Palladium Phthalocyanine Nanowire-Based Highly Sensitive Sensors for NO2(g) Detection
Abstract
1. Introduction
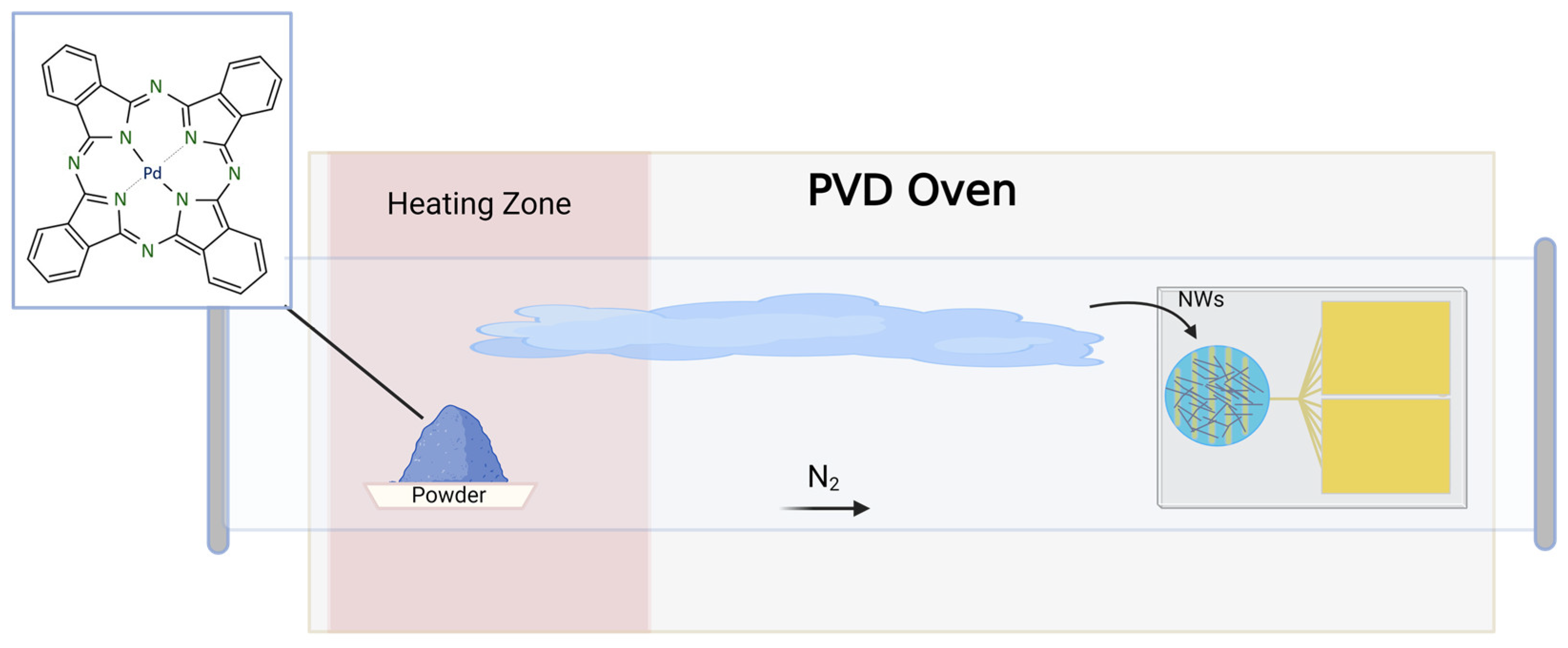
2. Materials and Methods
3. Results and Discussion
3.1. UV–Vis Spectroscopy
3.2. X-ray Diffraction
3.3. Raman Spectroscopy
3.4. Morphology and Elemental Analysis
