Biostrap Kairos Wristband Versus Electrocardiography for Resting Heart Rate Variability Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Electrocardiography
2.4. Wristband
2.5. Skin Pigmentation
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Agreement for Resting Heart Rate
4.2. Agreement for Time Domain Heart Rate Variability Parameters
4.3. Agreement for Frequency Domain Heart Rate Variability Parameters
4.4. Agreement for Non-Linear Heart Rate Variability Parameters
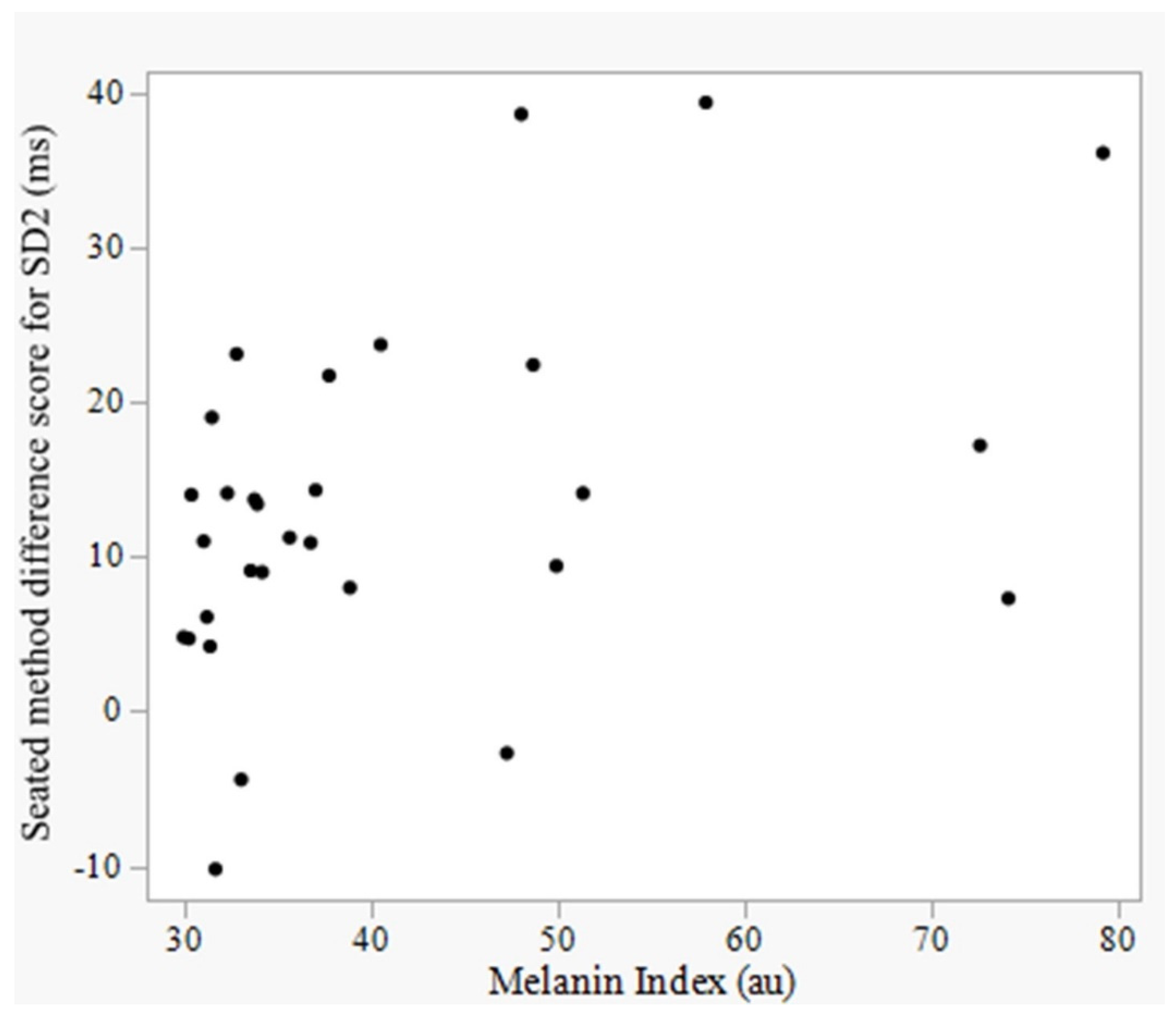
4.5. Effect of Skin Pigmentation on Device Accuracy
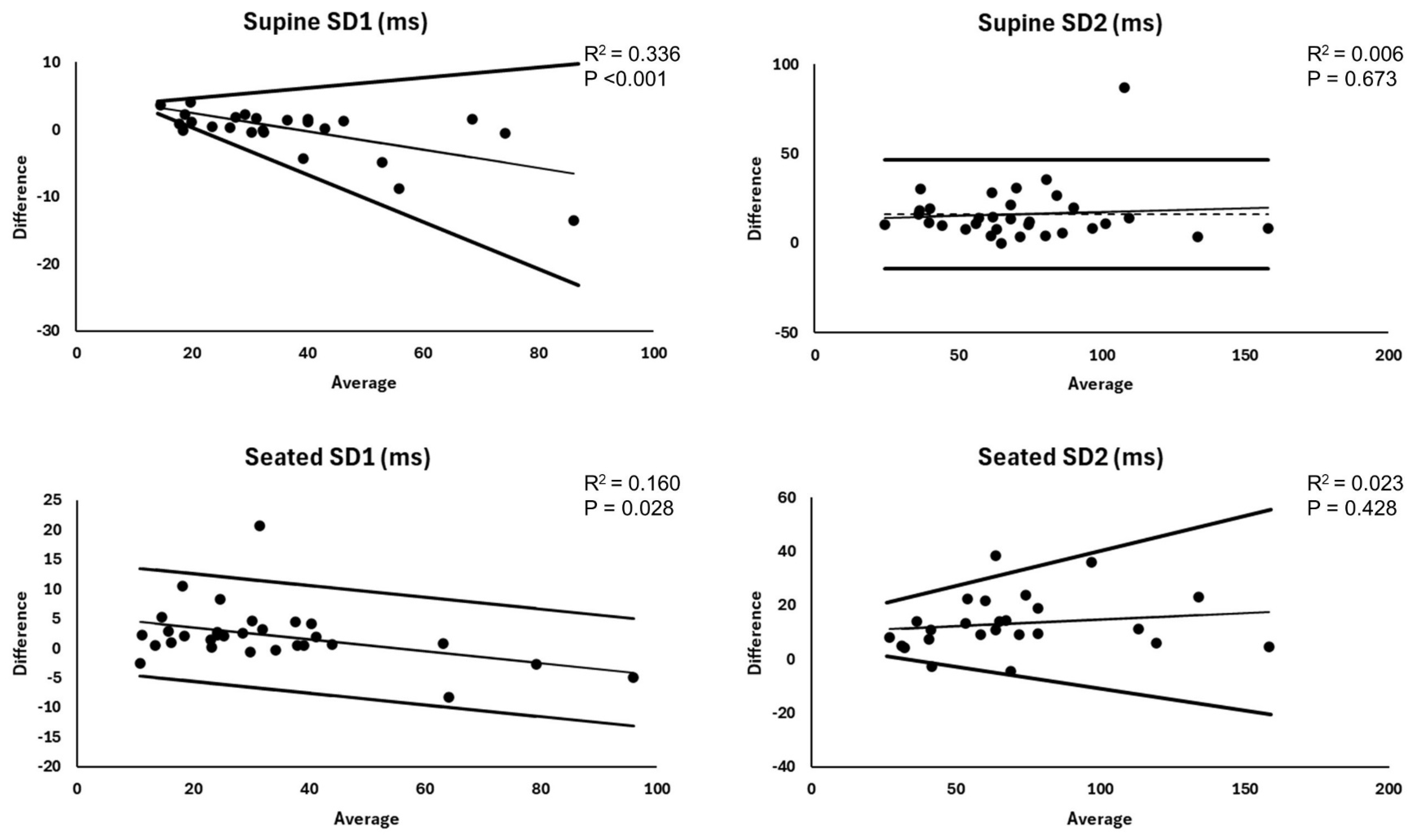
4.6. Heteroscedasticity and Proportional Bias
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Tulppo, M.P.; Kiviniemi, A.M.; Junttila, M.J.; Huikuri, H.V. Home monitoring of heart rate as a predictor of imminent cardiovascular events. Front. Physiol. 2019, 10, 347. [Google Scholar] [CrossRef]
- Flatt, A.A.; Allen, J.R.; Keith, C.M.; Martinez, M.W.; Esco, M.R. Season-long heart-rate variability tracking reveals autonomic imbalance in american college football players. Int. J. Sports Physiol. Perform. 2021, 16, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Heart rate variability in elite triathletes, is variation in variability the key to effective training? A case comparison. Eur. J. Appl. Physiol. 2012, 112, 3729–3741. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Wilkerson, G.B.; Allen, J.R.; Keith, C.M.; Esco, M.R. Daily heart rate variability before and after concussion in an american college football player. Sports 2019, 7, 97. [Google Scholar] [CrossRef]
- Natarajan, A.; Pantelopoulos, A.; Emir-Farinas, H.; Natarajan, P. Heart rate variability with photoplethysmography in 8 million individuals: A cross-sectional study. Lancet Digit. Health 2020, 2, e650–e657. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Culver, M.N.; McMillan, N.K.; Cross, B.L.; Montoye, A.H.; Riemann, B.L.; Flatt, A.A. Self-recorded heart rate variability profiles are associated with health and lifestyle markers in young adults. Clin. Auton. Res. 2022, 32, 507–518. [Google Scholar] [CrossRef]
- Dhingra, L.S.; Aminorroaya, A.; Oikonomou, E.K.; Nargesi, A.A.; Wilson, F.P.; Krumholz, H.M.; Khera, R. Use of wearable devices in individuals with or at risk for cardiovascular disease in the US, 2019 to 2020. JAMA Netw. Open 2023, 6, e2316634. [Google Scholar] [CrossRef]
- A’Naja, M.N.; Reed, R.; Sansone, J.; Batrakoulis, A.; McAvoy, C.; Parrott, M.W. 2024 ACSM worldwide fitness trends: Future directions of the health and fitness industry. ACSM’s Health Fit. J. 2024, 28, 14–26. [Google Scholar]
