Ventricular Arrhythmias in Left Ventricular Assist Device Patients—Current Diagnostic and Therapeutic Considerations
Abstract
1. Introduction
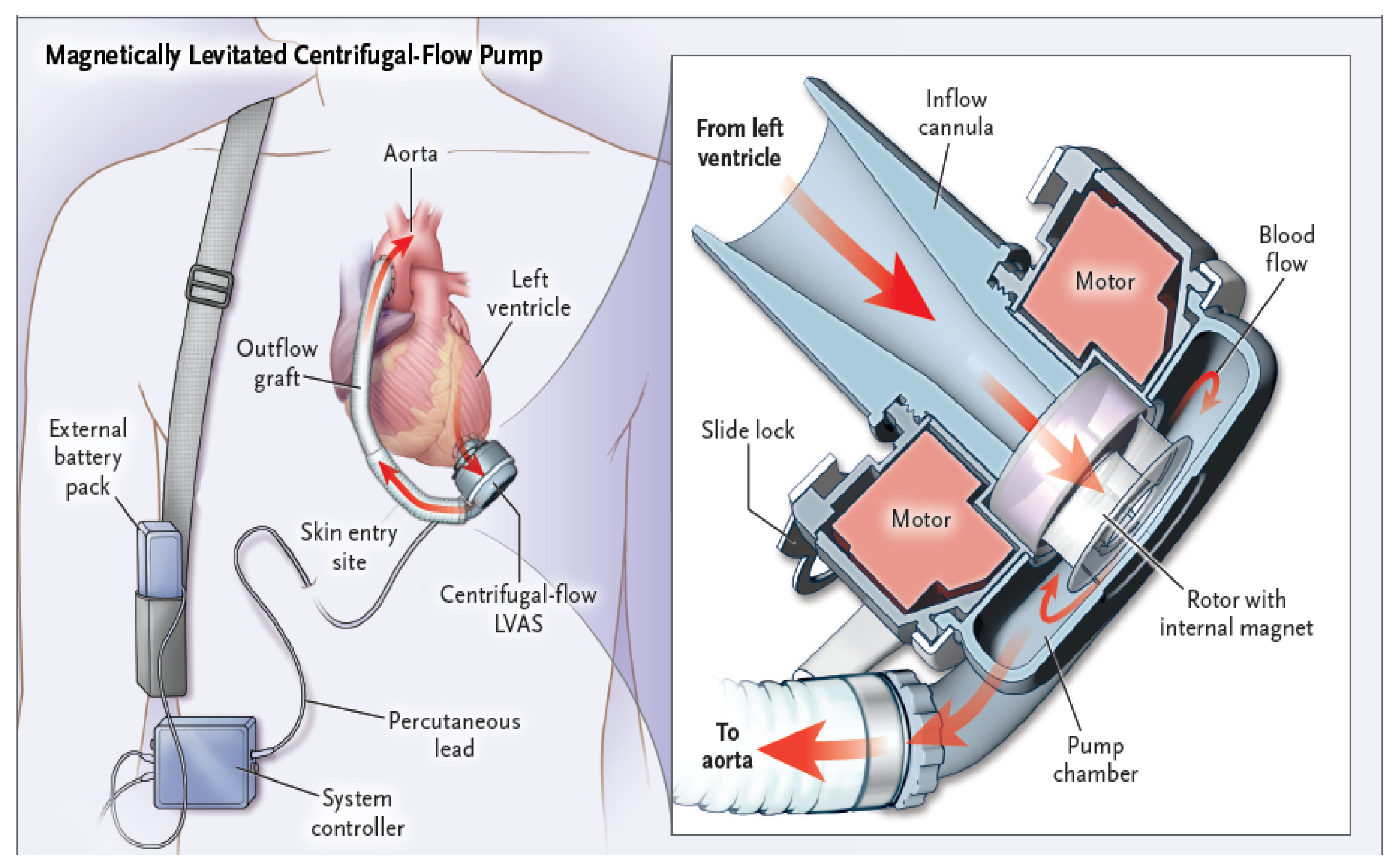
1.1. An Overview of Design Principles and Functional Mechanics of Left Ventricular Assist Devices
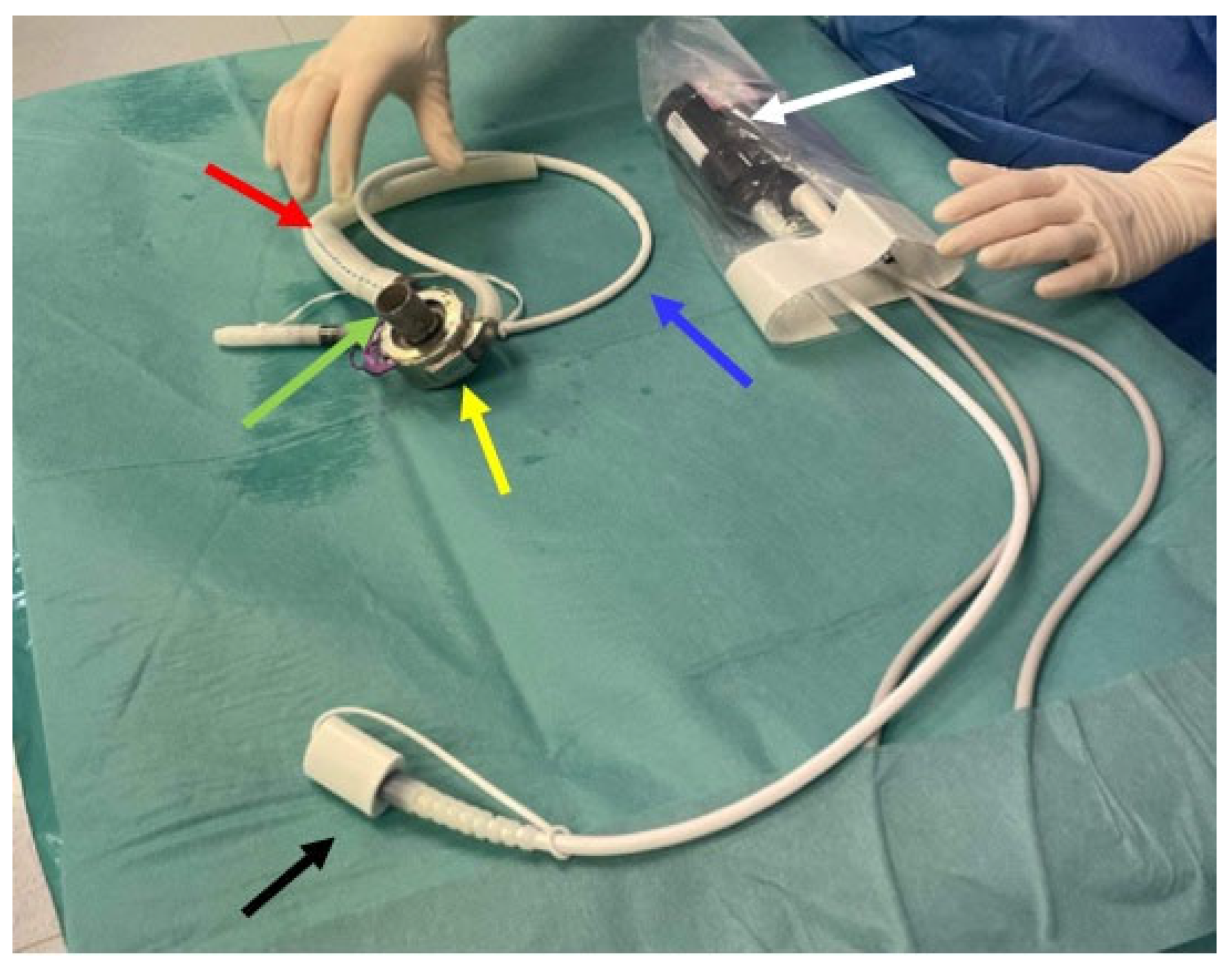
- − Inflow cannula: a tube which draws blood from the left ventricle (apex) of the heart and delivers it to the pump (Figure 2, green arrow);
- − Pump: the central component of the LVAD, assisting in pumping blood from the left ventricle of the heart to the circulatory system (Figure 2, yellow arrow);
- − Outflow cannula: a tube that carries the pumped blood to the aorta (Figure 2, red arrow);
- − Controller: the controller transmits information to the pump about the set rotational speed, measures the energy needed to operate the pump, and estimates the flow based on pump performance (Figure 2, white arrow).
1.2. Epidemiology of Ventricular Arrhythmias in LVAD Patients
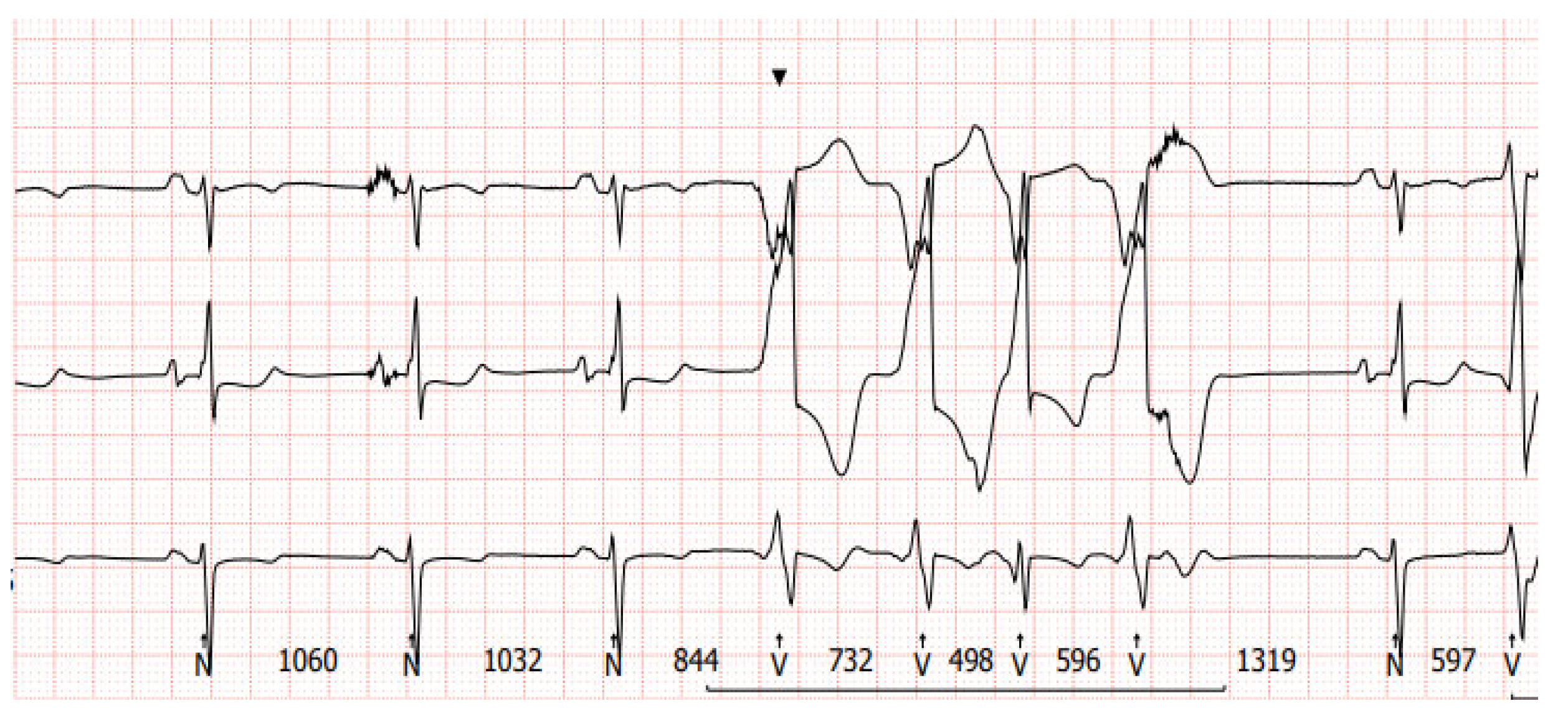
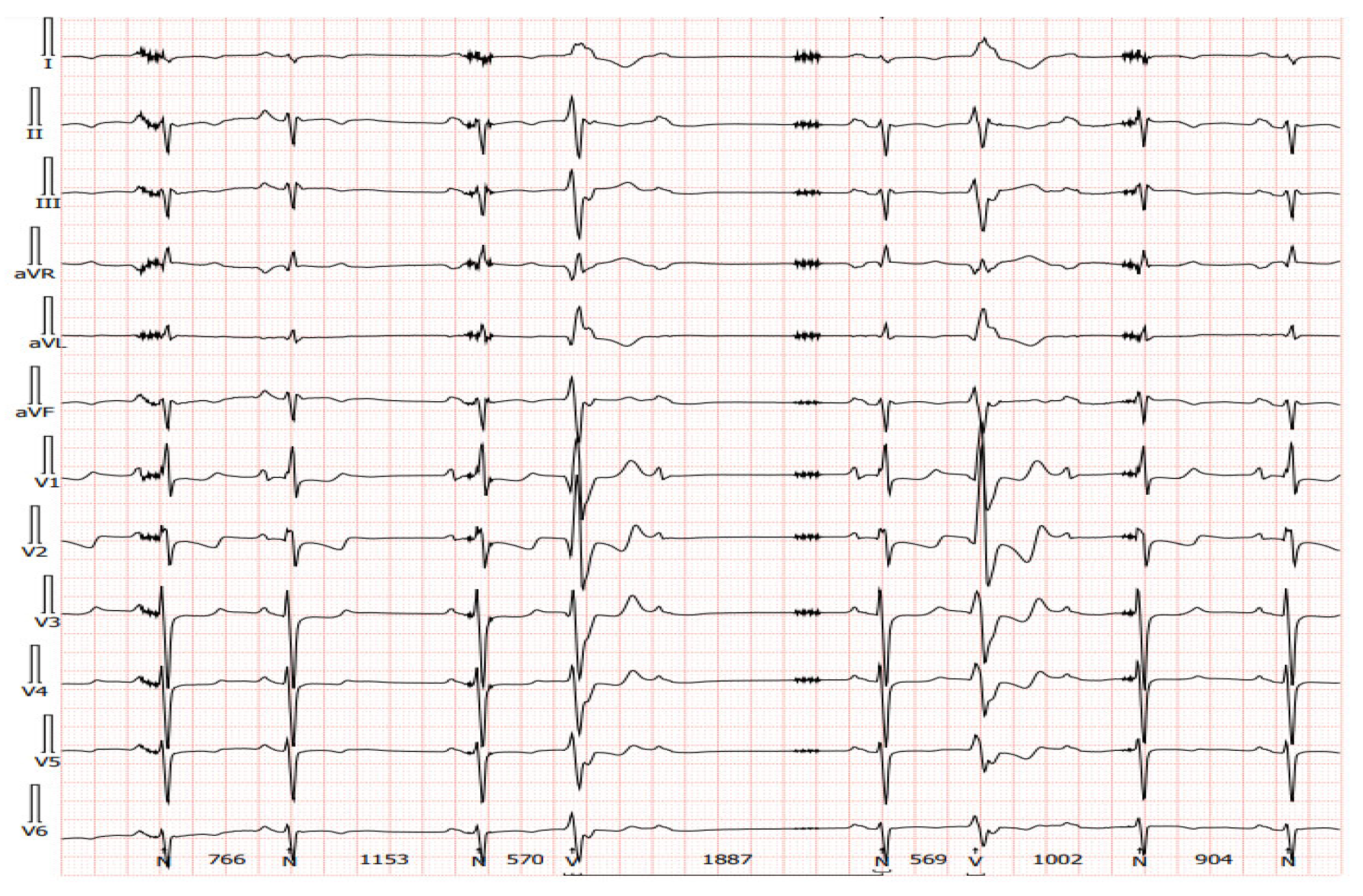
1.3. Potential Mechanisms of Ventricular Arrhythmia in LVAD Patients
2. Diagnostic Difficulties of Ventricular Arrhythmias in Patients with Left Ventricular Assist Device
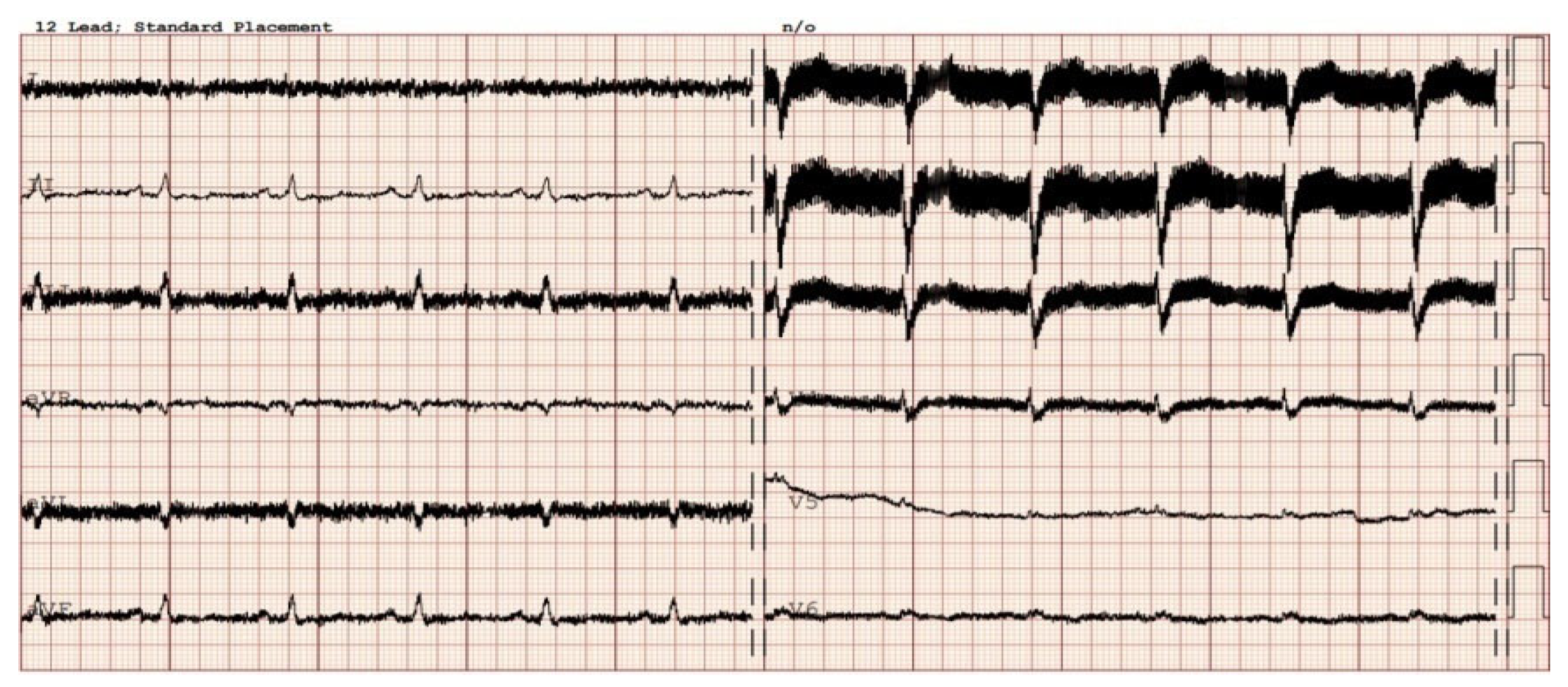
3. Electrocardiography in Patients with Implanted LVADs
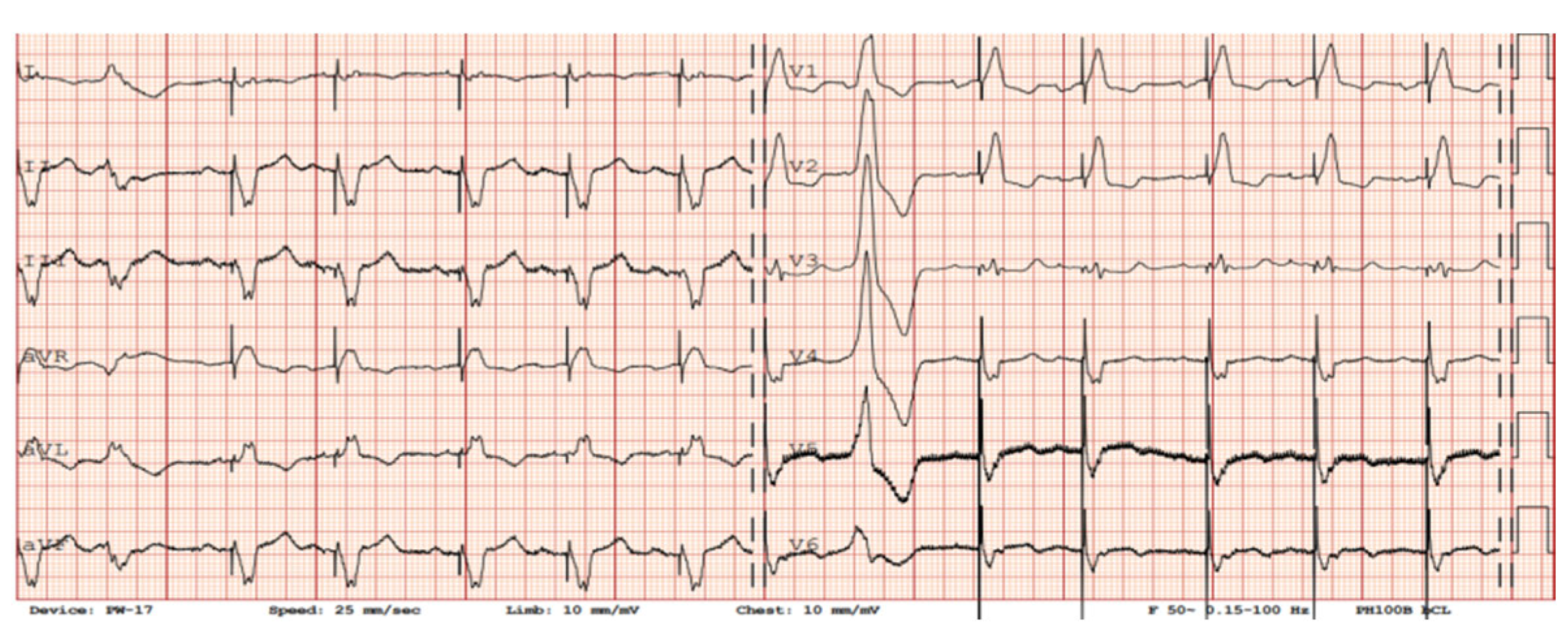
3.1. Twelve-Lead ECG Findings in Patients with LVADs
3.2. Alternative Diagnostic Methods of Noninvasive Electrocardiography in Patients with Implanted LVADs
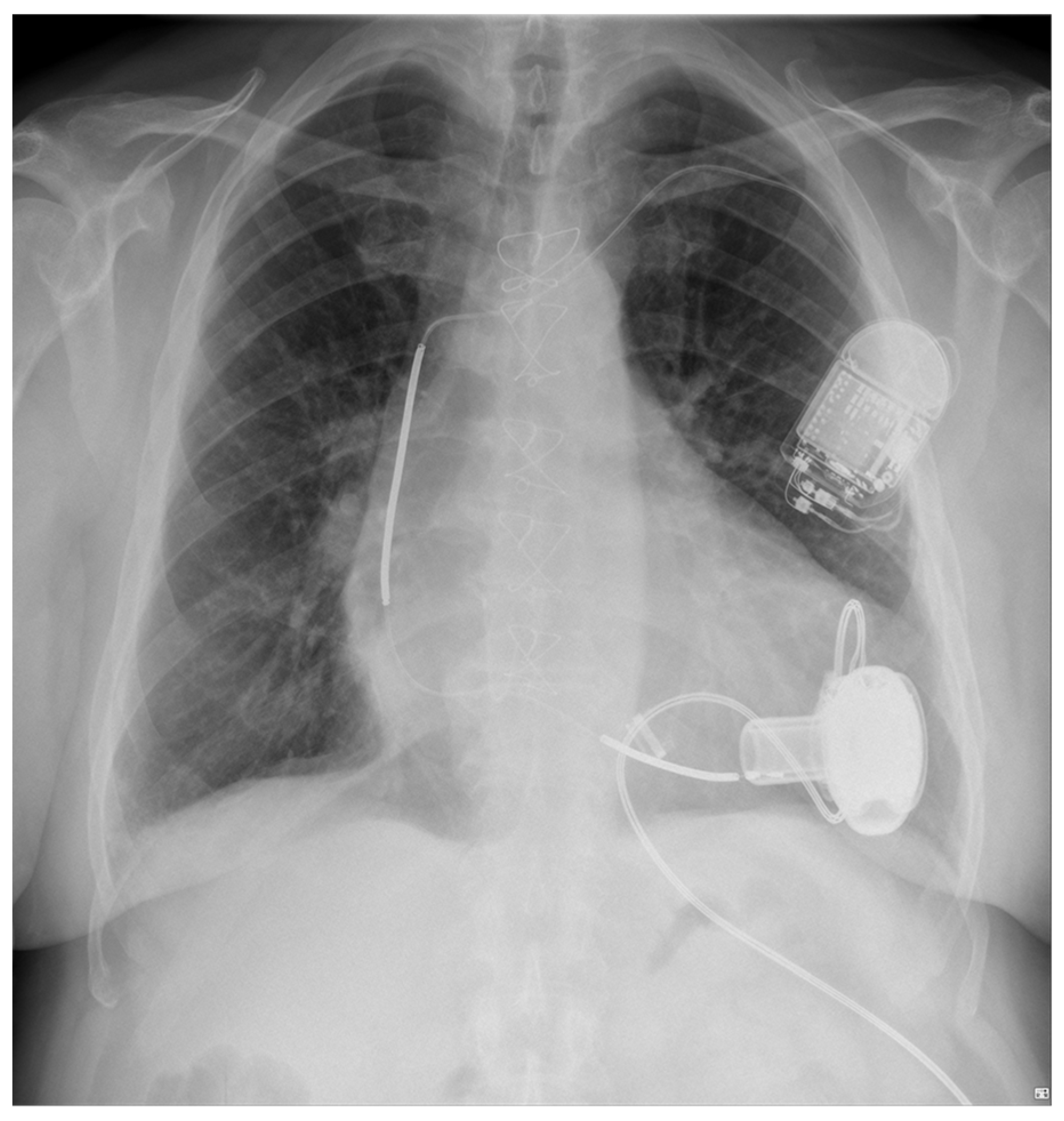
4. LVAD and Cardiac Implantable Electronic Devices
4.1. Pulsatile vs. Continuous Flow in Terms of ICD Outcomes in Patients with LVADs
4.2. ICD Implantation Recommendations for Patients with LVAD
4.3. Technical Considerations in ICD Programming in Patients with LVADs
5. Medical Therapy in Patients with LVAD and Ventricular Arrhythmias
6. Catheter Ablation in Patients with LVADs and Ventricular Arrhythmias
6.1. Ablation Post-LVAD Implantation: Technical Considerations
6.2. Results of Catheter Ablation of Ventricular Tachycardia in LVAD Patients
7. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J. Heart Lung Transpl. 2019, 38, 114–126. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Xie, R.; Cowger, J.; de By, T.M.M.H.; Nakatani, T.; Schueler, S.; Taylor, R.; Lannon, J.; Mohacsi, P.; Gummert, J.; et al. Second annual report from the ISHLT Mechanically Assisted Circulatory Support (IMACS) registry. J. Heart Lung Transpl. 2018, 37, 685–691. [Google Scholar] [CrossRef]
