Open Meter Duo: Low-Cost Instrument for Fluorimetric Determination of Cholinesterase Activity
Abstract
1. Introduction
2. Materials and Methods
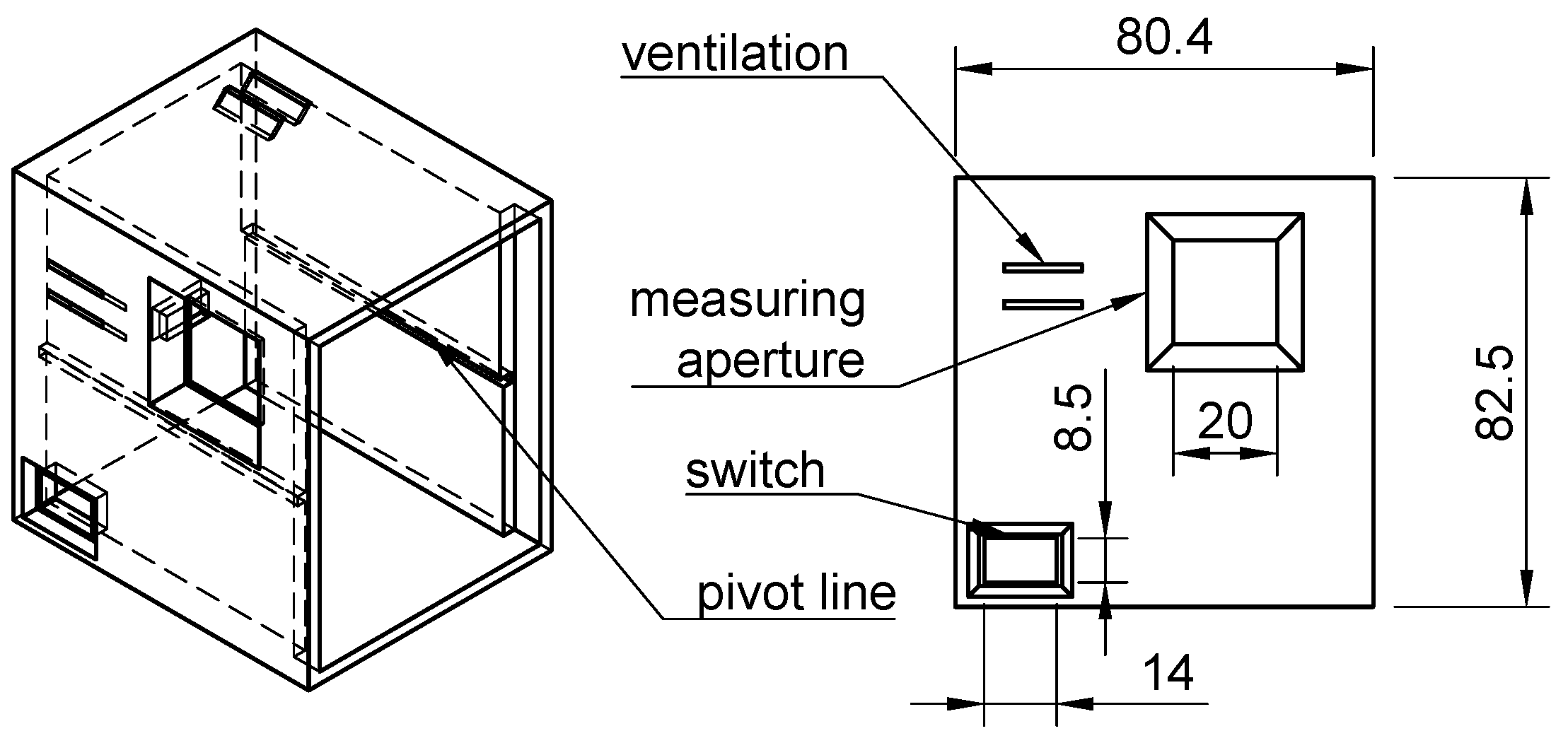
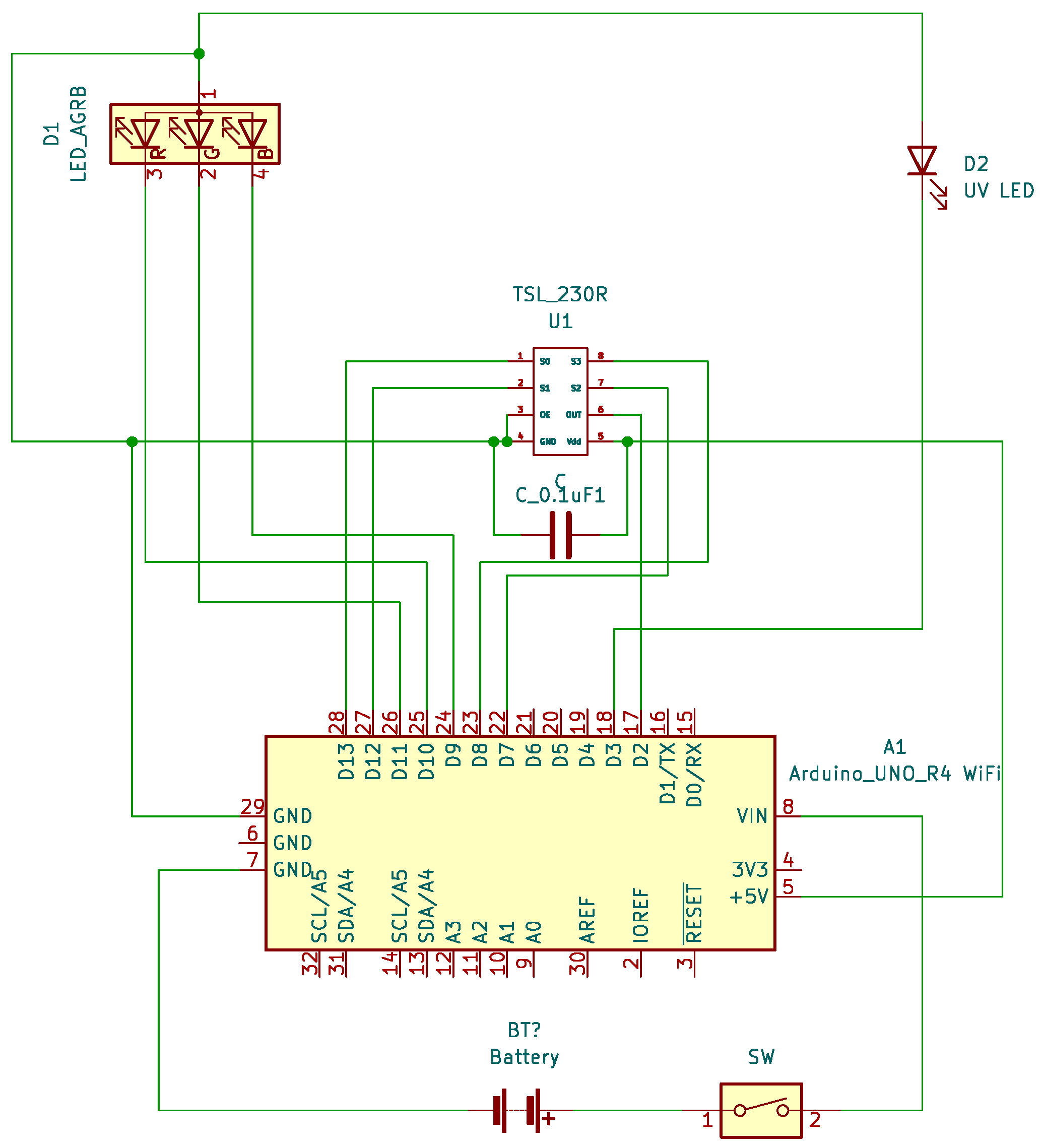
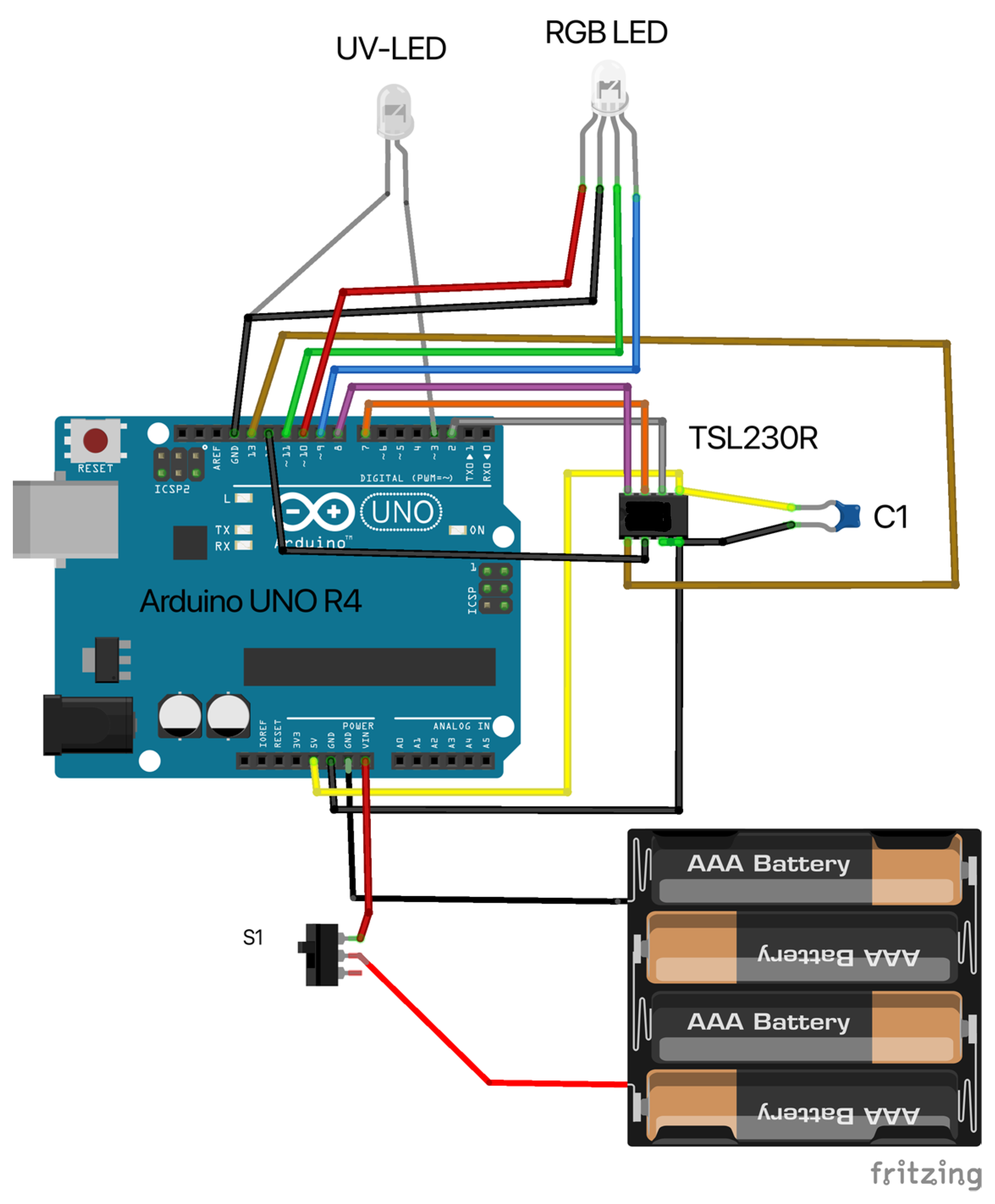
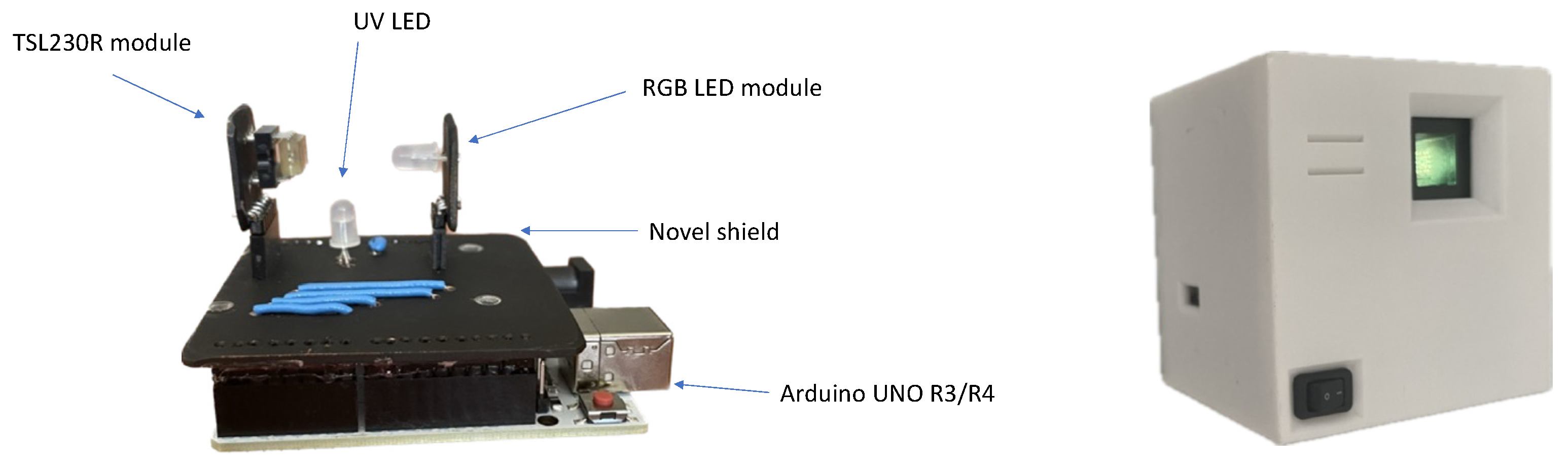
2.1. Design and Assembly of the Open Meter Duo (OMD)
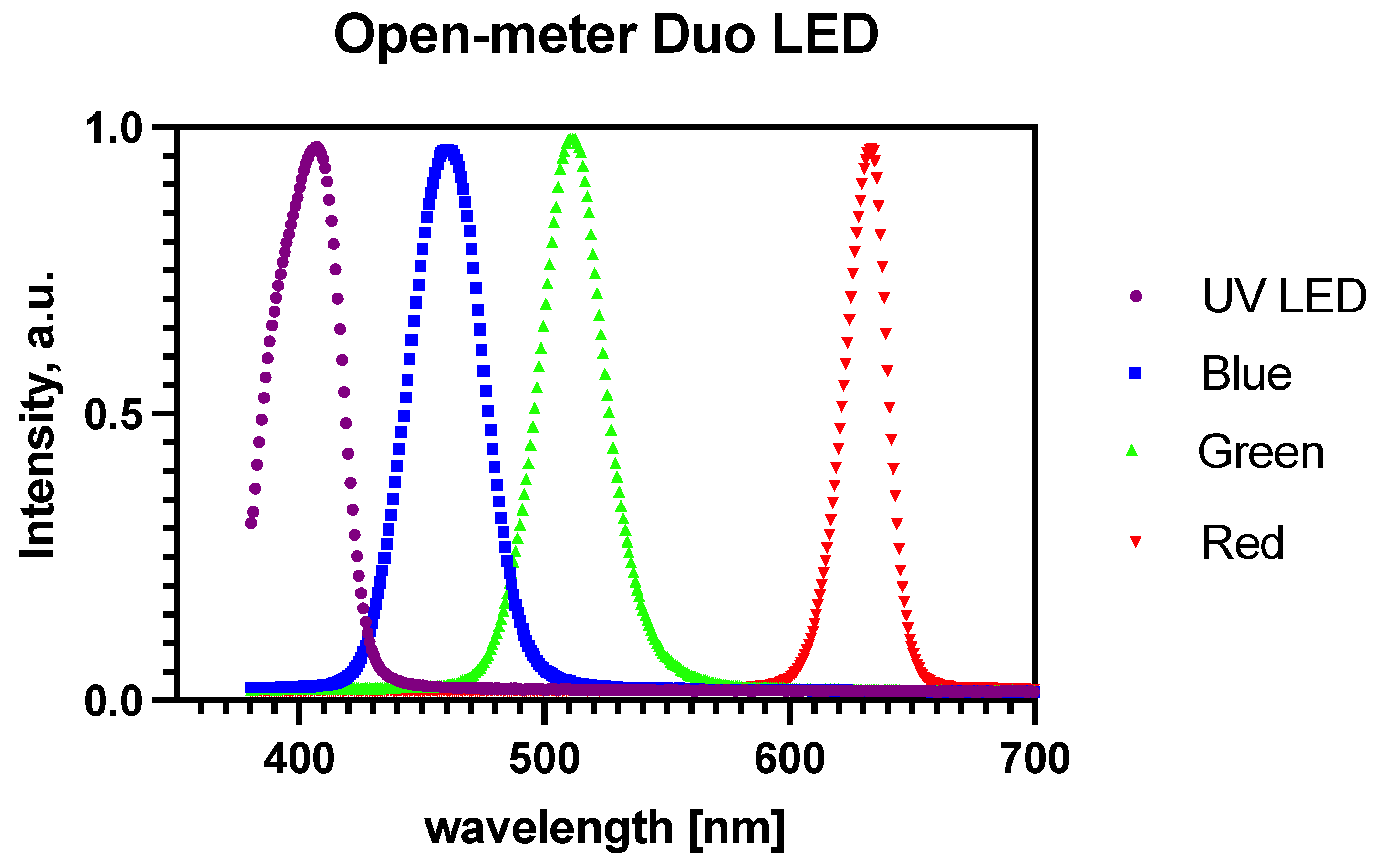
2.2. Characterization of Light Source
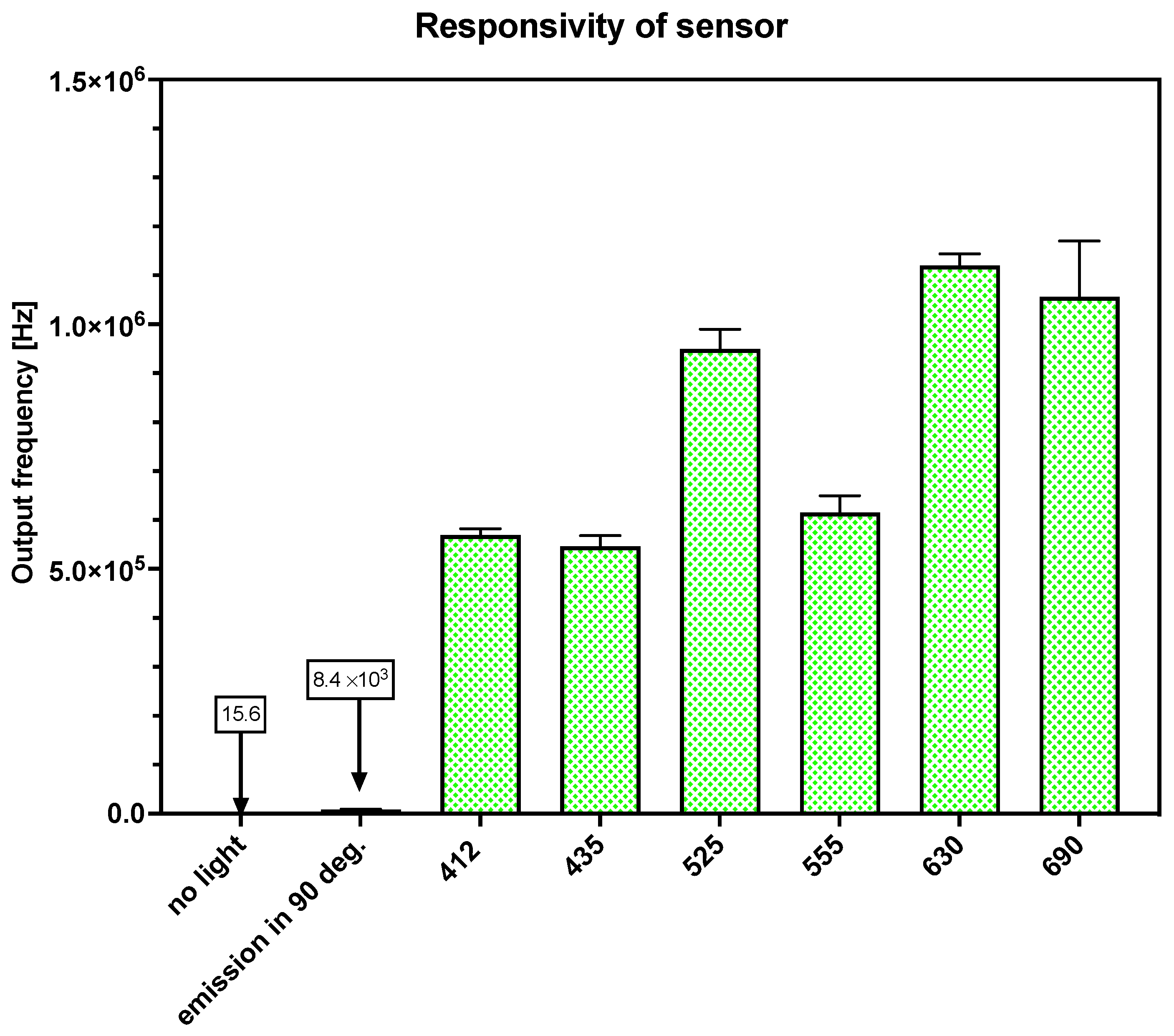
2.3. Testing of the TSL230R Sensor
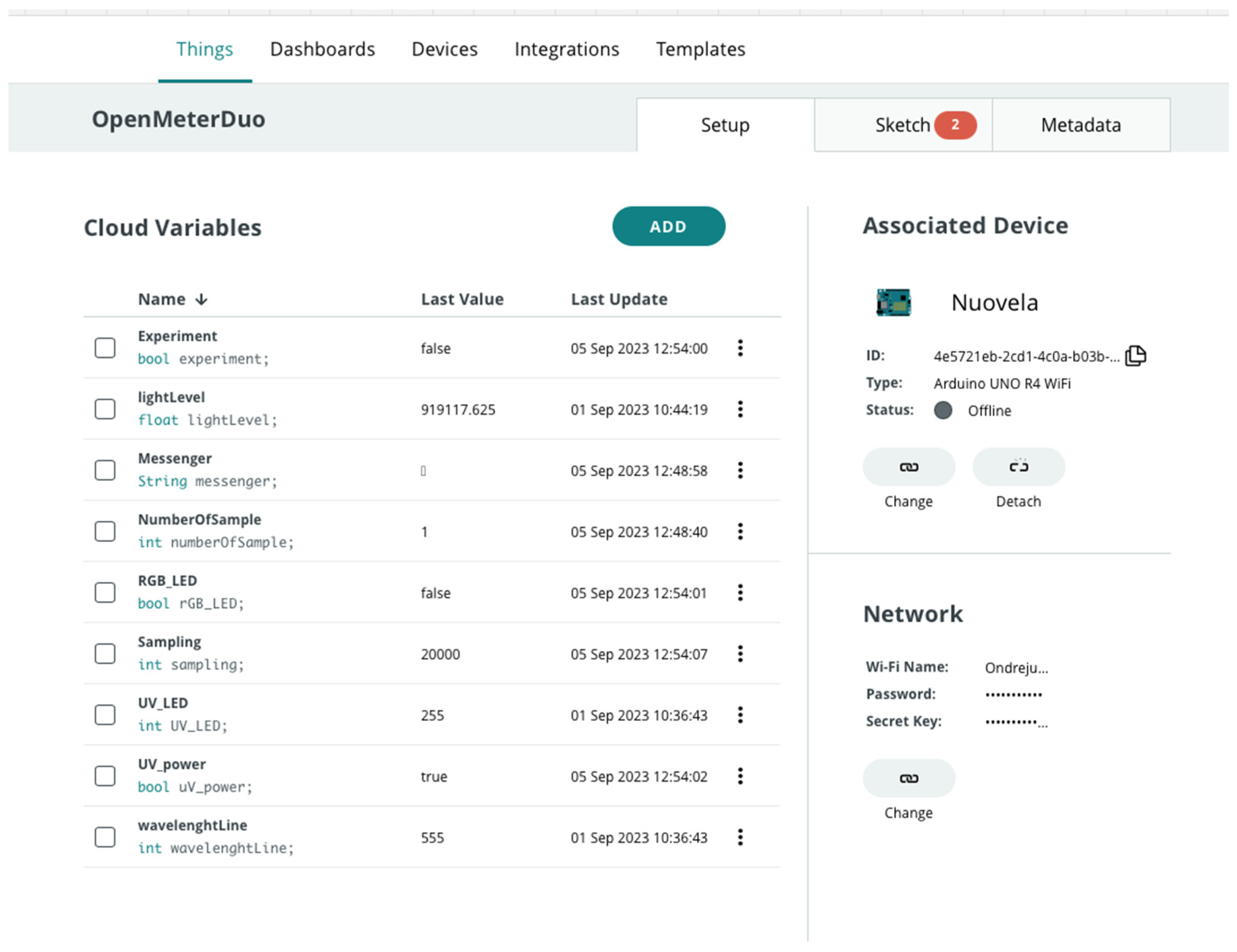
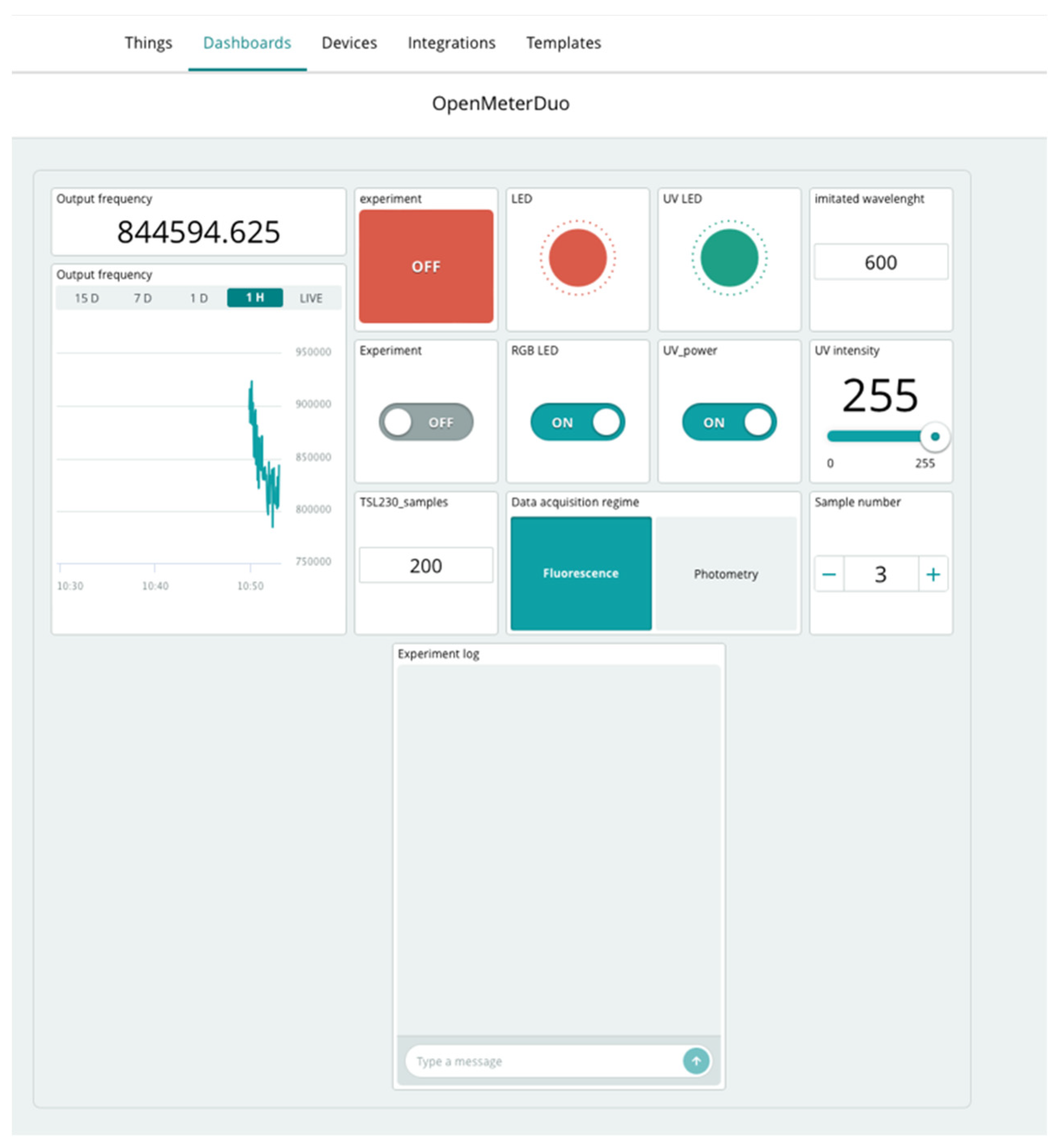
2.4. Control of the Open Meter Duo
2.4.1. MS Excel Data Streamer
2.4.2. Arduino IOT Cloud
2.5. Acetylcholinesterase Solution
2.6. Indoxyl Acetate Assay
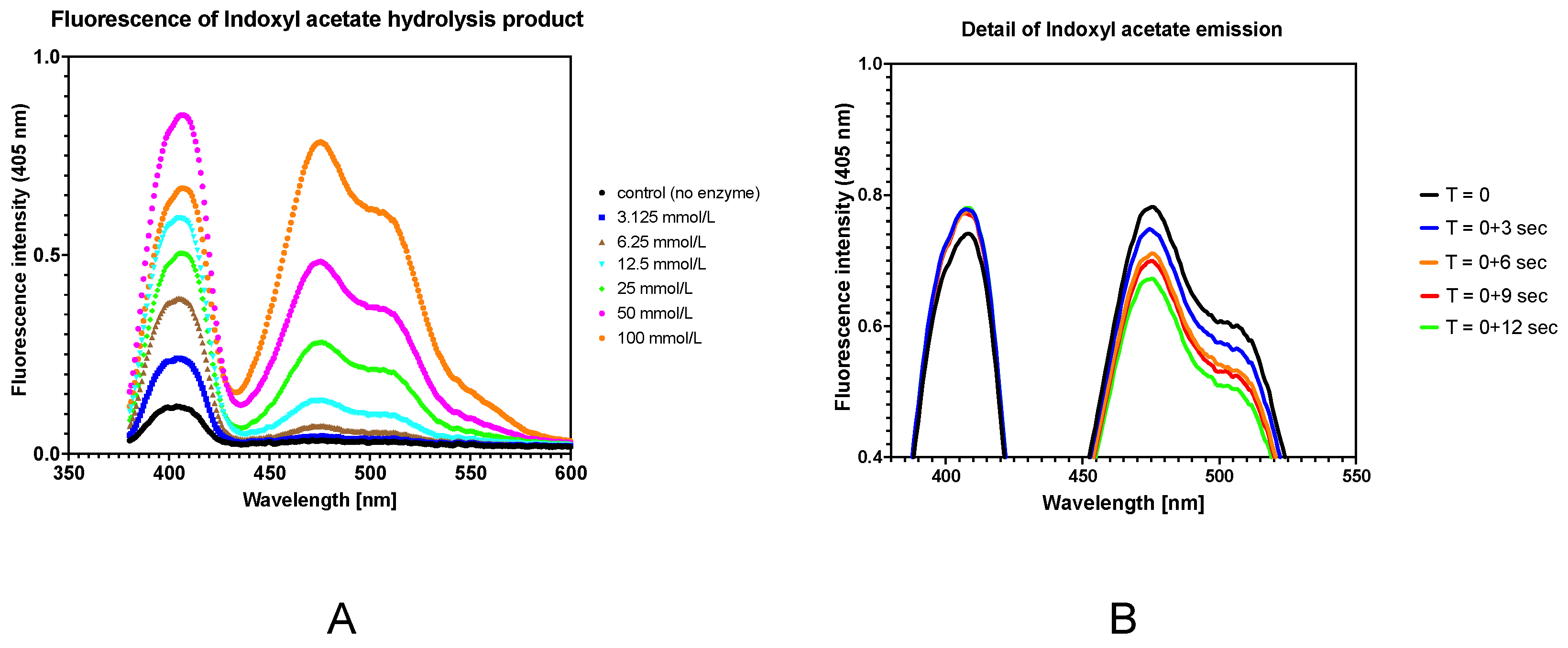
2.6.1. Spectral Characteristics of Indoxyl Acetate and Its Hydrolysis
2.6.2. Simple Fluorimetric Assay
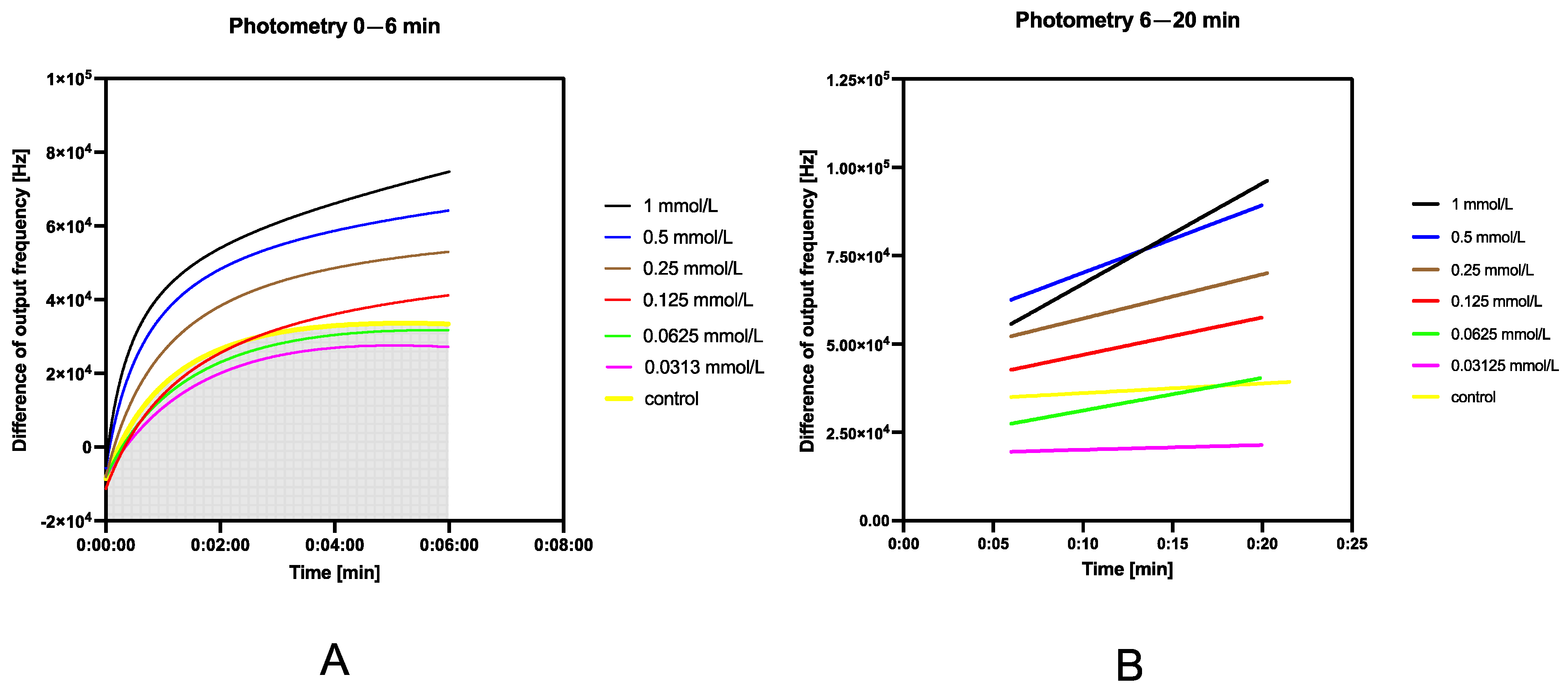
2.6.3. Study of Fluorescence Stability
2.6.4. Dual-Signal Concept
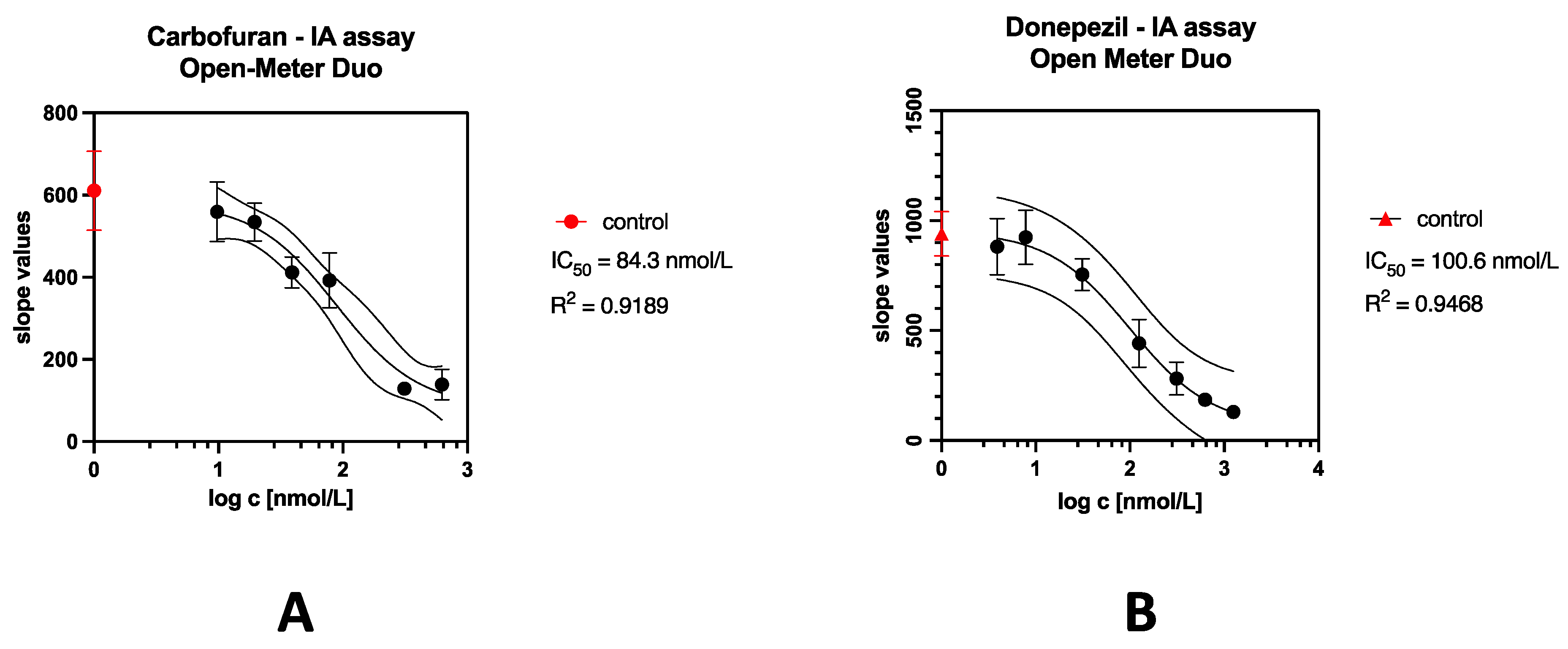
2.6.5. Determination of Model Cholinesterase Inhibitors
2.7. Acquisition and Evaluation of Data
2.7.1. Spectrovis Plus
2.7.2. Open Meter Duo
3. Results and Discussion
3.1. Design and Assembly of the Open Meter Duo (OMD)
3.2. Characterization of the Light Source
3.3. Response of Sensor
