FITA-Containing 2,4-Dinitrophenyl Alkylthioether-Based Probe for Detection and Imaging of GSH
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Probe (Scheme 2)
2.3. Mechanism Verification
2.4. Spectra Tests and Reaction Kinetics
2.5. Titration Experiments
2.6. Selective Tests
2.7. Cytotoxicity and Cell Imaging
3. Results
3.1. Reaction Mechanism Verification
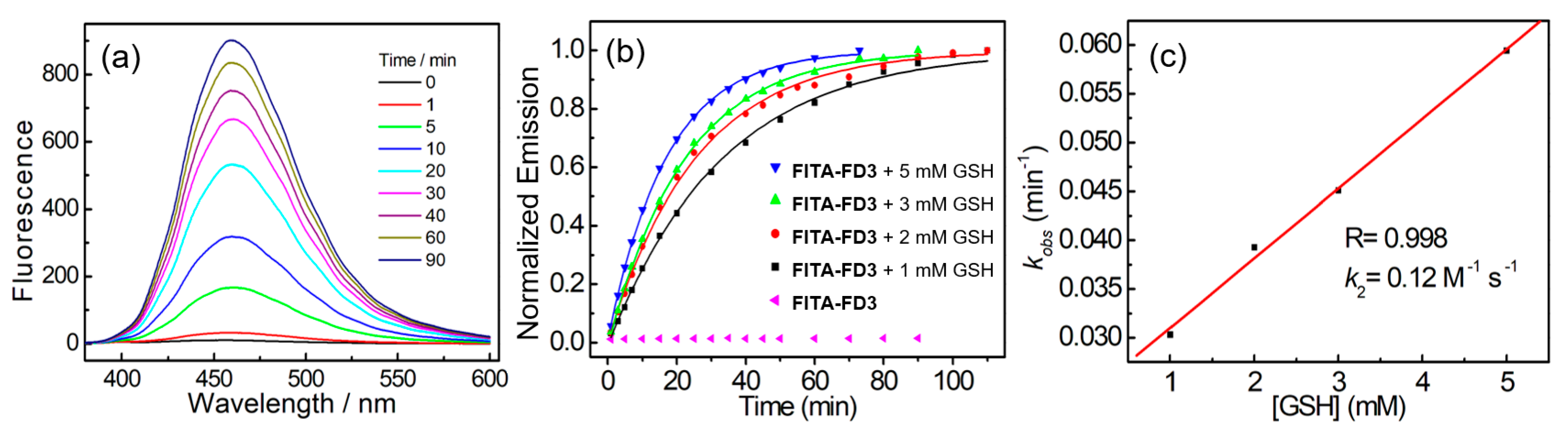
3.2. Reaction Kinetics
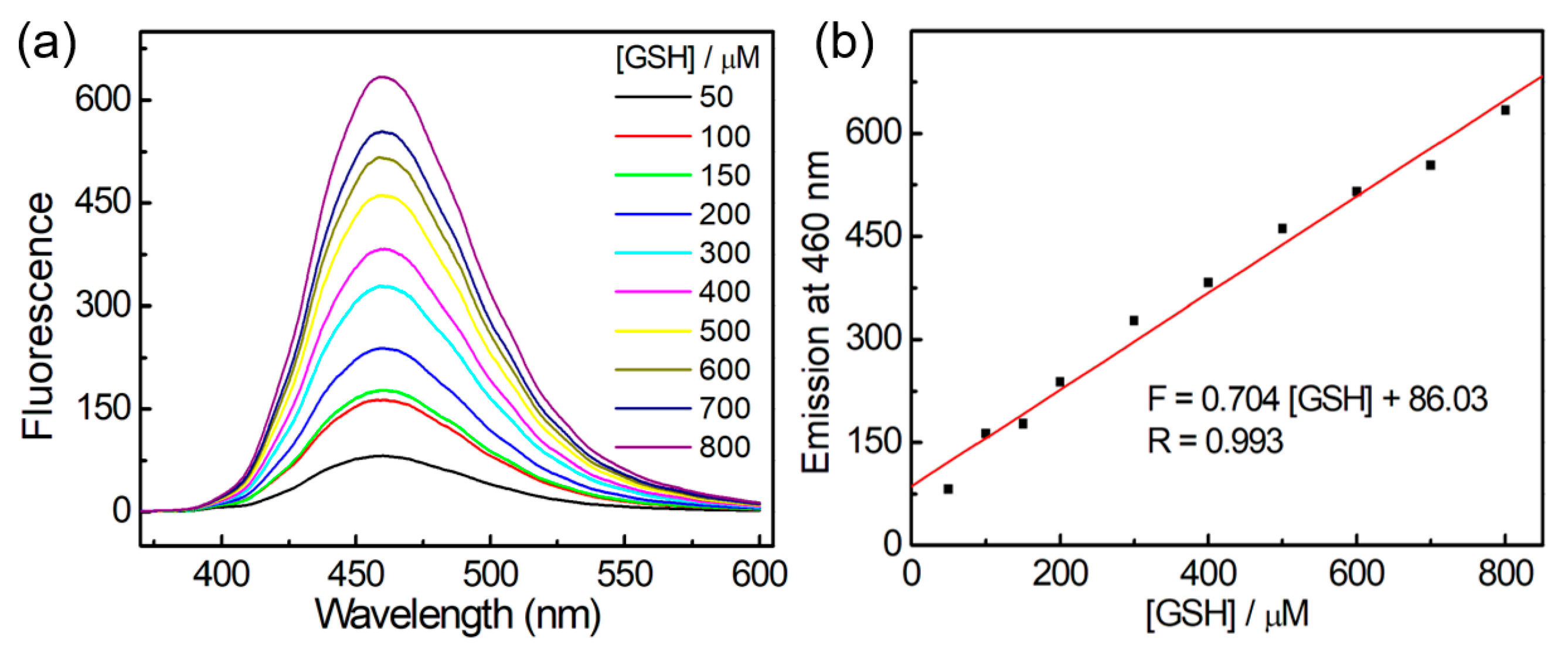
3.3. Titration Experiments of FITA-FD3
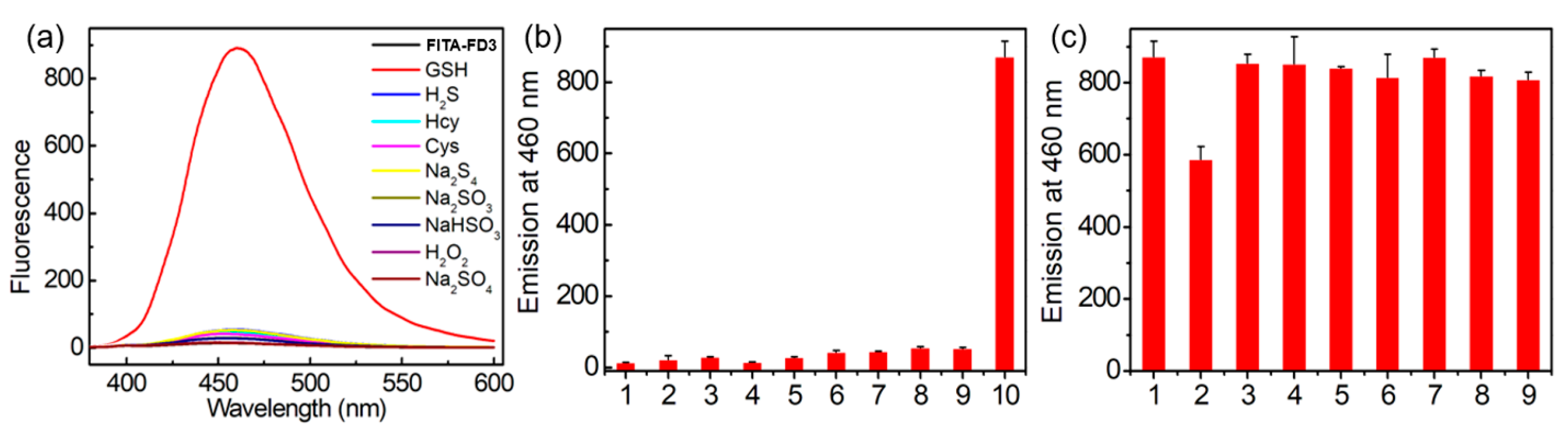
3.4. Selective Analysis Experiments
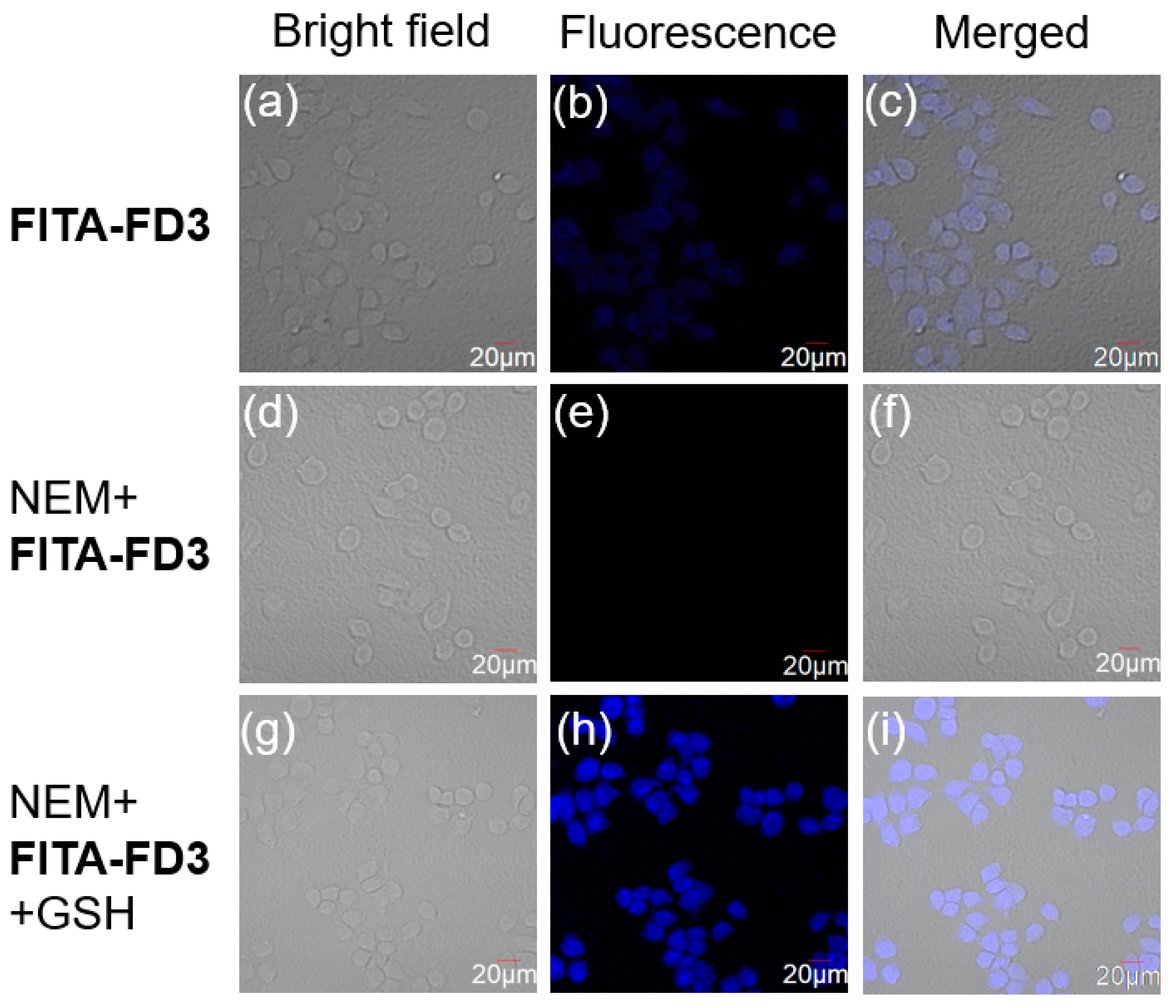
3.5. Potential Applications in Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oberkampf, M.; Guillerey, C.; Mouriѐs, J.; Rosenbaum, P.; Fayolle, C.; Bobard, A.; Savina, A.; Ogier-Denis, E.; Enninga, J.; Amigorena, S.; et al. Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat. Commun. 2018, 9, 2241. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.; Horvath, T. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, N.; Zhang, D.; Liu, H.; Li, L.; Wang, F.; Ren, J.; Wang, E. A mitochondria-targeted fluorescent probe for discrimination of biothiols by dual-channel imaging in living cells and zebrafish. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2024, 322, 124846. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Huang, S.; Chen, G.; Lin, Z.; Jiang, Y.; Qian, J.; Zhang, H.; Sun, H. A ratiometric fluorescent probe for cysteine and glutathione differentiation and its application for cysteine detection in foods. J. Mol. Struct. 2024, 1315, 138852. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, B.-B.; Si-Tu, X.-M.; Gao, T.; Wang, F.-F.; He, H.; Fan, X.-Y.; Jiang, F.-L.; Liu, Y. A lysosome-targeted fluorescent sensor for the detection of glutathione in cells with an extremely fast response. Chem. Commun. 2016, 52, 11579–11582. [Google Scholar] [CrossRef]
