Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor
Abstract
1. Introduction
2. Materials and Methods
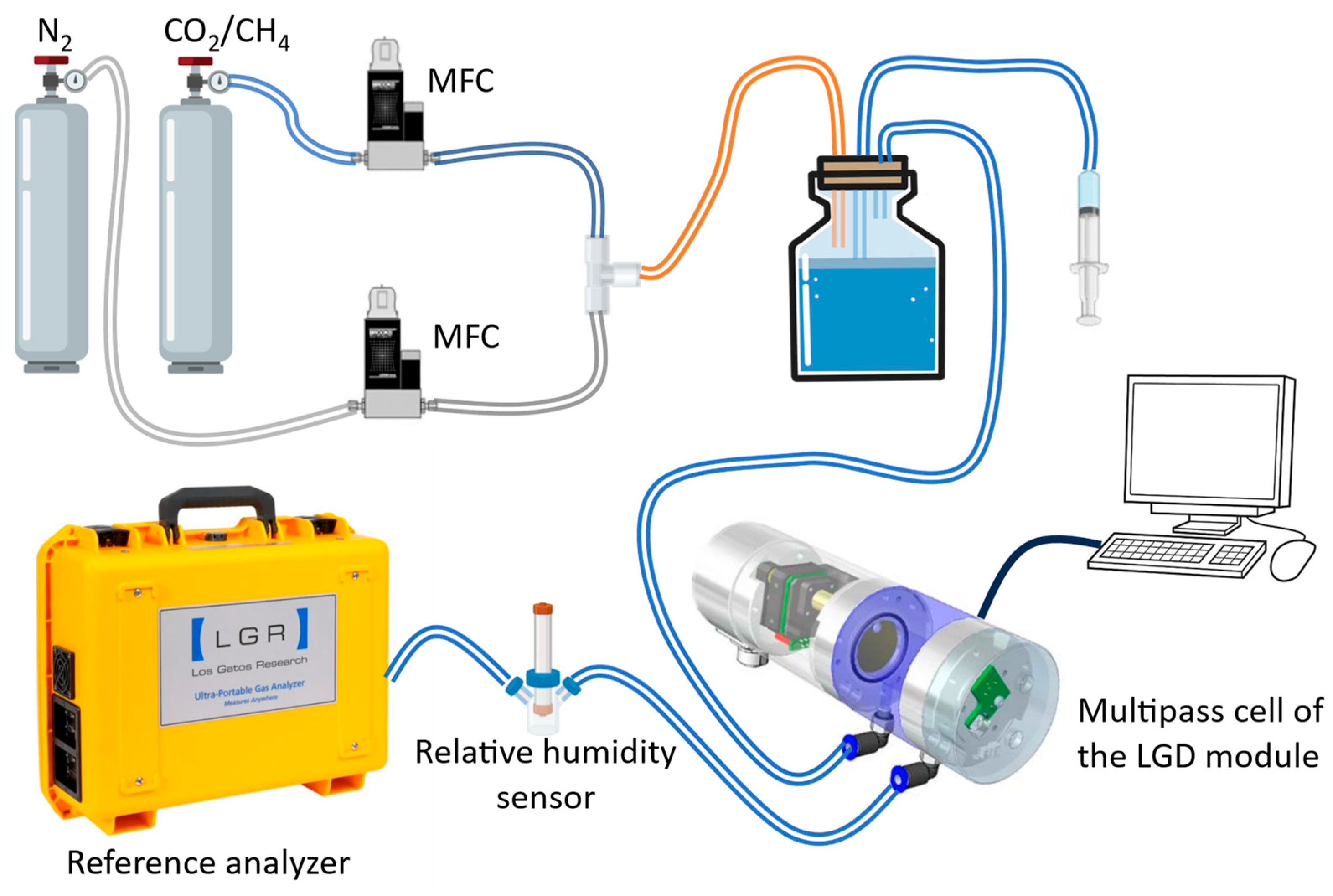
2.1. Experimental Set-Up for Investigating the Relative Humidity Influence
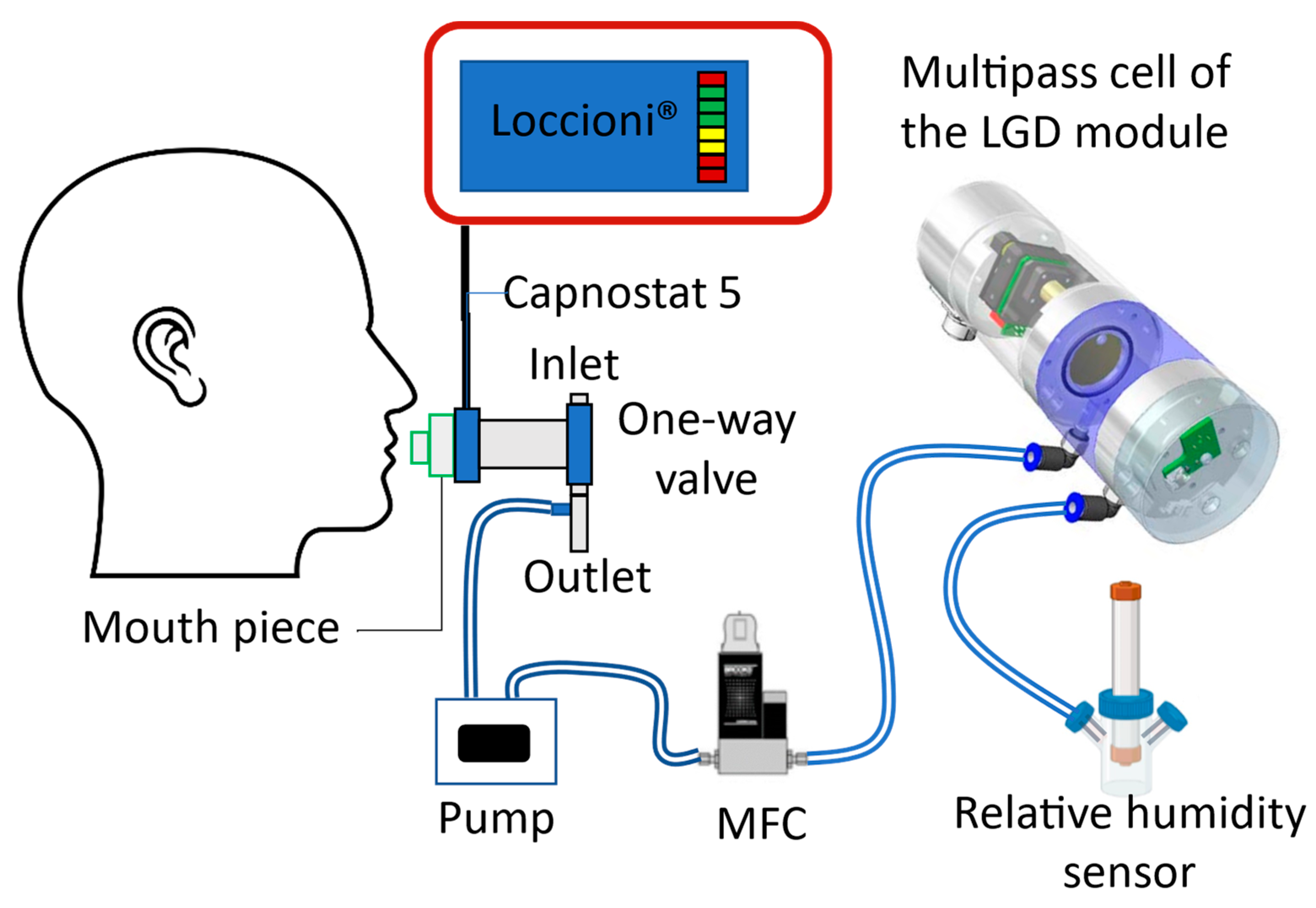
2.2. Experimental Setup for On-Line Breath Measurement
2.3. Sample Preparation and Data Processing
3. Results and Discussion
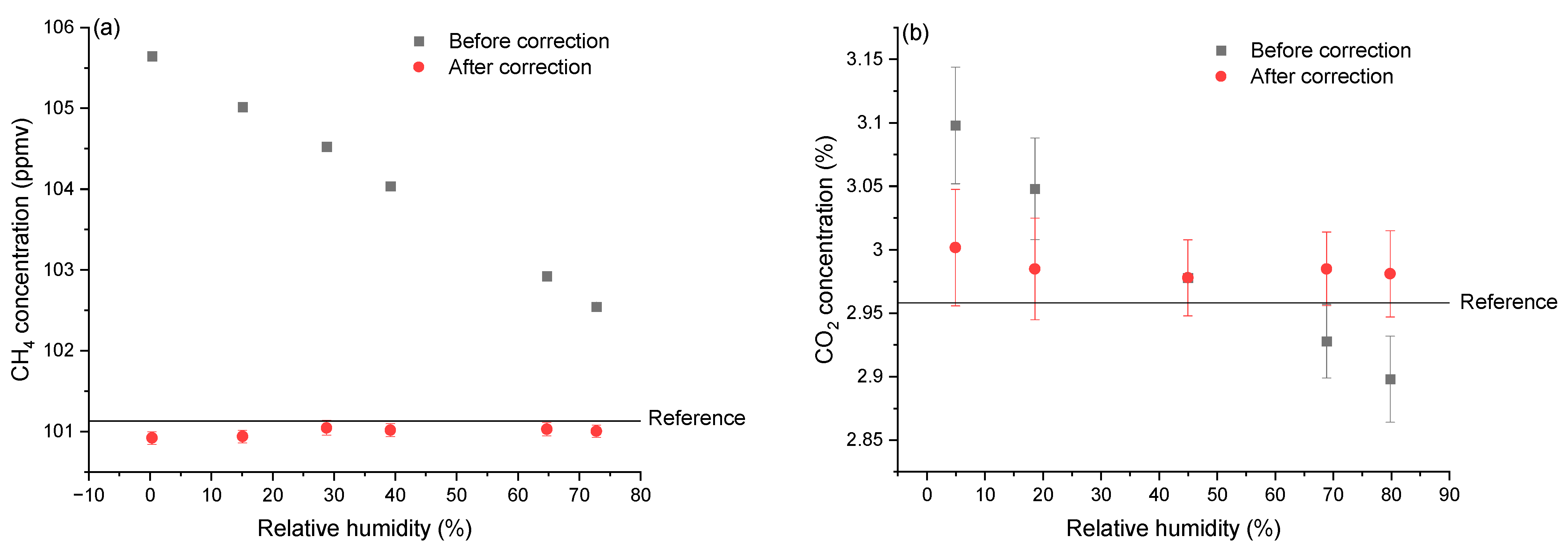
3.1. Effects of Relative Humidity
3.1.1. Results in Different Relative Humidity
3.1.2. Correction Function
3.2. Real-Time Breath Measurement
3.3. Effects of Environmental Temperature
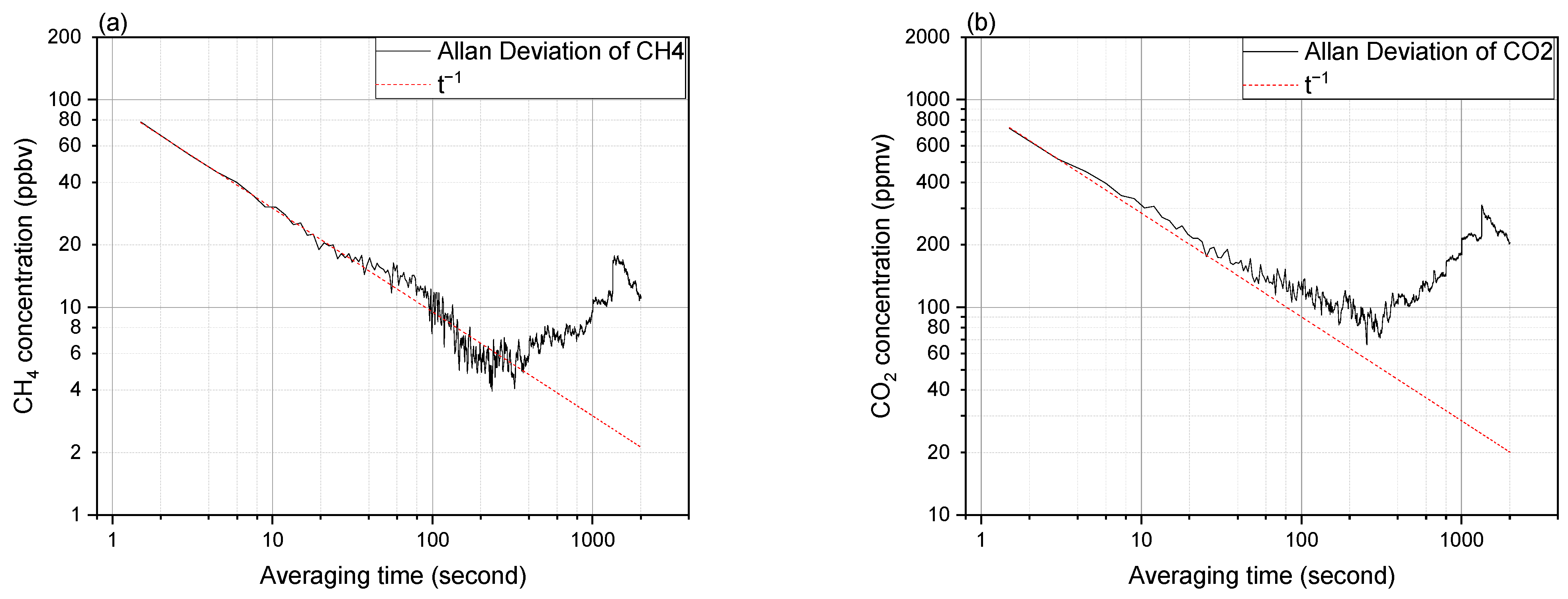
3.4. Sensitivity and Long-Term Stability
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, P.G.; Biswas, A.; Morales, S.E.; Greening, C.; Gaskins, H.R. H2 Metabolism Is Widespread and Diverse among Human Colonic Microbes. Gut Microbes 2016, 7, 235–245. [Google Scholar] [CrossRef]
- Chassard, C.; Lacroix, C. Carbohydrates and the Human Gut Microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 453. [Google Scholar] [CrossRef]
- de Lacy Costello, B.P.J.; Ledochowski, M.; Ratcliffe, N.M. The Importance of Methane Breath Testing: A Review. J. Breath Res. 2013, 7, 024001. [Google Scholar] [CrossRef]
- McKay, L.F.; Eastwood, M.A.; Brydon, W.G. Methane Excretion in Man—A Study of Breath, Flatus, and Faeces. Gut 1985, 26, 69–74. [Google Scholar] [CrossRef]
