Wearable Solutions Using Physiological Signals for Stress Monitoring on Individuals with Autism Spectrum Disorder (ASD): A Systematic Literature Review
Abstract
1. Introduction
2. Background
2.1. Stress
2.2. Physiological Signals
3. Objectives
- What kind of wearable solutions are most acceptable for people with ASD?
- What features extraction/parameters are used for stress detection?
- What techniques/processes are used for stress detection in individuals with ASD?
- What techniques are applied for multimodal physiological signal fusion?
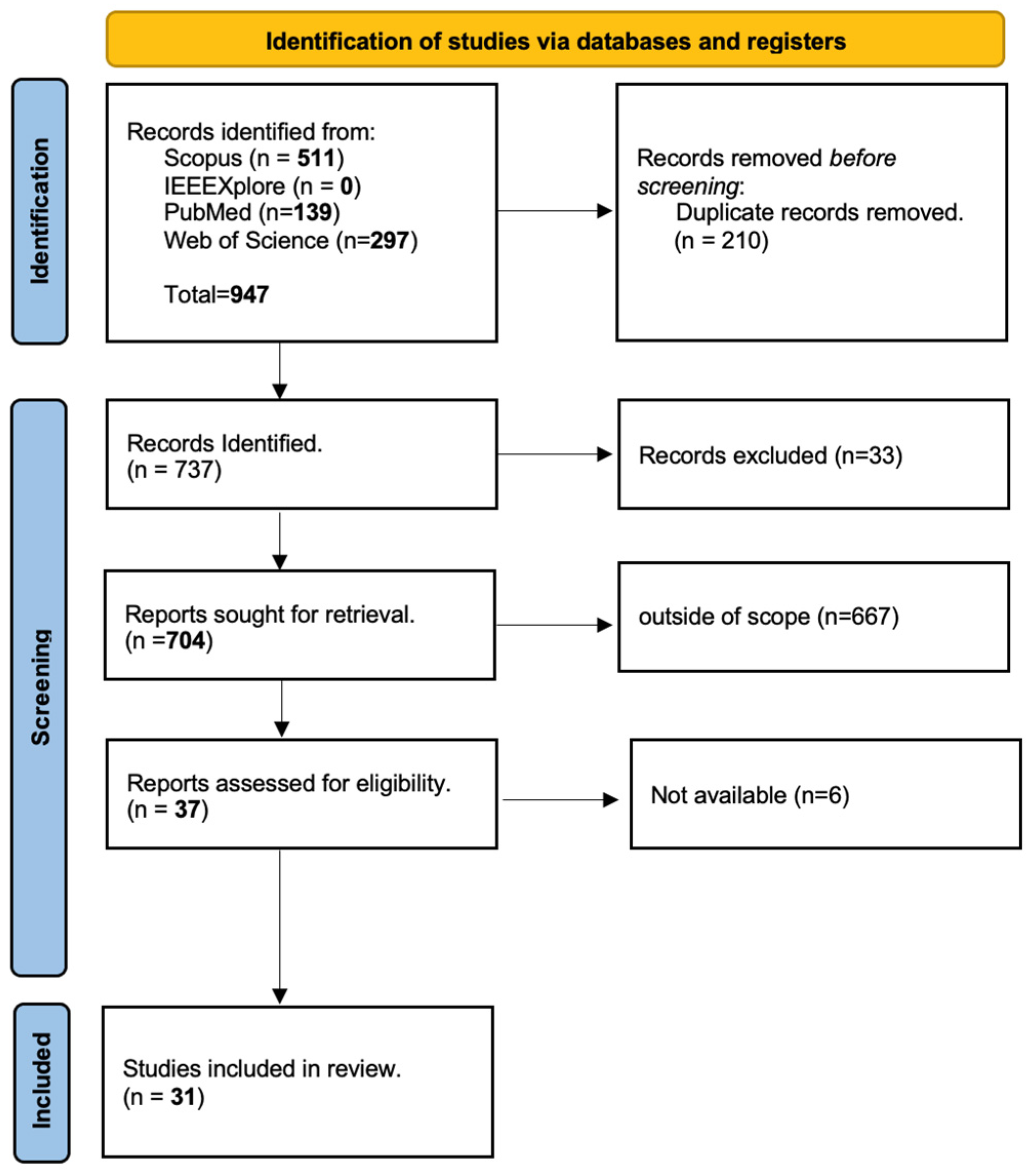
4. Methodology
4.1. Study Selection Process
- Participants: Only individuals with ASD children and adolescents.
