An Aquatic Treadmill Alters Lower Limb Walking Dynamics in Typically Developing Children and Children with Cerebral Palsy
Abstract
Highlights
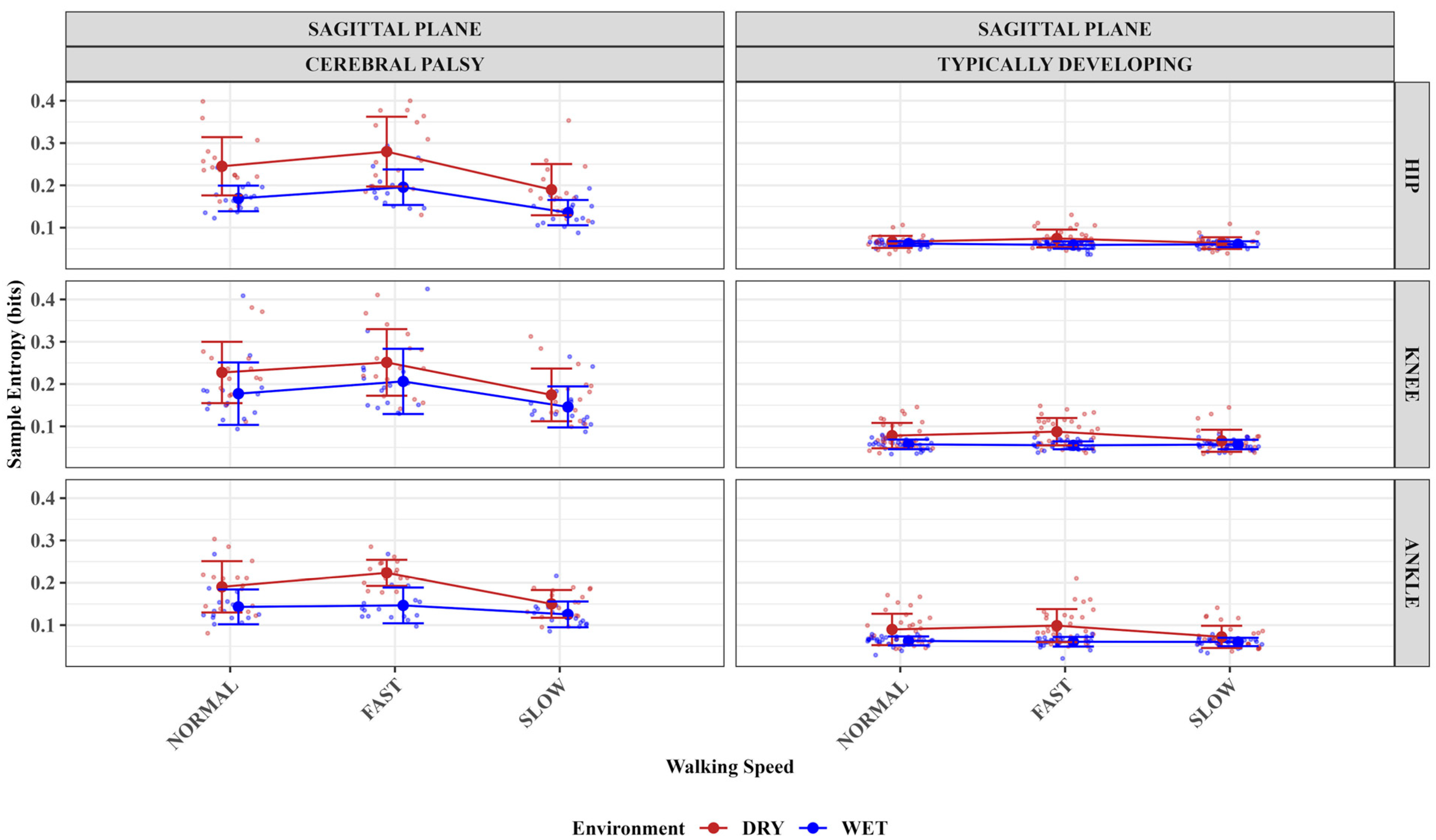
- This study showed improved sample entropy measures of the hip, knee, and ankle joints for the children with CP at slower than faster treadmill speeds in the aquatic treadmill compared to a dry treadmill environment.
- Sample entropy of the typically developing group’s knee and ankle joints improved in the aquatic treadmill environment compared to the dry treadmill.