- Knight, S.; Lipoth, J.; Namvari, M.; Gu, C.; Hedayati, M.; Syed-Abdul, S.; Spiteri, R.J. The accuracy of wearable photoplethysmography sensors for telehealth monitoring: A scoping review. Telemed. e-Health 2023, 29, 813–828. [Google Scholar] [CrossRef]
- Cao, R.; Azimi, I.; Sarhaddi, F.; Niela-Vilén, H.; Axelin, A.; Liljeberg, P.; Rahmani, A.M. Accuracy assessment of oura ring nocturnal heart rate and heart rate variability in comparison with electrocardiography in time and frequency domains: Comprehensive analysis. J. Med. Internet Res. 2022, 24, e27487. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Roca, S.; Sancho, J.; Alesanco, Á.; Bailón, R. Validation of the apple watch for heart rate variability measurements during relax and mental stress in healthy subjects. Sensors 2018, 18, 2619. [Google Scholar] [CrossRef]
- Theurl, F.; Schreinlechner, M.; Sappler, N.; Toifl, M.; Dolejsi, T.; Hofer, F.; Massmann, C.; Steinbring, C.; Komarek, S.; Mölgg, K.; et al. Smartwatch-derived heart rate variability: A head-to-head comparison with the gold standard in cardiovascular disease. Eur. Heart J. Digit. Health 2023, 4, 155–164. [Google Scholar] [CrossRef]
- Stuyck, H.; Dalla Costa, L.; Cleeremans, A.; Van den Bussche, E. Validity of the empatica E4 wristband to estimate resting-state heart rate variability in a lab-based context. Int. J. Psychophysiol. 2022, 182, 105–118. [Google Scholar] [CrossRef]
- Puranen, A.; Halkola, T.; Kirkeby, O.; Vehkaoja, A. Effect of skin tone and activity on the performance of wrist-worn optical beat-to-beat heart rate monitoring. In Proceedings of the 2020 IEEE SENSORS, Rotterdam, The Netherlands, 25–28 October 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Al-Halawani, R.; Qassem, M.; Kyriacou, P.A. Monte carlo simulation of the effect of melanin concentration on light-tissue interactions in transmittance and reflectance finger photoplethysmography. Sci. Rep. 2024, 14, 8145. [Google Scholar] [CrossRef] [PubMed]
- Christiani, M.; Grosicki, G.J.; Flatt, A.A. Cardiac-autonomic and hemodynamic responses to a hypertonic, sugar-sweetened sports beverage in physically active men. Appl. Physiol. Nutr. Metab. 2021, 46, 1189–1195. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Ranta-Aho, P.O.; Karjalainen, P.A. An advanced detrending method with application to HRV analysis. IEEE Trans. Biomed. Eng. 2002, 49, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chang, Y.; Kim, Y.; Shin, H.; Ryu, S. Ten-second heart rate variability, its changes over time, and the development of hypertension. Hypertension 2022, 79, 1308–1318. