Progress and Challenge of Sensors for Dairy Food Safety Monitoring
Abstract
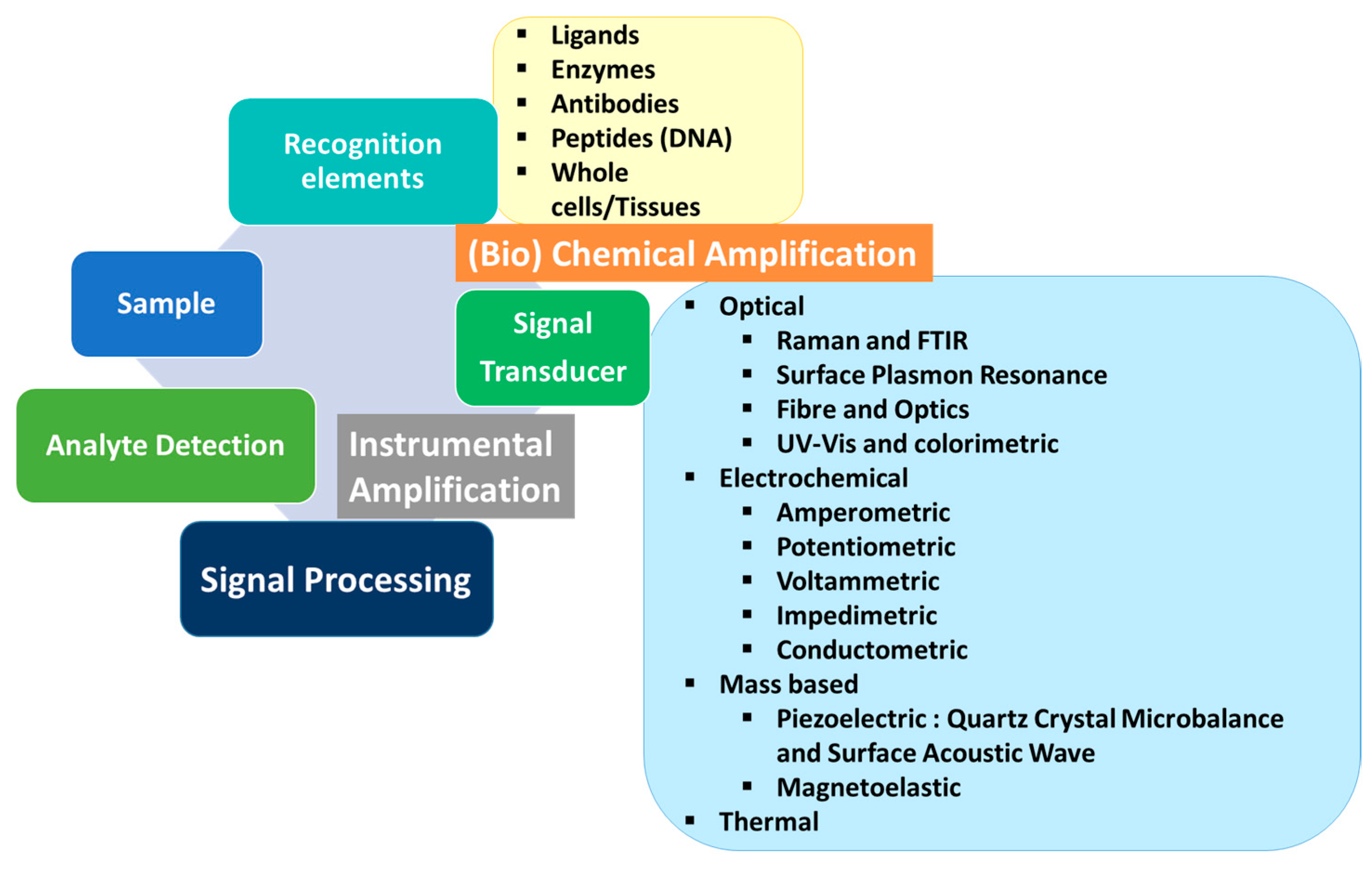
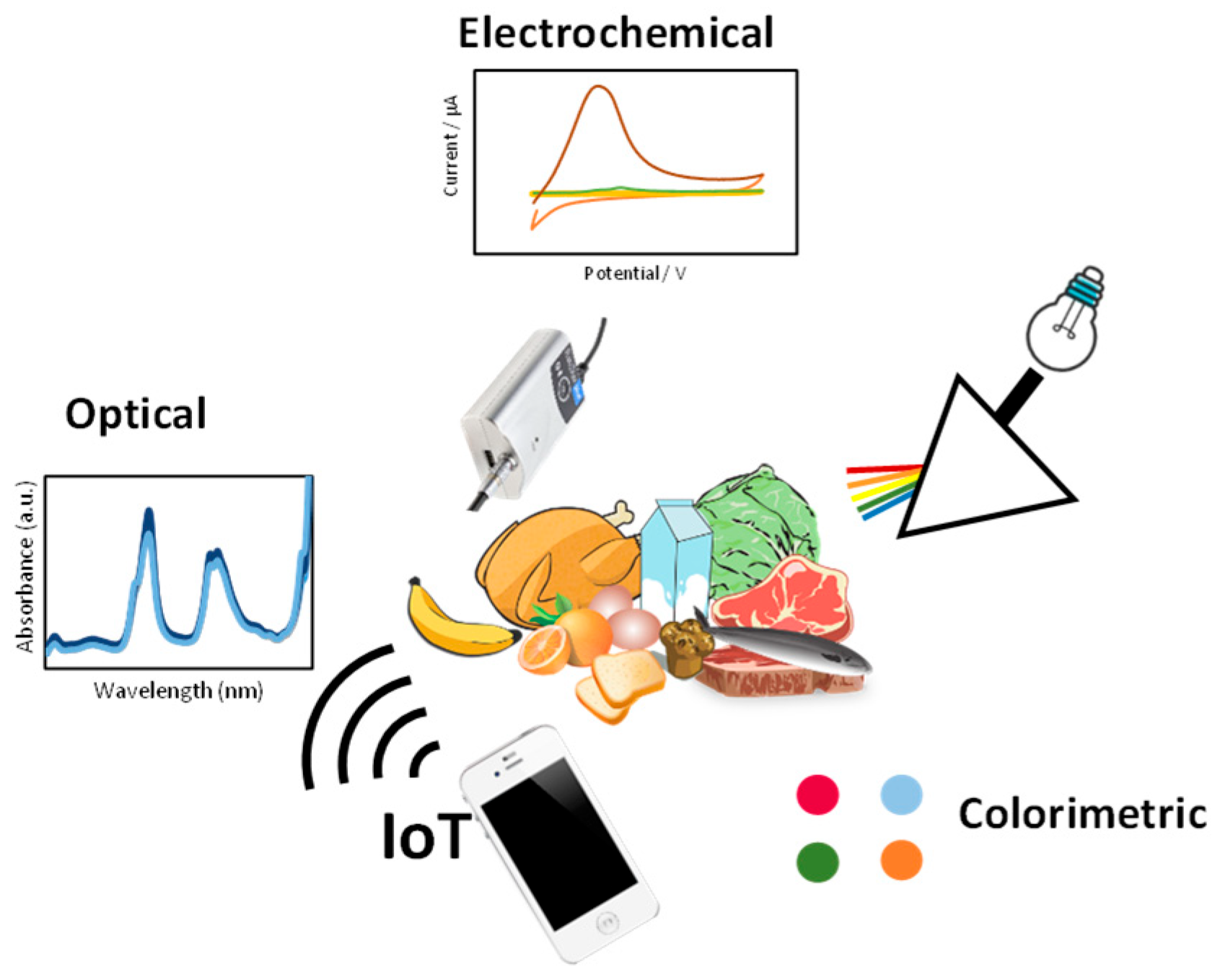
1. Introduction
2. Optical Sensors
2.1. Surface-Enhanced Raman Spectroscopy
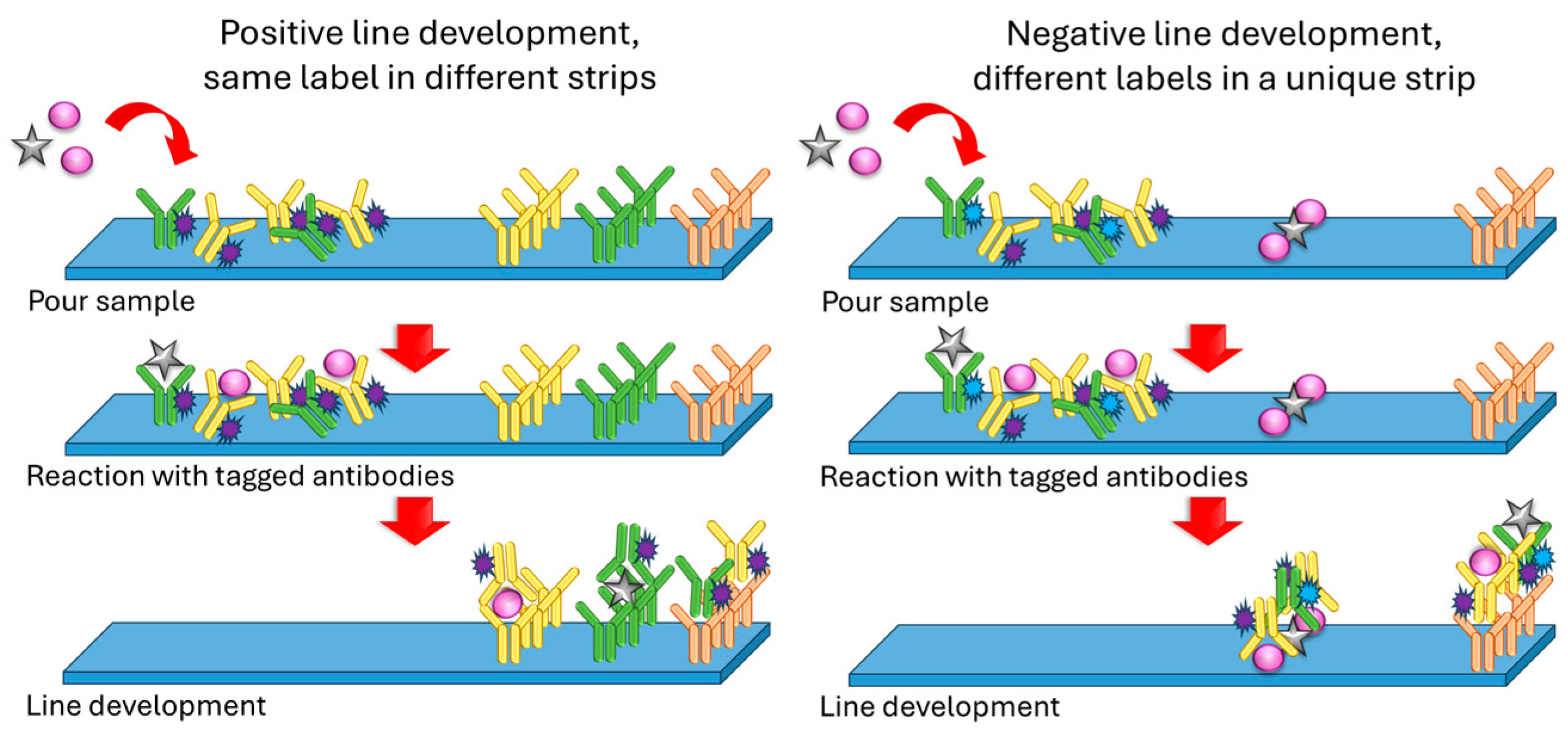
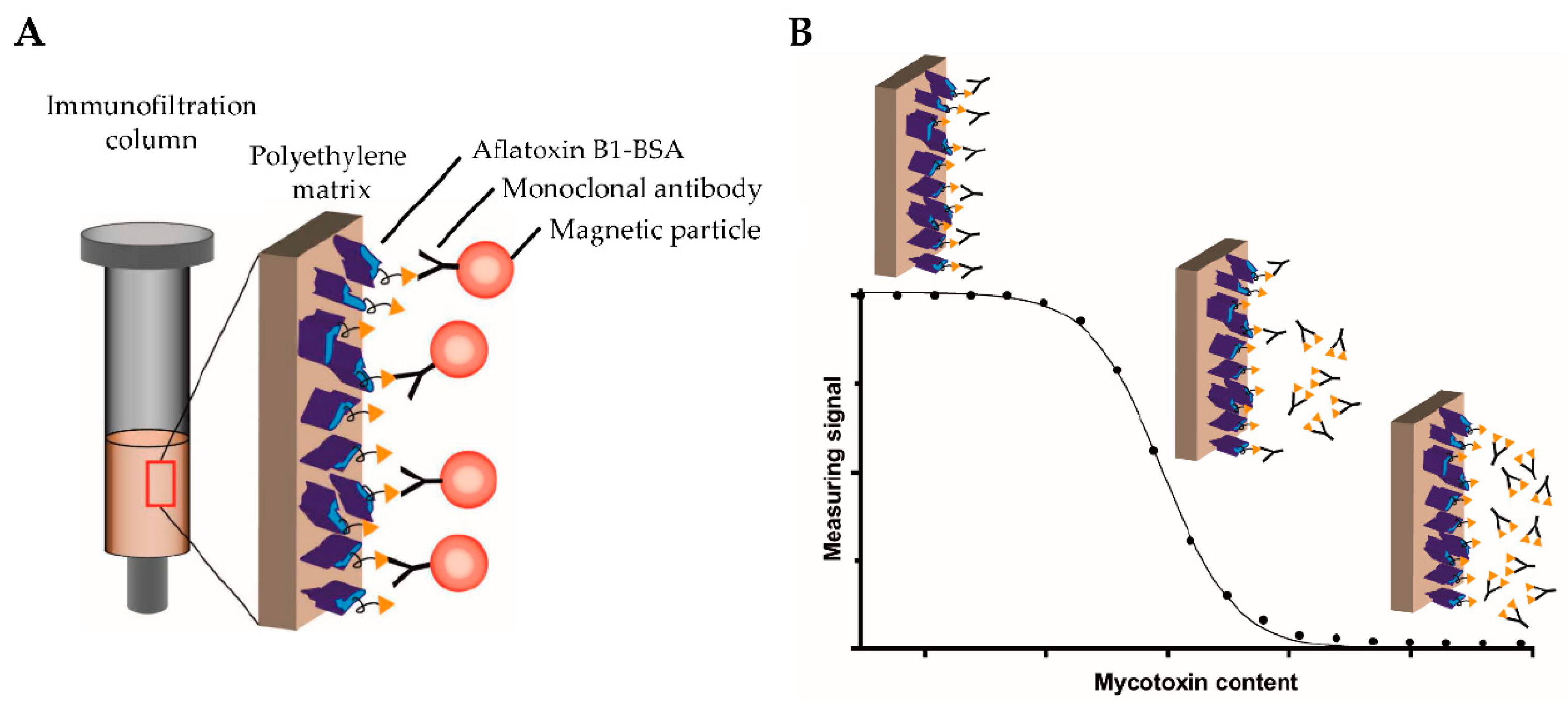
2.2. Lateral Flow Assays (LFAs)
2.3. Immunoassays with Optical Detection
2.4. Chemiluminescence
2.5. Label-Free Assays
2.6. Dairy and Allergens
3. Electrochemical Devices/Platforms
3.1. Multiplex Electrochemical Platform for Antibiotics
3.2. Multiplex Devices for Bacterial Recognition
3.3. Platforms for Other Targets of Interest
4. Other Sensing Platforms in Food Safety
4.1. Monitoring Temperature of the Chilled or Frozen Chain
4.2. Monitoring pH and Biogenic Amines
4.3. Oxygen, Carbon Dioxide, and Volatile Compounds
4.4. Pathogens
4.5. Mycotoxins
4.6. Multiplexed Sensors
4.7. Other Sensing Alternatives in Food Preservation and Sterilization
4.8. Sensor for Detecting Per- and Polyfluoroalkyl Substances (PFASs)
5. Conclusions and Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Codex Alimentarius, Milk and Milk Products, 2nd ed.; World Health Organization & Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-105837-4. Available online: https://www.fao.org/3/i2085e/i2085e00.pdf (accessed on 12 February 2024).
- Commission Regulation (EC) No 853/2004 of 29 April 2004, Official Journal L139. p. 55. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj (accessed on 12 February 2024).
- RD1086/2020, BOE-A-2020-15872. Available online: https://www.boe.es/buscar/pdf/2020/BOE-A-2020-15872-consolidado.pdf (accessed on 12 February 2024).
- Juronen, D.; Kuusk, A.; Kivirand, K.; Rinken, A.; Rinken, T. Immunosensing system for rapid multiplex detection of mastitis-causing pathogens in milk. Talanta 2018, 178, 949–954. [Google Scholar] [CrossRef]
- Duan, N.; Gong, W.; Wang, Z.; Wu, S. An aptasensor based on fluorescence resonance energy transfer for multiplexed pathogenic bacteria determination. Anal. Methods 2016, 8, 1390–1395. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Chen, X.; Wang, X.; Duan, N.; Wu, S.; Xu, B.; Wang, Z. A multicolor time-resolved fluorescence aptasensor for the simultaneous detection of multiplex Staphylococcus aureus enterotoxins in the milk. Biosens. Bioelectron. 