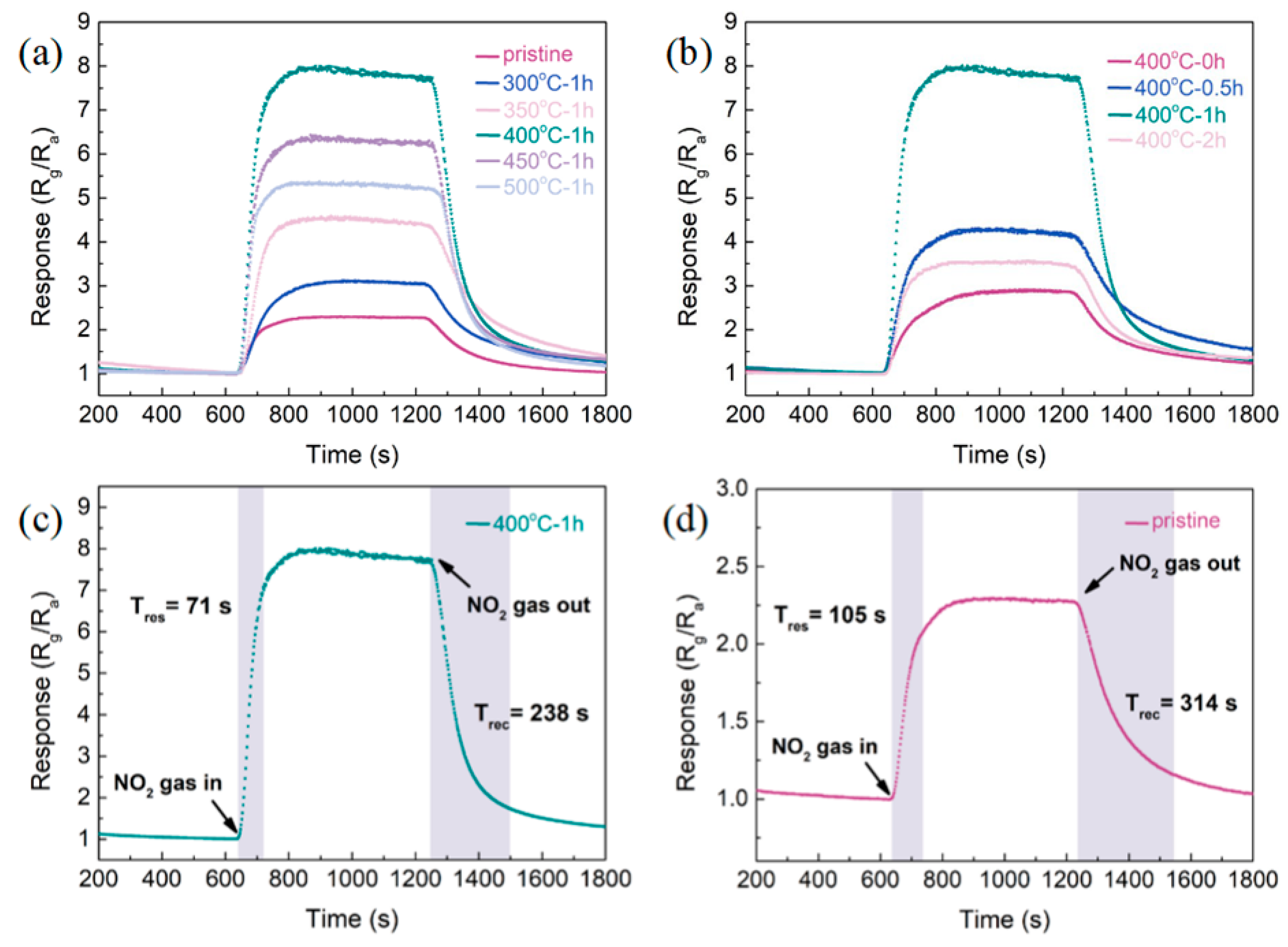
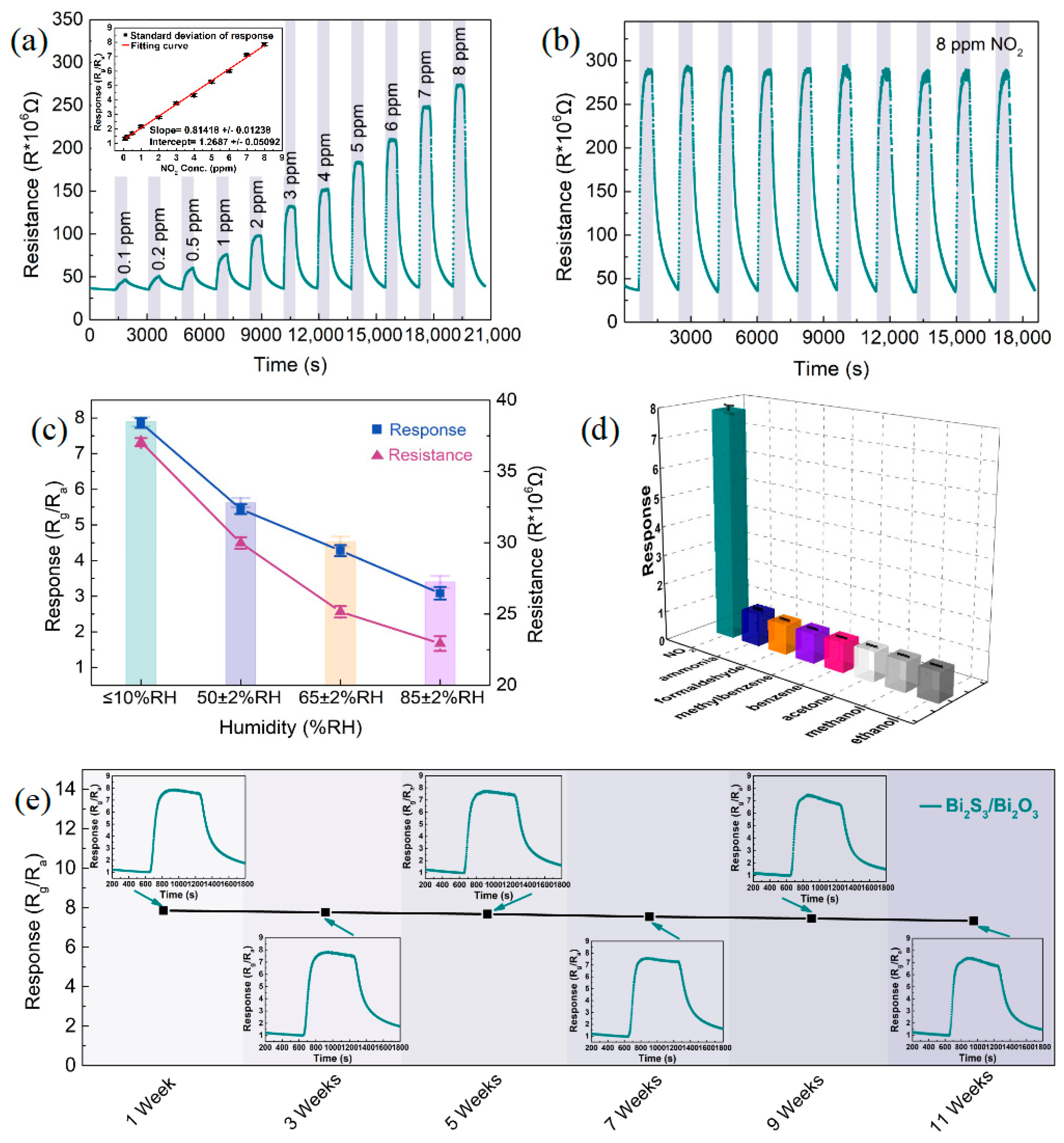
3.2. Gas-Sensitive Properties
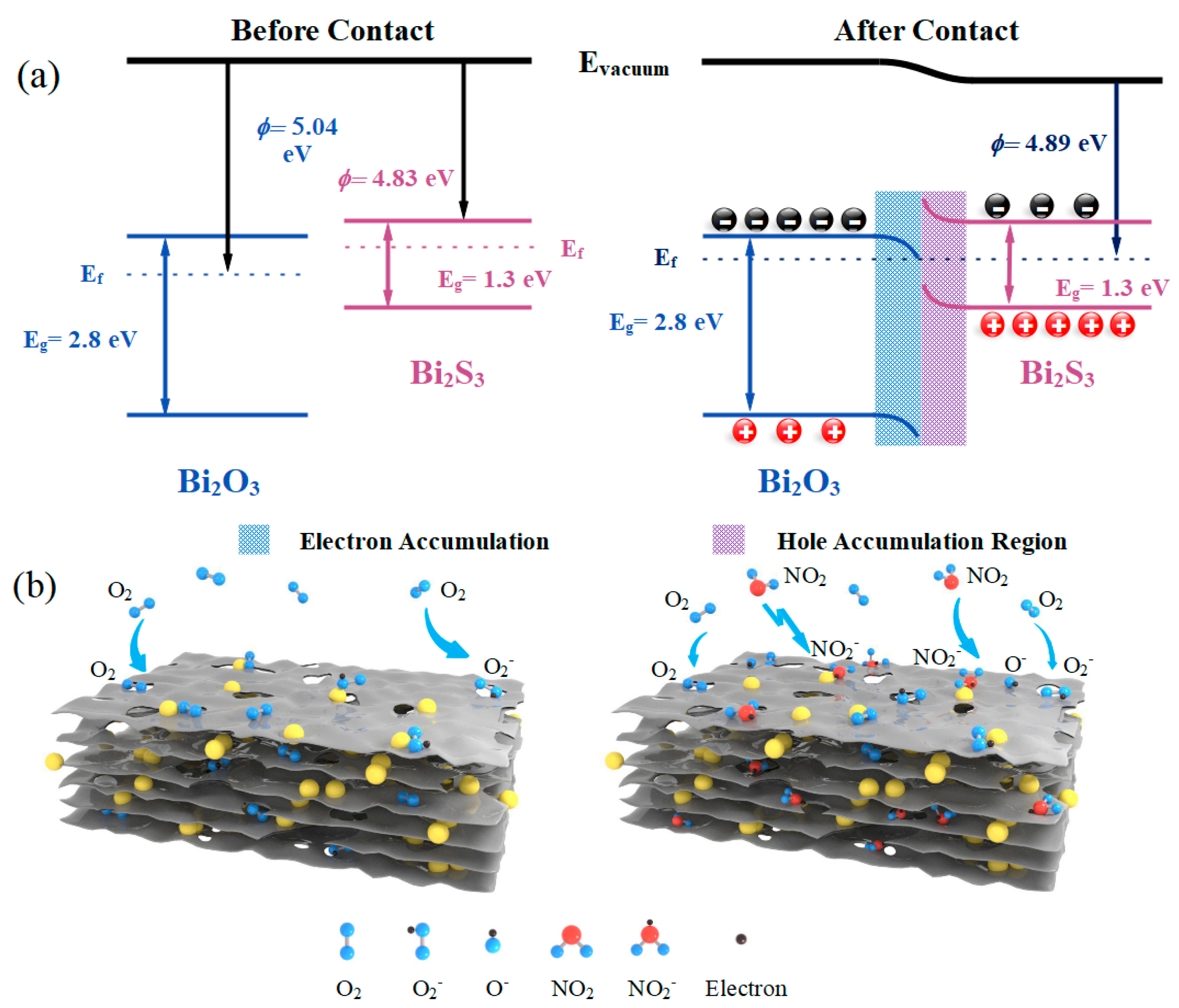
3.3. Sensitization Mechanisms
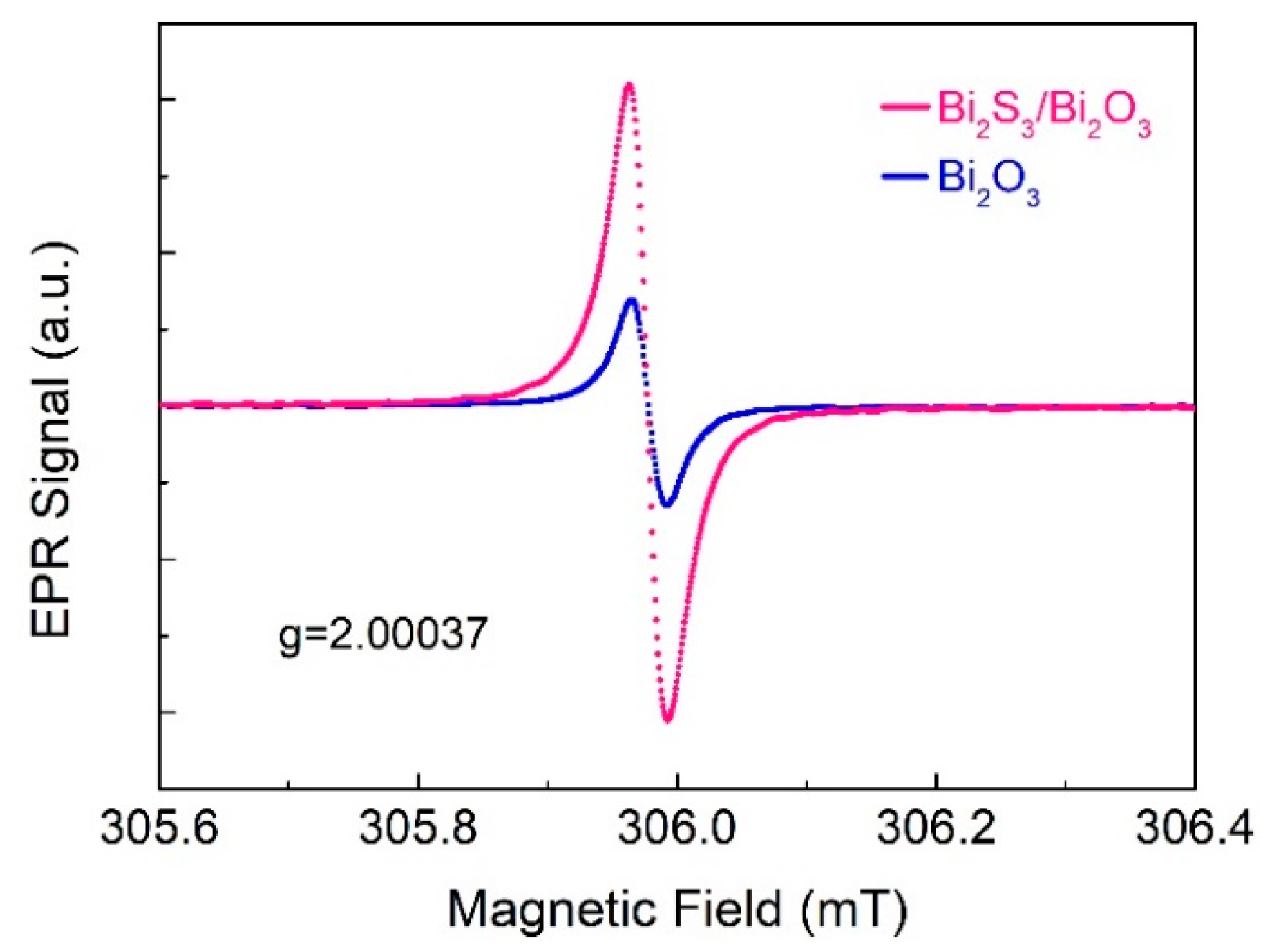
3.3.1. Roles of Bi2S3/Bi2O3 Heterostructure
3.3.2. Roles of Morphological Structure
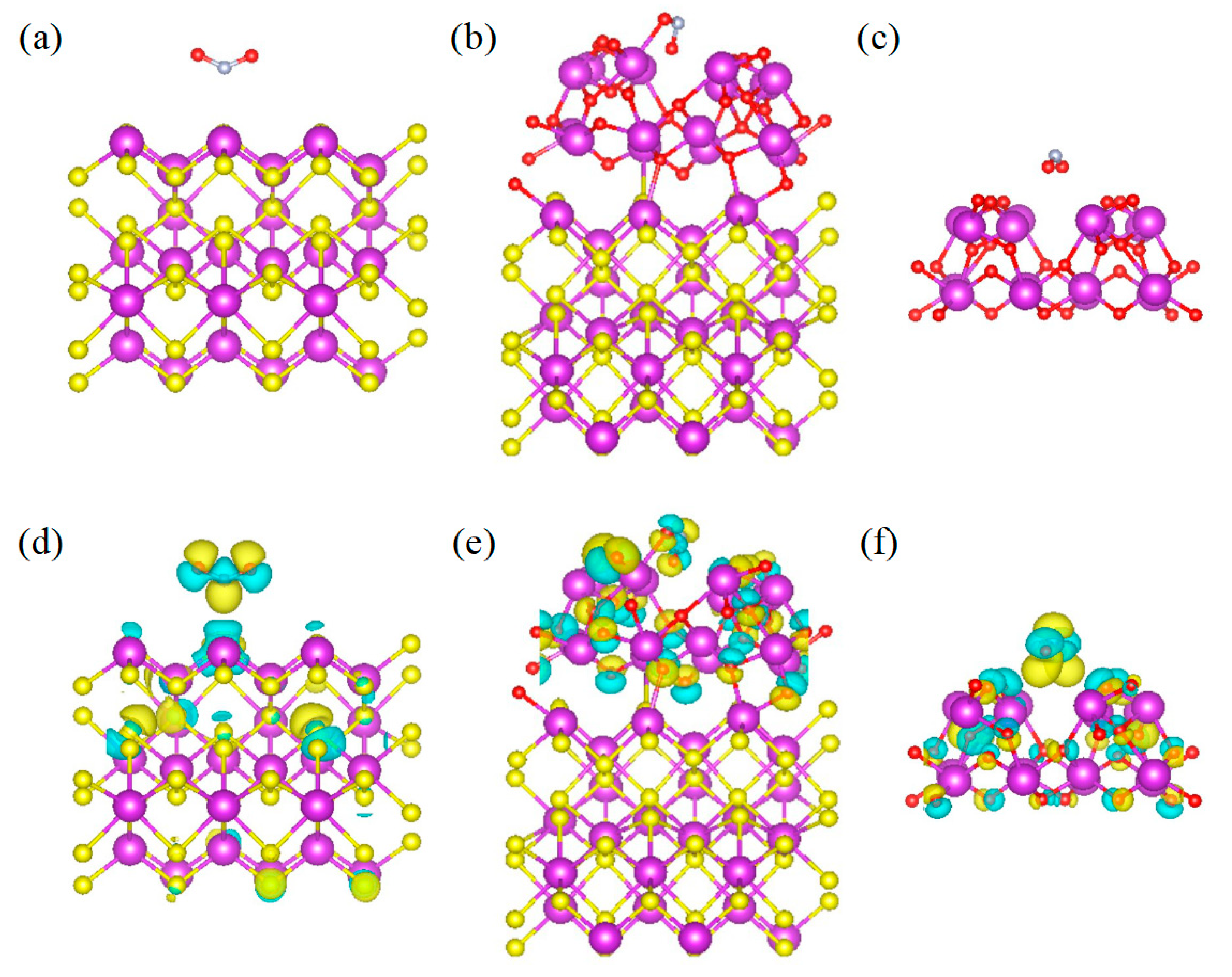
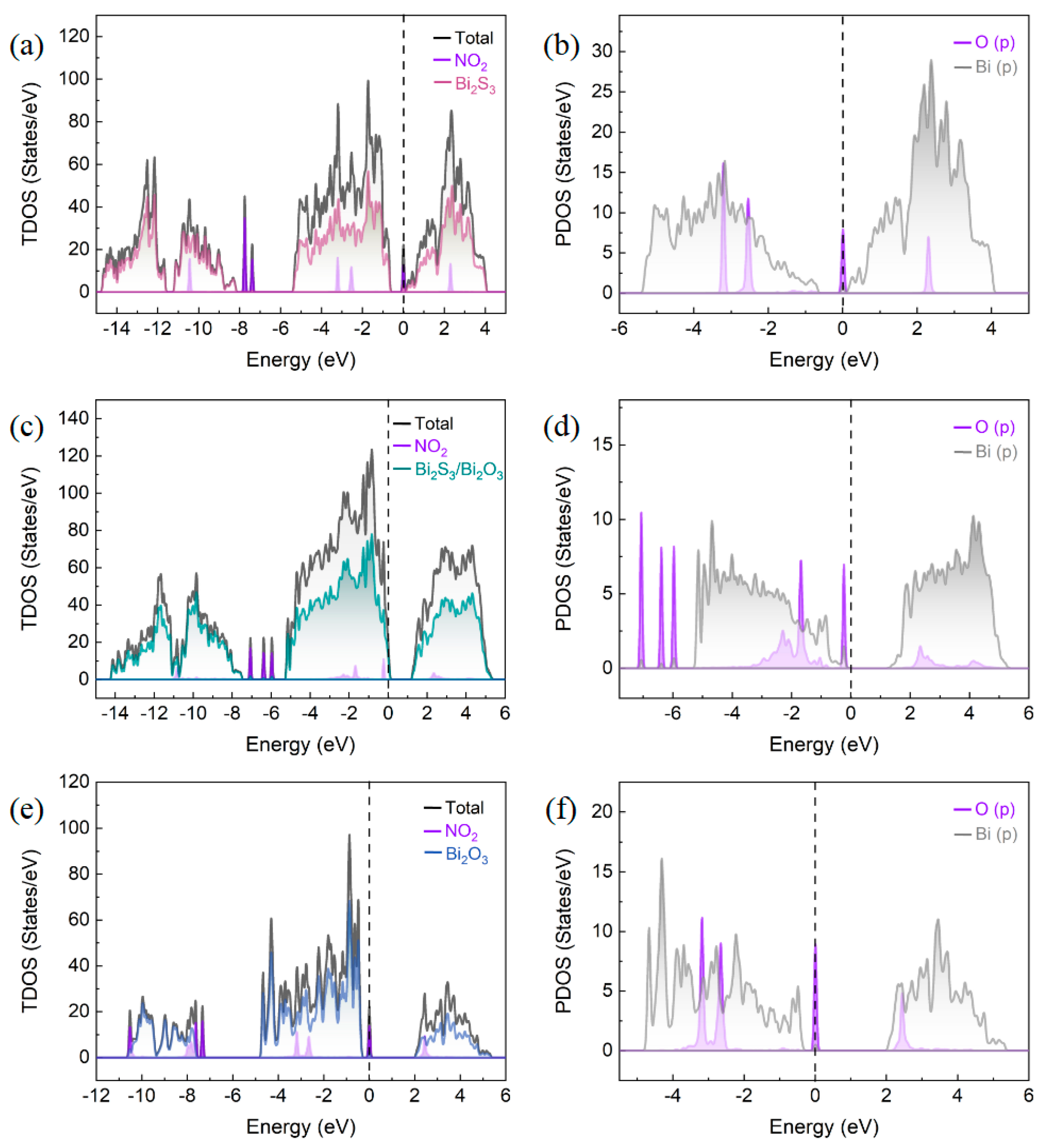
3.3.3. DFT Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Xu, Z.; Zhang, Z.; Liu, J.; Yan, X.; Zhu, Y.; Attfield, J.P.; Yang, M. Titanium Nitride Sensor for Selective NO2 Detection. Nat. Commun. 2025, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Y.; Yuan, Z.; Liu, B.; Zhao, Q.; Huang, Q.; Li, Z.; Zeng, W.; Duan, Z.; Tai, H. Synergistic Effect of Electron Scattering and Space Charge Transfer Enabled Unprecedented Room Temperature NO2 Sensing Response of SnO2. Small 2023, 19, 535–546. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Tang, T.; Cheng, Y.; Cheng, L.; Wang, X.; Haidry, A.A.; Jannat, A.; Ou, J.Z. Room-Temperature Optoelectronic NO2 Sensing Using Two-Dimensional Gallium Oxyselenides. ACS Appl. Nano Mater. 2024, 7, 3229–3238. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Sun, Y.; Wang, N.; Huang, B.; Li, X. Fabrication of TeNT/TeO2 Heterojunction Based Sensor for Ultrasensitive Detection of NO2. J. Hazard. Mater. 2025, 487, 137229. [Google Scholar] [CrossRef]
- Wang, B.; Gao, X.; He, J.; Xiao, Y.; Liu, Y.; Jia, X.; Zhang, K.; Wang, C.; Sun, P.; Liu, F.; et al. Room-Temperature Ppb-Level NO2 Sensor Based on Three-Dimensional Mo2CTx Nano-Crumpled Spheres. Sens. Actuators B Chem. 2024, 399, 134790. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Z.; Jiang, X.; Wang, J.; Yi, W.; Li, X.; Chu, J. Enhancement of NO2 Gas Sensing Properties of Polypyrrole by Polarization Doping with DBS: Experimental and DFT Studies. ACS Appl. Mater. Interfaces 2023, 15, 52961–52970. [Google Scholar] [CrossRef]