3.4. Remote Control Interface for Open Meter Duo
- Experiment: ON/OFF.
- Messenger—experiment marking—time stamp to distinguish experiments.
- NumberOfSamples—counter of various experimental samples based on user demand.
- Switch between fluorimetry/photometry (with automatic change of sample value). It was necessary to manually switch the light source on for fluorimetry or photometry using the button.
3.5. Indoxyl Acetate
3.5.1. Spectral Characteristics of Indoxyl Acetate and Its Hydrolysis
3.5.2. Simple Fluorimetric Approach
3.5.3. Study of Fluorescence Stability
3.5.4. Determination of Model Cholinesterase Inhibitor
3.5.5. Dual-Signal Approach for Indoxyl Acetate Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, C.; Fan, Y.; Zhao, H. Recent advances and perspectives of enzyme-based optical biosensing for organophosphorus pesticides detection. Talanta 2022, 240, 123145. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Thet Tun, W.S.; Saenchoopa, A.; Daduang, S.; Daduang, J.; Kulchat, S.; Patramanon, R. Electrochemical biosensor based on cellulose nanofibers/graphene oxide and acetylcholinesterase for the detection of chlorpyrifos pesticide in water and fruit juice. RSC Adv. 2023, 13, 9603–9614. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Wang, J.; Lao, S. Dual-mode detection of organophosphate pesticides in pear and Chinese cabbage based on fluorescence and AuNPs colorimetric assays. Food Chem. 2021, 364, 130326. [Google Scholar] [CrossRef]