- Interventions: Wearable sensors for detecting stress.
- Comparisons: The measured physiological signals for detection of stress or emotions.
- Outcome: qualitative and quantitative studies, excluding other systematic literature reviews or meta-analyses.
- Study Design: Observational and experimental studies.
4.2. Search Strategy
4.3. Screening and Eligibility
5. Results
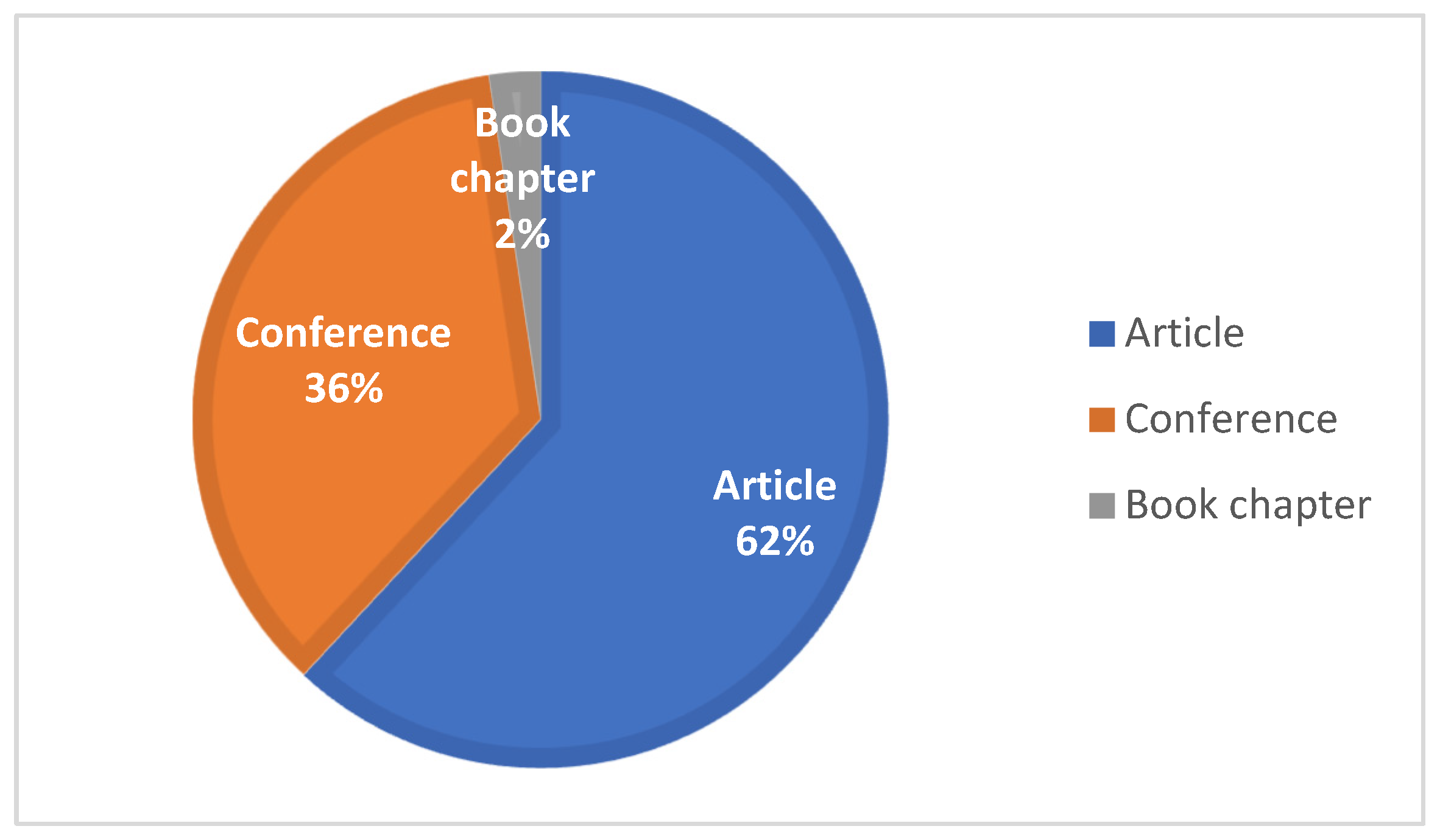
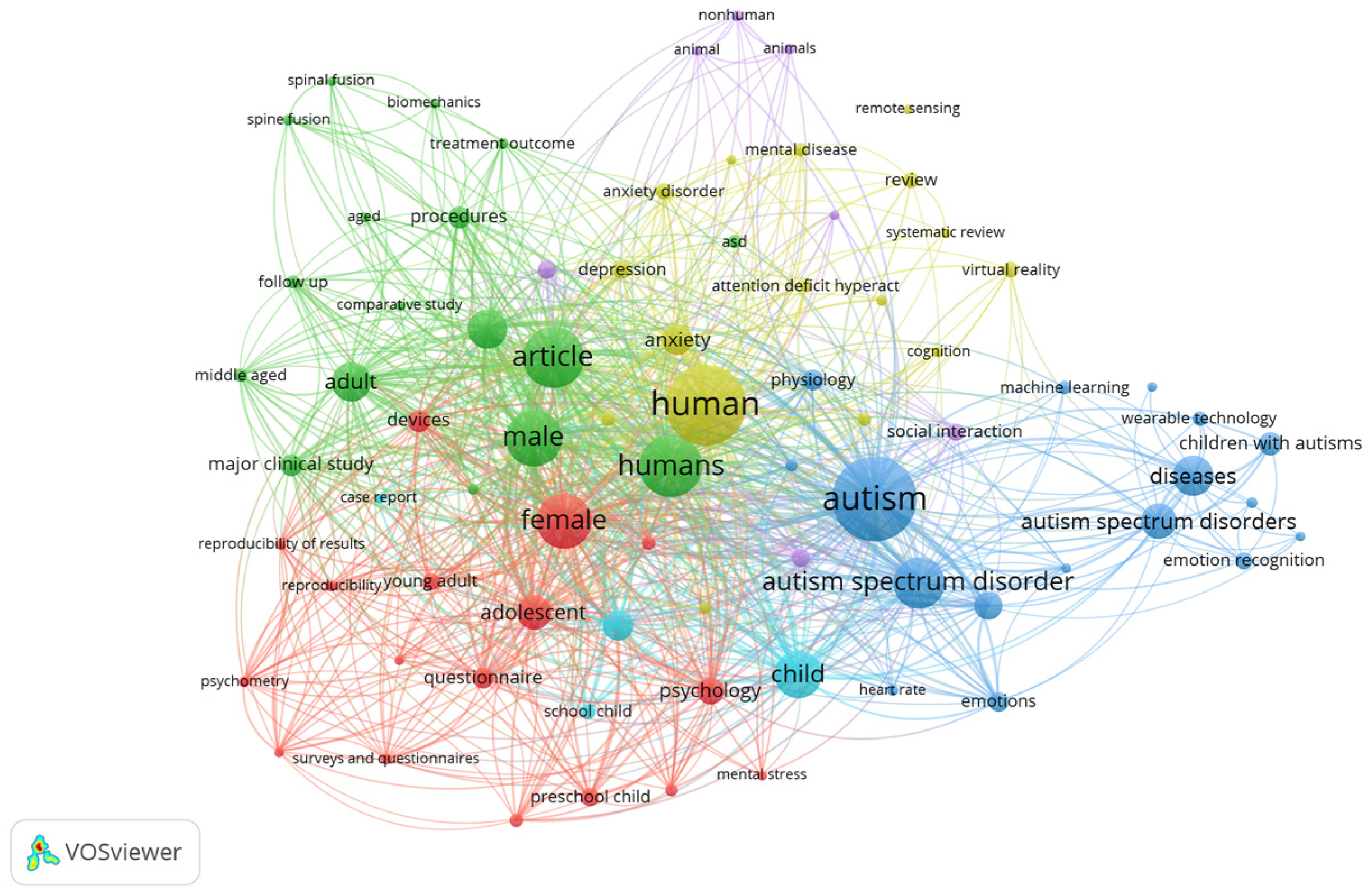
5.1. Data Synthesis
5.2. Answering the Research Questions
| Study/Year | Device Type | Sensors | Signals/Parameters | Outcomes | Purpose | Population |
| [80]/2014 | Affectiva Q Sensor; MindWave Mobile; Zephyr BioHarness | ACC; ST; GSR; EEG power spectrums; ECG | Arousal, alpha and beta waves, HRV. | - | App mobile for Monitoring emotions such as happiness, sadness, fear, disgust, surprise, and anger. | - |
| [105]/2014 | Mynplay Brainband | EEG; GSR; HR | EEG waves | - | Analyzing mental states | - |
| [91]/2015 | Zephyr BioHarness BT; two-lead cardiscope One. | ECG; SC; RT | RR-Intervals, HF and LF energies, time series SC and RT | Resulting stress induces a bilateral variation in HR of 60.8 ± 1.3 bpm and 5.2 ± 0.2, and SC ranges from 3.65 ± 15 μS to 3.31 ± 0.13 μS | A protocol was conducted for monitoring physiological responses for individuals with ASD | Thirty participants with ASD between 10 and 25 years |
| [57]/2016 | Shirt | ECG; ACC | R-R Intervals; Pan–Tompkins algorithm | Three participants accepted the wearable. The duration of the sessions was 60 min; different tasks were applied; tasks with high SD were reading, card selection, and motor imitation. | Design a smart wearable for monitoring | Four male children with ASD between 5 and 8 years |