- The findings imply that the aquatic treadmill environment could improve the walking regularity of children with cerebral palsy.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Data Processing
2.4. Data Analysis
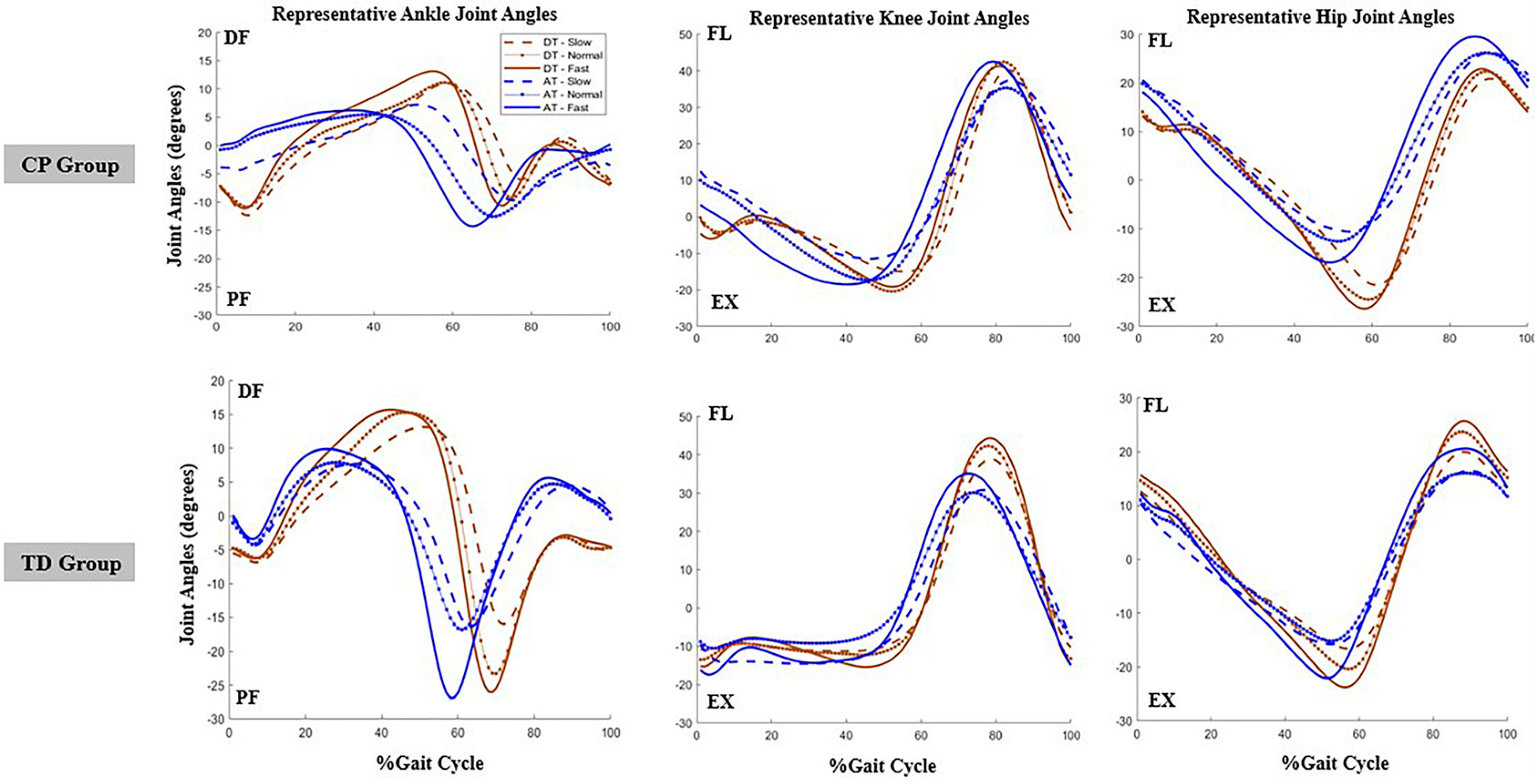
3. Results
3.1. The Impact of Treadmill Environment, Speed, and Group (Population) on Sagittal Plane Hip Angles Sample Entropy Measures
3.2. The Impact of Treadmill Environment, Speed, and Group (Population) on Sagittal Plane Knee Angles Sample Entropy Measures
3.3. The Impact of Treadmill Environment, Speed, and Group (Population) on Sagittal Plane Ankle Angles Sample Entropy Measures
3.4. Summaries
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, S.; Decoulon, G.; Bonnefoy-Mazure, A. Gait Analysis in Children with Cerebral Palsy. EFORT Open Rev. 2016, 1, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.; Goldstein, M.; Rosenbaum, P.; Leviton, A.; Paneth, N.; Dan, B.; Jacobsson, B.; Damiano, D. Executive Committee for the Definition of Cerebral Palsy Proposed Definition and Classification of Cerebral Palsy, April 2005. Dev. Med. Child Neurol. 2005, 47, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Tugui, R.D.; Antonescu, D. Cerebral Palsy Gait, Clinical Importance. Maedica 2013, 8, 388–393. [Google Scholar] [PubMed]
- Rab, G. Diplegic Gait: Is There More than Spasticity. In The Diplegic Child; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 1992; pp. 99–110. [Google Scholar]
- Rodda, J.; Graham, H.K. Classification of Gait Patterns in Spastic Hemiplegia and Spastic Diplegia: A Basis for a Management Algorithm. Eur. J. Neurol. 2001, 8, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.R.; Russman, B.S. Gait Analysis in Cerebral Palsy; Clinics in Developmental Medicine (Mac Keith Press); Cambridge University Press: Cambridge, UK, 1991; ISBN 978-0-521-41277-3. [Google Scholar]
- Stergiou, N.; Decker, L.M. Human Movement Variability, Nonlinear Dynamics, and Pathology: Is There a Connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- Pincus, S.M.; Goldberger, A.L. Physiological Time-Series Analysis: What Does Regularity Quantify? Am. J. Physiol. 1994, 266, H1643–H1656. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content Validity of the Expanded and Revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef]
- Palisano, R.J.; Avery, L.; Gorter, J.W.; Galuppi, B.; McCoy, S.W. Stability of the Gross Motor Function Classification System, Manual Ability Classification System, and Communication Function Classification System. Dev. Med. Child Neurol. 2018, 60, 1026–1032. [Google Scholar] [CrossRef]
- Johnson, D.C.; Damiano, D.L.; Abel, M.F. The Evolution of Gait in Childhood and Adolescent Cerebral Palsy. J. Pediatr. Orthop. 1997, 17, 392. [Google Scholar] [CrossRef]
