An Ultra-Sensitive Colorimetric Sensing Platform for Simultaneous Detection of Moxifloxacin/Ciprofloxacin and Cr(III) Ions Based on Ammonium Thioglycolate Functionalized Gold Nanoparticles
Abstract
Highlights
- ATG-functionalized AuNPs were prepared as a colorimetric chemosensor with ultra-sensitivity for the simultaneous detection of MOX/CIP and Cr(III).
- ATG-AuNPs sensor color transitions from wine-red to gray-blue indicated successful detection.
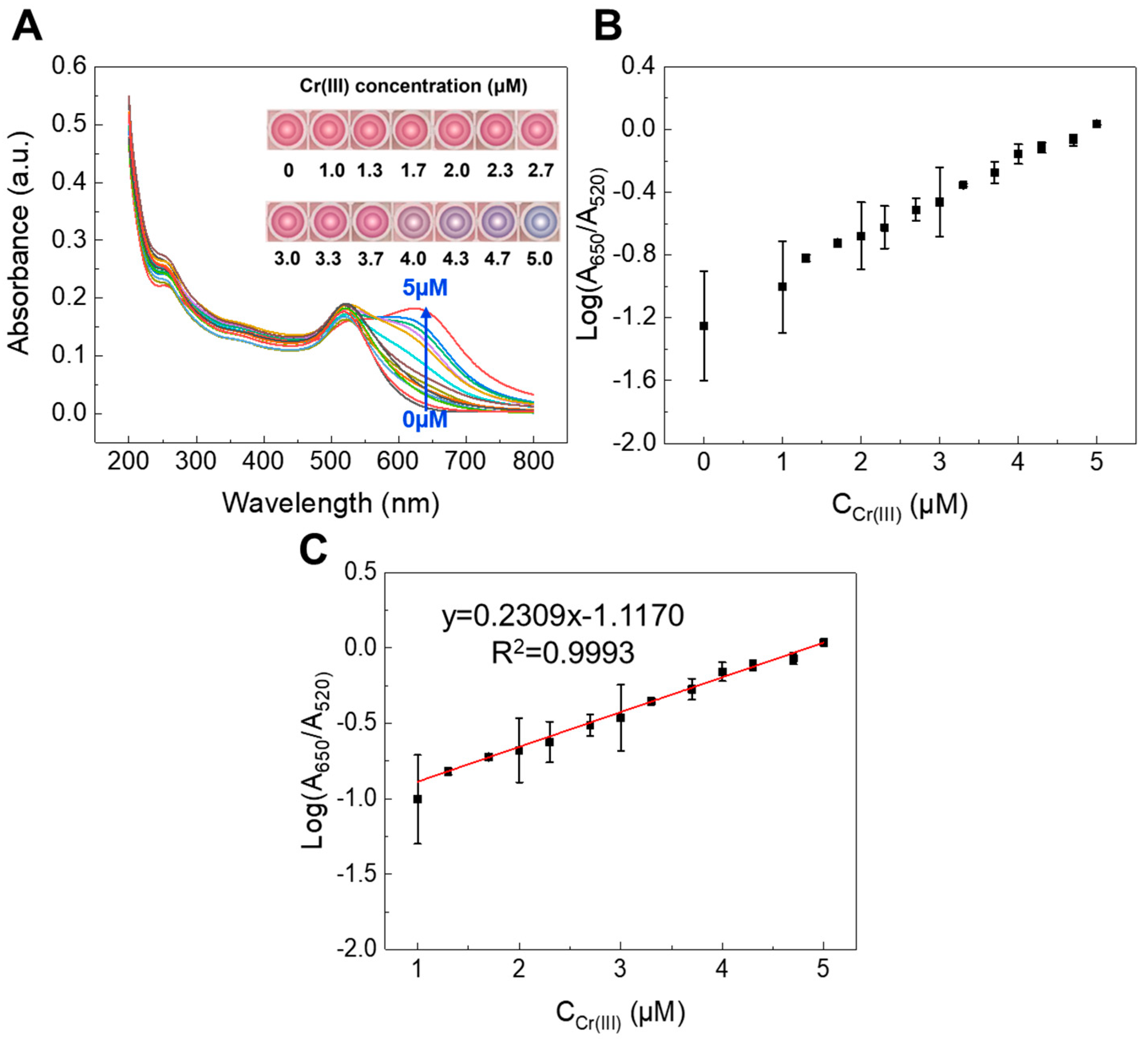
- The detection limits for MOX/CIP and Cr(III) are 1.57 µM, 1.30 µM, and 57.1 nM, respectively.
