Comparative Effects of Narrow vs. Wide Cuff Blood Flow Restriction on Muscle Synergy Dynamics: A Time-Frequency Decomposition Approach
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. 1RM Test
2.3. sEMG Select
2.4. Training Procedure
2.5. Data Analysis
2.5.1. Data Pre-Processing
2.5.2. NNMF Analysis
2.5.3. Outcome Variables
2.5.4. Statistical Analyses
3. Results
3.1. RMS Analysis of sEMG Signals During Non-BFRT, 5 cm-BFRT, and 10 cm-BFRT Squats
3.2. Muscle Synergy Number in BFRT Squats with Different Cuff Widths
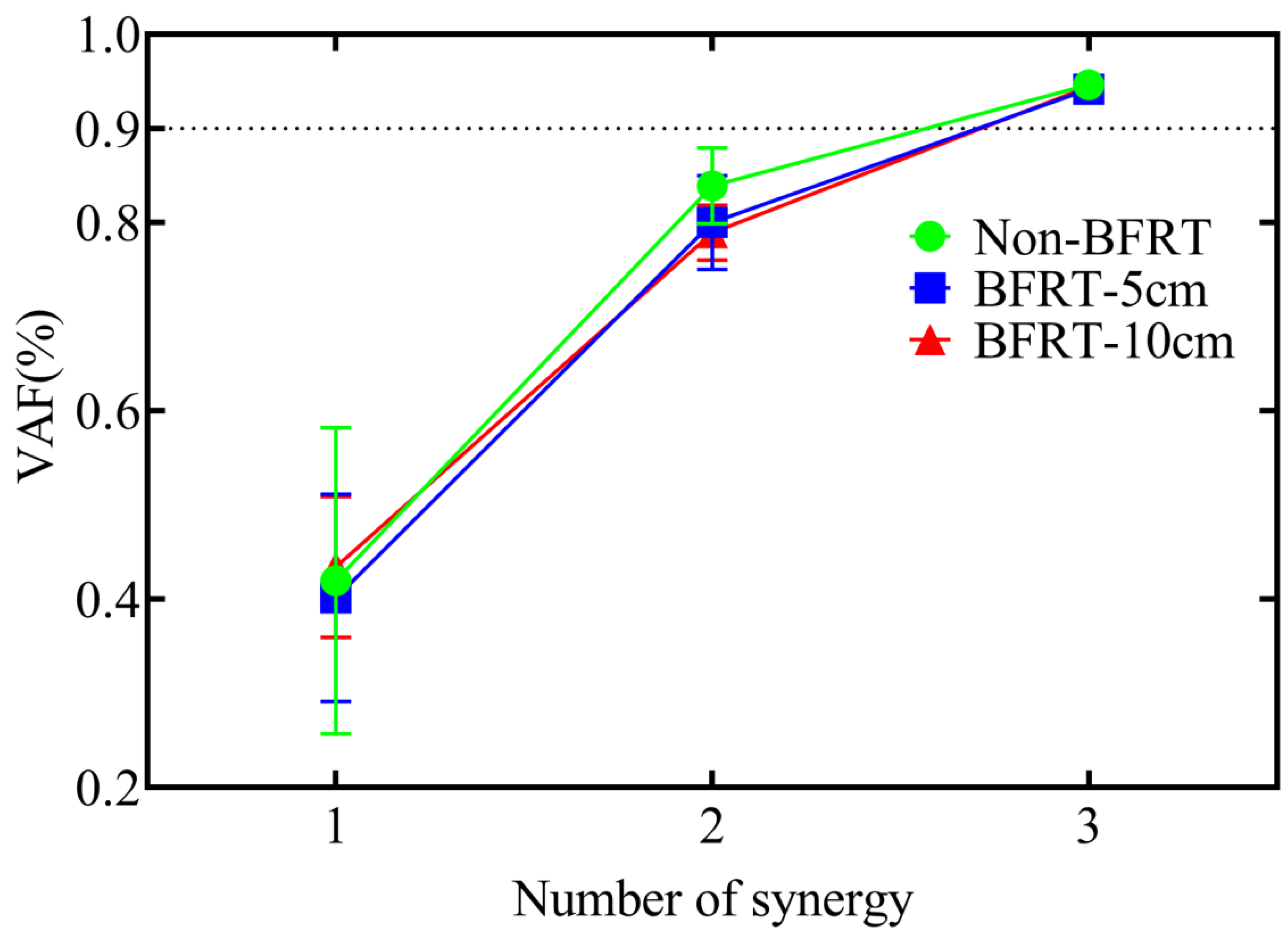
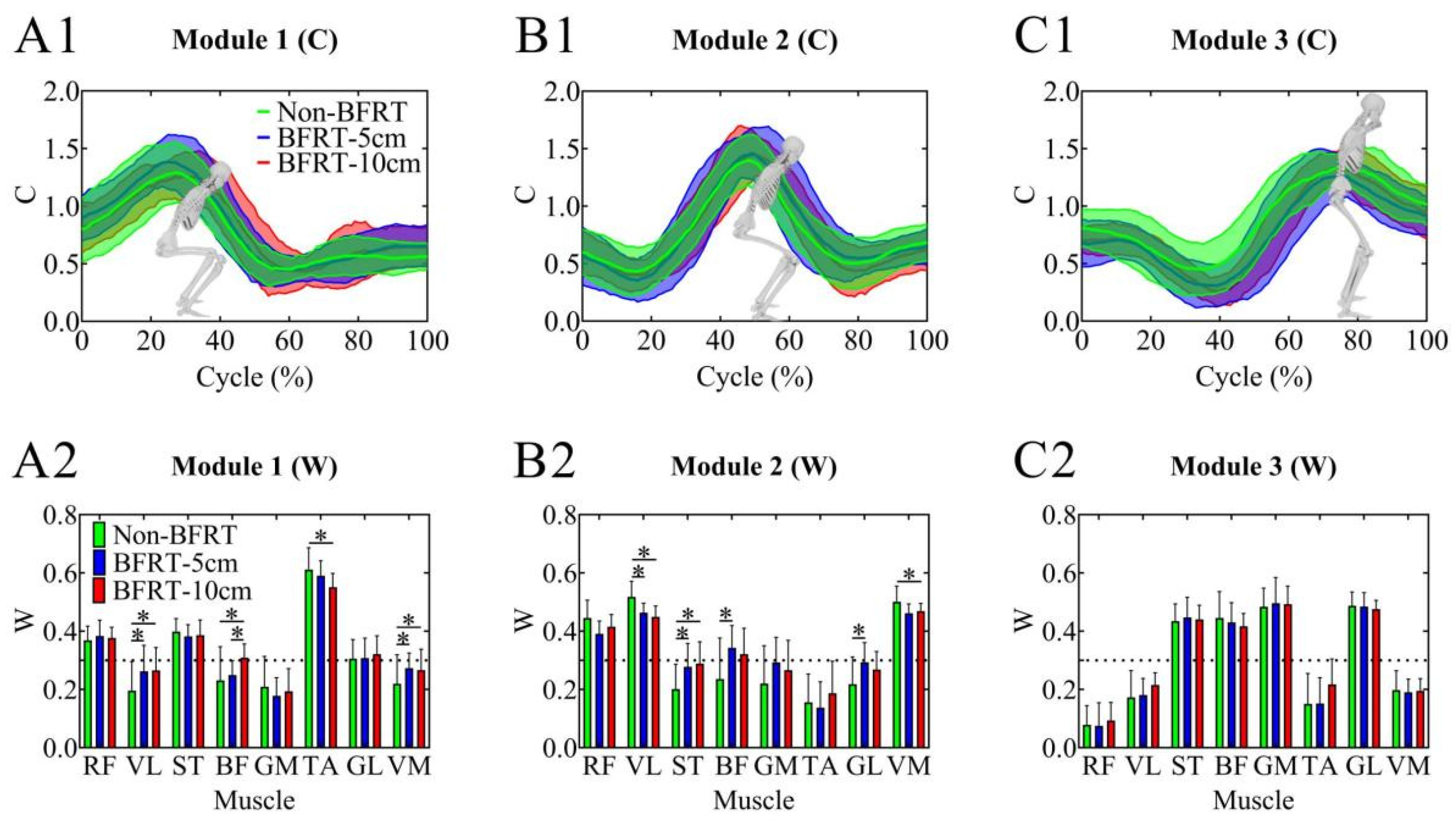
3.3. NNMF Results During BFRT Squats with Different Cuff Widths
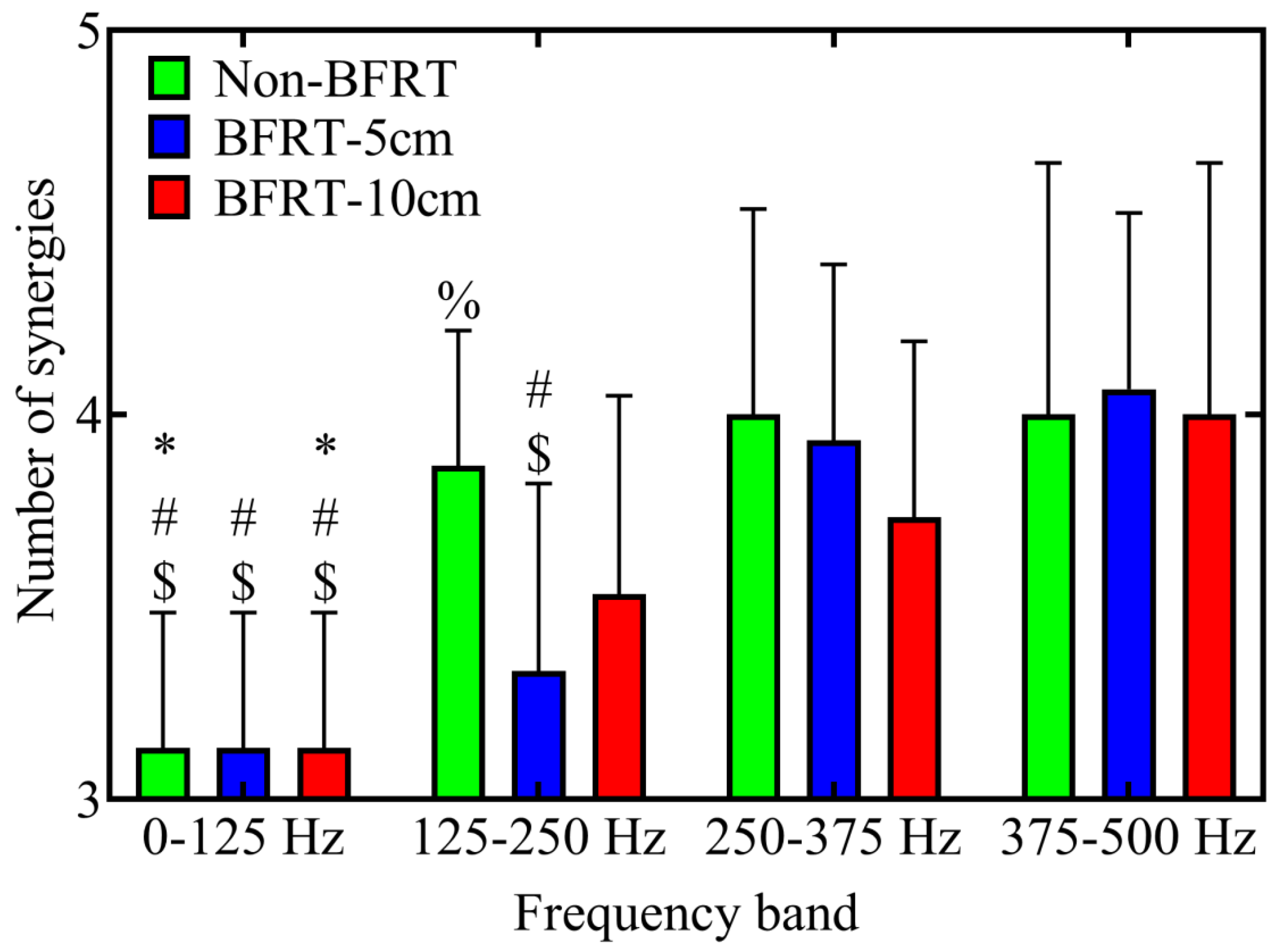
3.4. Muscle Synergy Analysis Within WPT-NNMF
4. Discussion
4.1. The RMS Results of Squat Movements Under Different Blood Flow Restriction Conditions
4.2. Changes in Muscle Synergy Numbers Based on NNMF
4.3. Changes in Muscle Synergy Modules Based on NNMF
4.4. Changes in Muscle Synergy Numbers Across Frequency Bands Under Different Training Conditions Based on Time-Frequency Synergy Analysis
4.5. Muscle Fatigue Considerations in BFRT
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wortman, R.J.; Brown, S.M.; Savage-Elliott, I.; Finley, Z.J.; Mulcahey, M.K. Blood Flow Restriction Training for Athletes: A Systematic Review. Am. J. Sports Med. 2021, 49, 1938–1944. [Google Scholar] [CrossRef]
- Lorenz, D.S.; Bailey, L.; Wilk, K.E.; Mangine, R.E.; Head, P.; Grindstaff, T.L.; Morrison, S. Blood Flow Restriction Training. J. Athl. Train. 2021, 56, 937–944. [Google Scholar] [CrossRef]
- Spranger, M.D.; Krishnan, A.C.; Levy, P.D.; O’Leary, D.S.; Smith, S.A. Blood flow restriction training and the exercise pressor reflex: A call for concern. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1440–H1452. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Morita, T.; Sato, Y. Key considerations when conducting KAATSU training. Int. J. KAATSU Train. Res. 2011, 7, 1–6. [Google Scholar] [CrossRef]