- De Geyter, C.; Van de Maele, K.; Hauser, B.; Vandenplas, Y. Hydrogen and Methane Breath Test in the Diagnosis of Lactose Intolerance. Nutrients 2021, 13, 3261. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, A.B.; Jee, S.-R.; Pimentel, M. Methane and the Gastrointestinal Tract. Dig. Dis. Sci. 2010, 55, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Roccarina, D.; Lauritano, E.C.; Gabrielli, M.; Franceschi, F.; Ojetti, V.; Gasbarrini, A. The Role of Methane in Intestinal Diseases. Am. J. Gastroenterol. 2010, 105, 1250–1256. [Google Scholar] [CrossRef]
- Hoegenauer, C.; Hammer, H.F.; Mahnert, A.; Moissl-Eichinger, C. Methanogenic Archaea in the Human Gastrointestinal Tract. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Kumpitsch, C.; Fischmeister, F.P.S.; Mahnert, A.; Lackner, S.; Wilding, M.; Sturm, C.; Springer, A.; Madl, T.; Holasek, S.; Högenauer, C.; et al. Reduced B12 Uptake and Increased Gastrointestinal Formate Are Associated with Archaeome-Mediated Breath Methane Emission in Humans. Microbiome 2021, 9, 193. [Google Scholar] [CrossRef]
- Di Francesco, F.; Loccioni, C.; Fioravanti, M.; Russo, A.; Pioggia, G.; Ferro, M.; Roehrer, I.; Tabucchi, S.; Onor, M. Implementation of Fowler’s Method for End-Tidal Air Sampling. J. Breath Res. 2008, 2, 037009. [Google Scholar] [CrossRef]
- Bhavani-Shankar, K.; Philip, J.H. Defining Segments and Phases of a Time Capnogram. Anesth. Analg. 2000, 91, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Hl, D. The Intestinal Microbiota and Chronic Disorders of the Gut. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 523–531. [Google Scholar] [CrossRef]
- Gould, O.; Drabińska, N.; Ratcliffe, N.; de Lacy Costello, B. Hyphenated Mass Spectrometry versus Real-Time Mass Spectrometry Techniques for the Detection of Volatile Compounds from the Human Body. Molecules 2021, 26, 7185. [Google Scholar] [CrossRef] [PubMed]
