Optimizing Epoch Length and Activity Count Threshold Parameters in Accelerometry: Enhancing Upper Extremity Use Quantification in Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
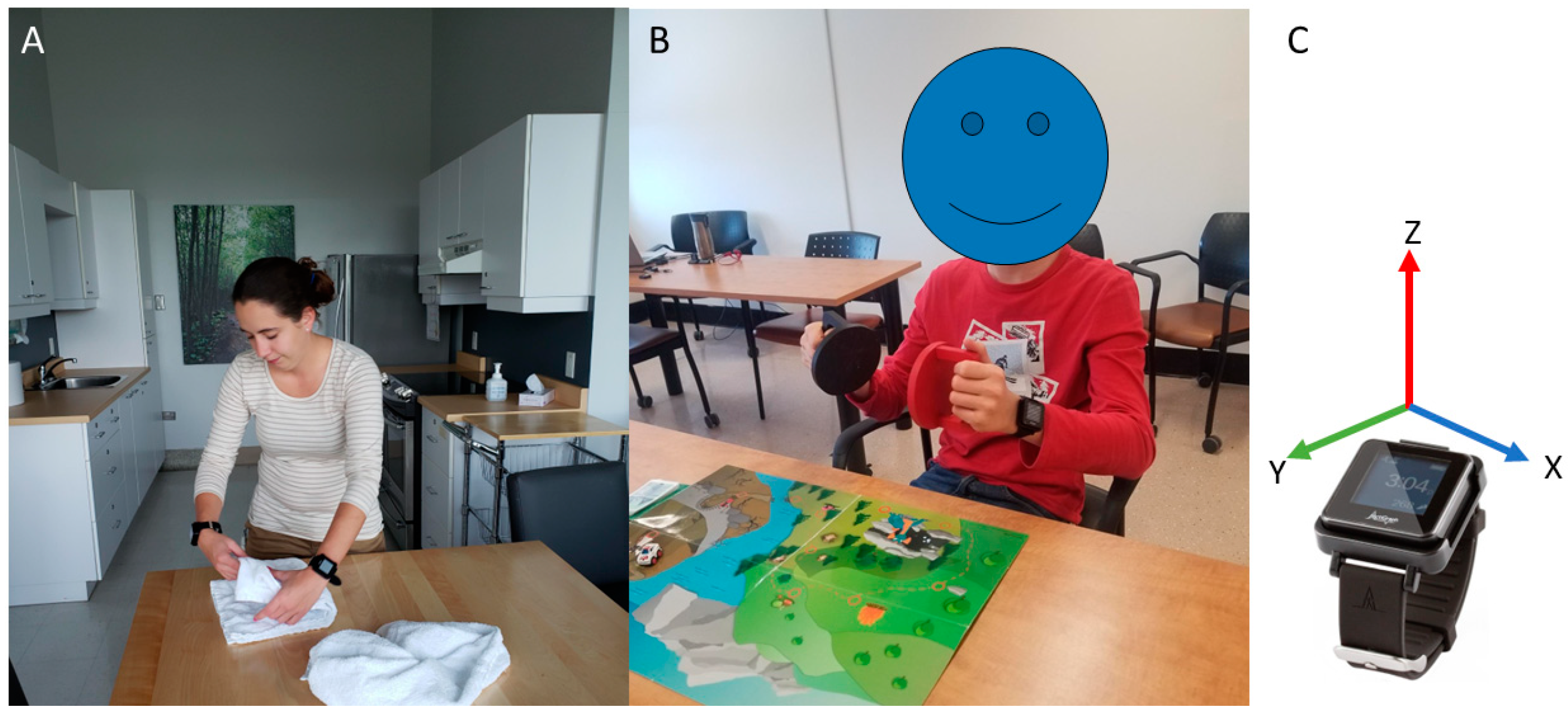
2.2. Experimental Setup
2.3. Video Processing
2.4. Accelerometry Processing
2.5. Use Ratio Calculation
2.6. Statistical Analysis
3. Results
3.1. Sample Description
3.2. Effect of Epoch Length and Activity Count Threshold on Use Ratios
3.3. Association between the UR Derived from Accelerometry and the One Derived from Video Rating
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailey, R.R. Assessment of Real-World Upper Limb Activity in Adults with Chronic Stroke; Washington University in St. Louis: St. Louis, MO, USA, 2015; p. 147. [Google Scholar]
- Rand, D.; Eng, J.J. Disparity between functional recovery and daily use of the upper and lower extremities during subacute stroke rehabilitation. Neurorehabil. Neural Repair 2012, 26, 76–84. [Google Scholar] [CrossRef]
- Hoyt, C.R.; Van, A.N.; Ortega, M.; Koller, J.M.; Everett, E.A.; Nguyen, A.L.; Lang, C.E.; Schlaggar, B.L.; Dosenbach, N.U.F. Detection of Pediatric Upper Extremity Motor Activity and Deficits With Accelerometry. JAMA Netw. Open 2019, 2, e192970. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Parnandi, A.; Eva, S.; Schambra, H. The use of wearable sensors to assess and treat the upper extremity after stroke: A scoping review. Disabil. Rehabil. 2022, 44, 6119–6138. [Google Scholar] [CrossRef]
- Waddell, K.J.; Lang, C.E. Comparison of Self-Report Versus Sensor-Based Methods for Measuring the Amount of Upper Limb Activity Outside the Clinic. Arch. Phys. Med. Rehabil. 2018, 99, 1913–1916. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Taub, E.; Morris, D.; Light, K.; Thompson, P.A. The Motor Activity Log-28: Assessing daily use of the hemiparetic arm after stroke. Neurology 2006, 67, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.A.; Eng, J.J.; Backman, C.L.; Miller, W.C. Rating of Everyday Arm-Use in the Community and Home (REACH) Scale for Capturing Affected Arm-Use after Stroke: Development, Reliability, and Validity. PLoS ONE 2013, 8, e83405. [Google Scholar] [CrossRef]
- Stone, A.A.; Bachrach, C.A.; Jobe, J.B.; Kurtzman, H.S.; Cain, V.S. The Science of Self-Report: Implications for Research and Practice; Psychology Press: London, UK, 1999. [Google Scholar]
- Sun, J.H.; Tan, L.; Yu, J.T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar] [CrossRef]
- Stadskleiv, K. Cognitive functioning in children with cerebral palsy. Dev. Med. Child Neurol. 2020, 62, 283–289. [Google Scholar] [CrossRef]
- Uswatte, G.; Giuliani, C.; Winstein, C.; Zeringue, A.; Hobbs, L.; Wolf, S.L. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: Evidence from the extremity constraint-induced therapy evaluation trial. Arch. Phys. Med. Rehabil. 2006, 87, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. An Accelerometry-Based Methodology for Assessment of Real-World Bilateral Upper Extremity Activity. PLoS ONE 2014, 9, e103135. [Google Scholar] [CrossRef]