| [92]/2016 | Wristband | GSR; PPG | SD-GSR; mean-GSR; HRV (LF, HF), IBI | SVM is employed for detection of emotions, with accuracy of 90%, with specificity of 100%, and sensitivity of 80%. | Monitoring emotions such as happy, neutral | Ten children with ASD |
| [86]/2017 | ECG chest belt | EEG; ECG | RMSSD; RSA; HR; | The children did not show sensory-motor and/or behavioral issues in wearing the devices and completing all the tasks. ECG patterns showed similar changes in five patients in a socio-cognitive task. | Propose a method for ECG analysis of data collected. | Five patients with ASD between 6 and 8 years; one session a week for six months |
| Study/Year | Device Type | Sensors | Signals/Parameters | Results | Purpose | Population |
| [86]/2017 | Empatica E4 | EDA; ST; HR | RMSSD; SD; SDNN | Happiness: HR (slight increase); EDA increase; EDA peaks (small and few); SKT (slight decrease). Sadness HR (decrease); EDA (Increase), EDA peaks (small and many) and SKT slight decrease) | Classifies emotions such as anger, happiness, pain and sadness | Ten subjects between 20 to 25 years. |
| [88]/2017 | LG Watch Urbane | PPG, ACC; barometer | - | User B experienced strong anxiety moments whose manifestation was not easily visible. User A manifested several signals of fear and tension. | A system for emotional self-regulation | Two individuals with ASD ages 10 years were tested 4 h a day over nine days |
| [87]/2018 | - | ACC; ECG; blood glucose | - | - | Designing a wireless body area network | - |
| [106]/2018 | - | EDA | SCL; SCR | Some data were missing due to equipment malfunction and/or removal of sensors. EDA were significantly related to externalizing behavior scores. | Testing electrodermal activity through 2 tasks | Forty children with ASD between ages of 4 and 11 years. |