- John, R. Current axial-flow devices–the HeartMate II and Jarvik 2000 left ventricular assist devices. Semin. Thorac. Cardiovasc. Surg. 2008, 20, 264–272. [Google Scholar] [CrossRef]
- Singhvi, A.; Trachtenberg, B. Left Ventricular Assist Devices 101: Shared Care for General Cardiologists and Primary Care. J. Clin. Med. 2019, 8, 1720. [Google Scholar] [CrossRef]
- Miller, L.W.; Pagani, F.D.; Russell, S.D.; John, R.; Boyle, A.J.; Aaronson, K.D.; Conte, J.V.; Naka, Y.; Mancini, D.; Delgado, R.M.; et al. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 2007, 357, 885–896. [Google Scholar] [CrossRef]
- Raasch, H.; Jensen, B.C.; Chang, P.P.; Mounsey, J.P.; Gehi, A.K.; Chung, E.H.; Sheridan, B.C.; Bowen, A.; Katz, J.N. Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation. Am. Heart J. 2012, 164, 373–378. [Google Scholar] [CrossRef]
- Sisti, N.; Santoro, A.; Carreras, G.; Valente, S.; Donzelli, S.; Mandoli, G.E.; Sciaccaluga, C.; Cameli, M. Ablation therapy for ventricular arrhythmias in patients with LVAD: Multiple faces of an electrophysiological challenge. J. Arrhythm. 2021, 37, 535–543. [Google Scholar] [CrossRef]
- Yoruk, A.; Sherazi, S.; Massey, H.T.; Kutyifa, V.; McNitt, S.; Hallinan, W.; Huang, D.T.; Chen, L.; Aktas, M.K. Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm 2016, 13, 1052–1056. [Google Scholar] [CrossRef]
- Ziv, O.; Dizon, J.; Thosani, A.; Naka, Y.; Magnano, A.R.; Garan, H. Effects of left ventricular assist device therapy on ventricular arrhythmias. J. Am. Coll. Cardiol. 2005, 45, 1428–1434. [Google Scholar] [CrossRef]
- Andersen, M.; Videbaek, R.; Boesgaard, S.; Sander, K.; Hansen, P.B.; Gustafsson, F. Incidence of ventricular arrhythmias in patients on long-term support with a continuous-flow assist device (HeartMate II). J. Heart Lung Transplant. 2009, 28, 733–735. [Google Scholar] [CrossRef]
- Sacher, F.; Reichlin, T.; Zado, E.S.; Field, M.E.; Viles-Gonzalez, J.F.; Peichl, P.; Ellenbogen, K.A.; Maury, P.; Dukkipati, S.R.; Picard, F.; et al. Characteristics of ventricular tachycardia abla-tion in patients with continuous flow left ventricular assist devices. Circ. Arrhythm. Electrophysiol. 2015, 8, 592–597. [Google Scholar] [CrossRef]
- Efimova, E.; Fischer, J.; Bertagnolli, L.; Dinov, B.; Kircher, S.; Rolf, S.; Sommer, P.; Bollmann, A.; Richter, S.; Meyer, A.; et al. Predictors of ventricular arrhythmia after left ventricular assist device implantation: A large single-center observational study. Heart Rhythm 2017, 14, 1812–1819. [Google Scholar] [CrossRef]
