Screening for Peripheral Vascular Stiffness in Lipedema Patients by Automatic Electrocardiogram-Based Oscillometric Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design and Cohort
2.3. Recruitment
2.4. Measuring Pulse Wave Speed Using Oscillometry
2.4.1. Preparation of Patients
2.4.2. Electrocardiogram (ECG) Recording and Recording of the Arterial Pulse Curves
2.5. Calculation of Pulse Wave Velocity (PWV)
2.6. Technical Details of the Oscillometric Sensor Technique
2.7. Standard Values for Pulse Wave Velocity of the Published Reference Cohort
2.8. Applied Statistical Methods
3. Results
Baseline Characteristics of Patients
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weissleder, H.; Brauer, J.W.; Schuchhardt, C.; Herpertz, U. Value of functional lymphoscintigraphy and indirect lymphangiography in lipedema syndrome. Z. Lymphol. 1995, 19, 38–41. [Google Scholar] [PubMed]
- Amann-Vesti, B.R.; Franzeck, U.K.; Bollinger, A. Microlymphatic aneurysms in patients with lipedema. Lymphology 2001, 34, 170–175. [Google Scholar] [PubMed]
- Szolnoky, G.; Nagy, N.; Kovacs, R. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology 2008, 41, 161–166. [Google Scholar] [PubMed]
- Wiedner, M.; Aghajanzadeh, D.; Richter, D.F. Lipödem—Grundlagen und aktuelle Thesen zum Pathomechanismus. Phlebologie 2021, 50, 288–293. [Google Scholar] [CrossRef]
- Schmeller, W.; Meier-Vollrath, I. Lipödem—Aktuelles zu einem weitgehend unbekanntem Krankheitsbild. Akt. Dermatol. 2007, 33, 251–260. [Google Scholar] [CrossRef][Green Version]
- Moulakakis, K.G.; Kadoglou, N.P.E.; Antonopoulos, C.N.; Mylonas, S.N.; Kakisis, J.; Papadakis, I.; Karakitsos, P.; Liapis, C.D. Changes in Arterial Stiffness and N-terminal pro-brain natriuretic peptide Levels after Endovascular Repair of Descending Thoracic Aorta. Ann. Vasc. Surg. 2017, 38, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Dumor, K.; Shoemaker-Moyle, M.; Nistala, R.; Whaley-Connell, A. Arterial Stiffness in Hypertension: An Update. Curr. Hypertens. Rep. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Szolnoky, G.; Nemes, A.; Gavallér, H.; Forster, T.; Kemény, L. Lipedema is associated with increased aortic stiffness. Lymphology 2012, 45, 71–79. [Google Scholar] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- The Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Troiani, C.; Gabutti, S.; Stefanelli, K.; Gabutti, L. Comparing oscillometric and tonometric methods to assess pulse wave velocity: A population-based study. Ann. Med. 2021, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Yudkin, J.S.; Stehouwer, C.D.; Schalkwijk, C.G.; Pratley, R.E.; Tataranni, P. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis 2002, 161, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.; Forner-Cordero, I.; Faerber, G.; Rapprich, S.; Cornely, M. Body mass index vs. waist-to-height-ratio in patients with lipohyperplasia dolorosa (vulgo lipedema). J. Der Dtsch. Dermatol. Ges. = J. Ger. Soc. Dermatol. JDDG 2023, 21, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormanyos, A.; Domsik, P.; Kalapos, A.; Kemeny, L.; Forster, T.; Szolnoky, G. Left ventricular rotational mechanics differ between lipedema and lymphedema: Insights from the three-dimensional speckle tracking echocardiographic MAGYAR-path study. Lymphology 2018, 51, 102–108. [Google Scholar] [PubMed]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ishizu, T.; Aonuma, K. Current status of 3-dimensional speckle tracking echocardiography: A review from our experiences. J. Cardiovasc. Ultrasound 2014, 22, 49–57. [Google Scholar] [CrossRef] [PubMed]

| Patients with Lipedema (Total n = 41, Only Females) | ||
|---|---|---|
| Baseline characteristics as mean ± standard deviation | ||
| Age (in years) | 40.2 ± 11.5 | |
| Height (in cm) | 167.6 ± 6.6 | |
| Weight (in kg) | 79.8 ± 14.2 | |
| Body mass index (BMI, in kg/m2) | 28.4 ± 4.9 | |
| Body surface (in m2) | 1.9 ± 0.2 | |
| Stage of lipedema disease | ||
| n= | % | |
| Stage I | 3 | 7.3 |
| Stage II | 8 | 19.5 |
| Stage III | 30 | 73.2 |
| Risk factors for lipedema disease | ||
| n= | % | |
| Family disposition | 11 | 22.8 |
| Previous pregnancy | 15 | 36.6 |
| Hormonal contraception or hormone replacement therapy | 19 | 46.3 |
| Patient Groups | |||
|---|---|---|---|
| Stage I and II lipedema | Stage III lipedema | p-value | |
| Age (in years) | |||
| Mean ± standard deviation | 34.7 ± 7.4 | 42.2 ± 12.3 | n.s. p = 0.066 |
| Minimum | 25 | 24 | |
| Q1 | 29 | 33 | |
| Median | 33 | 40.5 | |
| Q3 | 39.75 | 54 | |
| Maximum | 47 | 65 | |
| Height (in cm) | |||
| Mean ± standard deviation | 166.7 ± 6.1 | 167.9 ± 6.9 | n.s. p = 0.613 |
| Minimum | 157 | 153 | |
| Q1 | 162.5 | 163 | |
| Median | 166 | 168.5 | |
| Q3 | 172.5 | 173 | |
| Maximum | 175 | 185 | |
| Weight (in kg) | |||
| Mean ± standard deviation | 67 ± 11.0 kg | 84.5 ± 12.3 | p = 0.001 |
| Minimum | 55 | 54 | |
| Q1 | 58 | 80.3 | |
| Median | 68 | 88 | |
| Q3 | 70.1 | 92 | |
| Maximum | 92 | 107 | |
| Body mass index (in kg/m2) | |||
| Mean ± standard deviation | 24.1 ± 3.5 | 30.0 ± 4.3 | p = 0.001 |
| Minimum | 20.4 | 19.1 | |
| Q1 | 22.1 | 27.7 | |
| Median | 23.5 | 31.1 | |
| Q3 | 25.2 | 33.5 | |
| Maximum | 33.4 | 35.9 | |
| Body surface area (in m2) | |||