- Fabini, E.; Tramarin, A.; Bartolini, M. Combination of human acetylcholinesterase and serum albumin sensing surfaces as highly informative analytical tool for inhibitor screening. J. Pharm. Biomed. Anal. 2018, 155, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.J.; Roy, A.; Jung, H.J.; Ali, M.Y.; Min, B.-S.; Park, C.H.; Yokozawa, T.; Fan, T.-P.; Choi, J.S.; Jung, H.A. BACE1 molecular docking and anti-Alzheimer’s disease activities of ginsenosides. J. Ethnopharmacol. 2016, 190, 219–230. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pohanka, M.; Hrabinova, M.; Kuca, K.; Simonato, J.P. Assessment of acetylcholinesterase activity using indoxylacetate and comparison with the standard Ellman’s method. Int. J. Mol. Sci. 2011, 12, 2631–2640. [Google Scholar] [CrossRef]
- Pidany, F.; Kroustkova, J.; Al Mamun, A.; Suchankova, D.; Brazzolotto, X.; Nachon, F.; Chantegreil, F.; Dolezal, R.; Pulkrabkova, L.; Muckova, L.; et al. Highly selective butyrylcholinesterase inhibitors related to Amaryllidaceae alkaloids—Design, synthesis, and biological evaluation. Eur. J. Med. Chem. 2023, 252, 115301. [Google Scholar] [CrossRef]