- Španěl, P.; Smith, D. Quantification of Volatile Metabolites in Exhaled Breath by Selected Ion Flow Tube Mass Spectrometry, SIFT-MS. Clin. Mass Spectrom. 2020, 16, 18–24. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Unterkofler, K.; Teschl, S.; Amann, A.; Teschl, G. Volatile organic compounds in exhaled breath: Real-time measurements, modeling, and bio-monitoring applications. In The 1st International Workshop on Innovative Simulation for Health Care; Backfrieder, W., Bruzzone, A., Longo, F., Novak, V., Rosen, J., Eds.; DIME University of Genova: Genoa, Italy, 2012; pp. 139–144. [Google Scholar]
- Polag, D.; Keppler, F. Effect of Immune Responses on Breath Methane Dynamics. J. Breath Res. 2023, 17, 046005. [Google Scholar] [CrossRef]
- Dawson, B.; Drewer, J.; Roberts, T.; Levy, P.; Heal, M.; Cowan, N. Measurements of Methane and Nitrous Oxide in Human Breath and the Development of UK Scale Emissions. PLoS ONE 2023, 18, e0295157. [Google Scholar] [CrossRef]
- Kwaśny, M.; Bombalska, A. Optical Methods of Methane Detection. Sensors 2023, 23, 2834. [Google Scholar] [CrossRef]
- Szabó, A.; Ruzsanyi, V.; Unterkofler, K.; Mohácsi, Á.; Tuboly, E.; Boros, M.; Szabó, G.; Hinterhuber, H.; Amann, A. Exhaled Methane Concentration Profiles during Exercise on an Ergometer. J. Breath Res. 2015, 9, 016009. [Google Scholar] [CrossRef]
- Bársony, A.; Vida, N.; Gajda, Á.; Rutai, A.; Mohácsi, Á.; Szabó, A.; Boros, M.; Varga, G.; Érces, D. Methane Exhalation Can Monitor the Microcirculatory Changes of the Intestinal Mucosa in a Large Animal Model of Hemorrhage and Fluid Resuscitation. Front. Med. 2020, 7, 567260. [Google Scholar] [CrossRef]