- Garan, A.R.; Yuzefpolskaya, M.; Colombo, P.C.; Morrow, J.P.; Te-Frey, R.; Dano, D.; Takayama, H.; Naka, Y.; Garan, H.; Jorde, U.P.; et al. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: Need for primary prevention? J. Am. Coll. Cardiol. 2013, 61, 2542–2550. [Google Scholar] [CrossRef]
- Bedi, M.; Kormos, R.; Winowich, S.; McNamara, D.M.; Mathier, M.A.; Murali, S. Ventricular arrhythmias during left ventricular assist device support. Am. J. Cardiol. 2007, 99, 1151–1153. [Google Scholar] [CrossRef]
- Vollkron, M.; Voitl, P.; Ta, J.; Wieselthaler, G.; Schima, H. Suction events during left ventricular support and ventricular arrhythmias. J. Heart Lung Transplant. 2007, 26, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Galand, V.; Flécher, E.; Auffret, V.; Boulé, S.; Vincentelli, A.; Dambrin, C.; Mondoly, P.; Sacher, F.; Nubret, K.; Kindo, M.; et al. ASSIST-ICD Investigators. Predictors and Clinical Impact of Late Ventricular Arrhythmias in Patients with Continuous-Flow Left Ventricular Assist Devices. JACC Clin. Electrophysiol. 2018, 4, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, S.P.; Lachat, M.L.; Zünd, G.; Turina, M.I. Left ventricular assist device (LVAD) enables survival during 7 h of sustained ventricular fibrillation. Eur. J. Cardiothorac. Surg. 2004, 26, 444–446. [Google Scholar] [CrossRef]
- Ratman, K.; Biełka, A.; Kalinowski, M.E.; Herdyńska-Wąs, M.M.; Przybyłowski, P.; Zembala, M.O. Permanent cardiac arrest in a patient with a left ventricular assist device support. Kardiol Pol. 2022, 80, 709–710. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. MOMENTUM 3 Investigators. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Ho, G.; Braun, O.Ö.; Adler, E.D.; Feld, G.K.; Pretorius, V.G.; Birgersdotter-Green, U. Management of Arrhythmias and Cardiac Implantable Electronic Devices in Patients with Left Ventricular Assist Devices. JACC Clin. Electrophysiol. 2018, 4, 847–859. [Google Scholar] [CrossRef]
- Martins, R.P.; Leclercq, C.; Bourenane, H.; Auffret, V.; Boulé, S.; Loobuyck, V.; Dambrin, C.; Mondoly, P.; Sacher, F.; Bordachar, P.; et al. Incidence, predictors, and clinical impact of electrical storm in patients with left ventricular assist devices: New insights from the ASSIST-ICD study. Heart Rhythm 2019, 16, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.D.; Piacentino, V., 3rd; Gaughan, J.P.; Houser, S.R.; Margulies, K.B. Electroplysiological alternations after mechanicalcirculatory in patients with advanced cardiac failure. Circulation 2001, 104, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Cantillon, D.J.; Bianco, C.; Wazni, O.M.; Kanj, M.; Smedira, N.G.; Wilkoff, B.L.; Starling, R.C.; Saliba, W.I. Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm 2012, 9, 859–864. [Google Scholar] [CrossRef]