- Redondo-López, S.; León, A.C.; Jiménez, K.; Solano, K.; Blanco-Peña, K.; Mena, F. Transient exposure to sublethal concentrations of a pesticide mixture (chlorpyrifos–difenoconazole) caused different responses in fish species from different trophic levels of the same community. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2022, 251, 109208. [Google Scholar] [CrossRef]
- Pohanka, M.; Zakova, J. A Smartphone Camera Colorimetric Assay of Acetylcholinesterase and Butyrylcholinesterase Activity. Sensors 2021, 21, 1796. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, Y.; Park, S.J.; Kwon, C.; Noh, H. Development of Colorimetric Paper Sensor for Pesticide Detection Using Competitive-inhibiting Reaction. BioChip J. 2018, 12, 326–331. [Google Scholar] [CrossRef]
- Liu, D.M.; Xu, B.J.; Dong, C. Recent advances in colorimetric strategies for acetylcholinesterase assay and their applications. Trac-Trend Anal. Chem. 2021, 142, 116320. [Google Scholar] [CrossRef]
- Apilux, A.; Isarankura-Na-Ayudhya, C.; Tantimongcolwat, T.; Prachayasittikul, V. Paper-based acetylcholinesterase inhibition assay combining a wet system for organophosphate and carbamate pesticides detection. EXCLI J. 2015, 14, 307–319. [Google Scholar] [CrossRef]