| Mean ± standard deviation | 1.8 ± 0.2 | 1.9 ± 0.2 | p = 0.002 |
| Minimum | 1.6 vr | 1.6 | |
| Q1 | 1.6 | 1.9 | |
| Median | 1.8 | 2.0 | |
| Q3 | 1.9 | 2.0 | |
| Maximum | 2.0 | 2.2 | |
| Comparison of Hemodynamic Parameters | |||
|---|---|---|---|
| Stage I and II lipedema | Stage III lipedema | p-value | |
| Systolic blood pressure (mmHg) | |||
| Mean ± standard deviation | 115.8 ± 7.7 | 121.5 ± 9.2 | n.s. p = 0.074 |
| Minimum | 104.6 | 103.6 | |
| Q1 | 111.0 | 115.9 | |
| Median | 114.5 | 119.6 | |
| Q3 | 120.6 | 127.7 | |
| Maximum | 131.4 | 138.8 | |
| Diastolic blood pressure (mmHg) | |||
| Mean ± standard deviation | 75.1 ± 7.3 | 78.9 ± 9.8 | n.s. p = 0.245 |
| Minimum | 66.2 | 57.8 | |
| Q1 | 68.3 | 73.5 | |
| Median | 73.3 | 78.0 | |
| Q3 | 81.7 | 84.7 | |
| Maximum | 87.0 | 101.3 | |
| Heart rate (beats/minute) | |||
| Mean ± standard deviation | 70.9 ± 6.2 | 72.3 ± 11.2 | n.s. p = 0.706 |
| Minimum | 60 | 49 | |
| Q1 | 68.3 | 66 | |
| Median | 69 | 72.5 | |
| Q3 | 72.8 | 77 | |
| Maximum | 82 | 107 | |
| Heart rate variability (ms) | |||
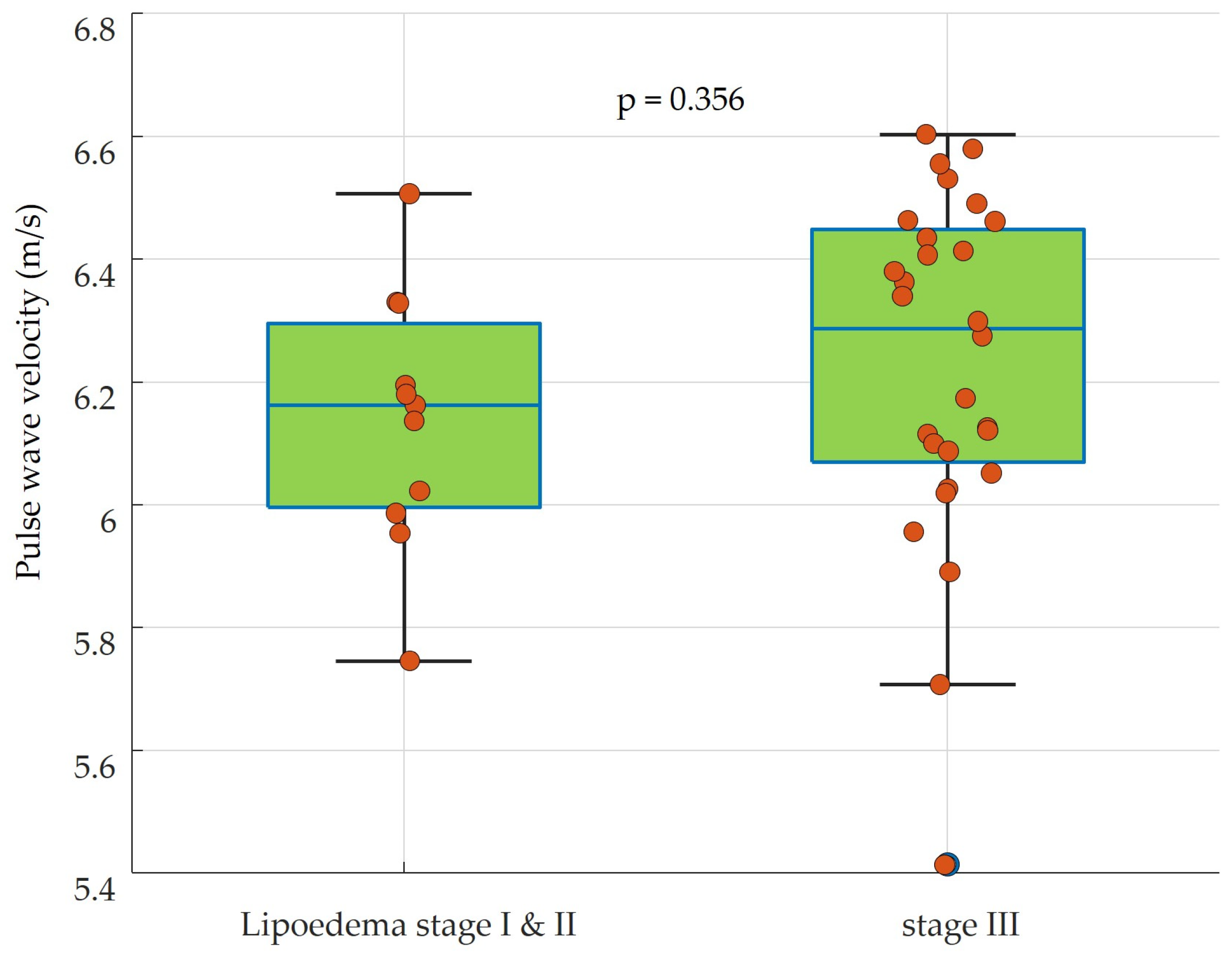
| Mean ± standard deviation | 830.9 ± 79.2 | 846.4 ± 121.9 | n.s. p = 0.356 |
| Minimum | 714 | 670 | |
| Q1 | 782.3 | 764 | |
| Median | 839 | 826.5 | |
| Q3 | 863 | 906 | |
| Maximum | 996 | 1204 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlmann, A.; Khorzom, Y.; Behrendt, C.-A.; Leip, J.L.; Bachler, M.; Wassertheurer, S.; Elzanaty, N.; Ghazy, T. Screening for Peripheral Vascular Stiffness in Lipedema Patients by Automatic Electrocardiogram-Based Oscillometric Detection. Sensors 2024, 24, 1673. https://doi.org/10.3390/s24051673
Mahlmann A, Khorzom Y, Behrendt C-A, Leip JL, Bachler M, Wassertheurer S, Elzanaty N, Ghazy T. Screening for Peripheral Vascular Stiffness in Lipedema Patients by Automatic Electrocardiogram-Based Oscillometric Detection. Sensors. 2024; 24(5):1673. https://doi.org/10.3390/s24051673
Chicago/Turabian StyleMahlmann, Adrian, Yazan Khorzom, Christian-Alexander Behrendt, Jennifer Lynne Leip, Martin Bachler, Siegfried Wassertheurer, Nesma Elzanaty, and Tamer Ghazy. 2024. "Screening for Peripheral Vascular Stiffness in Lipedema Patients by Automatic Electrocardiogram-Based Oscillometric Detection" Sensors 24, no. 5: 1673. https://doi.org/10.3390/s24051673
APA StyleMahlmann, A., Khorzom, Y., Behrendt, C.-A., Leip, J. L., Bachler, M., Wassertheurer, S., Elzanaty, N., & Ghazy, T. (2024). Screening for Peripheral Vascular Stiffness in Lipedema Patients by Automatic Electrocardiogram-Based Oscillometric Detection. Sensors, 24(5), 1673. https://doi.org/10.3390/s24051673