- Anderson, R.D.; Lee, G.; Virk, S.; Bennett, R.G.; Hayward, C.S.; Muthiah, K.; Kalman, J.; Kumar, S. Catheter Ablation of Ventricular Tachycardia in Patients with a Ventricular Assist Device: A Systematic Review of Procedural Characteristics and Outcomes. JACC Clin. Electrophysiol. 2019, 5, 39–51. [Google Scholar] [CrossRef]
- Hayward, C.S.; Salamonsen, R.; Keogh, A.M.; Woodard, J.; Ayre, P.; Prichard, R.; Walker, R.; Kotlyar, E.; Macdonald, P.S.; Jansz, P.; et al. Effect of alteration in pump speed on pump output and left ventricular filling with continuous-flow left ventricular assist device. ASAIO J. 2011, 57, 495–500. [Google Scholar] [CrossRef]
- Schettle, S.; Kassi, M.; Asleh, R.; Pereira, N.; Maltais, S.; Stulak, J.; Boilson, B. LVAD artifact reflecting heartware pump Speed. JACC 2018, 71, A816. [Google Scholar] [CrossRef]
- Zormpas, C.; Mueller-Leisse, J.; Koenig, T.; Schmitto, J.D.; Veltmann, C.; Duncker, D. Electrocardiographic changes after implantation of a left ventricular assist device—Potential implications for subcutaneous defibrillator therapy. J. Electrocardiol. 2019, 52, 29–34. [Google Scholar] [CrossRef]
- Martinez, S.C.; Fansler, D.; Lau, J.; Novak, E.L.; Joseph, S.M.; Kleiger, R.E. Characteristics of the electrocardiogram in patients with continuous-flow left ventricular assist devices. Ann. Noninvasive Electrocardiol. 2015, 20, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Loring, Z.; Sen, S.; Black-Maier, E.; Atwater, B.; Russell, S.; DeVore, A.; Piccini, J. Reducing ECG artifact from Left Ventricular Assist Device Electromagnetic Interference. JAHA 2020, 9, e017563. [Google Scholar] [CrossRef]
- Usman, M.S.; Minhas, A.M.K.; Greene, S.J.; Van Spall, H.G.; Mentz, R.J.; Fonarow, G.C.; Al-Khatib, S.M.; Butler, J.; Khan, M.S. Utilization of Implantable Cardioverter Defibrillators Among Patients with a Left Ventricular Assist Device: Insights from a National Database. Curr. Probl. Cardiol. 2022, 47, 101334. [Google Scholar] [CrossRef]