2015, 74, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Shriver-Lake, L.C.; Erickson, J.S.; Sapsford, K.E.; Ngundi, M.M.; Shaffer, K.M.; Kulagina, N.V.; Hu, J.E.; Gray III, S.A.; Golden, J.P.; Ligler, F.S.; et al. Blind laboratory trials for multiple pathogens in spiked food matrices. Anal. Lett. 2007, 40, 3219–3231. [Google Scholar] [CrossRef]
- Cho, I.H.; Mauer, L.; Irudayaraj, J. In-situ fluorescent immunomagnetic multiplex detection of foodborne pathogens in very low numbers. Biosens. Bioelectron. 2014, 57, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiao, R.; Wang, S.; Yang, X.; Bai, Z.; Li, X.; Rong, Z.; Shen, B.; Wang, S. Magnetic quantum dot based lateral flow assay biosensor for multiplex and sensitive detection of protein toxins in food samples. Biosens. Bioelectron. 2019, 146, 111754. [Google Scholar] [CrossRef]
- Shi, Q.; Tao, C.; Kong, D. Multiplex SERS-based lateral flow assay for one-step simultaneous detection of neomycin and lincomycin in milk. Eur. Food Res. Technol. 2022, 248, 2157–2165. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wang, L.; Yang, T.; Wang, D. Duplex Detection of Antibiotics in Milk Powder Using Lateral-Flow Assay Based on Surface-Enhanced Raman Spectroscopy. Food Anal. Methods 2021, 14, 165–171. [Google Scholar] [CrossRef]
- Li, Y.; Jin, G.; Liu, L.; Kuang, H.; Jing, X.; Chuanlai, X. A portable fluorescent microsphere-based lateral flow immunosensor for the simultaneous detection of colistin and bacitracin in milk. Analyst 2020, 145, 7884–7892. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zhao, D.; Wen, F.; Jiang, J.; Xu, D. Smartphone-based visualized microarray detection for multiplexed harmful substances in milk. Biosens. Bioelectron. 2017, 87, 874–880. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, C.; Zhang, H.; Yang, W.; Jiang, F.; Chen, G.; Liu, S.; Chen, Y. Machine-Learning-driven optical immunosensor based on microespheres-encoded signal transduction for the rapid and multiplexed detection of antibiotics in milk. Food Chem. 2024, 437, 137740. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, J.; Sun, Y.; Yin, M.; Hu, M.; Hu, X.; Zhang, Z.; Zhang, G. Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 107–113. [Google Scholar] [CrossRef]