- Negussie, E.; Lehtinen, J.; Mäntysaari, P.; Bayat, A.R.; Liinamo, A.-E.; Mäntysaari, E.A.; Lidauer, M.H. Non-Invasive Individual Methane Measurement in Dairy Cows. Animal 2017, 11, 890–899. [Google Scholar] [CrossRef]
- Tuboly, E.; Szabó, A.; Erős, G.; Mohácsi, Á.; Szabó, G.; Tengölics, R.; Rákhely, G.; Boros, M. Determination of Endogenous Methane Formation by Photoacoustic Spectroscopy. J. Breath Res. 2013, 7, 046004. [Google Scholar] [CrossRef] [PubMed]
- Keppler, F.; Schiller, A.; Ehehalt, R.; Greule, M.; Hartmann, J.; Polag, D. Stable Isotope and High Precision Concentration Measurements Confirm That All Humans Produce and Exhale Methane. J. Breath Res. 2016, 10, 016003. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Liu, W.; Luo, W.; Xiao, J.; He, L.; Huang, Y.; Zhu, D. Calibration-Free near-Infrared Methane Sensor System Based on BF-QEPAS. Infrared Phys. Technol. 2023, 133, 104784. [Google Scholar] [CrossRef]
- Menduni, G.; Zifarelli, A.; Sampaolo, A.; Patimisco, P.; Giglio, M.; Amoroso, N.; Wu, H.; Dong, L.; Bellotti, R.; Spagnolo, V. High-Concentration Methane and Ethane QEPAS Detection Employing Partial Least Squares Regression to Filter out Energy Relaxation Dependence on Gas Matrix Composition. Photoacoustics 2022, 26, 100349. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiao, S.; Ma, Y. Highly Sensitive Methane Detection Based on Light-Induced Thermoelastic Spectroscopy with a 2.33 Μm Diode Laser and Adaptive Savitzky-Golay Filtering. Opt. Express 2022, 30, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Tittel, F.K.; Ma, Y. Sensitive Methane Detection Based on Quartz-Enhanced Photoacoustic Spectroscopy with a High-Power Diode Laser and Wavelet Filtering. Opt. Lasers Eng. 2020, 132, 106155. [Google Scholar] [CrossRef]