- Elnaggar, R.K.; Alhowimel, A.; Alotaibi, M.; Abdrabo, M.S.; Elfakharany, M.S. Exploring Temporospatial Gait Asymmetry, Dynamic Balance, and Locomotor Capacity After a 12-Week Split-Belt Treadmill Training in Adolescents with Unilateral Cerebral Palsy: A Randomized Clinical Study. Phys. Occup. Ther. Pediatr. 2023, 43, 660–677. [Google Scholar] [CrossRef]
- Elnaggar, R.K. An Analytical Insight Into How Walking Speed and Spatial and Temporal Symmetry Are Related to Ankle Dysfunctions in Children with Hemiplegic Cerebral Palsy. Am. J. Phys. Med. Rehabil. 2021, 100, 458. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, H. Locomotor Training in Neurorehabilitation: Emerging Rehabilitation Concepts. Neurorehabil. Neural Repair 2003, 17, 3–11. [Google Scholar] [CrossRef]
- Hesse, S. Locomotor Therapy in Neurorehabilitation. NeuroRehabilitation 2001, 16, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Hill, M.; McAuliffe, K.; Watkins, P.; Wheeler, A.; Raynes, E. The Underwater Treadmill as a Physical Therapy Intervention for Children with Cerebral Palsy. FASEB J. 2015, 29, 983.10. [Google Scholar] [CrossRef]
- Becker, B.E. Aquatic Therapy in Contemporary Neurorehabilitation: An Update. PM&R 2020, 12, 1251–1259. [Google Scholar] [CrossRef]
- Kelly, M.; Darrah, J. Aquatic Exercise for Children with Cerebral Palsy. Dev. Med. Child Neurol. 2005, 47, 838–842. [Google Scholar] [CrossRef]
- Jung, T.; Kim, Y.; Lim, H.; Vrongistinos, K. The Influence of Water Depth on Kinematic and Spatiotemporal Gait Parameters during Aquatic Treadmill Walking. Sports Biomech. 2018, 18, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Badawy, W.M. Comparing the Effects of Aquatic and Land-Based Exercises on Balance and Walking in Spastic Diplegic Cerebral Palsy Children. Med. J. Cairo Univ. 2019, 84, 1–8. [Google Scholar]
- Ballington, S.J.; Naidoo, R. The Carry-over Effect of an Aquatic-Based Intervention in Children with Cerebral Palsy. Afr. J. Disabil. 2018, 7, 361. [Google Scholar] [CrossRef]
- Akinola, B.I.; Gbiri, C.A.; Odebiyi, D.O. Effect of a 10-Week Aquatic Exercise Training Program on Gross Motor Function in Children with Spastic Cerebral Palsy. Glob. Pediatr. Health 2019, 6, 2333794X19857378. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.; Dündar, Ü.; Demirdal, Ü.S.; Ulaşlı, A.M.; Toktaş, H.; Solak, Ö. The Effect of Aquatic Exercise on Spasticity, Quality of Life, and Motor Function in Cerebral Palsy. Turk. J. Phys. Med. Rehabil. 2017, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Pöyhönen, T.; Keskinen, K.L.; Hautala, A.; Mälkiä, E. Determination of Hydrodynamic Drag Forces and Drag Coefficients on Human Leg/Foot Model During Knee Exercise. Clin. Biomech. 2000, 15, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Narasaki-Jara, M.; Wagatsuma, M.; Holt, J.L.; Acuña, S.M.; Vrongistinos, K.; Jung, T. Aquatic Treadmill Walking at Three Depths of Water in People with Traumatic Brain Injury. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2020, 25, e1817. [Google Scholar] [CrossRef]