- Jiang, R.; Lin, D.; Zhang, Q.; Li, L.; Yang, L. Multiplex chroma-response based fluorescent smartphone sensing platform for rapid and visual quantitaive determination of antibiotic. Sens. Actuators B. Chem. 2022, 350, 130902. [Google Scholar] [CrossRef]
- Lu, L.; Xu, L.; Zhang, Y.; Jiang, T. Multiplexed surface-enhanced Raman scattering detection of melamine and dicyandiamide in dairy food enabled by three-dimensional polystyrene@silver@graphene oxide hybrid substrate. Appl. Surf. Sci. 2022, 603, 154419. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, S.; Jin, Y.; Yang, C.; He, L.; Wang, J.; Chen, Y. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J. Hazard. Mater. 2020, 393, 122348. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, M.L.; Zakaria, N.D.; Noordin, R.; Abdul Razak, K. Development of rapid gold nanoparticles based lateral flow assays for simultaneous detection of Shigella and Salmonella genera. Biotechnol. Appl. Biochem. 2021, 68, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, J.; Xie, Y.; Zuo, X.; Mo, F.; Zhou, S.; Li, H. Dual-Label Chemiluminescence Strategy for Multiplexed Immunoassay of 20 Fluoroquinolones, 15 β-Lactams. 15 Sulfonamides, and CAP in Milk. Food Anal. Methods 2017, 10, 3009–3022. [Google Scholar] [CrossRef]
- Yu, X.; Tao, X.; Shen, J.; Zhang, S.; Cao, X.; Chen, M.; Wang, W.; Wang, Z.; Wen, K. A one-step chemiluminescence immunoassay for 20 fluoroquinolone residues in fish and shrimp based on a single chain Fv–alkaline phosphatase fusion protein. Anal. Methods 2015, 7, 9032–9039. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, X.; Wang, X.; Tao, X. A quadruple-labeling luminescence strategy for multiplexed immunoassay of 51 drugs in milk with an automated pretreatment system. Anal. Methods 2019, 11, 5055–5063. [Google Scholar] [CrossRef]
- Singh, A.K.; Sun, X.; Bai, X.; Kim, H.; Abdalhaseib, M.U.; Bae, E.; Bhunia, A.K. Label-free, non-invasive light scattering sensor for rapid screening of Bacillus colonies. J. Microbiol. Methods 2015, 109, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Raz, S.R.; Bremer, M.G.E.G.; Haasnoot, W.; Norde, W. Label-Free and Multiplex Detection of Antibiotic Residues in Milk Using Imaging Surface Plasmon Resonance-Based Immunosensor. Anal. Chem. 2009, 81, 7743–7749. [Google Scholar] [CrossRef]