- Shrikrishna, N.S.; Kaushik, A.; Gandhi, S. Smartphone-assisted detection of monocrotophos pesticide using a portable nano-enabled chromagrid-lightbox system towards point-of-care application. Chemosphere 2023, 330, 138704. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.G.; Kramer, D.N. Resorufin Butyrate and Indoxyl Acetate as Fluorogenic Substrates for Cholinesterase. Anal. Chem. 1965, 37, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Tian, T.; Peng, L.J.; Zhou, H.Y.; Zhang, H.; Chen, H.; Yang, F.Q. A Paper-Based Analytical Device Integrated with Smartphone: Fluorescent and Colorimetric Dual-Mode Detection of beta-Glucosidase Activity. Biosensors 2022, 12, 893. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Barbieri, M.V.; Chino, M.; Manco, G.; Febbraio, F. A FRET Approach to Detect Paraoxon among Organophosphate Pesticides Using a Fluorescent Biosensor. Sensors 2022, 22, 561. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Barbieri, M.V.; Chino, M.; Manco, G.; Febbraio, F. A 3D printable adapter for solid-state fluorescence measurements: The case of an immobilized enzymatic bioreceptor for organophosphate pesticides detection. Anal. Bioanal. Chem. 2022, 414, 1999–2008. [Google Scholar] [CrossRef]
- Cetrangolo, G.P.; Rusko, J.; Gori, C.; Carullo, P.; Manco, G.; Chino, M.; Febbraio, F. Highly Sensitive Detection of Chemically Modified Thio-Organophosphates by an Enzymatic Biosensing Device: An Automated Robotic Approach. Sensors 2020, 20, 1365. [Google Scholar] [CrossRef]