- Miller, B.C.; Tirko, A.W.; Shipe, J.M.; Sumeriski, O.R.; Moran, K. The Systemic Effects of Blood Flow Restriction Training: A Systematic Review. Int. J. Sports Phys. Ther. 2021, 16, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Glynn, E.L.; Drummond, M.J.; Timmerman, K.L.; Fujita, S.; Abe, T.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010, 108, 1199–1209. [Google Scholar] [CrossRef]
- Manini, T.M.; Yarrow, J.F.; Buford, T.W.; Clark, B.C.; Conover, C.F.; Borst, S.E. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2012, 22, 167–172. [Google Scholar] [CrossRef]
- Telfer, S.; Calhoun, J.; Bigham, J.J.; Mand, S.; Gellert, J.M.; Hagen, M.S.; Kweon, C.Y.; Gee, A.O. Biomechanical Effects of Blood Flow Restriction Training after ACL Reconstruction. Med. Sci. Sports Exerc. 2021, 53, 115–123. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wong, C.M.; Chen, Y.C.; Chen, Y.; Hwang, I.S. Differential training benefits and motor unit remodeling in wrist force precision tasks following high and low load blood flow restriction exercises under volume-matched conditions. J. Neuroeng. Rehabil. 2024, 21, 123. [Google Scholar] [CrossRef]
- Gosselink, K.L.; Grindeland, R.E.; Roy, R.R.; Zhong, H.; Bigbee, A.J.; Grossman, E.J.; Edgerton, V.R. Skeletal muscle afferent regulation of bioassayable growth hormone in the rat pituitary. J. Appl. Physiol. 1998, 84, 1425–1430. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Manini, T.M.; Hoffman, R.L.; Williams, P.S.; Guiler, M.K.; Knutson, M.J.; McGlynn, M.L.; Kushnick, M.R. Relative safety of 4 weeks of blood flow-restricted resistance exercise in young, healthy adults. Scand. J. Med. Sci. Sports 2011, 21, 653–662. [Google Scholar] [CrossRef]
- Grønfeldt, B.M.; Lindberg Nielsen, J.; Mieritz, R.M.; Lund, H.; Aagaard, P. Effect of blood-flow restricted vs heavy-load strength training on muscle strength: Systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 837–848. [Google Scholar] [CrossRef]