- Poitras, I.; Clouâtre, J.; Campeau-Lecours, A.; Mercier, C. Accelerometry-Based Metrics to Evaluate the Relative Use of the More Affected Arm during Daily Activities in Adults Living with Cerebral Palsy. Sensors 2022, 22, 1022. [Google Scholar] [CrossRef]
- Heye, A.-L.; Kersting, C.; Kneer, M.; Barzel, A. Suitability of accelerometry as an objective measure for upper extremity use in stroke patients. BMC Neurol. 2022, 22, 220. [Google Scholar] [CrossRef]
- Hayward, K.S.; Eng, J.J.; Boyd, L.A.; Lakhani, B.; Bernhardt, J.; Lang, C.E. Exploring the Role of Accelerometers in the Measurement of Real World Upper-Limb Use After Stroke. Brain Impair. 2016, 17, 16–33. [Google Scholar] [CrossRef]
- Uswatte, G.; Miltner, W.H.; Foo, B.; Varma, M.; Moran, S.; Taub, E. Objective measurement of functional upper-extremity movement using accelerometer recordings transformed with a threshold filter. Stroke 2000, 31, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.R.; Birkenmeier, R.L.; Lang, C.E. Real-world affected upper limb activity in chronic stroke: An examination of potential modifying factors. Top. Stroke Rehabil. 2015, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.F.; Hayward, K.S.; Brauer, S. Upper limb use differs among people with varied upper limb impairment levels early post-stroke: A single-site, cross-sectional, observational study. Top. Stroke Rehabil. 2020, 27, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Geed, S.; Mitchell, A.; Lum, P.S.; Edwards, D.F.; Dromerick, A.W. Characterizing upper extremity motor behavior in the first week after stroke. PLoS ONE 2020, 15, e0221668. [Google Scholar] [CrossRef] [PubMed]
- Urbin, M.A.; Bailey, R.R.; Lang, C.E. Validity of body-worn sensor acceleration metrics to index upper extremity function in hemiparetic stroke. J. Neurol. Phys. Ther. JNPT 2015, 39, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Essers, B.; Biering Lundquist, C.; Verheyden, G.; Brunner, I.C. Determinants of Different Aspects of Upper-Limb Activity after Stroke. Sensors 2022, 22, 2273. [Google Scholar] [CrossRef] [PubMed]
- Haaland, K.Y.; Mutha, P.K.; Rinehart, J.K.; Daniels, M.; Cushnyr, B.; Adair, J.C. Relationship between arm usage and instrumental activities of daily living after unilateral stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Wagner, J.M.; Edwards, D.F.; Dromerick, A.W. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J. Neurol. Phys. Ther. 2007, 31, 56–63. [Google Scholar] [CrossRef]
- Pohl, J.; Ryser, A.; Veerbeek, J.; Verheyden, G.; Vogt, J.; Luft, A.; Easthope, C. Classification of functional and non-functional arm use by inertial measurement units in individuals with upper limb impairment after stroke. Front. Physiol. 2022, 13, 952757. [Google Scholar] [CrossRef] [PubMed]