- Suarez, G.; Jin, Y.H.; Auerswald, J.; Berchtold, S.; Knapp, H.F.; Diserens, J.M.; Leterrier, Y.; Manson, J.A.E.; Voirin, G. Lab-on-a-chip for multiplexed biosensing of residual antibiotics in milk. Lab Chip 2009, 9, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Petrou, P.S.; Makarona, E.; Haasnoot, W.; Moser, I.; Jobst, G.; Goustouridis, D.; Lees, M.; Kalatzi, K.; Raptis, I.; et al. Ultrafast Multiplexed-Allergen Detection through Advanced Fluidic Design and Monolithic Interferometric Silicon Chips. Anal. Chem. 2018, 90, 9559–9567. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, G.; Reboud, J.; Ali, S.A.; Kaur, G.; McGiven, J.; Boby, N.; Gupta, P.K.; Chaudhuri, P.; Cooper, J.M. Rapid Veterinary Diagnosis of Bovine Reproductive Infectious Diseases from Semen Using Paper-Origami DNA Microfluidics. ACS Sens. 2018, 3, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Tran, T.; Gartia, M.R. Multiplexed Paper Microfluidics for Titration and Detection of Ingredients in Beverages. Sensors 2019, 19, 1286. [Google Scholar] [CrossRef]
- Chen, X.; Yao, C.; Li, Z. Microarray-based chemical sensors and biosensors: Fundamentals and food safety applications, TrAC. Trends Anal. Chem. 2023, 158, 116785. [Google Scholar] [CrossRef]
- Ashley, J.; D’Aurelio, R.; Piekarska, M.; Temblay, J.; Pleasants, M.; Trinh, L.; Rodgers, T.L.; Tothill, I.E. Development of a β-Lactoglobulin Sensor Based on SPR for Milk Allergens Detection. Biosensors 2018, 8, 32. [Google Scholar] [CrossRef]
- Bojcukova, J.; Vlas, T.; Forstenlechner, P.; Panzner, P. Comparison of two multiplex arrays in the diagnostics of allergy. Clin. Transl. Allergy 2019, 9, 31. [Google Scholar] [CrossRef]
- Scala, E.; Caprini, E.; Abeni, D.; Meneguzzi, G.; Buzzulini, F.; Cecchi, L.; Villalta, D.; Asero, R. A qualitative and quantitative comparison of IgE antibody profiles with two multiplex platforms for component-resolved diagnostics in allergic patients. Clin. Exp. Allergy 2021, 51, 1603–1612. [Google Scholar] [CrossRef]
- ImmunoCAP ISAC Test. Available online: https://www.thermofisher.com/phadia/wo/en/our-solutions/immunocap-allergy-solutions/specific-ige-multiplex.html (accessed on 14 February 2024).
- ELISA Based In-Vitro Multiplex Allergy Test. Available online: https://www.macroarraydx.com/products/alex (accessed on 14 February 2024).