- Hu, Y.; Li, T.; Zhang, J.; Guo, J.; Wang, W.; Zhang, D. High-Sensitive NO2 Sensor Based on p-NiCo2O4/n-WO3 Heterojunctions. Sens. Actuators B Chem. 2022, 352, 130912. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, X.; Kuang, D.; Hu, X.; Zhou, Y.; He, Y.; Zang, Z. Hollow Cu2O Nanospheres Loaded with MoS2/Reduced Graphene Oxide Nanosheets for Ppb-Level NO2 Detection at Room Temperature. J. Hazard. Mater. 2021, 416, 126218. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Ge, Y.; Sun, L.; Zhou, C.; Sun, Y.; Hu, J. SnS2/Mo4/3B2 MBene Microcomposites for Highly Sensitive NO2 Sensor at Room Temperature. Sens. Actuators B Chem. 2024, 420, 136496. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, W.; Li, Y. Metal Oxide Gas Sensors for Detecting NO2 in Industrial Exhaust Gas: Recent Developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Zhou, M.; Zhang, S.; Cao, S.; Lei, G.; Lou, C.; Zhang, J. Plasma-Induced Oxygen Vacancies Enabled Ultrathin ZnO Films for Highly Sensitive Detection of Triethylamine. J. Hazard. Mater. 2021, 415, 125757. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Jo, Y.M.; Yoon, J.W.; Kim, J.S.; Lee, D.J.; Moon, Y.K.; Yoon, J.W.; Kim, J.H.; Choi, H.J.; Lee, J.H. A Transparent Nanopatterned Chemiresistor: Visible-Light Plasmonic Sensor for Trace-Level NO2 Detection at Room Temperature. Small 2021, 17, e2100438. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent Advances in Energy-Saving Chemiresistive Gas Sensors: A Review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Gupta, G. Recent Progress of Flexible NO2 and NH3 Gas Sensors Based on Transition Metal Dichalcogenides for Room Temperature Sensing. Mater. Today Chem. 2022, 23, 100726. [Google Scholar] [CrossRef]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent Advances in Two-Dimensional-Material-Based Sensing Technology toward Health and Environmental Monitoring Applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef]
- Galstyan, V.; Moumen, A.; Kumarage, G.W.C.; Comini, E. Progress towards Chemical Gas Sensors: Nanowires and 2D Semiconductors. Sens. Actuators B Chem. 2022, 357, 131466. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-Dimensional Materials in Biomedical, Biosensing and Sensing Applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Lashkov, A.V.; Lipatov, A.; Plugin, I.A.; Bruns, M.; Fuchs, D.; Varezhnikov, A.S.; Adib, M.; Sommer, M.; Sinitskii, A. UV-Light-Tunable p-/n-Type Chemiresistive Gas Sensors Based on Quasi-1D TiS3 Nanoribbons: Detection of Isopropanol at Ppm Concentrations. Sensors 2022, 22, 9815. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Wang, J.; Liu, J.; Yan, W. Hierarchical Heterojunctions of Metal Sulfide WS2 Nanosheets/Metal Oxide In2O3 Nanofibers for an Efficient Detection of Formaldehyde. Nanomaterials 2024, 14, 1702. [Google Scholar] [CrossRef]
- Meng, T.Y.; Hu, K.S.; Hsueh, T.J. A 2D Tin Disulfide Nanosheet Gas Sensor for Hydrogen Sulfide. IEEE Sens. J. 2025, 25, 3559181. [Google Scholar] [CrossRef]
- Harke, S.S.; Jadhav, Y.; Patil, V.B.; Gurnani, C. Facile Solution-Processed Deposition of Bi2S3 Nanostructures for a Highly Sensitive and Selective Room-Temperature NO2 Sensor. ACS Appl. Electron. Mater. 2025, 7, 1291–1304. [Google Scholar] [CrossRef]
- Russ, T.; Hu, Z.; Li, L.; Zhou, L.; Liu, H.; Weimar, U.; Barsan, N. In Operando Investigation of the Concentration Dependent NO2 Sensing Mechanism of Bi2S3 Nanorods at Low Temperatures and the Interference of O3. ACS Sens. 2022, 7, 3023–3031. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Wang, Y.; Hao, J. Nanorods Assembled Hierarchical Bi2S3 for Highly Sensitive Detection of Trace NO2 at Room Temperature. Chemosensors 2024, 12, 8. [Google Scholar] [CrossRef]
- Yang, Y.; Xin, T.; Liu, C.; Zhang, T.; Hao, W.; Wang, Y.; Hao, J. Urchin-like Bi2S3 Nanostructures with Rich Sulfur Vacancies for Ppb-Level NO2 Sensing. J. Alloys Compd. 2023, 930, 167467. [Google Scholar] [CrossRef]
- Kan, H.; Yang, W.; Guo, Z.; Li, M. Highly Sensitive Room-Temperture NO2 Gas Sensor Based on Bi2S3 Nanorods. J. Mater. Sci. Mater. Electron. 2024, 35, 331. [Google Scholar] [CrossRef]