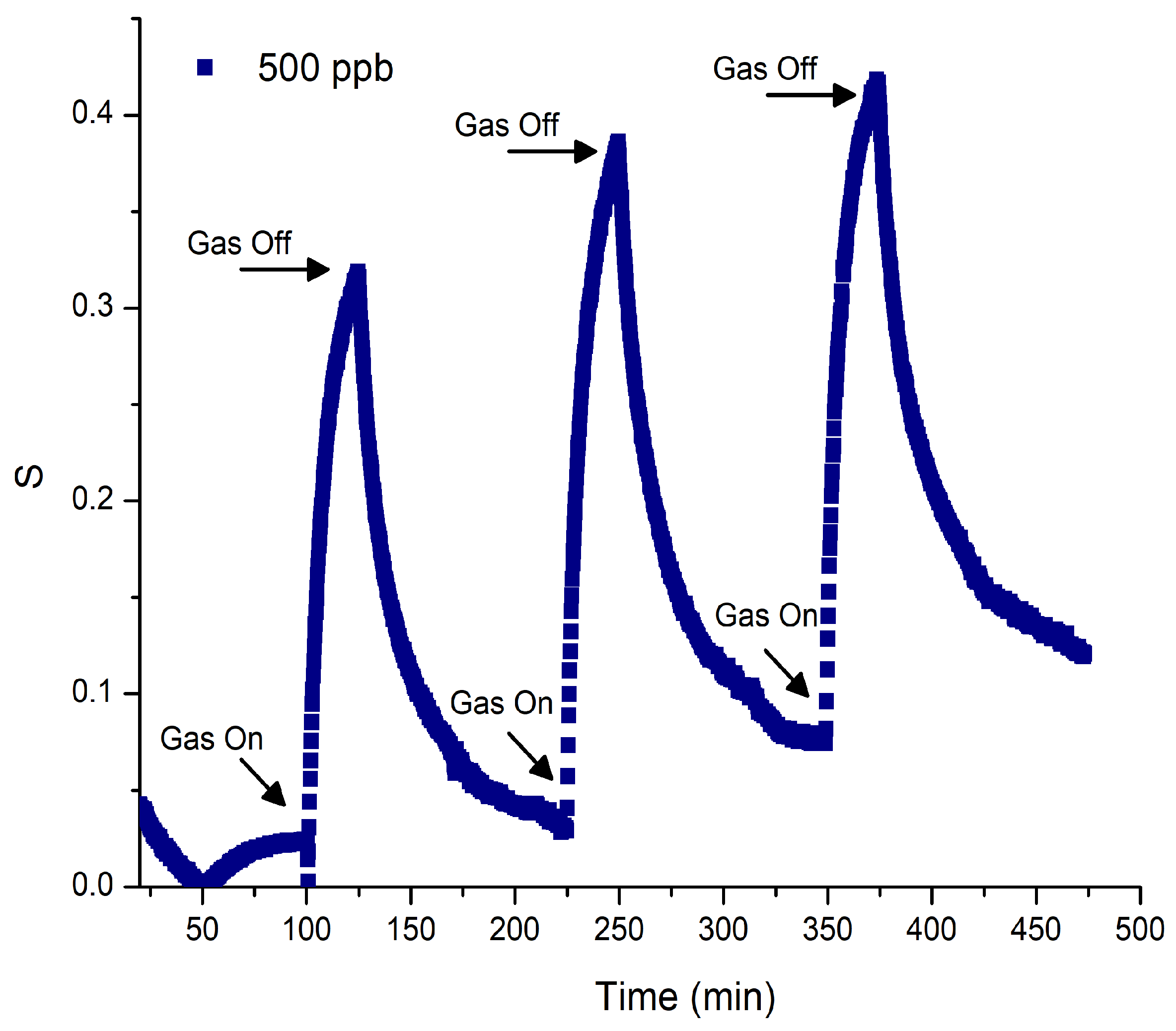
3.5. Gas-Sensing Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitrogen Dioxide’s Impact on Indoor Air Quality. Available online: https://www.epa.gov/indoor-air-quality-iaq/nitrogen-dioxides-impact-indoor-air-quality (accessed on 20 March 2023).