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Santos, I.S.; Dantas, E.M.; Andreão, R.V.; Schmidt, M.I.; Duncan, B.B.; Mill, J.G.; Lotufo, P.A.; Bensenor, I. Decreased heart rate variability as a predictor for diabetes—A prospective study of the brazilian longitudinal study of adult health. Diabetes Metab. Res. Rev. 2019, 35, e3175. [Google Scholar] [CrossRef]
- Jandackova, V.K.; Scholes, S.; Britton, A.; Steptoe, A. Midlife heart rate variability and cognitive decline: A large longitudinal cohort study. Int. J. Clin. Health Psychol. 2024, 24, 100518. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-B.; Lai, H.-Z.; Long, J.; Ma, Q.; Fu, X.; You, F.-M.; Xiao, C. Vagal nerve activity and cancer prognosis: A systematic review and meta-analysis. BMC Cancer 2025, 25, 579. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Stein, P.K. Heart rate variability in risk stratification of cardiac patients. Prog. Cardiovasc. Dis. 2013, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A.B.; Siedlik, J.A.; Wecht, J.M.; Deckert, J.A.; Nguyen, N.D.; Weir, J.P. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 2017, 56, 674–678. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Pastre, C.M.; Vanderlei, L.C.M.; Godoy, M.F. Poincaré plot indexes of heart rate variability: Relationships with other nonlinear variables. Auton. Neurosci. 2013, 177, 271–274. [Google Scholar] [CrossRef]
- Flatt, A.A.; Howells, D.; Williams, S. Effects of consecutive domestic and international tournaments on heart rate variability in an elite rugby sevens team. J. Sci. Med. Sport 2018, 22, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Esco, M.R.; Flatt, A.A.; Nakamura, F.Y. Agreement between a smartphone pulse sensor application and electrocardiography for determining lnRMSSD. J. Strength Cond. Res. 2017, 31, 380–385. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R. Heart rate variability stabilization in athletes: Towards more convenient data acquisition. Clin. Physiol. Funct. Imaging 2016, 36, 331–336. [Google Scholar] [CrossRef]
- Ludbrook, J. Confidence in altman–bland plots: A critical review of the method of differences. Clin. Exp. Pharmacol. Physiol. 2010, 37, 143–149. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Lawrence, I.; Lin, K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bennett, M.R.; Chen, C.; Shin, S.; Ghanbari, H.; Nelson, B.W. Impact of skin pigmentation on pulse oximetry blood oxygenation and wearable pulse rate accuracy: Systematic review and meta-analysis. J. Med. Internet Res. 2024, 26, e62769. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nuuttila, O.-P.; Korhonen, E.; Laukkanen, J.; Kyröläinen, H. Validity of the wrist-worn polar vantage V2 to measure heart rate and heart rate variability at rest. Sensors 2022, 22, 137. [Google Scholar] [CrossRef]