- Vakil, K.; Kazmirczak, F.; Sathnur, N.; Adabag, S.; Cantillon, D.J.; Kiehl, E.L.; Koene, R.; Cogswell, R.; Anand, I.; Roukoz, H. Implantable Cardioverter-Defibrillator Use in Patients with Left Ventricular Assist Devices: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2016, 4, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Cantillon, D.J.; Tarakji, K.G.; Kumbhani, D.J.; Smedira, N.G.; Starling, R.C.; Wilkoff, B.L. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter-defibrillator. Heart Rhythm 2010, 7, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Refaat, M.M.; Tanaka, T.; Kormos, R.L.; McNamara, D.; Teuteberg, J.; Winowich, S.; London, B.; Simon, M.A. Survival benefit of implantable cardioverter-defibrillators in left ventricular assist device-supported heart failure patients. J. Card. Fail. 2012, 18, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Cikes, M.; Jakus, N.; Claggett, B.; Brugts, J.J.; Timmermans, P.; Pouleur, A.C.; Rubis, P.; Van Craenenbroeck, E.M.; Gaizauskas, E.; Grundmann, S.; et al. PCHF-VAD registry. Cardiac implantable electronic devices with a defibrillator component and all-cause mortality in left ventricular assist device carriers: Results from the PCHF-VAD registry. Eur. J. Heart Fail. 2019, 21, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Topkara, V.K.; Demmer, R.T.; Dizon, J.M.; Yuzefpolskaya, M.; Fried, J.A.; Mai, X.; Mancini, D.M.; Takeda, K.; Takayama, H.; et al. Implantable Cardioverter-Defibrillators in Patients with a Continuous-Flow Left Ventricular Assist Device: An Analysis of the INTERMACS Registry. JACC Heart Fail. 2017, 5, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Garg, L.; Nanda, S.; Sharma, A.; Bhatia, N.; Manda, Y.; Singh, A.; Fegley, M.; Shirani, J. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices—A meta-analysis. Int. J. Cardiol. 2016, 222, 379–384. [Google Scholar] [CrossRef]
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; de Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardiothorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, M.A.; Charron, P.; Corrado, D.; Dagres, N.; De Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Richardson, T.D.; Hale, L.; Arteaga, C.; Xu, M.; Keebler, M.; Schlendorf, K.; Danter, M.; Shah, A.; Lindenfeld, J.; Ellis, C.R. Prospective Randomized Evaluation of Implantable Cardioverter-Defibrillator Programming in Patients with a Left Ventricular Assist Device. J. Am. Heart Assoc. 2018, 7, e007748. [Google Scholar] [CrossRef]
- Robinson, A.; Parikh, V.; Jazayeri, M.A.; Pierpoline, M.; Reddy, Y.M.; Emert, M.; Pimentel, R.; Dendi, R.; Berenbom, L.; Noheria, A.; et al. Impact of ultra-conservative ICD programming in patients with LVADs: Avoiding potentially unnecessary tachy-therapies. Pacing Clin. Electrophysiol. 2022, 45, 204–211. [Google Scholar] [CrossRef]