- Vechin, F.C.; Libardi, C.A.; Conceição, M.S.; Damas, F.R.; Lixandrão, M.E.; Berton, R.P.; Tricoli, V.A.; Roschel, H.A.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.; et al. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J. Strength Cond. Res. 2015, 29, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Jessee, M.B.; Buckner, S.L.; Dankel, S.J.; Counts, B.R.; Abe, T.; Loenneke, J.P. The Influence of Cuff Width, Sex, and Race on Arterial Occlusion: Implications for Blood Flow Restriction Research. Sports Med. 2016, 46, 913–921. [Google Scholar] [CrossRef]
- Mouser, J.G.; Laurentino, G.C.; Dankel, S.J.; Buckner, S.L.; Jessee, M.B.; Counts, B.R.; Mattocks, K.T.; Loenneke, J.P. Blood flow in humans following low-load exercise with and without blood flow restriction. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Et Metab. 2017, 42, 1165–1171. [Google Scholar] [CrossRef]
- Kacin, A.; Strazar, K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand. J. Med. Sci. Sports 2011, 21, e231–e241. [Google Scholar] [CrossRef]
- Rolnick, N.; Cerqueira, M.S. Comparison of blood flow restriction devices and their effect on quadriceps muscle activation: Letter to the editor. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2021, 49, 227–228. [Google Scholar] [CrossRef]
- Laurentino, G.C.; Loenneke, J.P.; Teixeira, E.L.; Nakajima, E.; Iared, W.; Tricoli, V. The Effect of Cuff Width on Muscle Adaptations after Blood Flow Restriction Training. Med. Sci. Sports Exerc. 2016, 48, 920–925. [Google Scholar] [CrossRef]
- Rossow, L.M.; Fahs, C.A.; Loenneke, J.P.; Thiebaud, R.S.; Sherk, V.D.; Abe, T.; Bemben, M.G. Cardiovascular and perceptual responses to blood-flow-restricted resistance exercise with differing restrictive cuffs. Clin. Physiol. Funct. Imaging 2012, 32, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Borzelli, D.; Vieira, T.M.M.; Botter, A.; Gazzoni, M.; Lacquaniti, F.; d’Avella, A. Synaptic inputs to motor neurons underlying muscle coactivation for functionally different tasks have different spectral characteristics. J. Neurophysiol. 2024, 131, 1126–1142. [Google Scholar] [CrossRef] [PubMed]