- Monroe, M.R.; Daaboul, G.G.; Tuysuzoglu, A.; López, C.A.; Little, F.F.; Ünlü, M.S. Single Nanoparticle Detection for Multiplexed Protein Diagnostics with Attomolar Sensitivity in Serum and Unprocessed Whole Blood. Anal. Chem. 2013, 85, 3698–3706. [Google Scholar] [CrossRef]
- Galan-Malo, P.; Pellicer, S.; Pérez, M.D.; Sánchez, L.; Razquin, P.; Mata, L. Development of a novel duplex lateral flow test for simultaneous detection of casein and β-lactoglobulin in food. Food Chem. 2019, 293, 41–48. [Google Scholar] [CrossRef]
- Masiri, J.; Barrios-López, B.; Benoit, L.; Tamayo, J.; Day, J.; Nadala, C.; Sung, S.L.; Samadpour, M. Development and Validation of a Lateral Flow Immunoassay Test Kit for Dual Detection of Casein and β-Lactoglobulin Residues. J. Food Prot. 2016, 79, 477–483. [Google Scholar] [CrossRef]
- Badran, A.A.; Morais, S.; Maquieira, Á. Simultaneous determination of four food allergens using compact disc immunoassaying technology. Anal. Bioanal. Chem. 2017, 409, 2261–2268. [Google Scholar] [CrossRef]
- Raz, S.R.; Liu, H.; Norde, W.; Bremer, M.G.E.G. Food Allergens Profiling with an Imaging Surface Plasmon Resonance-Based Biosensor. Anal. Chem. 2010, 82, 8485–8491. [Google Scholar] [CrossRef]
- Tortajada-Genaro, L.A.; Santiago-Felipe, S.; Morais, S.; Gabaldón, J.A.; Puchades, R.; Maquieira, Á. Multiplex DNA Detection of Food Allergens on a Digital Versatile Disk. J. Agric. Food Chem. 2012, 60, 36–43. [Google Scholar] [CrossRef]
- Blais, B.W.; Gaudreault, M.; Phillippe, L.M. Multiplex enzyme immunoassay system for the simultaneous detection of multiple allergens in foods. Food Control 2003, 14, 43–47. [Google Scholar] [CrossRef]
- Lin, H.Y.; Huang, C.H.; Park, J.; Pathania, D.; Castro, C.M.; Fasano, A.; Weissleder, R.; Lee, H. Integrated Magneto-Chemical Sensor for On-Site Food Allergen Detection. ACS Nano 2017, 11, 10062–10069. [Google Scholar] [CrossRef] [PubMed]
- Filep, S.C.; Black, K.R.; Smith, B.R.E.; Block, D.S.; Kuklinska-Pijanka, A.; Bermingham, M.; Oliver, M.A.; Thorpe, C.M.; Schuhmacher, Z.P.; Agah, S.; et al. Simultaneous quantification of specific food allergen proteins using a fluorescent multiplex array. Food Chem. 2022, 389, 132986. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.; Boye, J. Simultaneous detection of multi-allergens in an incurred food matrix using ELISA, multiplex flow cytometry and liquid chromatography mass spectrometry (LC–MS). Food Chem. 2015, 175, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.S.; Filippini, D.; Nielen, M.W.F.; Salentijn, G.I.J. Interconnectable solid-liquid protein extraction unit and chip-based dilution for multiplexed consumer immunodiagnostics. Anal. Chim. Acta 2020, 1140, 190–198. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Smits, N.G.E.; Blázquez, D.; Albero-Pérez, C.; Pallás-Tamarit, Y.; Maquieira, Á.; Morais, S. Simultaneous quantification of six major allergens in commercial foods for children using a multiplex array on a digital versatile disc. Food Chem. 2023, 404, 134570. [Google Scholar] [CrossRef]
- Grabowska, I.; Hepel, M.; Kurzątkowska-Adaszyńska, K. Advances in Design Strategies of Multiplex Electrochemical Aptasensors. Sensors 2022, 22, 161. [Google Scholar] [CrossRef] [PubMed]
- Rapini, R.; Marrazza, G. Electrochemical aptasensors for contaminants detection in food and environment: Recent advances. Bioelectrochemistry 2017, 118, 47–61. [Google Scholar] [CrossRef]
- Conzuelo, F.; Campuzano, S.; Gamella, M.; Pinacho, D.G.; Reviejo, A.J.; Marco, M.P.; Pingarrón, J.M. Integrated disposable electrochemical immunosensors for the simultaneous determination of sulfonamide and tetracycline antibiotics residues in milk. Biosens. Bioelectron. 2013, 50, 100–105. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.; Wang, C.; Tiana, Y.; Zhou, N. Simultaneous electrochemical detection of multiple antibiotic residues in milk based on aptamers and quantum dots. Anal. Methods 2016, 8, 1981–1988. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xua, Q.; Cao, Y.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection based on metal ions doped nanoscale MOFs as signal tracers and RecJf exonuclease assisted targets recycling amplification. Talanta 2016, 161, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xua, Q.; Cao, Y.; Chen, Y. A novel aptamer- metal ions- nanoscale MOF based electrochemicalbiocodes for multiple antibiotics detection and signal amplification. Sens. Actuators B Chem. 2017, 242, 1201–1209. [Google Scholar] [CrossRef]
- Yan, Z.; Gan, N.; Li, T.; Cao, Y.; Chen, Y. A sensitive electrochemical aptasensor for multiplex antibiotics detection based on high-capacity magnetic hollow porous nanotracers coupling exonuclease-assisted cascade target recycling. Biosens. Bioelectron. 2016, 78, 51–57. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Sun, X.; Guo, Y. Multiplex electrochemical aptasensor for detecting multipleantibiotics residues based on carbon fiber and mesoporouscarbon-gold nanoparticles. Sens. Actuators B Chem. 2018, 265, 217–226. [Google Scholar] [CrossRef]
- Li, F.; Wu, Y.; Chen, D.; Guo, Y.; Wang, X.; Sun, X. Sensitive dual-labeled electrochemical aptasensor for simultaneous detection of multi-antibiotics in milk. Int. J. Hydrogen Energy 2021, 46, 23301–23309. [Google Scholar] [CrossRef]