- Gulletta, S.; Scandroglio, A.M.; Pannone, L.; Falasconi, G.; Melisurgo, G.; Ajello, S.; D’Angelo, G.; Gigli, L.; Lipartiti, F.; Agricola, E.; et al. Clinical characteristics and outcomes of patients with ventricular arrhythmias after continuous-flow left ventricular assist device implant. Artif. Organs. 2022, 46, 1608–1615. [Google Scholar] [CrossRef]
- Chung, B.B.; Grinstein, J.S.; Imamura, T.; Kruse, E.; Nguyen, A.B.; Narang, N.; Holzhauser, L.H.; Burkhoff, D.; Lang, R.M.; Sayer, G.T.; et al. Biventricular Pacing versus Right Ventricular Pacing in Patients Supported with LVAD. JACC Clin. Electrophysiol. 2021, 7, 1003–1009. [Google Scholar] [CrossRef]
- Tomashitis, B.; Baicu, C.F.; Butschek, R.A.; Jackson, G.R.; Winterfield, J.; Tedford, R.J.; Zile, M.R.; Gold, M.R.; Houston, B.A. Acute Hemodynamic Effects of Cardiac Resynchronization Therapy versus Alternative Pacing Strategies in Patients with Left Ventricular Assist Devices. J. Am. Heart Assoc. 2021, 10, e018127. [Google Scholar] [CrossRef]
- Pausch, J.; Mersmann, J.; Bhadra, O.D.; Barten, M.J.; Tönnis, T.; Yildirim, Y.; Pecha, S.; Reichenspurner, H.; Bernhardt, A.M. Prognostic impact of implantable cardioverter defibrillators and associated adverse events in patients with Continuous flow left ventricular assist devices. Front. Cardiovasc. Med. 2023, 10, 1158248. [Google Scholar] [CrossRef]
- Tanawuttiwat, T.; Das, M.K.; Miller, J.M.; Guglin, M.E. Device-device interaction between cardiac implantable electronic devices and continuous-flow left ventricular assist devices. Heart Rhythm 2023, 20, 918–926. [Google Scholar] [CrossRef]
- Khetarpal, B.K.; Javaid, A.; Lee, J.Z.; Kusumoto, F.; Mulpuru, S.K.; Sorajja, D.; Cha, Y.M.; Srivathsan, K. Subcutaneous implantable cardioverter-defibrillator noise following left ventricular assist device implantation. J. Arrhythm. 2023, 39, 198–206. [Google Scholar] [CrossRef]
- Zormpas, C.; Eiringhaus, J.; Hillmann, H.A.K.; Hohmann, S.; Müller-Leisse, J.; Schmitto, J.D.; Veltmann, C.; Duncker, D. A novel screening tool to unmask potential interference between S-ICD and left ventricular assist device. J. Cardiovasc. Electrophysiol. 2020, 31, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Michalik, J.; Kozielski, J.; Węclewicz, M.; Moroz, R.; Sterliński, M.; Szołkiewicz, M. Leadless AV Pacemaker in Patient with Complete Heart Block and Bilaterally Implanted Two Deep Brain Stimulators Can Be Safe Therapeutic Option. Int. J. Environ. Res. Public Health 2022, 20, 388. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Dorian, P.; Roberts, R.; Gent, M.; Israel, C.W.; Fain, E.; Champagne, J.; Connolly, S.J. Effect of amiodarone and sotalol on ventricular defibrillation threshold: The optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation 2006, 114, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Saeed, D.; Feldman, D.; Banayosy, A.E.; Birks, E.; Blume, E.; Cowger, J.; Hayward, C.; Jorde, U.; Kremer, J.; MacGowan, G.; et al. The 2023 International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support: A 10-Year Update. J. Heart Lung Transplant. 2023, 42, e1–e222. [Google Scholar] [CrossRef]
- Toivonen, L.; Kadish, A.; Morady, F. A prospective comparison of class, I.A.; B, and C antiarrhythmic agents in combination with amiodarone in patients with inducible, sustained ventricular tachycardia. Circulation 1991, 84, 101–108. [Google Scholar] [CrossRef]
- Connolly, S.J.; Hallstrom, A.P.; Cappato, R.; Schron, E.B.; Kuck, K.H.; Zipes, D.P.; Greene, H.L.; Boczor, S.; Domanski, M.; Follmann, D.; et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur. Heart J. 2000, 21, 2071–2078. [Google Scholar] [CrossRef]
- Santangeli, P.; Muser, D.; Maeda, S.; Filtz, A.; Zado, E.S.; Frankel, D.S.; Dixit, S.; Epstein, A.E.; Callans, D.J.; Marchlinski, F.E. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: A systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016, 13, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, G.; Ghumman, W.S.; Das, M.K.; Miller, J.M. Endocardial catheter ablation of ventricular tachycardia in patients with ventricular assist devices. Heart Rhythm 2007, 4, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Grinstein, J.; Garan, A.R.; Oesterle, A.; Fried, J.; Imamura, T.; Mai, X.; Kalantari, S.; Sayer, G.; Kim, G.H.; Sarswat, N.; et al. Increased Rate of Pump Thrombosis and Cardioembolic Events Following Ventricular Tachycardia Ablation in Patients Supported with Left Ventricular Assist Devices. ASAIO J. 2020, 66, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.T.; Gosev, I.; Wood, K.L.; Vidula, H.; Stevenson, W.; Marchlinski, F.; Supple, G.; Zalawadiya, S.K.; Weiss, J.P.; Tung, R.; et al. Design and characteristics of the prophylactic intra-operative ventricular arrhythmia ablation in high-risk LVAD candidates (PIVATAL) trial. Ann. Noninvasive Electrocardiol. 2023, 28, e13073. [Google Scholar] [CrossRef]
- Moss, J.D.; Flatley, E.E.; Beaser, A.D.; Shin, J.H.; Nayak, H.M.; Upadhyay, G.A.; Burke, M.C.; Jeevanandam, V.; Uriel, N.; Tung, R. Characterization of Ventricular Tachycardia After Left Ventricular Assist Device Implantation as Destination Therapy: A Single-Center Ablation Experience. JACC Clin. Electrophysiol. 2017, 3, 1412–1424. [Google Scholar] [CrossRef]