- d’Avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef]
- Kupa, E.J.; Roy, S.H.; Kandarian, S.C.; De Luca, C.J. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J. Appl. Physiol. 1995, 79, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Merlo, E.; Pozzo, M.; Antonutto, G.; di Prampero, P.E.; Merletti, R.; Farina, D. Time-frequency analysis and estimation of muscle fiber conduction velocity from surface EMG signals during explosive dynamic contractions. J. Neurosci. Methods 2005, 142, 267–274. [Google Scholar] [CrossRef]
- Grosse, P.; Cassidy, M.J.; Brown, P. EEG-EMG, MEG-EMG and EMG-EMG frequency analysis: Physiological principles and clinical applications. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 1523–1531. [Google Scholar] [CrossRef]
- Xie, P.; Chang, Q.; Zhang, Y.; Dong, X.; Yu, J.; Chen, X. Estimation of Time-Frequency Muscle Synergy in Wrist Movements. Entropy 2022, 24, 707. [Google Scholar] [CrossRef]
- Lowery, M.; Nolan, P.; O’malley, M. Electromyogram median frequency, spectral compression and muscle fibre conduction velocity during sustained sub-maximal contraction of the brachioradialis muscle. J. Electromyogr. Kinesiol. 2002, 12, 111–118. [Google Scholar] [CrossRef]
- Frère, J.; Göpfert, B.; Slawinski, J.; Tourny-Chollet, C.J.H.m.s. Shoulder muscles recruitment during a power backward giant swing on high bar: A wavelet-EMG-analysis. Hum. Mov. Sci. 2012, 31, 472–485. [Google Scholar] [CrossRef]
- Chen, X.; Xie, P.; Liu, H.; Song, Y.; Du, Y. Local Band Spectral Entropy Based on Wavelet Packet Applied to Surface EMG Signals Analysis. Entropy 2016, 18, 41. [Google Scholar] [CrossRef]
- Zhu, F.; Li, Y.; Shi, Z.; Shi, W. TV-NARX and Coiflets WPT based time-frequency Granger causality with application to corticomuscular coupling in hand-grasping. Front. Neurosci. 2022, 16, 1014495. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Kim, D.; Fahs, C.A.; Thiebaud, R.S.; Abe, T.; Larson, R.D.; Bemben, D.A.; Bemben, M.G. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve 2015, 51, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Basili, T.; Meletani, S.; Scaradozzi, D.J.I.A. Wavelet-based assessment of the muscle-activation frequency range by EMG analysis. IEEE Access 2022, 10, 9793–9805. [Google Scholar] [CrossRef]