- Di Nonno, S.; Ulber, R. Portuino-A Novel Portable Low-Cost Arduino-Based Photo- and Fluorimeter. Sensors 2022, 22, 7916. [Google Scholar] [CrossRef]
- López-Pérez, G.; González-Arjona, D.; Roldán González, E.; Román-Hidalgo, C. Design of a Portable and Reliable Fluorimeter with High Sensitivity for Molecule Trace Analysis. Chemosensors 2023, 11, 389. [Google Scholar] [CrossRef]
- Laganovska, K.; Zolotarjovs, A.; Vazquez, M.; Mc Donnell, K.; Liepins, J.; Ben-Yoav, H.; Karitans, V.; Smits, K. Portable low-cost open-source wireless spectrophotometer for fast and reliable measurements. Hardwarex 2020, 7, e00108. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Shin, S.; Hwang, J.; Kim, Y.-J.; Choi, S. Open-Source Fluorescence Spectrometer for Noncontact Scientific Research and Education. J. Chem. Educ. 2021, 98, 3493–3501. [Google Scholar] [CrossRef]
- Mayer, M.; Baeumner, A.J. A Megatrend Challenging Analytical Chemistry: Biosensor and Chemosensor Concepts Ready for the Internet of Things. Chem. Rev. 2019, 119, 7996–8027. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Kitowski, I.; Łopucki, R.; Stachniuk, A.; Fornal, E. A pesticide banned in the European Union over a decade ago is still present in raptors in Poland. Environ. Conserv. 2020, 47, 310–314. [Google Scholar] [CrossRef]