- Liang, T.; Qiao, S.; Chen, Y.; He, Y.; Ma, Y. High-Sensitivity Methane Detection Based on QEPAS and H-QEPAS Technologies Combined with a Self-Designed 8.7 KHz Quartz Tuning Fork. Photoacoustics 2024, 36, 100592. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.M.; Conway, E.K.; Hill, C.; Kochanov, R.V.; Tan, Y.; et al. The HITRAN2020 Molecular Spectroscopic Database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar] [CrossRef]
- Henderson, B.; Meurs, J.; Lamers, C.R.; Batista, G.L.; Materić, D.; Bertinetto, C.G.; Bongers, C.C.W.G.; Holzinger, R.; Harren, F.J.M.; Jansen, J.J.; et al. Non-Invasive Monitoring of Inflammation in Inflammatory Bowel Disease Patients during Prolonged Exercise via Exhaled Breath Volatile Organic Compounds. Metabolites 2022, 12, 224. [Google Scholar] [CrossRef]
- Meyer, J.R.; Bewley, W.W.; Canedy, C.L.; Kim, C.S.; Kim, M.; Merritt, C.D.; Vurgaftman, I. The Interband Cascade Laser. Photonics 2020, 7, 75. [Google Scholar] [CrossRef]
- Gong, J.; Yang, R.Q.; Wang, Z.; He, J.-J. Single-Mode Tunable Interband Cascade Laser Emitting at 3.4 Μm with a Wide Tuning Range Over 100 Nm. IEEE Photonics Technol. Lett. 2023, 35, 309–312. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Z.; He, J.-J.; Li, L.; Yang, R.Q.; Gupta, J.A. Single-Mode Interband Cascade Laser Based on V-Coupled Cavity with 210 Nm Wavelength Tuning Range near 3 Μm. Opt. Express 2023, 31, 38409–38418. [Google Scholar] [CrossRef]