- Kokotilo, K.J.; Eng, J.J.; McKeown, M.J.; Boyd, L.A. Greater activation of secondary motor areas is related to less arm use after stroke. Neurorehabil. Neural Repair 2010, 24, 78–87. [Google Scholar] [CrossRef]
- Lakhani, B.; Hayward, K.S.; Boyd, L.A. Hemispheric asymmetry in myelin after stroke is related to motor impairment and function. NeuroImage Clin. 2017, 14, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lin, K.C.; Hsieh, Y.W.; Wu, C.Y.; Liing, R.J.; Chen, C.L. A study of predictive validity, responsiveness, and minimal clinically important difference of arm accelerometer in real-world activity of patients with chronic stroke. Clin. Rehabil. 2018, 32, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Narai, E.; Hagino, H.; Komatsu, T.; Togo, F. Accelerometer-Based Monitoring of Upper Limb Movement in Older Adults With Acute and Subacute Stroke. J. Geriatr. Phys. Ther. 2016, 39, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.A.; Danielsson, A.; Ohlsson, F.; Wipenmyr, J.; Alt Murphy, M. Arm impairment and walking speed explain real-life activity of the affected arm and leg after stroke. J. Rehabil. Med. 2021, 53, jrm00210. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Waddell, K.J.; Klaesner, J.W.; Bland, M.D. A Method for Quantifying Upper Limb Performance in Daily Life Using Accelerometers. J. Vis. Exp. JoVE 2017, 122, 55673. [Google Scholar] [CrossRef]
- Ayabe, M.; Kumahara, H.; Morimura, K.; Tanaka, H. Epoch length and the physical activity bout analysis: An accelerometry research issue. BMC Res. Notes 2013, 6, 20. [Google Scholar] [CrossRef]
- Trost, S.G.; McIver, K.L.; Pate, R.R. Conducting accelerometer-based activity assessments in field-based research. Med. Sci. Sports Exerc. 2005, 37, S531–S543. [Google Scholar] [CrossRef]
- Jebsen, R.H.; Taylor, N.; Trieschmann, R.B.; Trotter, M.J.; Howard, L.A. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969, 50, 311–319. [Google Scholar]
- Beagley, S.B.; Reedman, S.E.; Sakzewski, L.; Boyd, R.N. Establishing Australian Norms for the Jebsen Taylor Test of Hand Function in Typically Developing Children Aged Five to 10 Years: A Pilot Study. Phys. Occup. Ther. Pediatr. 2016, 36, 88–109. [Google Scholar] [CrossRef]
- Louwers, A.; Beelen, A.; Holmefur, M.; Krumlinde-Sundholm, L. Development of the Assisting Hand Assessment for adolescents (Ad-AHA) and validation of the AHA from 18 months to 18 years. Dev. Med. Child Neurol. 2016, 58, 1303–1309. [Google Scholar] [CrossRef]