- Merletti, R.; Di Torino, P.J.J.E.K. Standards for reporting EMG data. Int. Soc. Electrophysiol. Kinesiol. 1999, 9, 3–4. [Google Scholar]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef]
- Kim, H.; Palmieri-Smith, R.; Kipp, K. Muscle Synergies in People With Chronic Ankle Instability During Anticipated and Unanticipated Landing-Cutting Tasks. J. Athl. Train. 2023, 58, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fabero-Garrido, R.; Gragera-Vela, M.; del Corral, T.; Hernández-Martín, M.; Plaza-Manzano, G.; López-de-Uralde-Villanueva, I. Effects of Low-Load Blood Flow Restriction Training on Muscle Anabolism Biomarkers and Thrombotic Biomarkers Compared with Traditional Training in Healthy Adults Older Than 60 Years: Systematic Review and Meta-Analysis. Life 2024, 14, 411. [Google Scholar] [CrossRef]
- Clark, D.R.; Lambert, M.I.; Hunter, A.M. Muscle Activation in the Loaded Free Barbell Squat: A Brief Review. J. Strength Cond. Res. 2012, 26, 1169–1178. [Google Scholar] [CrossRef]
- Dionisio, V.C.; Almeida, G.L.; Duarte, M.; Hirata, R.P. Kinematic, kinetic and EMG patterns during downward squatting. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2008, 18, 134–143. [Google Scholar] [CrossRef]
- Jeong, H.; Haghighat, P.; Kantharaju, P.; Jacobson, M.; Jeong, H.; Kim, M. Muscle coordination and recruitment during squat assistance using a robotic ankle–foot exoskeleton. Sci. Rep. 2023, 13, 1363. [Google Scholar] [CrossRef]
- Matsunaga, N.; Aoki, K.; Kaneoka, K. Comparison of Modular Control during Side Cutting before and after Fatigue. Appl. Bionics Biomech. 2021, 2021, 8860207. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Goto, R.; Naka, T. Effects of orthoses on muscle activity and synergy during gait. PLoS ONE 2023, 18, e0281541. [Google Scholar] [CrossRef]