- Liu, J.; Dou, X.; Zhang, H. 2-Mercaptobenzimidazole Functionalized Copper Nanoparticles Fluorescence Probe for Sensitivity and Selectivity Detection of Cys in Serum. Sensors 2023, 23, 5814. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, Q.; Chao, J.; Tan, F.; Mao, G.; Hu, L.; Ouyang, J.; Li, C. Glutathione-activated biotin-targeted dual-modal imaging probe with improved PDT/PTT synergistic therapy. Anal. Chim. Acta 2024, 1316, 342860. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Wang, H.; Su, W.; Dong, S. A FRET Based Two-Photon Fluorescent Probe for Visualizing Mitochondrial Thiols of Living Cells and Tissues. Sensors 2020, 20, 1746. [Google Scholar] [CrossRef]
- Zampagni, M.; Wright, D.; Cascella, R.; D’Adamio, G.; Casamenti, F.; Evangelisti, E.; Cardona, F.; Goti, A.; Nacmias, B.; Sorbi, S.; et al. Novel S-acyl glutathione derivatives prevent amyloid oxidative stress and cholinergic dysfunction in Alzheimer disease models. Free Radic. Biol. Med. 2012, 52, 1362–1371. [Google Scholar] [CrossRef]
- Shahrokhian, S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal. Chem. 2001, 73, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Majmudar, C.; Pomerantz, W.; Gagnon, J.; Sadowsky, J.; Meagher, J.; Johnson, T.; Stuckey, J.; Brooks, C.; Wells, J.; et al. Ordering a dynamic protein via a small-molecule stabilizer. J. Am. Chem. Soc. 2013, 135, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.; Harper, M.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Tian, M.; Liu, Y.; Jiang, F.-L. On the Route to Quantitative Detection and Real-Time Monitoring of Glutathione in Living Cells by Reversible Fluorescent Probes. Anal. Chem. 2020, 92, 14285–14291. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Li, H.; Llangovan, G.; Cardounel, A.; Zweier, J.; Yamada, K.; Krishna, M. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002, 62, 307–312. [Google Scholar] [PubMed]
- Liu, Q.; Ding, X.; Xu, X.; Lai, H.; Zeng, Z.; Shan, T.; Zhang, T.; Chen, M.; Huang, Y.; Huang, Z.; et al. Tumor-targeted hyaluronic acid-based oxidative stress nanoamplifier with ROS generation and GSH depletion for antitumor therapy. Int. J. Biol. Macromol. 2022, 207, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, W.; Cai, M.; Ma, Y.; Yu, A.; Chen, S.; Zhang, S. Rational design of cascade reaction-assisted trinalsite fluorescent probe for simultaneous discrimination of Cys, Hcy, GSH, and H2S in living cells and zebrafish. Sens. Actuators B Chem. 2024, 418, 136151. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Li, W.; Chen, W.; Wang, Y.; Li, X.; Ye, Y.; Song, X. Selective and discriminative fluorescence sensing of Cys, Hcy, GSH and H2S with concise and distinct signals. Sens. Actuators B Chem. 2021, 331, 129394. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Chen, W.; Huang, J.; Huang, C.; Sheng, J.; Song, X. Simultaneous discrimination of cysteine, homocysteine, glutathione, and H2S in living cells through a multi signal combination strategy. Anal. Chem. 2019, 91, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Qu, Y.-C.; Shao, N.; Niu, L.-Y.; Yang, Q.-Z. Reversible Dual Fluorescence-Lifetime Imaging of Mitochondrial GSH and Microviscosity: Real-Time Evaluation of Ferroptosis Status. Anal. Chem. 2024, 96, 4570–4579. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Tang, D.; Xu, N.; Liu, H.; Dou, K.; Zhou, X.; Yu, F. Evaluation of erastin synergized cisplatin anti-nasopharyngeal carcinoma effect with a glutathione-activated near-infrared fluorescent probe. Chin. Chem. Lett. 2024, 35, 108658. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, Y.; Deng, C.; Zhu, N.; Shi, Y.; Wang, W.; Qing, L. GSH-specific fluorescent probe for sensing, bioimaging, rapid screening of natural inhibitor Celastrol and ccRCC theranostics. Anal. Chim. Acta 2023, 1248, 340933. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, T.; Ma, Y.; Lin, W. Discriminating Cys from GSH/H2S in vitro and in vivo with a NIR fluorescent probe. Sens. Actuators B Chem. 2019, 127, 127202. [Google Scholar] [CrossRef]
- Jiang, C.; Huang, H.; Kang, X.; Yang, L.; Xi, Z.; Sun, H.; Pluth, D.M.; Yi, L. NBD-based synthetic probes for sensing small molecules and proteins: Design, sensing mechanisms and biological applications. Chem. Soc. Rev. 2021, 50, 7436–7495. [Google Scholar] [CrossRef]
- Zhang, J.; Rakhimbekova, A.; Duan, X.; Yin, Q.; Foss, C.; Fan, Y.; Xu, Y.; Li, X.; Cai, X.; Kutil, Z.; et al. A prostate-specific membrane antigen activated molecular rotor for real-time fluorescence imaging. Nat. Commun. 2021, 12, 5460. [Google Scholar] [CrossRef]