- The presented method was used to detect multiple analytes in lake and tap water samples.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Synthesis of AuNPs
2.4. Modification of AuNPs by ATG
2.5. Colorimetric Detection
2.6. Detection of Real Samples
3. Results and Discussion
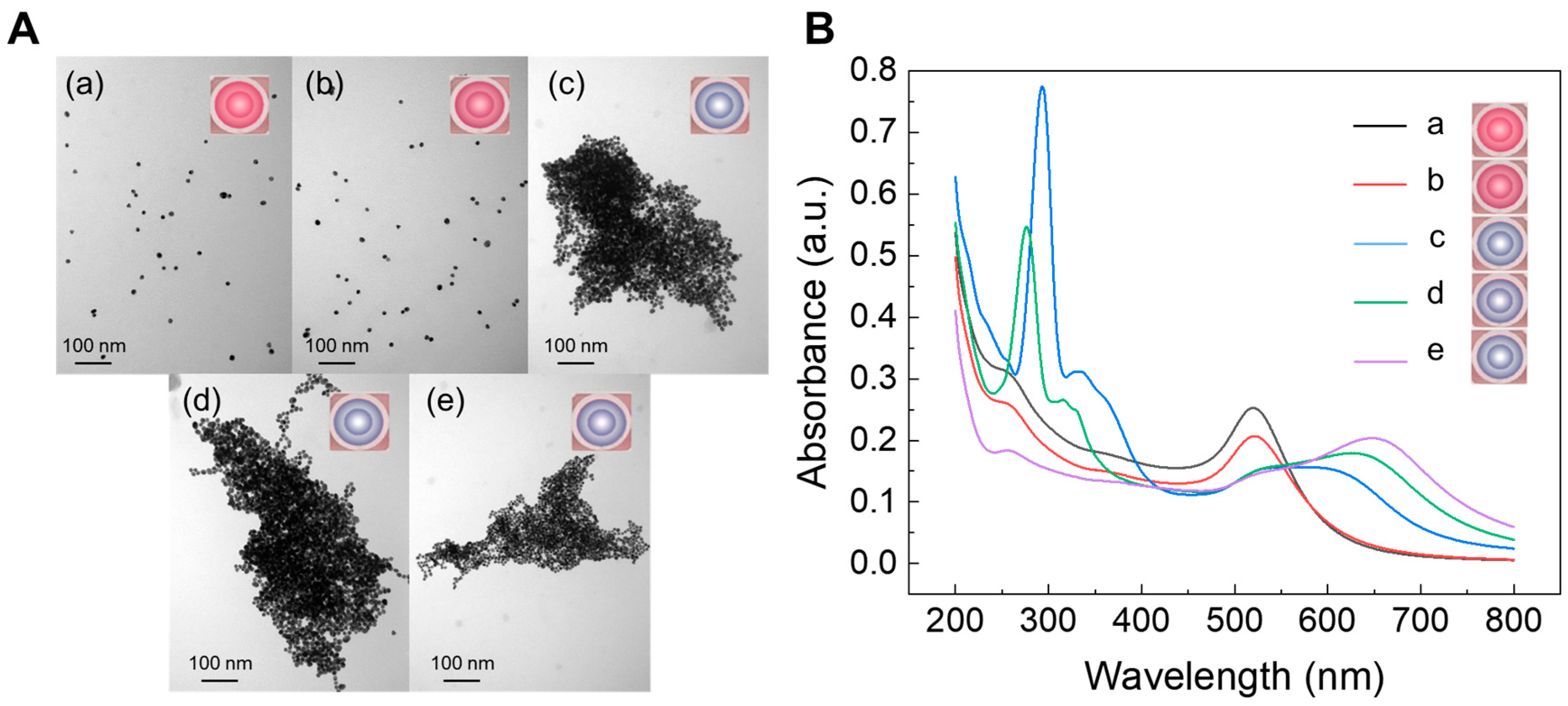
3.1. Preparation, Characterization, and Detection Mechanism of ATG-AuNPs
3.2. Optimization of the Detection System
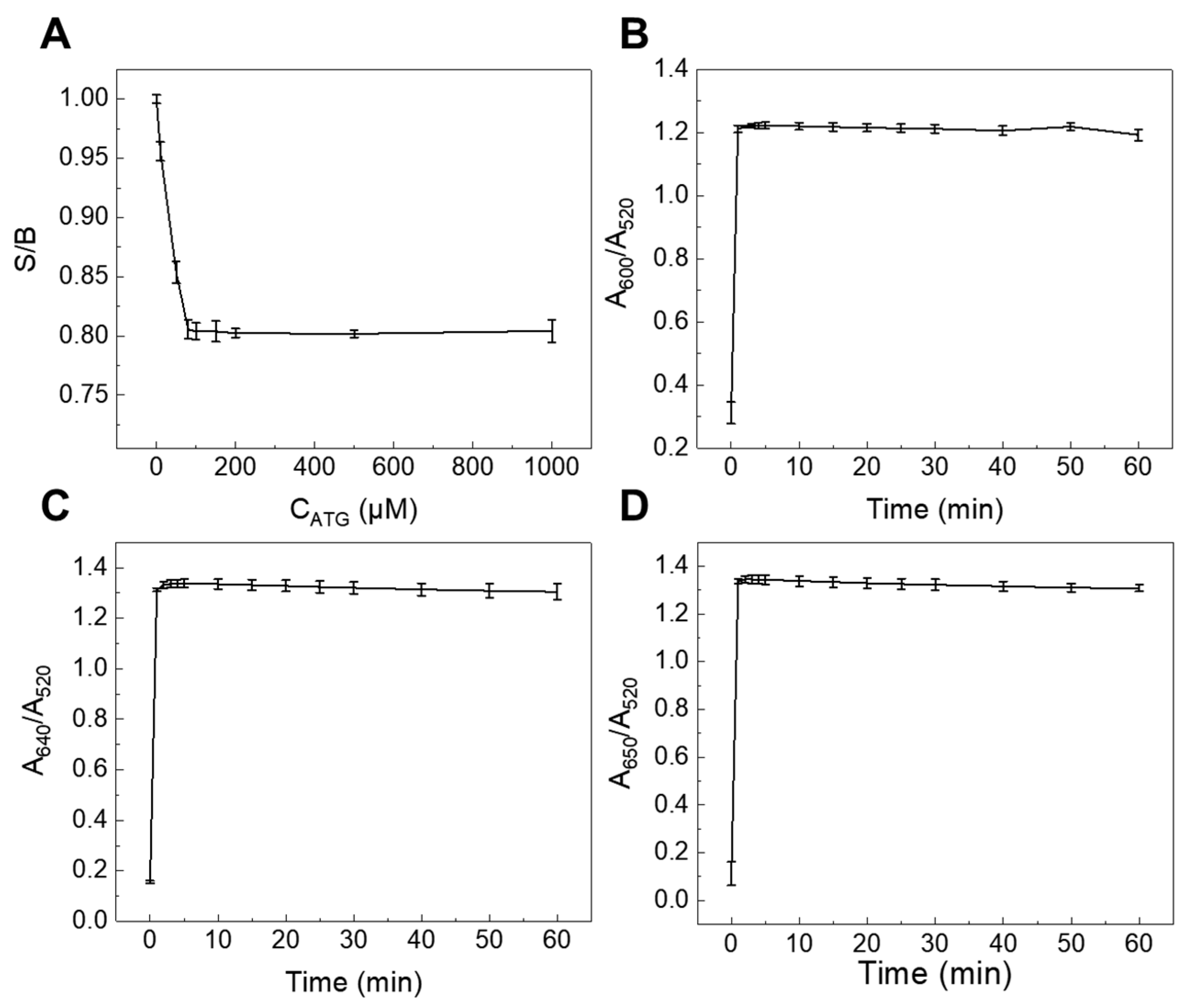
3.2.1. The Effect of Concentration of ATG as a Modifier
3.2.2. The Effect of the Reaction Time
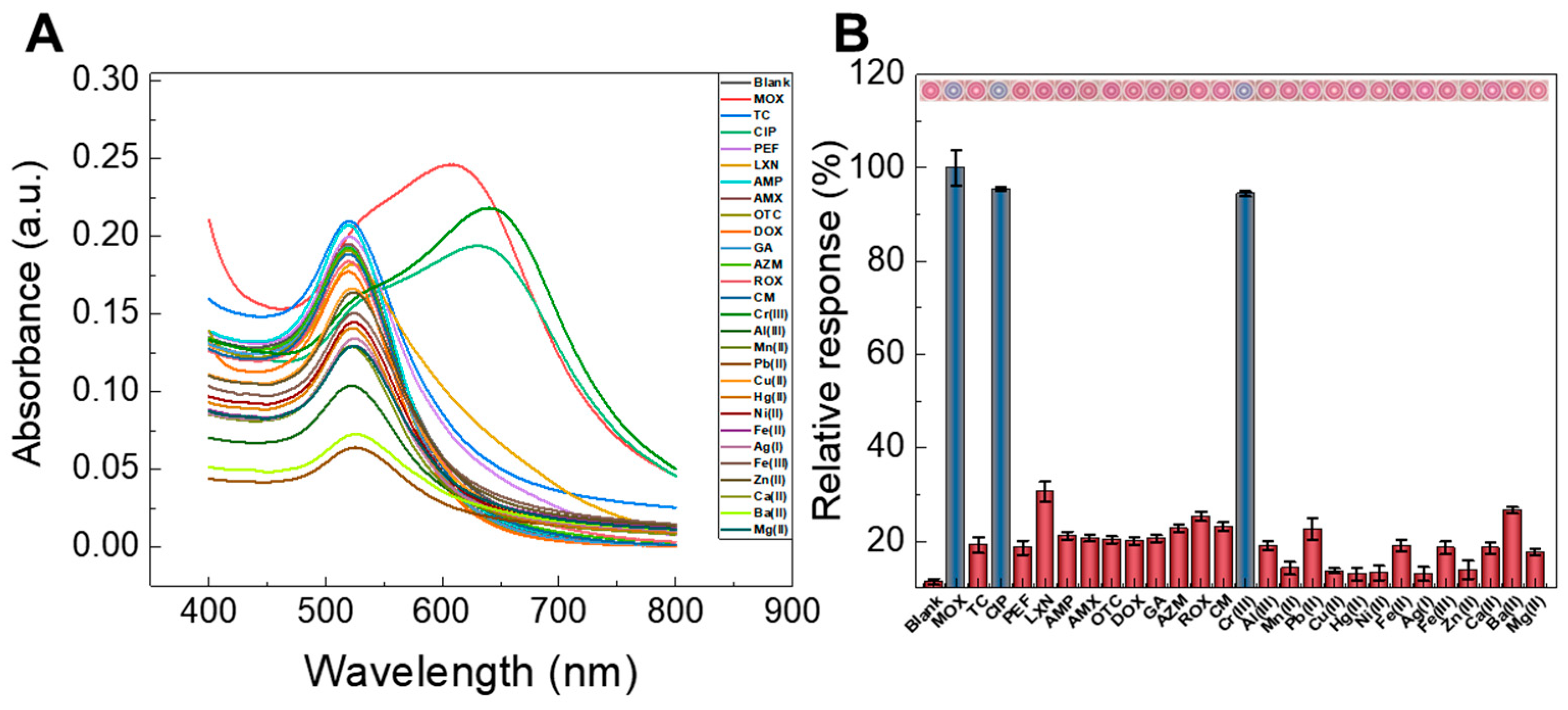
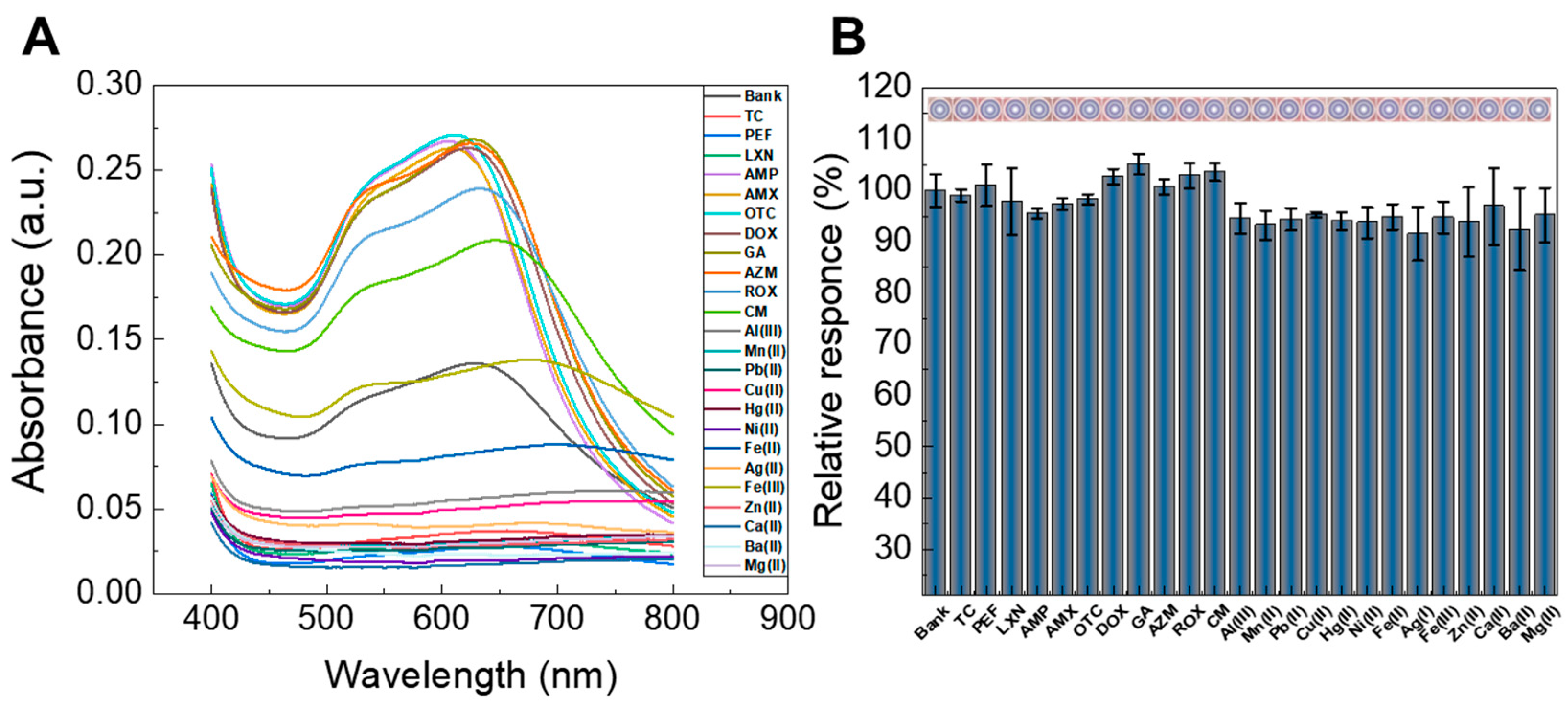
3.2.3. Selectivity Evaluation
3.2.4. Interference Study with Other Substances
3.2.5. Sensitive Detection
3.2.6. Investigation of the Performance of ATG-AuNPs Sensor in Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Long, N.; Liu, L.; Zhai, H.Y.; Zhu, M.F. Electrochemical determination of moxifloxacin hydrochloride based on molecularly imprinted polymer modified carbon paste electrode. Int. J. Electrochem. Sci. 2015, 10, 5069–5076. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Zhang, Z.L.; Lin, J.; Xu, J.C.; Wang, Q.X.; Hong, B.; Xia, Y.C.; Zeng, Y.X. Surface atom rearrangement enabling graphitic carbon nitride/sodium alginate gel monolith for ultrafast completely photodegrading ciprofloxacin under visible light. Chem. Eng. J. 2024, 489, 151218. [Google Scholar] [CrossRef]
- Singha, S.; Ahn, K.H. Detection of ciprofloxacin in urine through sensitized anthanide luminescence. Sensors 2016, 16, 2065. [Google Scholar] [CrossRef]
- Sajwan, R.K.; Shailja, P.; Kumar, R.; Dhiman, T.K.; Eremin, S.A.; Solanki, P.R. Enhanced fluorescence of mercaptopropionic acid-capped zinc sulfide quantum dots with moxifloxacin in food and water samples via reductive photoinduced electron transfer. Environ. Sci. Nano 2021, 8, 2693–2705. [Google Scholar] [CrossRef]
- Mohaghegh, F.; Tehrani, A.M.; Rana, D.; Winterhalter, M.; Materny, A. Detection and quantification of small concentrations of moxifloxacin using surface-enhanced Raman spectroscopy in a Kretschmann configuration. J. Raman Spectrosc. 2021, 52, 1617–1629. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D.; Yang, X.; He, J.-H. Novel 2D/2D S-scheme heterojunction photocatalyst for peroxymonosulfate activation enables ciprofloxacin ultrafast degradation: High-efficiency photogenerated carrier transport. Chem. Eng. J. 2024, 488, 150844. [Google Scholar] [CrossRef]