- Viswanathan, S.; Rani, C.; Ho, L.A. Electrochemical immunosensor for multiplexed detection of food-borne pathogens using nanocrystal bioconjugates and MWCNT screen-printed electrode. Talanta 2012, 94, 315–319. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. Ultrasensitive peptide-based multiplexed electrochemical biosensor for the simultaneous detection of Listeria monocytogenes and Staphylococcus aureus. Mikrochim. Acta 2020, 187, 486. [Google Scholar] [CrossRef]
- Viswanath, K.B.; Krithiga, N.; Jayachitra, A.; Mideen, A.K.S.; Amali, A.J.; Vasantha, V.S. Enzyme-Free Multiplex Detection of Pseudomonas aeruginosa and Aeromonas hydrophila with Ferrocene and Thionine-Labeled Antibodies Using ZIF-8/Au NPs as a Platform. ACS Omega 2018, 3, 17010–17022. [Google Scholar] [CrossRef]
- Viswanath, K.B.; Suganya, K.; Krishnamoorthy, G.; Marudhamuthu, M.; Selvan, S.T.; Vasantha, V.S. Enzyme-Free Multiplex Detection of Foodborne Pathogens Using Au Nanoparticles-Decorated Multiwalled Carbon Nanotubes. ACS Food Sci. Technol. 2021, 1, 1236–1246. [Google Scholar] [CrossRef]
- Ma, D.; Liu, J.; Liu, H.; Yi, J.; Xia, F.; Tian, D.; Zhou, C. Multiplexed electrochemical aptasensor based on mixed valence Ce (III, IV)-MOF for simultaneous determination of malathion and chlorpyrifos. Anal. Chim. Acta 2022, 1230, 340364. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, J.; Pan, M.; Yun, Y.; Wen, W.; Fang, G.; Wang, S. On-chip multiplex electrochemical immunosensor based on disposable 24-site fluidic micro-array screen printing analytical device for multi-component quantitative analysis. Sens. Actuat. B Chem. 2018, 260, 499–507. [Google Scholar] [CrossRef]
- Kokkinos, C.; Angelopoulou, M.; Economou, A.; Prodromidis, M.; Florou, A.; Haasnoot, W.; Petrou, P.; Kakabakos, S. Lab-on-a-Membrane Foldable Devices for Duplex Drop-Volume Electrochemical Biosensing Using Quantum Dot Tags. Anal. Chem. 2016, 88, 6897–6904. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Povedano, E.; Benedé, S.; Mata, L.; Galán-Malo, P.; Gamella, M.; Reviejo, A.J.; Campuzano, S.; Pingarrón, J.M. Disposable Amperometric Immunosensor for the Detection of Adulteration in Milk through Single or Multiplexed Determination of Bovine, Ovine, or Caprine Immunoglobulins G. Anal. Chem. 2019, 91, 11266–11274. [Google Scholar] [CrossRef] [PubMed]
- Chand, R.; Ramalingam, S.; Neethirajan, S. A 2D transition-metal dichalcogenide MoS2 based novel nanocomposite and nanocarrier for multiplex miRNA detection. Nanoscale 2018, 10, 8217–8225. [Google Scholar] [CrossRef]
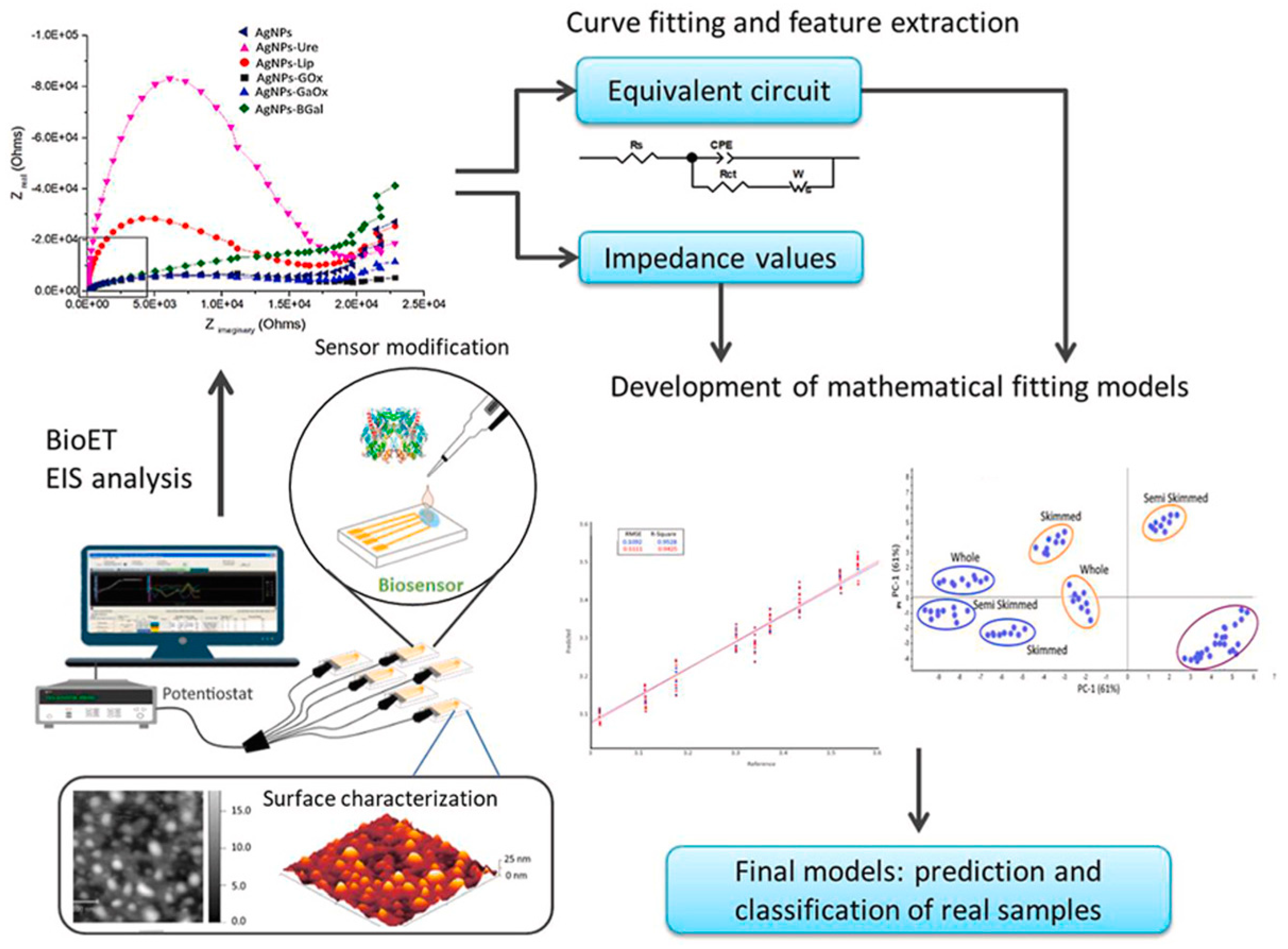
- Perez-Gonzalez, C.; Salvo-Comino, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L.; García-Cabezón, C. A new data analysis approach for an AgNPs-modified impedimetric bioelectronic tongue for dairy analysis. Food Control 2024, 156, 110136. [Google Scholar] [CrossRef]
- Park, L.S.; Park, H.J.; Jeong, W.; Nam, J.; Kang, Y.; Shin, K.; Chung, H.; Kim, J.M. Low temperature thermocromic polydiacetylenes: Design, colorimetric properties, and nanofiber formation. Macromolecules 2016, 49, 1270–1278. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.; Shang, M.; Zhong, Y. Research progress of packaging indicating materials for real time monitoring of food quality. Mater. Express 2019, 9, 377–396. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with pH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Kannan, S.K.; Ambrose, B.; Sudalaimani, S.; Pandiaraj, M.; Giribabu, K.; Kathiresan, M. A review on chemical and electrochemical methodologies for the sensing of biogenic amines. Anal. Methods 2020, 12, 3438–3453. [Google Scholar] [CrossRef]
- Du, L.; Lao, Y.; Sasaki, Y.; Lyu, X.; Gao, P.; Wu, S.; Minami, T.; Liu, Y. Freshness monitoring of raw fish by detecting biogenic amines using a gold nanoparticle-based colorimetric sensor array. RSC Adv. 2022, 12, 6803–6810. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality Yousefi. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/Corn Starch Blend Films with Extract from Brassica Oleraceae (Red Cabbage) as a Visual Indicator of Fish Deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Roberts, L.; Lines, R.; Reddy, M.; Hay, J. Investigation of Polyviologens as Oxygen Indicators in Food Packaging. Sens. Actuators B 2011, 152, 63–67. [Google Scholar] [CrossRef]
- Mohamed, Z.; Barbara, R.; Todd, C.; Gülhan, Ü.; Shyam, S.S. Colorimetric detection of volatile organic compounds for shelf-life monitoring of milk. Food Control 2019, 100, 220–226. [Google Scholar] [CrossRef]
- Ronil, J.R.; Syamak, F.; Farshad, O.; Fariba, D.; Sina, N. Chemiresistive Sensor Arrays for Gas/Volatile Organic Compounds Monitoring: A Review. Adv. Eng. Mater. 2022, 25, 2200830. [Google Scholar] [CrossRef]
- Xu, H.; Xia, A.; Wang, D.; Zhang, Y.; Deng, S.; Lu, W.; Luo, J.; Zhong, Q.; Zhang, F.; Zhou, L.; et al. An ultraportable and versatile point-of-care DNA testing platform. Sci. Adv. 2020, 6, eaaz7445. [Google Scholar] [CrossRef]
- Mazur, F.; Tran, H.; Kuchel, R.P.; Chandrawati, R. Rapid Detection of Listeriolysin O Toxin Based on a Nanoscale Liposome–Gold Nanoparticle Platform, ACS Appl. Nano Mater. 2020, 3, 7270–7280. [Google Scholar] [CrossRef]