- O’Grady, B.; Lambe, R.; Baldwin, M.; Acheson, T.; Doherty, C. The validity of apple watch series 9 and ultra 2 for serial measurements of heart rate variability and resting heart rate. Sensors 2024, 24, 6220. [Google Scholar] [CrossRef]
- Nissen, M.; Slim, S.; Jäger, K.; Flaucher, M.; Huebner, H.; Danzberger, N.; Fasching, P.A.; Beckmann, M.W.; Gradl, S.; Eskofier, B.M. Heart rate measurement accuracy of fitbit charge 4 and samsung galaxy watch active2: Device evaluation study. JMIR Form. Res. 2022, 6, e33635. [Google Scholar] [CrossRef]
- Williams, K.; Jamieson, A.; Chaturvedi, N.; Hughes, A.; Orini, M. Validation of wearable derived heart rate variability and oxygen saturation from the garmin’s health snapshot. In Proceedings of the 2023 Computing in Cardiology (CinC), Atlanta, GA, USA, 1–4 October 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–4. [Google Scholar]
- Vondrasek, J.D.; Riemann, B.L.; Grosicki, G.J.; Flatt, A.A. Validity and efficacy of the elite HRV smartphone application during slow-paced breathing. Sensors 2023, 23, 9496. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef]
- Gisselman, A.S.; D’Amico, M.; Smoliga, J.M. Optimizing intersession reliability of heart rate variability—The effects of artifact correction and breathing type. J. Strength Cond. Res. 2020, 34, 3199–3207. [Google Scholar] [CrossRef]
- Penttilä, J.; Helminen, A.; Jartti, T.; Kuusela, T.; Huikuri, H.V.; Tulppo, M.P.; Coffeng, R.; Scheinin, H. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clin. Physiol. 2001, 21, 365–376. [Google Scholar] [CrossRef]
- Heathers, J.A.J. Everything hertz: Methodological issues in short-term frequency-domain HRV. Front. Physiol. 2014, 5, 177. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Esco, M.R. Validity of the ithleteTM smart phone application for determining ultra-short-term heart rate variability. J. Hum. Kinet. 2013, 39, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Miller, D.J.; Halson, S.L.; Roach, G.D.; Sargent, C. Wrist-based photoplethysmography assessment of heart rate and heart rate variability: Validation of WHOOP. Sensors 2021, 21, 3571. [Google Scholar] [CrossRef]