- Poitras, I.; Clouâtre, J.; Bouyer, L.J.; Routhier, F.; Mercier, C.; Campeau-Lecours, A. Development and Validation of Open-Source Activity Intensity Count and Activity Intensity Classification Algorithms from Raw Acceleration Signals of Wearable Sensors. Sensors 2020, 20, 6767. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.R.; Lang, C.E. Upper-limb activity in adults: Referent values using accelerometry. J. Rehabil. Res. Dev. 2013, 50, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.A.; McMahon, N.E.; Simpson, L.A.; Watkins, C.L.; Eng, J.J. Investigating measures of intensity during a structured upper limb exercise program in stroke rehabilitation: An exploratory study. Arch. Phys. Med. Rehabil. 2014, 95, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Rand, D.; Givon, N.; Weingarden, H.; Nota, A.; Zeilig, G. Eliciting upper extremity purposeful movements using video games: A comparison with traditional therapy for stroke rehabilitation. Neurorehabil. Neural Repair 2014, 28, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Odding, E.; Roebroeck, M.E.; Stam, H.J. The epidemiology of cerebral palsy: Incidence, impairments and risk factors. Disabil. Rehabil. 2006, 28, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, D.K.; Gripenstedt, U.; Welmer, A.-K. Spasticity After Stroke: An Overview of Prevalence, Test Instruments, and Treatments. Am. J. Phys. Med. Rehabil. 2012, 91, 814–820. [Google Scholar] [CrossRef]
- Regterschot, G.R.H.; Selles, R.W.; Ribbers, G.M.; Bussmann, J.B.J. Whole-Body Movements Increase Arm Use Outcomes of Wrist-Worn Accelerometers in Stroke Patients. Sensors 2021, 21, 4353. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Pelletier, L.; Poitras, I.; Flamand, V.H.; Mercier, C. Reliability of an observation-based scoring grid to assess bimanual performance during unstandardized tasks in adults living with cerebral palsy. Disabil. Rehabil. 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Foo, W.L.; Olmstead, H.; Lopez, K.; Holand, A.; Simms, L.B. Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1498–1501. [Google Scholar] [CrossRef] [PubMed]



| Adults (n = 15) | Children (n = 14) | |
|---|---|---|
| Age (years; Mean ± SD) | 34.5 ± 12.2 | 10.6 ± 1.9 |
| Sex | 6 males/9 females | 7 males/7 females |
| Manual Ability Classification Scale (MACS) level | I = 4 | I = 4 |
| II = 6 | II = 5 | |
| III = 5 | III = 5 | |
| Handedness | 10 left-handed | 8 left-handed |
| 5 right-handed * | 6 right-handed | |
| Side of lesion | Right = 11 * | Right = 6 |
| Left = 4 | Left = 8 | |
| Jebsen Taylor Hand Function Test (Z-score; Mean ± SD) | MA: 23.9 ± 35.0 | MA: 50.9 ± 59.5 |
| LA: 1.4 ± 4.4 | LA: 1.61 ± 2.0 | |
| Use ratio based on video rating (Mean ± SD) | 0.58 ± 0.21 | 0.62 ± 0.18 |
| Epoch | AC | Adults | Children | ||
|---|---|---|---|---|---|
| Mean ΔUR | 95% CI | Mean ΔUR | 95% CI | ||
| 1 | 2 | 0.33 | 0.22–0.43 | 0.23 | 0.15–0.30 |
| 25 | 0.20 | 0.12–0.28 | 0.08 | 0.02–0.13 | |
| 50 | 0.11 | 0.04–0.17 | −0.05 | −0.11–0.006 | |
| 75 | 0.06 | 0.004–0.11 | −0.16 | −0.21–(−0.10) | |
| 100 | 0.02 | −0.03–0.06 | −0.24 | −0.30–(−0.17) | |
| 125 | 0.01 | −0.03–0.05 | −0.29 | −0.36–(−0.22) | |
| 150 | 0.02 | −0.01–0.05 | −0.33 | −0.41–(−0.26) | |
| 1.5 | 2 | 0.34 | 0.24–0.44 | 0.24 | 0.16–0.31 |