- Rubab, M.; Shahbaz, H.M.; Olaimat, A.N.; Oh, D.H. Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens. Bioelectron. 2018, 105, 49–57. [Google Scholar] [CrossRef]
- Chen, I.H.; Horikawa, S.; Bryant, K.; Riggs, R.; Chin, B.A.; Barbaree, J.M. Bacterial assessment of phage magnetoelastic sensors for Salmonella enterica Typhimurium detection in chicken meat. Food Control 2017, 71, 273–278. [Google Scholar] [CrossRef]
- Plata, M.R.; Contento, A.M.; Ríos, A. Simplified determination of bacterial contamination by Escherichia coli using a flow injection system with piezoelectric detection. Microchim. Acta 2011, 172, 447–454. [Google Scholar] [CrossRef]
- Kim, N.; Park, I.S. Application of a flow-type antibody sensor to the detection of Escherichia coli in various foods. Biosens. Bioelectron. 2003, 18, 1101–1107. [Google Scholar] [CrossRef]
- Minunni, M.; Mascini, M.; Carter, R.M.; Jacobs, M.B.; Lubrano, G.J.; Guilbault, G.G. A quartz crystal microbalance displacement assay for Listeria monocytogenes. Anal. Chim. Acta 1996, 325, 169–174. [Google Scholar] [CrossRef]
- Hou, K.J.; Zhao, P.S.; Chen, Y.R.; Li, G.P.; Lin, Y.; Chen, D.J.; Zhu, D.; Wu, Z.Z.; Lian, D.C.; Huang, X.J.; et al. Rapid detection of Bifidobacterium bifidum in feces sample by highly sensitive quartz crystal microbalance immunosensor. Front. Chem. 2020, 8, 548. [Google Scholar] [CrossRef]
- Pietschmann, J.; Spiegel, H.; Krause, H.-J.; Schillberg, S.; Schröper, F. Sensitive Aflatoxin B1 Detection Using Nanoparticle-Based Competitive Magnetic Immunodetection. Toxins 2020, 12, 337. [Google Scholar] [CrossRef]
- Weston, M.; Geng, S.; Chandrawati, R. Food Sensors: Challenges and Opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Vahidpour, F.; Oberländer, J.; Schöning, M.J. Flexible Calorimetric Gas Sensors for Detection of a Broad Concentration Range of Gaseous Hydrogen Peroxide: A Step Forward to Online Monitoring of Food-Package Sterilization Processes. Phys. Status Solidi A 2018, 215, 1800044. [Google Scholar] [CrossRef]
- Jia, Z.; Luo, Y.; Wang, D.; Dinh, Q.N.; Lin, S.; Sharma, A.; Block, E.M.; Yang, M.; Gu, T.; Pearlstein, A.J.; et al. Nondestructive multiplex detection of foodborne pathogens with background microflora and symbiosis using a paper chromogenic array and advanced neural network. Biosens. Bioelectron. 2021, 183, 113209. [Google Scholar] [CrossRef]
- Escobedo, P.; Bhattacharjee, M.; Nikbakhtnasrabadi, F.; Dahiya, R. Flexible Strain and Temperature Sensing NFC Tag for Smart Food Packaging Applications. IEEE Sens. J. 2021, 21, 26406–26414. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Z.; Liu, Q. Smartphone-based biosensors for portable food evaluation. Curr. Opin. Food Sci. 2019, 28, 74–81. [Google Scholar] [CrossRef]
- Amani, H.; Badak-Kerti, K.; Khaneghah, A.M. Current progress in the utilization of smartphone-based imaging for quality assessment of food products: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3631–3643. [Google Scholar] [CrossRef]
- Jiménez-Skrzypek, G.; González-Sálamo, J.; Hernández-Borges, J. Analytical methodologies and occurrence of per- and polyfluorinated alkyl substances—A review. J. Chromatogr. Open 2023, 4, 100089. [Google Scholar] [CrossRef]
- Ruth, F.; Menger, E.F.; Charles, S.H.; Thomas, B. Sensors for detecting per- and polyfluoroalkyl substances (PFAS): A critical review of development challenges, current sensors, and commercialization obstacles. J. Chem. Eng. 2021, 417, 129133. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, X.; Dong, Z.; Wang, L.; Megharaj, M.; Naidu, R. Smartphone app-based/portable sensor for the detection of fluoro-surfactant PFOA. Chemosphere 2018, 191, 381–388. [Google Scholar] [CrossRef]
- Liang, X.; Deng, X.; Tan, K. An eosin Y-based “turn-on” fluorescent sensor for detection of perfluorooctane sulfonate. Spectrochim. Acta Part A 2015, 150, 772–777. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, Y.; Liang, J.; Long, S.; Chen, X.; Tan, K. A simple and highly sensitive assay of perfluorooctanoic acid based on resonance light scattering technique. Spectrochim. Acta Part A 2016, 159, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Dharmarajan, R.; Megharaj, M.; Naidu, R. Gold nanoparticle-based optical sensors for selected anionic contaminants. Trends Anal. Chem. 2017, 86, 143–154. [Google Scholar] [CrossRef]
- Moro, G.; Bottari, F.; Liberi, S.; Covaceuszach, S.; Cassetta, A.; Angelini, A.; De Wael, K.; Moretto, L.M. Covalent immobilization of delipidated human serum albumin on poly(pyrrole-2-carboxylic) acid film for the impedimetric detection of perfluorooctanoic acid. Bioelectrochemistry 2020, 134, 107540. [Google Scholar] [CrossRef]

| Sample | Detection | |||||
|---|---|---|---|---|---|---|
| Food | Allergens | Technique | Sensing System | Total Assay Time | LOD | Ref. |
| Multiplex detection of allergens in milk, milk-containing products, and dairy products | ||||||
| Milk, (*) cookies, ice cream | Gliadin, Ara h 1, Cor a1 (hazelnut), casein, ovalbumin | Amperometry | Magnet/SPE antibody-tagged immunomagnetic beads | <10 min | Ranging from 0.003 to 0.170 mg/kg | [43] |