- Ruiz-Suarez, N.; Boada, L.D.; Henriquez-Hernandez, L.A.; Gonzalez-Moreo, F.; Suarez-Perez, A.; Camacho, M.; Zumbado, M.; Almeida-Gonzalez, M.; Del Mar Travieso-Aja, M.; Luzardo, O.P. Continued implication of the banned pesticides carbofuran and aldicarb in the poisoning of domestic and wild animals of the Canary Islands (Spain). Sci. Total Environ. 2015, 505, 1093–1099. [Google Scholar] [CrossRef]
- Alvarez, J.L.; Mozo, J.D.; Duran, E. Analysis of Single Board Architectures Integrating Sensors Technologies. Sensors 2021, 21, 6303. [Google Scholar] [CrossRef]
- TAOS TSL230R Datasheet. Available online: https://pdf1.alldatasheet.com/datasheet-pdf/view/153439/ETC1/TSL230R.html (accessed on 30 April 2023).
- Centropen. Security UV-SET 2699. Available online: https://www.centropen.cz/en/security-uv-set-2699-pg-1909-c-190/ (accessed on 5 September 2023).
- Keresteš, O.; Pohanka, M. Affordable Portable Platform for Classic Photometry and Low-Cost Determination of Cholinesterase Activity. Biosensors 2023, 13, 599. [Google Scholar] [CrossRef]
- Söderby, K. Getting Started with the Arduino IoT Cloud. Available online: https://docs.arduino.cc/arduino-cloud/getting-started/iot-cloud-getting-started (accessed on 20 September 2023).
- Söderby, K. IoT Cloud Dashboards & Widgets. Available online: https://docs.arduino.cc/arduino-cloud/getting-started/dashboard-widgets (accessed on 20 September 2023).