- Jia, Y.-H.; Sun, Y.-X.; Gao, L.-L.; Sun, Y.; Deng, Z.-P.; Li, J.-G.; Zhao, B.; Ji, B.-T. A highly selective and sensitive rhodamine B-based chemosensor for Sn4+ in water-bearing and biomaging and biosensing in zebrafish. Spectrochim. Acta Part A 2024, 317, 124385. [Google Scholar] [CrossRef]
- Deng, Z.-P.; Hu, W.-Q.; Yuan, J.-L.; Sun, Y.; Wang, Q.; Sun, Y.-X.; Wang, J.-J.; Zhang, S.-Z.; Xu, L. Dual-ligand Zn-based MOF as a fluorescent probe for the detection of HSO4−. J. Mol. Struct. 2025, 1319, 139607. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Meng, J.; Bao, W.; Li, M.; Wang, X.; Tian, Z. Mitochondrion-Targeting near-Infrared Fluorescent Probe for Detecting Intracellular Nanomolar Level Hydrogen Sulfide with High Recognition Rate. Anal. Bioanal. Chem. 2021, 413, 1215–1224. [Google Scholar] [CrossRef]
- Fosnacht, K.; Pluth, M.D. Activity-Based Fluorescent Probes for Hydrogen Sulfide and Related Reactive Sulfur Species. Chem. Rev. 2024, 124, 4124–4257. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Song, B.; Zhang, W.; Duan, C.; Wang, Y.-L.; Liu, C.; Zhang, R.; Yuan, J. Quantitative Monitoring and Visualization of Hydrogen Sulfide in Vivo Using a Luminescent Probe Based on a Ruthenium (II) Complex. Angew. Chem. Int. Ed. 2018, 57, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, X.; Yang, S.; Hu, S.; Zheng, X.; Mao, G.; Li, Y.; Zhou, Y. Monitoring H2S Fluctuation During Autophagic Fusion of Lysosomes and Mitochondria Using a Lysosome-Targeting Fluorogenic Probe. Anal. Chim. Acta 2023, 1265, 341356. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Wang, J.; Lu, J. Efficient Strategy for the Synthesis and Modification of 2-Hydroxyethylluciferin for Highly Sensitive Bioluminescence Imaging of Endogenous Hydrogen Sulfide in Cancer Cells and Nude Mice. Anal. Chem. 2019, 91, 15703–15708. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Hou, B.; Ji, K.; Song, J.; Lu, F.; Plalanisamy, K.; Kanagaraj, R.; Selvaraj, M. A coumarin-based fluorescent probe for imaging H2S and distinguishing breast cancer cells from normal ones. J. Mol. Liq. 2024, 414, 126158. [Google Scholar] [CrossRef]