- Xia, J.; Feng, C.; Zhu, F.; Ye, S.; Zhang, S.; Kolomenskii, A.; Wang, Q.; Dong, J.; Wang, Z.; Jin, W.; et al. A Sensitive Methane Sensor of a Ppt Detection Level Using a Mid-Infrared Interband Cascade Laser and a Long-Path Multipass Cell. Sens. Actuators B Chem. 2021, 334, 129641. [Google Scholar] [CrossRef]
- Zheng, C.; Ye, W.; Sanchez, N.P.; Li, C.; Dong, L.; Wang, Y.; Griffin, R.J.; Tittel, F.K. Development and Field Deployment of a Mid-Infrared Methane Sensor without Pressure Control Using Interband Cascade Laser Absorption Spectroscopy. Sens. Actuators B Chem. 2017, 244, 365–372. [Google Scholar] [CrossRef]
- Davis, N.M.; Francis, D.; Hodgkinson, J.; Tatam, R.P. Compact Methane Sensor Using an Integrating Sphere and Interband Cascade Laser at 3313 Nm. Sens. Actuators B Chem. 2023, 389, 133866. [Google Scholar] [CrossRef]
- Schliesser, A.; Picqué, N.; Hänsch, T.W. Mid-Infrared Frequency Combs. Nat. Photonics 2012, 6, 440–449. [Google Scholar] [CrossRef]
- Vasilyev, S.; Muraviev, A.; Konnov, D.; Mirov, M.; Smolski, V.; Moskalev, I.; Mirov, S.; Vodopyanov, K. Longwave Infrared (6.6–11.4 Μm) Dual-Comb Spectroscopy with 240,000 Comb-Mode-Resolved Data Points at Video Rate. Opt. Lett. 2023, 48, 2273–2276. [Google Scholar] [CrossRef] [PubMed]
- Konnov, D.; Muraviev, A.; Vasilyev, S.; Vodopyanov, K. High-Resolution Frequency-Comb Spectroscopy with Electro-Optic Sampling and Instantaneous Octave-Wide Coverage across Mid-IR to THz at a Video Rate. APL Photonics 2023, 8, 110801. [Google Scholar] [CrossRef]
- Krebbers, R.; Van Kempen, K.; Harren, F.J.; Vasilyev, S.; Peterse, I.; Lücker, S.; Khodabakhsh, A.; Cristescu, S. Ultra-Broadband Spectroscopy Using a 2–11.5 Μm IDFG-Based Supercontinuum Source. Optica Open, Preprint. [CrossRef]