- Li, H.; Huang, H.; Huang, W.; Zhang, X.; Hai, G.; Lai, F.; Zhu, T.; Bai, S.; Zhang, N.; Liu, T. Interfacial Accumulation and Stability Enhancement Effects Triggered by Built-in Electric Field of SnO2/LaOCl Nanofibers Boost Carbon Dioxide Electroreduction. Small 2024, 2402654, e2402654. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Song, J.G.; Ko, K.Y.; Woo, W.J.; Lee, S.W.; Park, M.; Lee, H.; Lee, Z.; Choi, H.; et al. 2D Transition Metal Dichalcogenide Heterostructures for P- and n-Type Photovoltaic Self-Powered Gas Sensor. Adv. Funct. Mater. 2020, 30, 2003360. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Yin, D.; Zhou, Z.; Wei, C.; Wang, Y.; Hao, J. Enhanced Oxygen Anions Generation on Bi2S3/Sb2S3 Heterostructure by Visible Light for Trace H2S Detection at Room Temperature. J. Hazard. Mater. 2024, 476, 134932. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Shi, J.; Lv, W.; Han, Y.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; Wei, H.; et al. A Novel Artificial Neuron-Like Gas Sensor Constructed from CuS Quantum Dots/Bi2S3 Nanosheets. Nano-Micro Lett. 2022, 14, 8. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Hu, Q. Controllable Epitaxial-like Growth of Bi2S3/MoS2 Hybrid Aerogel Nanostructures for Sensitive NO2 Detection at Room Temperature. ACS Appl. Nano Mater. 2023, 6, 8260–8269. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.; Zhang, H.; Wang, B.; Chen, C.; Li, J.; Wang, Y.; Hao, J. Room Temperature Gas Sensor Based on rGO/Bi2S3 Heterostructures for Ultrasensitive and Rapid NO2 Detection. Chem. Eng. J. 2024, 490, 151872. [Google Scholar] [CrossRef]
- Zhao, X.; Zhi, M.; Hang, D.; Ren, Q.; Zhang, P.; Chen, C.; Chen, Q.; Li, Q.; Zhang, Z.; Yan, J.; et al. Ultrasensitive NO2 Gas Sensor Based on MoS2 Modified Urchin-like Bi2S3 Heterojunction. Phys. E Low-Dimens. Syst. Nanostructures. 2023, 147, 115575. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Wang, N.; Huang, B.; Li, X. SnTe/SnSe Heterojunction Based Ammonia Sensors with Excellent Withstand to Ambient Humidities. Small 2024, 20, 2309831. [Google Scholar] [CrossRef]
- Xing, H.; Li, X.; Sun, S.; Huang, B.; Li, X. Low-Temperature NO2 Sensor Based on γ-In2Se3/In2O3 Nanoflower Heterojunction. Sens. Actuators B Chem. 2024, 415, 136034. [Google Scholar] [CrossRef]