- Vaidya, V.R.; Desimone, C.V.; Madhavan, M.; Noheria, A.; Shahid, M.; Walters, J.; Ladewig, D.J.; Mikell, S.B.; Johnson, S.B.; Suddendorf, S.H.; et al. Compatibility of electroanatomical mapping systems with a concurrent percutaneous axial flow ventricular assist device. J. Cardiovasc. Electrophysiol. 2014, 25, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Gopinathannair, R.; Cornwell, W.K.; Dukes, J.W.; Ellis, C.R.; Hickey, K.T.; Joglar, J.A.; Pagani, F.D.; Roukoz, H.; Slaughter, M.S.; Patton, K.K. Device Therapy and Arrhythmia Management in Left Ventricular Assist Device Recipients: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e967–e989. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, N.; Imamura, T. Catheter Ablation for Tachyarrhythmias in Left Ventricular Assist Device Recipients: Clinical Significance and Technical Tips. J. Clin. Med. 2023, 12, 7111. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.D.; Chrispin, J. Ventricular Tachycardia Ablation in Patients with Left Ventricular Assist Devices. J. Innov. Card. Rhythm Manag. 2019, 10, 3913–3918. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Załucka, L.; Świerżyńska, E.; Orczykowski, M.; Dutkowski, K.; Szymański, J.; Kuriata, J.; Dąbrowski, R.; Kołsut, P.; Szumowski, Ł.; Sterliński, M. Ventricular Arrhythmias in Left Ventricular Assist Device Patients—Current Diagnostic and Therapeutic Considerations. Sensors 2024, 24, 1124. https://doi.org/10.3390/s24041124
Załucka L, Świerżyńska E, Orczykowski M, Dutkowski K, Szymański J, Kuriata J, Dąbrowski R, Kołsut P, Szumowski Ł, Sterliński M. Ventricular Arrhythmias in Left Ventricular Assist Device Patients—Current Diagnostic and Therapeutic Considerations. Sensors. 2024; 24(4):1124. https://doi.org/10.3390/s24041124
Chicago/Turabian StyleZałucka, Laura, Ewa Świerżyńska, Michał Orczykowski, Krzysztof Dutkowski, Jarosław Szymański, Jarosław Kuriata, Rafał Dąbrowski, Piotr Kołsut, Łukasz Szumowski, and Maciej Sterliński. 2024. "Ventricular Arrhythmias in Left Ventricular Assist Device Patients—Current Diagnostic and Therapeutic Considerations" Sensors 24, no. 4: 1124. https://doi.org/10.3390/s24041124
APA StyleZałucka, L., Świerżyńska, E., Orczykowski, M., Dutkowski, K., Szymański, J., Kuriata, J., Dąbrowski, R., Kołsut, P., Szumowski, Ł., & Sterliński, M. (2024). Ventricular Arrhythmias in Left Ventricular Assist Device Patients—Current Diagnostic and Therapeutic Considerations. Sensors, 24(4), 1124. https://doi.org/10.3390/s24041124