- Effects Associated with Nitrogen Dioxide. Available online: https://www.epa.gov/indoor-air-quality-iaq/nitrogen-dioxides-impact-indoor-air-quality#:~:text=Standards%20or%20Guidelines,-No%20standards%20have&text=EPA%20National%20Ambient%20Air%20Quality,NO2%20in%20outdoor%20air (accessed on 20 March 2023).
- Bodner, M.; Senn, J.; Hacker, V. Degradation mechanisms and their lifetime. In Fuel Cells and Hydrogen: From Fundamentals to Applied Research; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shi, Y.; Xia, Y.; Lu, B.; Liu, N.; Zhang, L.; Li, S.; Li, W. Emission Inventory and Trends of NOx for China, 2000–2020. J. Zhejiang Univ. Sci. A 2014, 15, 454–464. [Google Scholar] [CrossRef]
- Space Shuttle Use of Propellants and Fluids Facts-NASA. Available online: http://www.nasa-klass.com/Curriculum/Get_Training%201/ET/RDG_ET-Additional/Shuttles%20Propellants%20and%20Fluids.pdf (accessed on 20 March 2023).
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro-and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Flores, S.Y.; Gonzalez-Espiet, J.; Cintrón, J.; Villanueva, N.D.J.; Camino, F.E.; Kisslinger, K.; Cruz, D.M.P.; Rivera, R.D.; Fonseca, L.F. Fluorinated Iron and Cobalt Phthalocyanine Nanowire Chemiresistors for Environmental Gas Monitoring at Parts-per-Billion Levels. ACS Appl. Nano Mater. 2022, 5, 4688–4699. [Google Scholar] [CrossRef]
- Gounden, D.; Nombona, N.; Van Zyl, W.E. Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 2020, 420, 213359. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Zhu, P.; Ng, D.K.P.; Kobayashi, N.; Jiang, J. Electron-Donating or -Withdrawing Nature of Substituents Revealed by the Electrochemistry of Metal-Free Phthalocyanines. Inorg. Chem. 2006, 45, 1636–1637. [Google Scholar] [CrossRef] [PubMed]
- Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V. Thin Films of Tetrafluorosubstituted Cobalt Phthalocyanine: Structure and Sensor Properties. Appl. Surf. Sci. 2016, 372, 79–86. [Google Scholar] [CrossRef]
- Wilson, R.L.; Simion, C.E.; Blackman, C.S.; Carmalt, C.J.; Stanoiu, A.; Di Maggio, F.; Covington, J.A. The effect of film thickness on the gas sensing properties of ultra-thin TiO2 films deposited by atomic layer deposition. Sensors 2018, 18, 735. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Meng, F. Metal Oxide Semiconductor Sensors for Triethylamine Detection: Sensing Performance and Improvements. Chemosensors 2022, 10, 231. [Google Scholar] [CrossRef]
- Liu, F.; Sun, J.; Xiao, S.; Huang, W.; Tao, S. Controllable Fabrication of Copper Phthalocyanine Nanostructure Crystals. Nanotechnology 2015, 26, 225601. [Google Scholar] [CrossRef]
- Chen, H.; Hsiao, C.; Chen, W.; Chang, C.; Chou, T.; Liu, I.; Lin, K.; Liu, W. Characteristics of a Pt/NiO thin filmbased ammonia gas sensor. Sens. Actuators B 2018, 256, 962–967. [Google Scholar] [CrossRef]
- Kim, T.H.; Hasani, A.; Quyet, L.V.; Kim, Y.; Park, S.Y.; Lee, M.G.; Sohn, W.; Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; et al. NO2 sensing properties of porous Au-incorporated tungsten oxide thin films prepared by solution process. Sens. Actuators B 2019, 286, 512–520. [Google Scholar] [CrossRef]