- Lan, M.; Dong, X.; Zheng, N.; Zhang, X.; Wang, Y.; Zhang, X. In-Situ Construction of Novel Sulfur-Vacancy-Rich Bi/Bi2S3/SnS2 Z-Scheme Heterostructure Photocatalysts for Efficient Cr(VI) Reduction and Nitrogen Fixation. J. Mater. Sci. Technol. 2023, 167, 237–247. [Google Scholar] [CrossRef]
- Chen, D.N.; Wang, G.Q.; Mei, L.P.; Feng, J.J.; Wang, A.J. Dual II-Scheme Nanosheet-like Bi2S3/Bi2O3/Ag2S Heterostructures for Ultrasensitive PEC Aptasensing of Aflatoxin B1 Coupled with Catalytic Signal Amplification by Dendritic Nanorod-like Au@Pd@Pt Nanozyme. Biosens. Bioelectron. 2023, 223, 115038. [Google Scholar] [CrossRef]
- Wang, X.; Du, C.; Yu, Y.; Li, W.; Li, T.; Wang, S.; Mao, S.; Wang, Y.; Zhao, J.; Xiong, C. Efficient Photocatalytic Degradation with a Lattice-Matched α-Bi2O3/Co3O4 Z-Scheme Heterojunction: An Integrated Experimental and DFT Study. J. Water Process Eng. 2024, 65, 105829. [Google Scholar] [CrossRef]
- Cheng, X.; Xiao, X.; Wang, F.; Lu, T.; Zhang, Y. Heterojunctions Based on BiOBr Nanosheets Decorated on α-Bi2O3 for Photodegradation of Rhodamine B. ACS Appl. Nano Mater. 2024, 7, 4413–4422. [Google Scholar] [CrossRef]
- Tang, Y.; Gan, S.; Zhong, L.; Sun, Z.; Xu, L.; Liao, C.; Lin, K.; Cui, X.; He, D.; Ma, Y.; et al. Lattice Proton Intercalation to Regulate WO3-Based Solid-Contact Wearable PH Sensor for Sweat Analysis. Adv. Funct. Mater. 2022, 32, 2107653. [Google Scholar] [CrossRef]
- Bai, Y.; Fu, H.; Yang, X.; Xiong, S.; Li, S.; An, X. Conductometric Isopropanol Gas Sensor: Ce-Doped In2O3 Nanosheet-Assembled Hierarchical Microstructure. Sens. Actuators B Chem. 2023, 377, 133007. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, G.; Duan, M.; Liu, Y.; Cai, Y.; Xu, M.; Zhao, K.; Tai, H.; Jiang, Y.; Su, Y. Zinc Oxide Nanorods for Light-Activated Gas Sensing and Photocatalytic Applications. ACS Appl. Nano Mater. 2023, 6, 17445–17456. [Google Scholar] [CrossRef]
- Cheng, L.; Li, Y.; Sun, G.; Cao, J.; Wang, Y. Modification of Bi2O3 on ZnO Porous Nanosheets-Assembled Architecture for Ultrafast Detection of TEA with High Sensitivity. Sens. Actuators B Chem. 2023, 376, 132986. [Google Scholar] [CrossRef]