- European Economic Community. EEC Commission Regulation (EEC) No. 2901/93 of 23/10/93; European Economic Community: Brussels, Belgium, 1993. [Google Scholar]
- Zhang, L.X.; Tang, S.Y.; He, F.X.; Liu, Y.; Mao, W.; Guan, Y.T. Highly efficient and selective capture of heavy metals by poly(acrylic acid) grafted chitosan and biochar composite for wastewater treatment. Chem. Eng. J. 2019, 378, 122215. [Google Scholar] [CrossRef]
- Zheng, P.; Li, M.; Jurevic, R.; Cushing, S.K.; Liu, Y.X.; Wu, N.Q. A gold nanohole array based surface-enhanced Raman scattering biosensor for detection of silver(I) and mercury(II) in human saliva. Nanoscale 2015, 7, 11005–11012. [Google Scholar] [CrossRef]
- Jahrman, E.P.; Seidler, G.T.; Sieber, J.R. Determination of hexavalent chromium fractions in plastics using laboratory-based, high-resolution X-ray emission spectroscopy. Anal. Chem. 2018, 90, 6587–6593. [Google Scholar] [CrossRef]
- Eastmond, D.A.; Macgregor, J.T.; Slesinski, R.S. Trivalent chromium: Assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit. Rev. Toxicol. 2008, 38, 173–190. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Elbanna, T.E.; Gamaleldeen, N.M. Validated microbiological and HPLC methods for the determination of moxifloxacin in pharmaceutical preparations and human plasma. Braz. J. Microbiol. 2012, 43, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Pereira, J.; Firme, J.; Lemos, M.; Lemos, M. Determination of Ciprofloxacin in Pharmaceutical Formulations Using HPLC Method with UV Detection. Indian J. Pharm. Sci. 2014, 76, 541–544. [Google Scholar] [PubMed]
- Ahmed, R.M.; Hadad, G.M.; El-Gendy, A.E.; Ibrahim, A. Development of HPLC method for determination of sitagliptin in human plasma using fluorescence detector by experimental design approach. Anal. Chem. Lett. 2018, 8, 813–828. [Google Scholar] [CrossRef]
- Zhao, X.X.; Yuan, Y.J.; Shao, Q.; Qiao, H.Q. Simultaneous determination of moxifloxacin hydrochloride and dexamethasone sodium phosphate in rabbit ocular tissues and plasma by LC-MS/MS: Application for pharmacokinetics studies. Molecules 2022, 27, 7934. [Google Scholar] [CrossRef]
- Ramadan, L.; Ozturk-Ufuk, I.; Yuksel, E.; Topuz, E. Development of LC–MS/MS Method for the Simultaneous Detection of Emerging Contaminants in Aquatic Matrices. Water Air Soil Pollut. 2024, 235, 537. [Google Scholar] [CrossRef]