- Mani, G.K.; Rayappan, J.B.B. Influence of copper doping on structural, optical and sensing properties of spray deposited zinc oxide thin films. J. Alloys Compd. 2014, 582, 414–419. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Yi, G.; Zhou, L.; Cao, J.; Sun, G.; Chen, Z.; Bala, H.; Zhang, Z. Hydrothermal synthesis of Ag modified ZnO nanorods and their enhanced ethanol- sensing properties. Mater. Sci. Semicond. Process. 2018, 75, 327–333. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Tong, W.Y.; Djuris, A.B.; Lin, H.W.; Gwo, S. Metal Phthalocyanine Nanoriboons and Nanowires. J. Phys. Chem. B 2006, 110, 17406–17413. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, K.; Liu, H.; Chen, L.; Jin, Z.; Yan, S. Rapid and Efficient NO2 Sensing Performance of TeO2 Nanowires. Sensors 2023, 23, 9097. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. Synthesis of WO3 nanorods by thermal oxidation technique for NO2 gas sensing application. Mater. Sci. Semicond. Process. 2018, 86, 79–84. [Google Scholar] [CrossRef]
- Ndaya, C.C.; Javahiraly, N.; Brioude, A. Recent Advances in Palladium Nanoparticles-Based Hydrogen Sensors for Leak Detection. Sensors 2019, 19, 4478. [Google Scholar] [CrossRef]
- Liu, S.; Xue, J.; Liu, Y.; Cui, Y.; Qiu, J.; Xu, R.; Chen, G. The Sensitizing Effect of Enhanced NO2 Gas Sensing at Near-Room Temperature Using Pd-Decorated VO2 Nanowires. J. Electron. Mater. 2024, 53, 1461–1475. [Google Scholar] [CrossRef]
- Liangruksa, M.; Laomettachit, T.; Siriwong, C. Enhancing Gas Sensing Properties of Novel Palladium-Decorated Zinc Oxide Surface: A First-Principles Study. Mater. Res. Express 2021, 8, 045004. [Google Scholar] [CrossRef]
- Nikolaeva, N.S.; Parkhomenko, R.G.; Klyamer, D.D.; Shushanyan, A.D.; Asanov, I.P.; Morozova, N.B.; Basova, T.V. Bilayer Structures Based on Metal Phthalocyanine and Palladium Layers for Selective Hydrogen Detection. Int. J. Hydrogen Energy 2017, 42, 28640–28646. [Google Scholar] [CrossRef]
- Parkhomenko, R.G.; Sukhikh, A.S.; Klyamer, D.D.; Krasnov, P.O.; Gromilov, S.; Kadem, B.; Hassan, A.K.; Basova, T.V. Thin Films of Unsubstituted and Fluorinated Palladium Phthalocyanines: Structure and Sensor Response toward Ammonia and Hydrogen. J. Phys. Chem. C 2017, 121, 1200–1209. [Google Scholar] [CrossRef]
- Denekamp, I.M.; Veenstra FL, P.; Jungbacker, P.; Rothenberg, G. A simple synthesis of symmetric phthalocyanines and their respective perfluoro and transition-metal complexes. Appl. Organomet. Chem. 2019, 33, e4872. [Google Scholar] [CrossRef]
- Comini, E. Metal oxides nanowires chemical/gas sensors: Recent advances. Mater. Today Adv. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Y.; Li, R.; Shi, C.; Moro, R.; Ma, Y.; Ma, L. High-Performance UV-Assisted NO2 Sensor Based on Chemical Vapor Deposition Graphene at Room Temperature. ACS Omega 2019, 4, 14179–14187. [Google Scholar] [CrossRef]
- Claessens, C.G.; Hahn, U.; Torres, T. Phthalocyanines: From outstanding electronic properties to emerging applications. Chem. Rec. 2008, 8, 75–97. [Google Scholar] [CrossRef]
- Kempa, A.; Dobrowolski, J. Palladium phthalocyanine and its polymorphic forms. Can. J. Chem. 1988, 66, 2553–2555. [Google Scholar] [CrossRef]