- Kashmery, H.A.; El-Hout, S.I. Bi2S3/Bi2O3 Nanocomposites as Effective Photocatalysts for Photocatalytic Degradation of Tetracycline under Visible-Light Exposure. Opt. Mater. 2023, 135, 113231. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Liu, F.; Jia, M.; Zhang, Z.; Liu, M.; Jiang, L. Renewable WO3/Bi2O3 Heterojunction for Photoelectrochemical and Visual Dual-Mode Detection of Hydrogen Sulfide. Sens. Actuators B Chem. 2022, 369, 132274. [Google Scholar] [CrossRef]
- Guang, Q.; Huang, B.; Yu, J.; Bonyani, M.; Moaddeli, M.; Kanani, M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Li, X. PtS-Decorated WS2 Microflakes Based Sensors for Selective Ammonia Detection at Room Temperature. Sens. Actuators B Chem. 2023, 394, 134399. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, X.; Sun, R.; Yang, J.; Jiang, X.; Liu, Z.; Xie, M.; Xie, E.; Han, W. Z-Scheme Bi2O2.33/Bi2S3 Heterojunction Nanostructures for Photocatalytic Overall Water Splitting. Chem. Eng. J. 2020, 382, 123020. [Google Scholar] [CrossRef]
- Cheng, D.; Wu, H.; Feng, C.; Ding, Y.; Mei, H. Bifunctional Photoelectrochemical Sensor Based on Bi/Bi2S3/BiVO4 for Detecting Hexavalent Chromium and Hydrogen Peroxide. Sens. Actuators B Chem. 2022, 353, 131108. [Google Scholar] [CrossRef]
- Ou, L.X.; Liu, M.Y.; Zhu, L.Y.; Zhang, D.W.; Lu, H.L. Recent Progress on Flexible Room-Temperature Gas Sensors Based on Metal Oxide Semiconductor. Nano-Micro Lett. 2022, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cui, X.; Zhou, Y.; Zhang, H.; Cao, L.; Gao, L.; Yin, H.; Ai, S. MXene Enhanced Photoactivity of Bi2O3/Bi2S3 Heterojunction with G-Wire Superstructure for Photoelectrochemical Detection of TET1 Protein. ACS Sens. 2022, 7, 3116–3125. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Liu, L.; Lv, H.; Liu, Y.; Ur Rehman, A.; Kan, K.; Zhang, W.J.; He, L.; Wang, Y.; Wang, R.; et al. Intercalation of Bi2O3/Bi2S3 Nanoparticles into Highly Expanded MoS2 Nanosheets for Greatly Enhanced Gas Sensing Performance at Room Temperature. J. Hazard. Mater. 2019, 363, 335–345. [Google Scholar] [CrossRef]