- Keresteš, O. iPhone 12 Mini Cuvette Stand for Photogrammetry; NIH 3D; National Institutes of Health: Bethesda, MD, USA, 2023. [Google Scholar] [CrossRef]
- Sun, Z.W.; Tian, L.Y.; Guo, M.; Xu, X.T.; Li, Q.; Weng, H.B. A double-film screening card for rapid detection of organophosphate and carbamate pesticide residues by one step in vegetables and fruits. Food Control 2017, 81, 23–29. [Google Scholar] [CrossRef]
- Guo, X.S.; Zhang, X.Y.; Cai, Q.; Shen, T.; Zhu, S.M. Developing a novel sensitive visual screening card for rapid detection of pesticide residues in food. Food Control 2013, 30, 15–23. [Google Scholar] [CrossRef]
- Matoušek, J.; Fischer, J.; Cerman, J. A new fluorimetric method for determination of submicrogram amounts of cholinesterase inhibitors. Chem. Pap. 1968, 22, 184–189. [Google Scholar]
- Díaz, A.N.; Sánchez, F.G.; Bracho, V.; Lovillo, J.; Aguilar, A. Enzymatic determination of fenitrothion by cholinesterase and acetylcholinesterase on fluorogenic substrates. Fresenius’ J. Anal. Chem. 1997, 357, 958–961. [Google Scholar] [CrossRef]
- Díaz, A.N.; Sánchez, F.G.; del Río, V.B. Kinetic Enzymatic Determination of Chlorpyriphos in Apples. Anal. Lett. 1995, 28, 1071–1082. [Google Scholar] [CrossRef]
- Valek, T.; Kostelnik, A.; Valkova, P.; Pohanka, M. Indoxyl Acetate as a Substrate for Analysis of Lipase Activity. Int. J. Anal. Chem. 2019, 2019, 8538340. [Google Scholar] [CrossRef]
- Kostelnik, A.; Cegan, A.; Pohanka, M. Acetylcholinesterase Inhibitors Assay Using Colorimetric pH Sensitive Strips and Image Analysis by a Smartphone. Int. J. Anal. Chem. 2017, 2017, 3712384. [Google Scholar] [CrossRef]
- Ramallo, I.A.; Garcia, P.; Furlan, R.L.E. A reversed-phase compatible thin-layer chromatography autography for the detection of acetylcholinesterase inhibitors. J. Sep. Sci. 2015, 38, 3788–3794. [Google Scholar] [CrossRef]
- Antela, K.U.; Sáez-Hernández, R.; Cervera, M.L.; Morales-Rubio, Á.; Luque, M.J. Development of an automated colorimeter controlled by Raspberry Pi4. Anal. Methods 2023, 15, 512–518. [Google Scholar] [CrossRef]
- de Carvalho Oliveira, G.; Machado, C.C.S.; Inácio, D.K.; Silveira Petruci, J.F.d.; Silva, S.G. RGB color sensor for colorimetric determinations: Evaluation and quantitative analysis of colored liquid samples. Talanta 2022, 241, 123244. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Halder, N.; Singh, B.; Singh, J.; Sharma, S.; Shacham-Diamand, Y. Smart Farming Revolution: Portable and Real-Time Soil Nitrogen and Phosphorus Monitoring for Sustainable Agriculture. Sensors 2023, 23, 5914. [Google Scholar] [CrossRef] [PubMed]


| Component | Number | Cost per Unit | Total Cost | Source of Materials | Material Type |
|---|---|---|---|---|---|
| One-sided positive pre-sensitized PCB card | 1 | USD 2 | USD 2 | https://www.amazon.com/Aoje-Link-Single-Copper-Laminate-Circuit/dp/B091T93HKS/ (accessed on 20 January 2024) | Composite |
| Arduino UNO R3/R4 Wi-Fi | 1 | USD 24 (offline) / USD 25 (remote) | USD 24 (offline) / USD 25 (remote) | https://store.arduino.cc/products/arduino-uno-rev3 /https://store.arduino.cc/products/uno-r4-wifi (accessed on 20 January 2024) | Semi-conductor |
| Header 4 × 1, 2.54 mm, female, straight | 1 | USD 1 | USD 1 | Local hardware store | Metal |
| Header 7 × 1, 2.54 mm, female, straight | 1 | USD 1 | USD 1 | Local hardware store | Metal |
| Male header 20 × 1, 2.54 mm, angle | 1 | USD 1 | USD 1 | Local hardware store | Metal |
| Male header, 20 × 1, 2.54 mm, straight | 1 | USD 1 | USD 1 | Local hardware store | Metal |
| TSL230R light-frequency sensor | 1 | USD 28 (2022) USD 51 (2024) | USD 28 (2022) USD 51 (2024) | https://www.aliexpress.com/item/1005006332337743.html (accessed on 20 January 2024) | Semi-conductor |
| RGB LED common cathode, diffuse | 1 | USD 1 | USD 1 | Local hardware store | Semi-conductor |
| Jumper wires | 5 | USD 0.2 | USD 1 | Local hardware store | Metal |
| Printing material (ASA) | 100 g | USD 4 | https://www.prusa3d.com/product/prusament-asa-signal-white-850g/ (accessed on 20 January 2024) | Polymer | |
| Capacitor 0.1 μF | 1 | USD 1 | USD 1 | Local hardware store | Composite |
| Socket 4 × 2, DIP | 1 | USD 1 | USD 1 | Local hardware store | Other |
| UV LED from Centropen secure kit | 1 | USD 1 | USD 1 | https://www.amazon.de/dp/B01AUI4VTM (similar emission, accessed on 20 January 2024)) | Semi-conductor |
| Battery holder for 4 × AAA | 1 | USD 2 | USD 2 | https://www.amazon.com/Pack-AA-AAA-Battery-Holders/dp/B08Q3DLY39 (accessed on 20 January 2024) | Other |
| Screw terminal 3-pin | 1 | USD 1 | USD 1 | Local hardware store | Other |
| Cable 2-wire (black, red; 50 cm/approx. 2 ft) | 1 | USD 1 | USD 1 | Local hardware store | metal |
| Analyte | Principle | Method | IC50 [nmol/L] | LOD [nmol/L] | Ref. |
|---|---|---|---|---|---|
| Donepezil | Spectrofluorimetry | IA assay | 80.59 | 33.7 | This work |
| Fluorimetry (OMD) | 100.6 | 54.45 | |||
| Photogrammetry | Dipstick pH-metry | --- | 22.3 | [43] | |
| Carbofuran | Spectrofluorimetry | Ellman assay | 102.9 | 17.15 | [33] |
| Fluorimetry (OMD) | IA assay | 84.30 | 94.42 | This work | |
| Photogrammetry | --- | 22.55 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keresteš, O.; Mozo, J.D.; Pohanka, M. Open Meter Duo: Low-Cost Instrument for Fluorimetric Determination of Cholinesterase Activity. Sensors 2024, 24, 1774. https://doi.org/10.3390/s24061774
Keresteš O, Mozo JD, Pohanka M. Open Meter Duo: Low-Cost Instrument for Fluorimetric Determination of Cholinesterase Activity. Sensors. 2024; 24(6):1774. https://doi.org/10.3390/s24061774
Chicago/Turabian StyleKeresteš, Ondřej, Juan Daniel Mozo, and Miroslav Pohanka. 2024. "Open Meter Duo: Low-Cost Instrument for Fluorimetric Determination of Cholinesterase Activity" Sensors 24, no. 6: 1774. https://doi.org/10.3390/s24061774
APA StyleKeresteš, O., Mozo, J. D., & Pohanka, M. (2024). Open Meter Duo: Low-Cost Instrument for Fluorimetric Determination of Cholinesterase Activity. Sensors, 24(6), 1774. https://doi.org/10.3390/s24061774