- Thorpe, M.J.; Balslev-Clausen, D.; Kirchner, M.S.; Ye, J. Cavity-Enhanced Optical Frequency Comb Spectroscopy: Application to Human Breath Analysis. Opt. Express 2008, 16, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Chan, Y.-C.; Changala, P.B.; Nesbitt, D.J.; Ye, J.; Toscano, J. Ultrasensitive Multispecies Spectroscopic Breath Analysis for Real-Time Health Monitoring and Diagnostics. Proc. Natl. Acad. Sci. USA 2021, 118, e2105063118. [Google Scholar] [CrossRef] [PubMed]



| Gas | Applied Concentration | Error before Correction | Error after Correction |
|---|---|---|---|
| CH4 (ppmv) | 100 | 1.4~4.4% | <0.2% |
| 75 | 1.9~4.7% | <0.2% | |
| 50 | 2.8~5.8% | <0.7% | |
| 25 | 4.3~7.5% | <1.5% | |
| 10 | 6.4~9.2% | <0.5% | |
| 8 | 6.2~9.1% | <0.5% | |
| 6 | 6.7~9.8% | <1% | |
| 4 | 6.4~8.7% | <0.2% | |
| 2 | 4.1~5.7% | <2.8% | |
| CO2 (%) | 2 | 1~3.4% | <1.4% |
| 3 | 0.6~4.7% | <1.4% | |
| 5 | 0.1~3.9% | <1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Manalili, D.; Khodabakhsh, A.; Cristescu, S.M. Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor. Sensors 2024, 24, 1077. https://doi.org/10.3390/s24041077
Lin Y, Manalili D, Khodabakhsh A, Cristescu SM. Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor. Sensors. 2024; 24(4):1077. https://doi.org/10.3390/s24041077
Chicago/Turabian StyleLin, Yueyu, Dexter Manalili, Amir Khodabakhsh, and Simona M. Cristescu. 2024. "Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor" Sensors 24, no. 4: 1077. https://doi.org/10.3390/s24041077
APA StyleLin, Y., Manalili, D., Khodabakhsh, A., & Cristescu, S. M. (2024). Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor. Sensors, 24(4), 1077. https://doi.org/10.3390/s24041077