- Li, H.; Jing, P.; He, C.; Pan, Z.; Liu, J.; Cui, Y.; Wang, J. Interfacial Configuration and Mechanism Insights of an All-Solid-State Z-Scheme BaTiO3/Bi/Bi2O3 Heterojunctions for Rapid Removal of Tetracycline Antibiotics. Appl. Surf. Sci. 2023, 615, 156416. [Google Scholar] [CrossRef]
- Chang, J.; Liang, W.; Wang, W.; Wu, D.; Jiang, K.; Wang, G.; Xu, F.; Gao, Z. Oxygen Vacancies Enriched Bi2O3 as High Capacity and High Rate Negative Material for Aqueous Alkali Battery. Appl. Surf. Sci. 2022, 601, 154296. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Sun, S.; Wang, N.; Huang, B.; Li, X. Hierarchically In2S3@In2O3 Nanorods Heterojunctions for Enhanced NO2 Sensing at Lower Operating Temperature. Sens. Actuators B Chem. 2024, 419, 136360. [Google Scholar] [CrossRef]
- Chen, X.; Shi, J.; Wang, T.; Zheng, S.; Lv, W.; Chen, X.; Yang, J.; Zeng, M.; Hu, N.; Su, Y.; et al. High-Performance Wearable Sensor Inspired by the Neuron Conduction Mechanism through Gold-Induced Sulfur Vacancies. ACS Sens. 2022, 7, 816–826. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zeng, Z.; Yan, M.; Jia, X.; Hu, P.; Xu, J.; Xue, Z.; Xu, J. Tailoring the Injection Action of Oxygen over Top-Surface of Bismuth Sulfide to Change Reactive Electron Transfer Path for Flexible NO2 Sensors. Mater. Sci. Eng. R. Rep. 2024, 160, 100819. [Google Scholar] [CrossRef]
- Chang, F.; Peng, S.; Yan, W.; Yang, C.; Li, S.; Liu, X. A Novel and Facile Procedure to Decorate Bi2O3 with Bi2S3 Nanocrystals: Composites Synthesis, Analyses, and Photocatalytic Performance Assessment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125640. [Google Scholar] [CrossRef]
- Gasso, S.; Sohal, M.K.; Mahajan, A. MXene Modulated SnO2 Gas Sensor for Ultra-Responsive Room-Temperature Detection of NO2. Sens. Actuators B Chem. 2022, 357, 131427. [Google Scholar] [CrossRef]
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A Room-Temperature NO2 Gas Sensor Based on CuO Nanoflakes Modified with rGO Nanosheets. Sens. Actuators B Chem. 2021, 337, 129783. [Google Scholar] [CrossRef]
- Hau, H.H.; Duong, T.T.H.; Man, N.K.; Thi Viet Nga, T.; Thi Xuan, C.; Thi Thanh Le, D.; Van Toan, N.; Hung, C.M.; Van Duy, N.; Van Hieu, N.; et al. Enhanced NO2 Gas-Sensing Performance at Room Temperature Using Exfoliated MoS2 Nanosheets. Sens. Actuators A Phys. 2021, 332, 113137. [Google Scholar] [CrossRef]