| Powdered milk, (*) cookies, sponge cake | Hazelnut, peanut, soybean | Optical (laser) | Digoxin-labeled PCR products detected by hybridization on modified DVD surface | 5 h 20 min | 1 μg/g | [37] |
| MoniQA milk, NIST SRM 1549a milk, (*) Nutella hazelnut spread, 2% milk | Ana o 3 (cashew), Ara h 3/Ara h 6 (peanut), Cor a 9 (hazelnut), Gal d 1/Gal d 2 (egg), Gly m 5 (soy), Bos d 5 (milk), tropomyosin (shrimp) | Fluorescent multiplex array | Monoclonal or polyclonal antibodies covalently coupled to Luminex xMAP® system | 30 min | Ranging from 0.02 to 1.95 ng/mL | [44] |
| (*) Cookies | Casein, soy protein, gluten | Flow cytometry | Fluorescent microsphere-based immunoassay | 1 h 10 min | 0.4 ppm | [45] |
| (*) Oatmeal cookies, milk chocolate, chocolate ice cream | Hazelnut, Brazil nut, peanut | Colorimetry | Enzyme immunoassay system with chromogenic substrate | 4 h 10 min | Ranging from 0.1 to 1.0 μg/g | [38] |
| (*) Cookies | Hazelnut, peanut | Colorimetry | Lab-on-chip/Carbon dot label for lateral flow immunoassay | 15 min | 0.1 ppm | [46] |
| Multiplex detection of milk allergens in food stuff | ||||||
| Allergen-free probiotics | Gliadin, β-lactoglobulin, hazelnut, almond, peanut, soy | Colorimetry | DVD functionalized with the capture bioreceptors in microarray format | 20 samples in 70 min | Ranging from 0.1 to 143.4 ng/mL | [47] |
| A panel of 38 food commodities based on AOAC recommendations and milk from six different animal sources | Casein, β-lactoglobulin | Colorimetry/Visual | Antibodies coupled to red and blue Carboxyl-dyed, antibody-modified latex beads | 10 min | 0.5 ppm β-lactoglobulin, 2 ppm for caseins | [34] |
| Infant jar food, apple juice | Gliadin, casein, β-lactoglobulin, ovalbumin | Optical (laser) | Immunoassay developed on DVD surface | 1 h 25 min | 31 μg/L (casein), 120 μg/L (β-lactoglobulin) | [36] |
| Target | Analyte | Electrode/Modification/Label | Analytical Signal | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| Antibiotics | Sulphapyridine (SPY), Oxytetracycline (OTC) | SPCE/protein G/– | Amperometric | 0.39, 1.93 nM | 1.92–454 nM, – | [46] |
| Streptomycin (STR), Chloramphenicol (CHL), Tetracycline (TC) | Gold electrode | SWV | – – | 10 nM, 5 nM, 20 nM | [47] | |
| Kanamycin (KAN), Streptomycin (STR) | SPCE/carbon nanofibers, carbon–gold nanoparticles/CdS, PbS | DPV | 10−1–103 nM | 87.3 pM, 45.0 pM | [51] | |
| Kanamycin (KAN), Tobramycin (TOB) | Au electrode/gold nanoshells/SCd, SPb | DPV | 1–4 × 102 nM, 1–1 × 104 nM | 0.12 nM, 0.49 nM | [52] | |
| Pathogens | Escherichia coli, Campylobacter, Salmonella | SPCE/multiwall carbon nanotube–polyallylamine/CdS, PbS, CuS QDs | SWASV | 103–5 × 105 cells/mL | 400 cells/mL, 400 cells/mL, 800 cells/mL | [53] |
| Listeria monocytogenes, Staphylococcus aureus | SPCE/gold nanoparticle–streptavidin/magnetic nanoparticles | SWV | 10–107 CFU/mL, 10–107 CFU/mL | 9 CFU/mL, 3 CFU/mL | [54] | |
| Aeromonas hydrophile (Ah), Pseudomonas aeruginosa (Ps) | GCE/ZIF-8-gold nanoparticles/thionine, ferrocene | SWV | 101–103 CFU/mL, 101–105 CFU/mL | 3.60 CFU/mL, 8095 CFU/mL | [55] | |
| Listeria monocytogenes (Lm), Enterobacter cloacae (Ec) | GCE electrodes/carbon nanotubes, Au nanoparticles anti-Lm, anti-Ec/thionine ferrocene | SWV | 101–107 CFU/mL, 101–106 CFU/mL | 3.22 CFU/mL, 4.17 CFU/mL | [56] | |
| Pesticide | Malathion (MAL), chlorpyrifos (CLO) | GCE/Au nanoparticles, cDNA/Ce(III)–Ce(V)–MOF | SWV | 1−1 pM–1 μM | 0.045 pM, 0.038 pM | [57] |
| Immunoglobulins | Bovine casein, bovine immunoglobulin G | Graphite/bismuth layer/CdS, PbS QDs | ASV | 1–102% v/v | 0.04 μg/mL, 0.02 μg/mL | [59] |
| Bovine immunoglobulin G, bovine immunoglobulin G, caprine immunoglobulin G | SPCE/–/HRP, hydrogen peroxide, hydroquinone | Amperometric | 2.6–250 ng/mL, 2.7–250 ng/mL, 2.2–250 ng/mL | 0.74 ng/mL, 0.82 ng/mL, 0.66 ng/mL | [60] | |
| mi-RNA | mi-RNAs | SPCE/MoS2 nanosheets, CuFe2O4/MoS2 nanosheets, ferrocene | SWV | 1 pM to 1.5 nM | 0.48 pM | [61] |
| Others | Glucose, galactose, lactose, urea | Gold thin-film interdigitated sensors/AgNPs/enzymes | Impedance spectroscopy | PCA discrimination of milks with different nutritional characteristics | [66] | |
| Enrofloxacin Melamine | SPCE into fluidic microarray/Au@PtNPs | Impedance spectroscopy/Cyclic voltammetry | 0.1–1000 ng/mL 0.1–500 ng/mL | 18.97 pg/mL 26.80 pg/mL | [58] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández González, A.; Badía Laíño, R.; Costa-Fernández, J.M.; Soldado, A. Progress and Challenge of Sensors for Dairy Food Safety Monitoring. Sensors 2024, 24, 1383. https://doi.org/10.3390/s24051383
Fernández González A, Badía Laíño R, Costa-Fernández JM, Soldado A. Progress and Challenge of Sensors for Dairy Food Safety Monitoring. Sensors. 2024; 24(5):1383. https://doi.org/10.3390/s24051383
Chicago/Turabian StyleFernández González, Alfonso, Rosana Badía Laíño, José M. Costa-Fernández, and Ana Soldado. 2024. "Progress and Challenge of Sensors for Dairy Food Safety Monitoring" Sensors 24, no. 5: 1383. https://doi.org/10.3390/s24051383
APA StyleFernández González, A., Badía Laíño, R., Costa-Fernández, J. M., & Soldado, A. (2024). Progress and Challenge of Sensors for Dairy Food Safety Monitoring. Sensors, 24(5), 1383. https://doi.org/10.3390/s24051383