- Roubinet, B.; Renard, P.-Y.; Romieu, A. New insights into the water-solubilization of thiol-sensitive fluorogenic probes based on long-wavelength 7-hydroxycoumarin scaffolds. Dye. Pigment. 2014, 110, 270–284. [Google Scholar] [CrossRef]
- Roubinet, B.; Massif, C.; Moreau, M.; Boschetti, F.; Ulrich, G.; Ziessel, R.; Renard, P.-Y.; Romieu, A. New 3-(Heteroaryl)-2-iminocoumarin-based Borate Complexes: Synthesis, Photophysical Properties, and Rational Functionalization for Biosensing/Biolabeling Application. Chem. Eur. J. 2015, 25, 14589–14601. [Google Scholar] [CrossRef]
- Tso, K.; Liu, H.; Lo, K. Phosphorogenic sensors for biothiols derived from cyclometalated iridium(III) polypyridine complexes containing a dinitrophenyl ether moiety. J. Inorg. Biochem. 2017, 177, 412–422. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, Y.; Li, Z.; Wang, Y.; Du, H.; Liu, L.; Ding, R.; Liu, G. A GSH Fluorescent Probe with a Large Stokes Shift and Its Application in Living Cells. Sensors 2019, 19, 5348. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Wang, H.; Wang, F.; Kambam, S.; Wang, Y.; Zhao, W.; Chen, X. A fluorescent probe with high selectivity to glutathione over cysteine and homocysteine based on positive effect of carboxyl on nucleophilic substitution in CTAB. Sens. Actuators B Chem. 2014, 192, 708–713. [Google Scholar] [CrossRef]
- Yang, R.; Tang, Y.; Zhu, W. Ratiometric Fluorescent Probe for the Detection of Glutathione in Living Cells. Chem. J. Chin. Univ.-Chin. 2016, 37, 643–647. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Liu, B.; Lu, X.; Li, M.; Ji, L.; Mao, Z. Mitochondria-targeted two-photon fluorescent probe for the detection of biothiols in living cells. Sens. Actuators B Chem. 2018, 255, 193–202. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Wang, B.; Gao, L.; Jiang, F.; Liu, Y. A BODIPY-based mitochondria-targeted turn-on fluorescent probe with dual response units for the rapid detection of intracellular biothiols. Dye. Pigment. 2018, 152, 29–35. [Google Scholar] [CrossRef]