- Jafari, M.J.; Azim-Araghi, M.E.; Barhemat, S.; Riyazi, S. Effect of post-deposition annealing on surface morphology and gas sensing properties of palladium phthalocyanine thin films. Surf. Interface Anal. 2012, 44, 601–608. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation. In Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elservier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Klyamer, D.D.; Basova, T.V.; Krasnov, P.O.; Sukhikh, A.S. Effect of fluorosubstitution and central metals on the molecular structure and vibrational spectra of metal phthalocyanines. J. Mol. Struct. 2019, 1189, 73–80. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N. Improved textile functionality through surface modifications. In Functional Textiles for Improved Performance, Protection and Health; Woodhead Publishing Limited: Southampton, UK, 2011. [Google Scholar]
- Zdorovets, M.V.; Kozlovskiy, A.L. Study of phase transformations in Co/CoCo2O4 nanowires. J. Alloys Compd. 2020, 815, 152450. [Google Scholar] [CrossRef]
- Rashid, A.A.; Mara, U.T.; Berhad, N. Hydrothermal Synthesis of Tungsten Oxide (WO3) Nanostructures Using Sodium Chloride as Structure Directing Agent. J. Multidiscip. Eng. Sci. Technol. (JMEST) 2015, 2, 1572–1576. [Google Scholar]
- Karan, S.; Mallik, B. Nanoflowers grown from phthalocyanine seeds: Organic nanorectifiers. J. Phys. Chem. C 2008, 112, 2436–2447. [Google Scholar] [CrossRef]
- Padma, N.; Joshi, A.; Singh, A.; Deshpande, S.K.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. NO2 Sensors with Room Temperature Operation and Long Term Stability Using Copper Phthalocyanine Thin Films. Sens. Actuators B Chem. 2009, 143, 246–252. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, T.; Chen, X.; Li, B.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; Yang, Z. Enhancing Room-Temperature NO2 detection of Cobalt Phthalocyanine Based Gas Sensor at an Ultralow Laser Exposure. Phys. Chem. Chem. Phys. 2020, 22, 18499–18506. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jiang, M.; Huang, J.; Zhou, X.; Li, K.; Zheng, Y.; Jiang, W.; Zhang, T.; Yan, H.; Xia, H. Study on NO2 gas Sensitivity of Metal Phthalocyanine Enhanced by Graphene Quantum Dots. J. Phys. Conf. Ser. 2022, 2369, 012083. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero Vélez, C.; Flores, S.Y.; Fonseca, L.F.; Piñero Cruz, D.M. Palladium Phthalocyanine Nanowire-Based Highly Sensitive Sensors for NO2(g) Detection. Sensors 2024, 24, 1819. https://doi.org/10.3390/s24061819
Otero Vélez C, Flores SY, Fonseca LF, Piñero Cruz DM. Palladium Phthalocyanine Nanowire-Based Highly Sensitive Sensors for NO2(g) Detection. Sensors. 2024; 24(6):1819. https://doi.org/10.3390/s24061819
Chicago/Turabian StyleOtero Vélez, Crystal, Soraya Y. Flores, Luis F. Fonseca, and Dalice M. Piñero Cruz. 2024. "Palladium Phthalocyanine Nanowire-Based Highly Sensitive Sensors for NO2(g) Detection" Sensors 24, no. 6: 1819. https://doi.org/10.3390/s24061819
APA StyleOtero Vélez, C., Flores, S. Y., Fonseca, L. F., & Piñero Cruz, D. M. (2024). Palladium Phthalocyanine Nanowire-Based Highly Sensitive Sensors for NO2(g) Detection. Sensors, 24(6), 1819. https://doi.org/10.3390/s24061819