- Raju, B.; Ramesh, M.; Borkar, R.M.; Padiya, R.; Banerjee, S.K.; Srinivas, R. Development and validation of liquid chromatography-mass spectrometric method for simultaneous determination of moxifloxacin and ketorolac in rat plasma: Application to pharmacokinetic study. Biomed. Chromatogr. 2012, 26, 1341–1347. [Google Scholar] [CrossRef]
- Loh, G.O.K.; Wong, E.Y.L.; Tan, Y.T.F.; Lee, Y.L.; Pang, L.H.; Chin, M.C.; Damenthi, N.; Peh, K.K. Simple and rapid LC-MS/MS method for determination of sitagliptin in human plasma and application to bioequivalence study. J. Chromatogr. B 2020, 1159, 122337. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Timerbaev, A.R. Direct seawater analysis by high-resolution ICP-MS provides insights into toxic metal accumulation in marine sediments. Atomic Spectrosc. 2021, 42, 85–90. [Google Scholar] [CrossRef]
- Aydin, F.A.; Soylak, M. Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium(IV), titanium(IV), iron(III), lead(II) and chromium(III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin. J. Hazard. Mater. 2010, 173, 669–674. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, Y.; Jarmal, R.; Abdiryim, T. Highly Sensitive electrochemical sensing of moxifloxacin based on molecularly imprinted and Au nanoparticle functionalized PEDOT composites. Chem. Eng. J. 2024, 498, 155314. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.J.; Wu, T.H.; Liu, R.J.; Sun, J.; Ma, J. Fabrication of a novel HKUST-1/CoFe2O4/g-C3N4 electrode for the electrochemical detection of ciprofloxacin in physiological samples. Talanta 2024, 273, 125882. [Google Scholar] [CrossRef]
- Panagopoulou, C.; Skotadis, E.; Aslanidis, E.; Tzourmana, G.; Rapesi, A.; Tsioustas, C.; Kainourgiaki, M.; Kleitsiotis, G.; Tsekenis, G.; Tsoukalas, D. Non-Faradaic Impedimetric Detection of Heavy Metal Ions via a Hybrid Nanoparticle-DNAzyme Biosensor. Biosensors 2024, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghannam, S.M. Atomic absorption spectroscopic, conductometric and colorimetric methods for determination of some fluoroquinolone antibacterials using ammonium reineckate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 1188–1194. [Google Scholar] [CrossRef]