- Stone, J.D.; Ulman, H.K.; Tran, K.; Thompson, A.G.; Halter, M.D.; Ramadan, J.H.; Stephenson, M.; Finomore, V.S., Jr.; Galster, S.M.; Rezai, A.R.; et al. Assessing the accuracy of popular commercial technologies that measure resting heart rate and heart rate variability. Front. Sports Act. Living 2021, 3, 585870. [Google Scholar] [CrossRef] [PubMed]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef]
- Koerber, D.; Khan, S.; Shamsheri, T.; Kirubarajan, A.; Mehta, S. Accuracy of heart rate measurement with wrist-worn wearable devices in various skin tones: A systematic review. J. Racial Ethn. Health Disparities 2023, 10, 2676–2684. [Google Scholar] [CrossRef]
- Mühlen, J.M.; Stang, J.; Skovgaard, E.L.; Judice, P.B.; Molina-Garcia, P.; Johnston, W.; Sardinha, L.B.; Ortega, F.B.; Caulfield, B.; Bloch, W. Recommendations for determining the validity of consumer wearable heart rate devices: Expert statement and checklist of the INTERLIVE network. Br. J. Sports Med. 2021, 55, 767–779. [Google Scholar] [CrossRef]



| Descriptor | Supine Comparison (n = 32) | Seated Comparison (n = 30) |
|---|---|---|
| Male/Female | 13/19 | 12/18 |
| Age (years) | 21.7 ± 3.3 | 21.4 ± 3.0 |
| Height (cm) | 169.1 ± 10.5 | 169.5 ± 9.5 |
| Mass (kg) | 76.3 ± 15.3 | 75.0 ± 13.3 |
| Melanin Index (au) | 35.3 (16.6) | 34.9 (16.6) |
| Metric | Device | Mean ± SD or Median (IQR) | β | Bias | LOA (95%) | MAE | MAPE% | CCC |
|---|---|---|---|---|---|---|---|---|
| RHR (bpm) | Wristband | 71.1 ± 9.5 | −0.266 | 0.0 ± 0.7 | −1.3–1.3 | 0.3 ± 0.6 | 0.5 ± 0.9 | 0.99 |
| ECG | 71.1 ± 9.7 | |||||||
| RMSSD (ms) | Wristband | 53.3 ± 22.3 | −0.762 *** | 9.95–0.23 * A | Bias ± 2.46 * (−0.16 + 0.073 * A) | 5.1 ± 6.5 | 8.0 ± 7.2 | 0.95 |
| ECG | 56.3 ± 28.2 | |||||||
| SDNN (ms) | Wristband | 54.0 ± 23.4 | −0.241 | 0.4 ± 4.7 | Bias ± 2.46 * (−0.35 + 0.06 * A) | 3.0 ± 3.6 | 5.8 ± 6.4 | 0.98 |
| ECG | 53.5 ± 24.5 | |||||||
| HF (ms2) | Wristband | 606.5 (1038.0) | −0.839 *** | 81.3–0.540 * A | Bias ± 2.46 * (36.5 + 0.14 * A) | 482.8 ± 536.6 | 30.2 ± 16.5 | 0.72 |
| ECG | 957.0 (1521.1) | |||||||
| LF (ms2) | Wristband | 497.0 (519.3) | −0.954 *** | 16.1–0.56 * A | Bias ± 2.46 * (97.4 + 0.09 * A) | 703.2 ± 1121.8 | 42.7 ± 21.9 | 0.74 |
| ECG | 749.6 (1221.4) | |||||||
| SD1 (ms) | Wristband | 39.7 ± 17.4 | −0.580 ** | 5.17–0.14 * A | Bias ± 2.46 * (−0.86 + 0.87 * A) | 2.8 ± 3.4 | 7.2 ± 7.5 | 0.97 |
| ECG | 39.9 ± 20.0 | |||||||
| SD2 (ms) | Wristband | 79.8 ± 30.8 | 0.078 | 16.2 ± 15.6 *** | −14.3–46.7 | 16.2 ± 15.6 | 33.5 ± 33.9 | 0.76 |
| ECG | 63.6 ± 29.6 |