- Kenville, R.; Clauß, M.; Maudrich, T. Investigating the impact of external load on muscle synergies during bipedal squats. Eur. J. Appl. Physiol. 2024, 124, 2035–2044. [Google Scholar] [CrossRef]
- van der Kruk, E.; Silverman, A.K.; Koizia, L.; Reilly, P.; Fertleman, M.; Bull, A.M.J. Age-related compensation: Neuromusculoskeletal capacity, reserve & movement objectives. J. Biomech. 2021, 122, 110385. [Google Scholar] [CrossRef]
- Bowman, E.N.; Elshaar, R.; Milligan, H.; Jue, G.; Mohr, K.; Brown, P.; Watanabe, D.M.; Limpisvasti, O. Proximal, Distal, and Contralateral Effects of Blood Flow Restriction Training on the Lower Extremities: A Randomized Controlled Trial. Sports Health 2019, 11, 149–156. [Google Scholar] [CrossRef]
- Yavuz, H.U.; Erdağ, D.; Amca, A.M.; Aritan, S. Kinematic and EMG activities during front and back squat variations in maximum loads. J. Sports Sci. 2015, 33, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Ellefsen, S.; Hammarström, D.; Strand, T.A.; Zacharoff, E.; Whist, J.E.; Rauk, I.; Nygaard, H.; Vegge, G.; Hanestadhaugen, M.; Wernbom, M.; et al. Blood flow-restricted strength training displays high functional and biological efficacy in women: A within-subject comparison with high-load strength training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R767–R779. [Google Scholar] [CrossRef]
- Taleshi, M.; Bubeck, F.; Brunner, P.; Gizzi, L.; Vujaklija, I. Observing changes in motoneuron characteristics following distorted sensorimotor input via blood flow restriction. J. Appl. Physiol. 2025, 138, 559–570. [Google Scholar] [CrossRef]
- Iskarevskii, G.V.; Pekonidi, A.A.; Beknazarova, A.M.; Pozdnyakova, A.E.; Onishchenko, D.A.; Kirsanov, A.S.; Baltin, M.E.; Bravyy, Y.R. Effect of Blood Flow Restriction on Recruitment Threshold and Amplitude- Frequency Characteristics of Motor Units During Exercise. In Proceedings of the 2024 Sixth International Conference Neurotechnologies and Neurointerfaces (CNN), Kaliningrad, Russia, 19–21 September 2024; pp. 44–46. [Google Scholar]