| 25 | 0.21 | 0.13–0.29 | 0.08 | 0.02–00.14 | |
| 50 | 0.11 | 0.06–0.18 | −0.06 | −0.11–0.0007 | |
| 75 | 0.05 | −0.002–0.11 | −0.17 | −0.22–(−0.11) | |
| 100 | 0.02 | −0.03–0.07 | −0.25 | −0.32–(−0.19) | |
| 125 | 0.02 | −0.02–0.06 | −0.31 | −0.38–(−0.24) | |
| 150 | 0.03 | 0.001–0.07 | −0.36 | −0.44–(−0.28) | |
| 2 | 2 | 0.37 | 0.26–0.48 | 0.28 | 0.20–0.36 |
| 25 | 0.28 | 0.19–0.38 | 0.19 | 0.12–0.25 | |
| 50 | 0.21 | 0.13–0.29 | 0.09 | 0.03–0.15 | |
| 75 | 0.16 | 0.08–0.23 | 0.009 | −0.05–0.096 | |
| 100 | 0.12 | 0.05–0.18 | −0.07 | −0.12–(−0.01) | |
| 125 | 0.08 | 0.02–0.15 | −0.13 | −0.19–(−0.07) | |
| 150 | 0.05 | −0.01–0.11 | −0.20 | −0.27–(−0.13) | |
| Adults | Children | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epoch 1 | Epoch 1.5 | Epoch 2 | Epoch 1 | Epoch 1.5 | Epoch 2 | |||||||
| ICC | p-Value | ICC | p-Value | ICC | p-Value | ICC | p-Value | ICC | p-Value | ICC | p-Value | |
| 2 | 0.31 | 0.12 | 0.31 | 0.12 | 0.18 | 0.25 | 0.58 | 0.01 | 0.56 | 0.01 | 0.40 | 0.07 |
| 25 | 0.66 | 0.003 | 0.65 | 0.003 | 0.48 | 0.03 | 0.80 | <0.001 | 0.78 | <0.001 | 0.68 | 0.003 |
| 50 | 0.79 | <0.001 | 0.79 | <0.001 | 0.64 | 0.004 | 0.85 | <0.001 | 0.86 | <0.001 | 0.80 | <0.001 |
| 75 | 0.88 | <0.001 | 0.87 | <0.001 | 0.72 | <0.001 | 0.87 | <0.001 | 0.87 | <0.001 | 0.84 | <0.001 |
| 100 | 0.93 | <0.001 | 0.91 | <0.001 | 0.79 | <0.001 | 0.84 | <0.001 | 0.85 | <0.001 | 0.86 | <0.001 |
| 125 | 0.95 | <0.001 | 0.91 | <0.001 | 0.82 | <0.001 | 0.84 | <0.001 | 0.85 | <0.001 | 0.86 | <0.001 |
| 150 | 0.96 | <0.001 | 0.97 | <0.001 | 0.85 | <0.001 | 0.83 | <0.001 | 0.80 | <0.001 | 0.84 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poitras, I.; Gagné-Pelletier, L.; Clouâtre, J.; Flamand, V.H.; Campeau-Lecours, A.; Mercier, C. Optimizing Epoch Length and Activity Count Threshold Parameters in Accelerometry: Enhancing Upper Extremity Use Quantification in Cerebral Palsy. Sensors 2024, 24, 1100. https://doi.org/10.3390/s24041100
Poitras I, Gagné-Pelletier L, Clouâtre J, Flamand VH, Campeau-Lecours A, Mercier C. Optimizing Epoch Length and Activity Count Threshold Parameters in Accelerometry: Enhancing Upper Extremity Use Quantification in Cerebral Palsy. Sensors. 2024; 24(4):1100. https://doi.org/10.3390/s24041100
Chicago/Turabian StylePoitras, Isabelle, Léandre Gagné-Pelletier, Jade Clouâtre, Véronique H. Flamand, Alexandre Campeau-Lecours, and Catherine Mercier. 2024. "Optimizing Epoch Length and Activity Count Threshold Parameters in Accelerometry: Enhancing Upper Extremity Use Quantification in Cerebral Palsy" Sensors 24, no. 4: 1100. https://doi.org/10.3390/s24041100
APA StylePoitras, I., Gagné-Pelletier, L., Clouâtre, J., Flamand, V. H., Campeau-Lecours, A., & Mercier, C. (2024). Optimizing Epoch Length and Activity Count Threshold Parameters in Accelerometry: Enhancing Upper Extremity Use Quantification in Cerebral Palsy. Sensors, 24(4), 1100. https://doi.org/10.3390/s24041100