| Metric | Device | Mean ± SD or Median (IQR) | β | Bias | LOA (95%) | MAE | MAPE% | CCC |
|---|---|---|---|---|---|---|---|---|
| RHR (bpm) | Wristband | 75.9 ± 9.7 | 0.085 | 0.1 ± 0.6 | −1.3–1.3 | 0.5 ± 0.4 | 0.6 ± 0.5 | 0.99 |
| ECG | 75.8 ± 9.7 | |||||||
| RMSSD (ms) | Wristband | 46.3 ± 24.6 | −0.646 *** | 9.95–0.23 * A | Bias ± 1.96 * 6.10 | 5.2 ± 5.9 | 14.4 ± 18.3 | 0.96 |
| ECG | 45.1 ± 29.7 | |||||||
| SDNN (ms) | Wristband | 52.5 ± 26.0 | −0.515 * | 3.34–0.07 * A | Bias ± 1.96 * 3.52 | 2.9 ± 2.8 | 6.1 ± 6.4 | 0.99 |
| ECG | 53.2 ± 28.1 | |||||||
| HF (ms2) | Wristband | 494.0 (710.3) | −0.967 *** | 208.9–(0.70 * A) | Bias ± 2.46 * (103.6 + 0.07 * A) | 483.9 ± 878.5 | 41.4 ± 47.6 | 0.70 |
| ECG | 609.1 (1092.6) | |||||||
| LF (ms2) | Wristband | 542.5 (1006.5) | −0.926 *** | 209.3–(0.80 * A) | Bias ± 2.46 * (226.1 + 0.10 * A) | 791.8 ± 1461.1 | 34.1 ± 19.4 | 0.65 |
| ECG | 698.5 (1084.8) | |||||||
| SD1 (ms) | Wristband | 34.2 ± 19.1 | −0.400 * | 5.50–0.10 * A | Bias ± 1.96 * 4.64 | 3.5 ± 4.1 | 15.9 ± 22.7 | 0.96 |
| ECG | 32.0 ± 21.0 | |||||||
| SD2 (ms) | Wristband | 80.7 ± 36.4 | 0.150 | 13.4 ± 11.4 *** | Bias ± 2.46 * (1.84 + 0.09 * A) | 14.6 ± 9.8 | 25.5 ± 17.9 | 0.89 |
| ECG | 67.3 ± 34.8 |
| Supine | Seated | |||
|---|---|---|---|---|
| Parameter | ρ | p | ρ | p |
| RHR | −0.034 | 0.854 | −0.132 | 0.486 |
| RMSSD | −0.195 | 0.284 | −0.067 | 0.727 |
| SDNN | −0.339 | 0.058 | 0.203 | 0.281 |
| HF | 0.057 | 0.757 | −0.214 | 0.257 |
| LF | 0.282 | 0.118 | 0.213 | 0.258 |
| SD1 | −0.325 | 0.070 | −0.081 | 0.670 |
| SD2 | 0.131 | 0.474 | 0.450 | 0.013 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flatt, A.A.; Blalock, A.C.E.; Wade, A.N.; Riemann, B.L. Biostrap Kairos Wristband Versus Electrocardiography for Resting Heart Rate Variability Assessment. Sensors 2025, 25, 3165. https://doi.org/10.3390/s25103165
Flatt AA, Blalock ACE, Wade AN, Riemann BL. Biostrap Kairos Wristband Versus Electrocardiography for Resting Heart Rate Variability Assessment. Sensors. 2025; 25(10):3165. https://doi.org/10.3390/s25103165
Chicago/Turabian StyleFlatt, Andrew A., Ann Claire E. Blalock, Allison N. Wade, and Bryan L. Riemann. 2025. "Biostrap Kairos Wristband Versus Electrocardiography for Resting Heart Rate Variability Assessment" Sensors 25, no. 10: 3165. https://doi.org/10.3390/s25103165
APA StyleFlatt, A. A., Blalock, A. C. E., Wade, A. N., & Riemann, B. L. (2025). Biostrap Kairos Wristband Versus Electrocardiography for Resting Heart Rate Variability Assessment. Sensors, 25(10), 3165. https://doi.org/10.3390/s25103165