- Harrington, J.W.; Anguiano-Hernandez, J.G.; Kingston, D.C. Muscle Activation and Rating of Perceived Exertion of Typically Developing Children During DRY and Aquatic Treadmill Walking. J. Electromyogr. Kinesiol. 2023, 68, 102737. [Google Scholar] [CrossRef]
- McCamley, J.D.; Denton, W.; Arnold, A.; Raffalt, P.C.; Yentes, J.M. On the Calculation of Sample Entropy Using Continuous and Discrete Human Gait Data. Entropy 2018, 20, 764. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means 2017, 1.10.7. Available online: https://cran.r-project.org/package=emmeans (accessed on 14 May 2025).
- Raudenbush, S.W.; Bryk, A.S. Hierarchical Linear Models: Applications and Data Analysis Methods; SAGE: Newcastle upon Tyne, UK, 2002; Volume 485. [Google Scholar]
- Kwon, J.W.; Son, S.M.; Lee, N.K. Changes of Kinematic Parameters of Lower Extremities with Gait Speed: A 3D Motion Analysis Study. J. Phys. Ther. Sci. 2015, 27, 477–479. [Google Scholar] [CrossRef]
- Prosser, L.A.; Lauer, R.T.; VanSant, A.F.; Barbe, M.F.; Lee, S.C.K. Variability and Symmetry of Gait in Early Walkers with and without Bilateral Cerebral Palsy. Gait Posture 2010, 31, 522–526. [Google Scholar] [CrossRef]
- Katz-Leurer, M.; Rotem, H.; Keren, O.; Meyer, S. Balance Abilities and Gait Characteristics in Post-Traumatic Brain Injury, Cerebral Palsy and Typically Developed Children. Dev. Neurorehabilit. 2009, 12, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Wingert, J.R.; Burton, H.; Sinclair, R.J.; Brunstrom, J.E.; Damiano, D.L. Joint-Position Sense and Kinesthesia in Cerebral Palsy. Arch. Phys. Med. Rehabil. 2009, 90, 447–453. [Google Scholar] [CrossRef]
- Gormley, M.E., Jr. Treatment of Neuromuscular and Musculoskeletal Problems in Cerebral Palsy. Pediatr. Rehabil. 2001, 4, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Miller, M.; Richard, M.; Ritenour, D.; Chapman, B. Comparisons of Static and Dynamic Balance Following Training in Aquatic and Land Environments. J. Sport Rehabil. 2006, 15, 299–311. [Google Scholar] [CrossRef]
- Pöyhönen, T.; Sipilä, S.; Keskinen, K.L.; Hautala, A.; Savolainen, J.; Mälkiä, E. Effects of Aquatic Resistance Training on Neuromuscular Performance in Healthy Women. Med. Sci. Sports Exerc. 2002, 34, 2103–2109. [Google Scholar] [CrossRef]
- Rosello, M.; Caro-Llopis, A.; Orellana, C.; Oltra, S.; Alemany-Albert, M.; Marco-Hernandez, A.V.; Monfort, S.; Pedrola, L.; Martinez, F.; Tomás, M. Hidden Etiology of Cerebral Palsy: Genetic and Clinical Heterogeneity and Efficient Diagnosis by next-Generation Sequencing. Pediatr. Res. 2021, 90, 284–288. [Google Scholar] [CrossRef]
- Becker, B.E. Aquatic Therapy: Scientific Foundations and Clinical Rehabilitation Applications. PM&R 2009, 1, 859–872. [Google Scholar] [CrossRef]
| Joint-Direction | Best Model | Model Index | AIC | BIC | logLik | p |
|---|---|---|---|---|---|---|