- Altunay, N.; Yildirim, E.; Gurkan, R. Extraction and preconcentration of trace Al and Cr from vegetable samples by vortex-assisted ionic liquid-based dispersive liquid–liquid microextraction prior to atomic absorption spectrometric determination. Food Chem. 2018, 245, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Sajwan, R.K.; Himanshu, J.K.; Solanki, P.R. Polyvinyl alcohol-derived-carbon quantum dots based fluorometric “On-Off” probe for moxifloxacin detection in milk and egg samples. Food Chem. 2024, 439, 138038. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wu, X.; Li, Z.; Mao, S. Dual-lanthanide cyclodextrin-polyoxometalate fluorescent assembly for rapid and sensitive detection of ciprofloxacin. Sens. Actuat. B Chem. 2024, 418, 136297. [Google Scholar] [CrossRef]
- Chen, J.; Yin, C.; Zhao, B.; Cheng, X.Y. Strategies for preparation of chitosan based water-soluble fluorescent probes to detect Cr3+ and Cu2+ ions. Int. J. Biol. Macromol. 2024, 276, 133915. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.C.; Yin, W.J.; Retout, M.; Housel, E.; Zhong, W.B.; Zhou, J.J.; Strano, M.S.; Jokerst, J.V. Colorimetric sensing for translational applications: From colorants to mechanisms. Chem. Soc. Rev. 2024, 15, 7681–7741. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Yue, G.Z.; Su, S.; Li, N.; Shuai, M.; Lai, X.C.; Astruc, D.; Zhao, P.X. Gold nanoparticles as sensors in the colorimetric and fluorescence detection of chemical warfare agents. Coordin. Chem. Rev. 2016, 311, 75–84. [Google Scholar] [CrossRef]
- Chen, H.; Cai, S.T.; Luo, J.X.; Liu, X.H.; Ou, L.J.; Zhang, Q.W.; Liedberg, B.; Wang, Y. Colorimetric biosensing assays based on gold nanoparticles functionalized/combined with non-antibody recognition elements. TrAC Trends Anal. Chem. 2024, 173, 117654. [Google Scholar] [CrossRef]
- Zhai, R.Q.; Chen, G.; Liu, G.Y.; Huang, X.D.; Xu, X.M.; Li, L.Y.; Zhang, Y.G.; Wang, J.; Jin, M.J.; Xu, D.H.; et al. Enzyme inhibition methods based on Au nanomaterials for rapid detection of organophosphorus pesticides in agricultural and environmental samples: A review. J. Adv. Res. 2021, 28, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Song, Z.R.; Peng, J.; Yang, M.L.; Zhi, H.; He, H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020, 127, 115880. [Google Scholar] [CrossRef]