- Xia, X.; Qian, Y.; Shen, B. Synthesis of a BODIPY disulfonate near-infrared fluorescence-enhanced probe with high selectivity to endogenous glutathione and two-photon fluorescent turn-on through thiol-induced SNAr substitution. J. Mater. Chem. B 2018, 6, 3023–3029. [Google Scholar] [CrossRef]
- Zhan, C.; Zhang, G.; Zhang, D. Zincke’s Salt-Substituted Tetraphenylethylenes for Fluorometric Turn-On Detection of Glutathione and Fluorescence Imaging of Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 12141–12149. [Google Scholar] [CrossRef]
- Kang, J.; Xu, S.; Radford, M.N.; Zhang, W.; Kelly, S.S.; Day, J.J.; Xian, M. O→S relay deprotection: A general approach to controllable donors of reactive sulfur species. Angew. Chem. Int. Ed. 2018, 57, 5893–5897. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Kelly, S.S.; Xu, S.; Xian, M. The Path to Controlled Delivery of Reactive Sulfur Species. Acc. Chem. Res. 2021, 54, 3968–3978. [Google Scholar] [CrossRef]
- Dong, Y.; Liang, W.; Yi, L. Fast Intramolecular Thiol-Activated Arylselenoamides Provide Access to Triggered, Fluorogenic H2Se Donors. J. Am. Chem. Soc. 2024, 146, 24776–24781. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; He, L.; Huang, H.; Ye, H.; Sun, L.; Yi, L. Thiolysis of CBD arylethers for development of highly GSH-selective fluorescent probes. Chem. Commun. 2021, 57, 8802–8805. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Wang, L.; Liang, W.; Zhu, J.; Sun, L.; Yi, L. FITA-Containing 2,4-Dinitrophenyl Alkylthioether-Based Probe for Detection and Imaging of GSH. Sensors 2025, 25, 34. https://doi.org/10.3390/s25010034
Dong Y, Wang L, Liang W, Zhu J, Sun L, Yi L. FITA-Containing 2,4-Dinitrophenyl Alkylthioether-Based Probe for Detection and Imaging of GSH. Sensors. 2025; 25(1):34. https://doi.org/10.3390/s25010034
Chicago/Turabian StyleDong, Yalun, Liyue Wang, Wenfang Liang, Jiqin Zhu, Lu Sun, and Long Yi. 2025. "FITA-Containing 2,4-Dinitrophenyl Alkylthioether-Based Probe for Detection and Imaging of GSH" Sensors 25, no. 1: 34. https://doi.org/10.3390/s25010034
APA StyleDong, Y., Wang, L., Liang, W., Zhu, J., Sun, L., & Yi, L. (2025). FITA-Containing 2,4-Dinitrophenyl Alkylthioether-Based Probe for Detection and Imaging of GSH. Sensors, 25(1), 34. https://doi.org/10.3390/s25010034