| Sagittal Hip | Model 6: +Environment:Speed | 7 | −1116.71 | −1073.27 | 570.36 | <0.001 |
| Sagittal Knee | Model 5: +group:Speed | 6 | −944.75 | −908.55 | 482.38 | <0.001 |
| Sagittal Ankle | Model 6: +Environment:Speed | 7 | −1167.07 | −1123.63 | 595.54 | <0.001 |
| Joint | Group | Speed | Environment | Mean | Std_dev |
|---|---|---|---|---|---|
| Hip | CP | Fast | DT | 0.28 | 0.08 |
| Hip | CP | Fast | AT | 0.2 | 0.04 |
| Hip | TD | Fast | DT | 0.07 | 0.02 |
| Hip | TD | Fast | AT | 0.06 | 0.01 |
| Hip | CP | Normal | DT | 0.25 | 0.07 |
| Hip | CP | Normal | AT | 0.17 | 0.03 |
| Hip | TD | Normal | DT | 0.07 | 0.01 |
| Hip | TD | Normal | AT | 0.06 | 0.01 |
| Hip | CP | Slow | DT | 0.19 | 0.06 |
| Hip | CP | Slow | AT | 0.14 | 0.03 |
| Hip | TD | Slow | DT | 0.06 | 0.01 |
| Hip | TD | Slow | AT | 0.06 | 0.01 |
| Knee | CP | Fast | DT | 0.25 | 0.08 |
| Knee | CP | Fast | AT | 0.21 | 0.08 |
| Knee | TD | Fast | DT | 0.09 | 0.03 |
| Knee | TD | Fast | AT | 0.06 | 0.01 |
| Knee | CP | Normal | DT | 0.23 | 0.07 |
| Knee | CP | Normal | AT | 0.18 | 0.07 |
| Knee | TD | Normal | DT | 0.08 | 0.03 |
| Knee | TD | Normal | AT | 0.06 | 0.01 |
| Knee | CP | Slow | DT | 0.17 | 0.06 |
| Knee | CP | Slow | AT | 0.15 | 0.05 |
| Knee | TD | Slow | DT | 0.07 | 0.03 |
| Knee | TD | Slow | AT | 0.06 | 0.01 |
| Ankle | CP | Fast | DT | 0.22 | 0.03 |
| Ankle | CP | Fast | AT | 0.15 | 0.04 |
| Ankle | TD | Fast | DT | 0.1 | 0.04 |
| Ankle | TD | Fast | AT | 0.06 | 0.01 |
| Ankle | CP | Normal | DT | 0.19 | 0.06 |
| Ankle | CP | Normal | AT | 0.14 | 0.04 |
| Ankle | TD | Normal | DT | 0.09 | 0.04 |
| Ankle | TD | Normal | AT | 0.06 | 0.01 |
| Ankle | CP | Slow | DT | 0.15 | 0.03 |
| Ankle | CP | Slow | AT | 0.13 | 0.03 |
| Ankle | TD | Slow | DT | 0.07 | 0.03 |
| Ankle | TD | Slow | AT | 0.06 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odanye, O.; Harrington, J.; Likens, A.; Kingston, D.; Knarr, B. An Aquatic Treadmill Alters Lower Limb Walking Dynamics in Typically Developing Children and Children with Cerebral Palsy. Sensors 2025, 25, 3220. https://doi.org/10.3390/s25103220
Odanye O, Harrington J, Likens A, Kingston D, Knarr B. An Aquatic Treadmill Alters Lower Limb Walking Dynamics in Typically Developing Children and Children with Cerebral Palsy. Sensors. 2025; 25(10):3220. https://doi.org/10.3390/s25103220
Chicago/Turabian StyleOdanye, Oluwaseye, Joseph Harrington, Aaron Likens, David Kingston, and Brian Knarr. 2025. "An Aquatic Treadmill Alters Lower Limb Walking Dynamics in Typically Developing Children and Children with Cerebral Palsy" Sensors 25, no. 10: 3220. https://doi.org/10.3390/s25103220
APA StyleOdanye, O., Harrington, J., Likens, A., Kingston, D., & Knarr, B. (2025). An Aquatic Treadmill Alters Lower Limb Walking Dynamics in Typically Developing Children and Children with Cerebral Palsy. Sensors, 25(10), 3220. https://doi.org/10.3390/s25103220