- Crenshaw, A.G.; Hargens, A.R.; Gershuni, D.H.; Rydevik, B. Wide tourniquet cuffs more effective at lower inflation pressures. Acta Orthop. Scand. 1988, 59, 447–451. [Google Scholar] [CrossRef]
- Mouser, J.G.; Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Buckner, S.L.; Counts, B.R.; Loenneke, J.P. A tale of three cuffs: The hemodynamics of blood flow restriction. Eur. J. Appl. Physiol. 2017, 117, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Spitz, R.W.; Chatakondi, R.N.; Bell, Z.W.; Wong, V.; Viana, R.B.; Dankel, S.J.; Abe, T.; Yamada, Y.; Loenneke, J.P. Blood Flow Restriction Exercise: Effects of Sex, Cuff Width, and Cuff Pressure on Perceived Lower Body Discomfort. Percept. Mot. Ski. 2021, 128, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Jiang, X.; Dai, C. Exploring Control Mechanism of Motoneuron Pools for the Forearm Antagonist Synergistic Muscles. IEEE Trans. Neural Syst. Rehabil. Eng. 2025, 33, 750–759. [Google Scholar] [CrossRef]
- Carlowitz-Ghori, K. Optimizing the Detection of Corticomuscular Coherence (CMC) with the Use of EEG and EMG Recordings. Doctoral Dissertation, Technische Universitat Berlin University, Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- L’Abbate, T.; Armonaite, K.; Gianni, E.; Bertoli, M.; Conti, L.; Grifoni, J.; Cancelli, A.; Cottone, C.; Trombetta, E.; Padalino, M.; et al. Corticomuscular Coherence Dependence on Body Side and Visual Feedback. Neuroscience 2022, 490, 144–154. [Google Scholar] [CrossRef]
- Stray-Gundersen, S.; Wooten, S.; Tanaka, H. Walking With Leg Blood Flow Restriction: Wide-Rigid Cuffs vs. Narrow-Elastic Bands. Front. Physiol. 2020, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, S.; Montesanto, P.; Lanzoni, I.M.; Gatta, G.; Cortesi, M.; Fantozzi, S. A Comparison between High and Low Cuff Pressures on Muscle Oxygen Saturation and Recovery Responses Following Blood-Flow Restriction Resistance Exercise. Sensors 2022, 22, 9138. [Google Scholar] [CrossRef]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef]

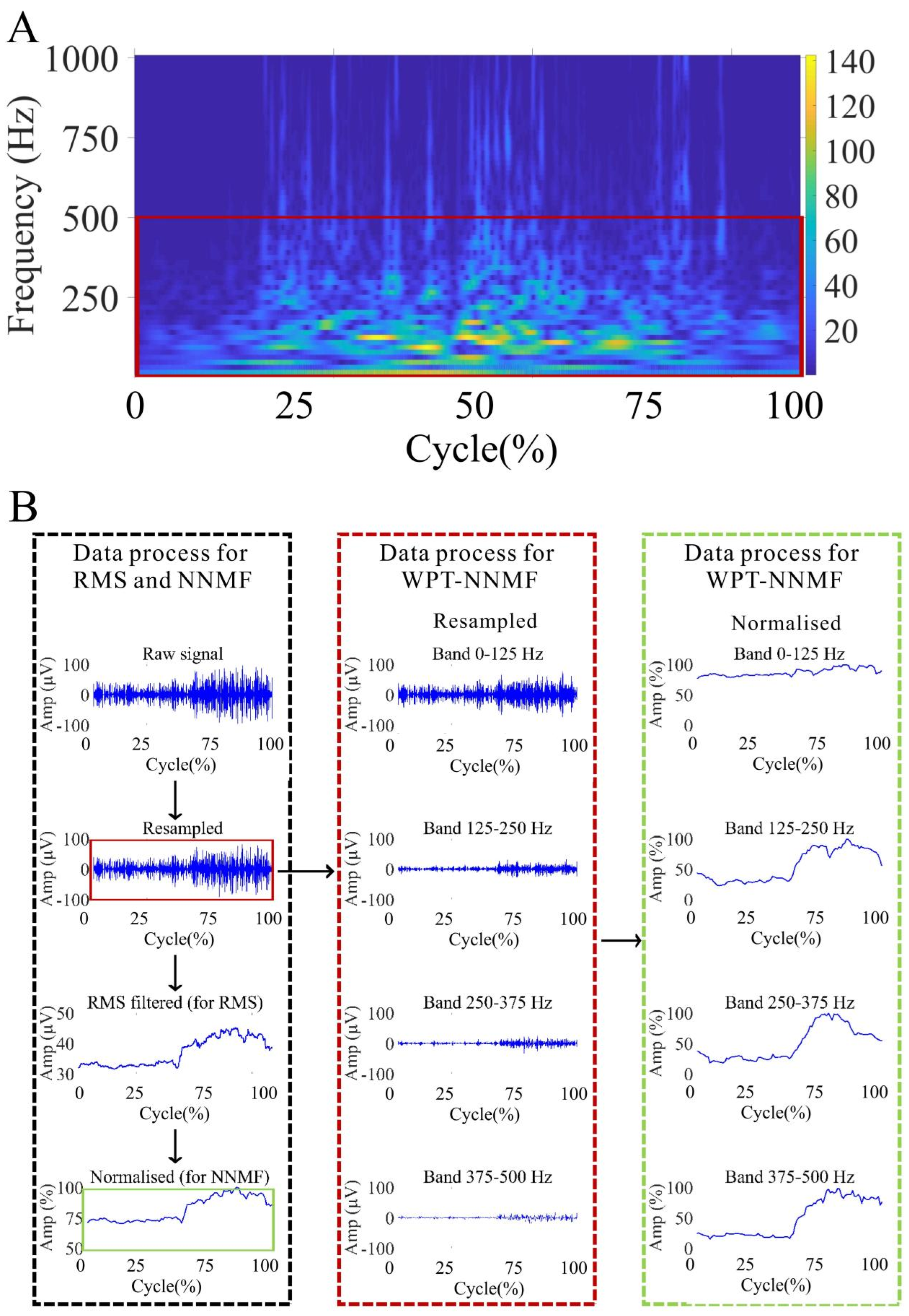