- Yakout, S.M. New Efficient Sunlight Photocatalysts Based on Gd, Nb, V and Mn Doped Alpha-Bi2O3 Phase. J. Environ. Chem. Eng. 2020, 8, 103644. [Google Scholar] [CrossRef]
- Motaung, M.P.; Onwudiwe, D.C.; Lei, W. Microwave-Assisted Synthesis of Bi2S3 and Sb2S3 Nanoparticles and Their Photoelectrochemical Properties. ACS Omega 2021, 6, 18975–18987. [Google Scholar] [CrossRef] [PubMed]
| Materials | Gas | Temp. (°C) | Conc. (ppm) | Tres/Tres (s) | LOD (ppm) | Refs. | |
|---|---|---|---|---|---|---|---|
| Bi2S3 | NO2 | RT | 100 | 1.39 | 14/257 | 1 | [21] |
| Bi2S3 | NO2 | RT | 10 | 12.2 | 39/696 | 2 | [25] |
| Au/Bi2S3 | NO2 | RT | 5 | 5.6 | 21/371 | 0.25 | [57] |
| CuS/Bi2S3 | NO2 | RT | 10 | 3.4 | 18/338 | 0.5 | [29] |
| Bi2S3/Bi2O3 | NO2 | RT | 10 | 1.28 | 26.6/69.9 | 0.1 | [58] |
| Bi2S3/Bi2O3 | NO2 | RT | 8 | 7.85 | 71/238 | 0.1 | this work |
| Configurations | (eV) | (e) | (Å) |
|---|---|---|---|
| Bi2S3/Bi2O3 | −1.26 | −0.67 | 2.41 |
| Bi2S3 | −0.21 | −0.17 | 2.97 |
| Bi2O3 | −0.39 | −0.08 | 2.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Chen, J.; Gu, D.; Sun, S.; Li, X.; Li, X. Mesoporous Bi2S3/Bi2O3 Heterostructure-Based Sensors for Sub-ppm NO2 Detection at Room Temperature. Sensors 2025, 25, 3612. https://doi.org/10.3390/s25123612
Liu W, Chen J, Gu D, Sun S, Li X, Li X. Mesoporous Bi2S3/Bi2O3 Heterostructure-Based Sensors for Sub-ppm NO2 Detection at Room Temperature. Sensors. 2025; 25(12):3612. https://doi.org/10.3390/s25123612
Chicago/Turabian StyleLiu, Wei, Jiashuo Chen, Ding Gu, Shupeng Sun, Xinlei Li, and Xiaogan Li. 2025. "Mesoporous Bi2S3/Bi2O3 Heterostructure-Based Sensors for Sub-ppm NO2 Detection at Room Temperature" Sensors 25, no. 12: 3612. https://doi.org/10.3390/s25123612
APA StyleLiu, W., Chen, J., Gu, D., Sun, S., Li, X., & Li, X. (2025). Mesoporous Bi2S3/Bi2O3 Heterostructure-Based Sensors for Sub-ppm NO2 Detection at Room Temperature. Sensors, 25(12), 3612. https://doi.org/10.3390/s25123612