- Liu, D.M.; Dong, C. Gold nanoparticles as colorimetric probes in food analysis: Progress and challenges. Food Chem. 2023, 429, 136887. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Qiao, J.; Zhang, H.Y.; Zhao, Z.W.; Qi, L. Polymer-capped gold nanoparticles as nanozymes with improved catalytic activity for the monitoring of serum ciprofloxacin. Analyst 2022, 147, 1509–1514. [Google Scholar] [CrossRef]
- Zehra, T.; Ahsan, F.; Versiani, M.A.; Wahid, S.; Jahangir, S.; Shah, M.R. Puerarin-coated gold nanoparticles (PUE-AuNPs) synthesized via green synthetic route: A new colorimetric probe for the detection of ciprofloxacin. Turk. J. Chem. 2021, 45, 1814–1827. [Google Scholar] [PubMed]
- Trivedi, M.U.; Sharma, P.; Kaur, A.; Sharma, R.K. Unraveling the β-Cyclodextrin-Driven Inclusion Complexation with Phenylalanine-Capped AuNPs for Colorimetric Detection of Cr(III) and Fe(III). ACS Appl. Nano Mater. 2024, 7, 4377–4387. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Dong, Q.; Cao, Y.P.; Liu, Y.M.; Zhao, Y. Colorimetric Detection of Chromium Ions in Aqueous Solution Based on Functionalized Gold Nanoparticles with 4-Mercaptobenzoic Acid and Polyethyleneimine. Nano 2022, 17, 2250105. [Google Scholar] [CrossRef]
- Liu, J.F.; Wang, X.K.; Zhu, X.F.; Zhao, Y.F.; Ye, Y. Advances in dual-function bioprobes for simultaneous detection of transition metal ions (Fe, Cu, Zn) and bioactive species. Coord. Chem. Rev. 2025, 526, 216352. [Google Scholar] [CrossRef]
- Zhu, J.P.; Graziotto, M.E.; Cottam, V.; Hawtrey, T.; Adair, L.D.; Trist, B.G.; Pham, N.T.H.; Rouaen, J.R.C.; Ohno, C.; Heisler, M.; et al. Near-Infrared Ratiometric Fluorescent Probe for Detecting Endogenous Cu2+ in the Brain. ACS Sens. 2024, 9, 2858–2868. [Google Scholar] [CrossRef]
- Zhang, H.T.; Feng, L.; Jiang, Y.; Wong, Y.T.; He, Y.H.; Zheng, G.S.; He, J.; Tan, Y.; Sun, H.Y.; Ho, D. A reaction-based near-infrared fluorescent sensor for Cu2+ detection in aqueous buffer and its application in living cells and tissues imaging. Biosens. Bioelectron. 2017, 94, 24–29. [Google Scholar] [CrossRef]
- Zhang, L.M.; Han, Y.Y.; Zhao, F.; Shi, G.Y.; Tian, Y. A Selective and Accurate Ratiometric Electrochemical Biosensor for Monitoring of Cu2+ Ions in a Rat Brain. Anal. Chem. 2015, 87, 2931–2936. [Google Scholar] [CrossRef]