| Factor Type | Factor | Levels | Purpose |
|---|---|---|---|
| Independent | Cuff condition | Non-BFRT, 5 cm-BFRT, 10 cm-BFRT | To determine how cuff width modulates muscle activation and synergy patterns |
| Independent | Muscle | RF, VL, VM, ST, BF, GM, TA, GL | To compare activation (RMS) and synergy contributions (W) across eight lower-limb muscles |
| Independent | Synergy module (NNMF) | Module 1, Module 2, Module 3 | To examine changes in each module’s muscle weights (W) under different cuff conditions |
| Independent | Frequency band (WPT-NNMF) | 0–125 Hz, 125–250 Hz, 250–375 Hz, 375–500 Hz | To assess how time-frequency decomposition influences the number of synergies (K) |
| Dependent | RMS value | – | Reflects overall muscle activation |
| Dependent | Synergy weight (W) | – | NNMF-derived contribution of each muscle to its module |
| Dependent | Synergy module number (NNMF) | – | Number of NNMF modules needed to reach VAF > 0.9 in each frequency band |
| Dependent | Synergy module number (WPT-NNMF) | – | Number of WPT-NNMF modules extracted (time-domain synergies) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.; Hu, C. Comparative Effects of Narrow vs. Wide Cuff Blood Flow Restriction on Muscle Synergy Dynamics: A Time-Frequency Decomposition Approach. Sensors 2025, 25, 3154. https://doi.org/10.3390/s25103154
Chang S, Hu C. Comparative Effects of Narrow vs. Wide Cuff Blood Flow Restriction on Muscle Synergy Dynamics: A Time-Frequency Decomposition Approach. Sensors. 2025; 25(10):3154. https://doi.org/10.3390/s25103154
Chicago/Turabian StyleChang, Shuai, and Chenxi Hu. 2025. "Comparative Effects of Narrow vs. Wide Cuff Blood Flow Restriction on Muscle Synergy Dynamics: A Time-Frequency Decomposition Approach" Sensors 25, no. 10: 3154. https://doi.org/10.3390/s25103154
APA StyleChang, S., & Hu, C. (2025). Comparative Effects of Narrow vs. Wide Cuff Blood Flow Restriction on Muscle Synergy Dynamics: A Time-Frequency Decomposition Approach. Sensors, 25(10), 3154. https://doi.org/10.3390/s25103154