- Chen, S.Y.; Li, Z.; Li, K.; Yu, X.Q. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 213691. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wu, S.Y.; Xue, Y.; Xie, Q.; Danzeng, Q.Z.; Liu, C.; Zhou, C.H. Fluorescence/colorimetric dual-signal sensor based on carbon dots and gold nanoparticles for visual quantification of Cr3+. Microchim. Acta 2024, 191, 571. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Bahram, M.; Dadashi, R. Colorimetric detection of sitagliptin and moxifloxacin using AgNPs modified by 4-mercaptophenylbronic acid. Inorg. Chem. Commun. 2024, 167, 112834. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.H.; Wu, S.P. Nanodiamonds conjugated to gold nanoparticles for colorimetric detection of clenbuterol and chromium(III) in urine. Microchim. Acta 2017, 185, 74. [Google Scholar] [CrossRef]
- Li, S.; Wei, T.; Ren, G.; Chai, F.; Wu, H.; Qu, F. Gold nanoparticles based colorimetric probe for Cr(III) and Cr(VI) detection. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 215–224. [Google Scholar] [CrossRef]
- Karn-Orachai, K.; Wattanasin, P.; Ngamaroonchote, A. Colorimetric Sensor for Cr(VI) Ion Detection in Tap Water Using a Combination of AuNPs and AgNPs. ACS Omega 2024, 9, 26472–26483. [Google Scholar] [CrossRef]
- Huang, W.W.; Wang, Y.C.; Wang, L.M.; Pan, C.Q.; Shen, G.Q. Colorimetric detection of ciprofloxacin in aqueous solution based on an unmodified aptamer and the aggregation of gold nanoparticles. Anal. Methods 2021, 13, 90–98. [Google Scholar] [CrossRef]
- Nawaz, A.; Ali, S.M.; Rana, N.F.; Tanweer, T.; Batool, A.; Webster, T.J.; Menaa, F.; Riaz, S.; Rehman, Z.; Batool, F.; et al. Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment. Nanomaterials 2021, 11, 3152. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, H.; He, Y.J.; Ding, N.; Cao, Q. Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 391, 179–183. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Safavi, A.; Honarasa, F. Determination of nanoparticles concentration by multivariate curve resolution. Chemom. Intell. Lab. Syst. 2015, 141, 88–93. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, Y.S.; Zhao, Y.; Zhu, Q.S.; Wu, Y. Cr3+/COO− complexation induced aggregation of gelatin in dilute solution. Macromolecules 2002, 35, 7407–7413. [Google Scholar] [CrossRef]
- Shadab, F.; Gholamreza, K.; Hamed, G.; Raouf, G. A paper-based optical probe for chromium by using gold nanoparticles modified with 2,2′-thiodiacetic acid and smartphone camera readout. Microchim. Acta 2018, 185, 374. [Google Scholar]
- Heo, D.N.; Yang, D.H.; Moon, H.-J.; Lee, J.B.; Bae, M.S.; Lee, S.C.; Lee, W.J.; Sun, I.-C.; Kwon, I.K. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012, 33, 856–866. [Google Scholar] [CrossRef]
- Yılmaz, D.D.; Demirezen, D.A.; Mıhçıokur, H. Colorimetric detection of mercury ion using chlorophyll functionalized green silver nanoparticles in aqueous medium. Surfaces Interfaces 2021, 22, 100840. [Google Scholar] [CrossRef]



| Samples | Added (μM) | Tap Water | Lake Water | |||||
|---|---|---|---|---|---|---|---|---|
| Measured (μM) | Recovery (%) | RSD (%) | Measured (μM) | Recovery (%) | RSD (%) | |||
| 1 | 20 | 19.29 | 97.87 | 3.40 | 19.35 | 96.77 | 3.40 | |
| MOX | 2 | 30 | 28.92 | 96.42 | 3.53 | 29.59 | 98.77 | 3.34 |
| 3 | 40 | 38.95 | 97.38 | 3.07 | 38.93 | 97.33 | 2.20 | |
| 1 | 20 | 19.61 | 98.05 | 0.46 | 19.37 | 96.85 | 1.96 | |
| CIP | 2 | 30 | 29.84 | 99.46 | 2.50 | 29.89 | 99.62 | 3.30 |
| 3 | 40 | 39.85 | 99.63 | 1.30 | 39.88 | 99.71 | 2.70 | |
| 1 | 1 | 1.00 | 99.54 | 2.37 | 0.99 | 99.08 | 0.93 | |
| Cr(III) | 2 | 2 | 1.95 | 97.70 | 2.70 | 1.94 | 97.00 | 0.40 |
| 3 | 3 | 2.98 | 99.50 | 1.60 | 2.93 | 97.62 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, J.; Wang, J.; Yan, X.; Song, J.; Feng, F. An Ultra-Sensitive Colorimetric Sensing Platform for Simultaneous Detection of Moxifloxacin/Ciprofloxacin and Cr(III) Ions Based on Ammonium Thioglycolate Functionalized Gold Nanoparticles. Sensors 2025, 25, 3228. https://doi.org/10.3390/s25103228
Zhang L, Li J, Wang J, Yan X, Song J, Feng F. An Ultra-Sensitive Colorimetric Sensing Platform for Simultaneous Detection of Moxifloxacin/Ciprofloxacin and Cr(III) Ions Based on Ammonium Thioglycolate Functionalized Gold Nanoparticles. Sensors. 2025; 25(10):3228. https://doi.org/10.3390/s25103228
Chicago/Turabian StyleZhang, Lihua, Jiang Li, Juan Wang, Xu Yan, Jinping Song, and Feng Feng. 2025. "An Ultra-Sensitive Colorimetric Sensing Platform for Simultaneous Detection of Moxifloxacin/Ciprofloxacin and Cr(III) Ions Based on Ammonium Thioglycolate Functionalized Gold Nanoparticles" Sensors 25, no. 10: 3228. https://doi.org/10.3390/s25103228
APA StyleZhang, L., Li, J., Wang, J., Yan, X., Song, J., & Feng, F. (2025). An Ultra-Sensitive Colorimetric Sensing Platform for Simultaneous Detection of Moxifloxacin/Ciprofloxacin and Cr(III) Ions Based on Ammonium Thioglycolate Functionalized Gold Nanoparticles. Sensors, 25(10), 3228. https://doi.org/10.3390/s25103228