| [82]/2018 | Thoracic belt | Piezoelectric sensors, ECG | SD; mean | Three game activities were evaluated, where in the first game activity, the HR is higher than mean frequency of 96 bpm. The last game activity, in which the child plays with a balloon inflated by the operator, is characterized by a great variability in HR, i.e., 102.9 bpm. | Testing the device during the designed activities | Five children with ASD between 2 and 5 years |
| [103]/2018 | Affective Q Sensors | EDA | SCL; SCR | Four simple rules, such as (1) EDA is out of range (not within 0.05–60 μS); (2) EDA changes too quickly; (3) Temperature is out of range (not within 30–40 °C); (4) EDA data are surrounding (within 5 s) | Testing data quality of EDA considering 4 rules. | Fifteen males, 5 females, ages range 5–13 years for 8 weeks |
| [99]/2018 | - | GSR | The lowest value of EDA was observed when the sensors were placed on the middle and index fingers. The data collected on the wrist and forearm were comparable. | Validation in four parts of the body such as (1) on the arm; (2) on the leg; (3) on the leg, 1—index and middle fingers, 2—wrist, 3—forearm, and 4—ankle. | Sixteen subjects between 15 and 50 years (8 males and 8 females) | |
| [97]/2019 | Biopac AcqKnowledge versión 4.4.2 | ECG | RMSSD; QRS; BPM; HRV | BPM before challenging behavior was higher (SD = 16.10). HR increase was significantly associated with challenging behavior. | Testing HR activity in tasks designed to induce low-level stress. | Forty-one children with ASD between 2–4 years (32 males, 9 females) sessions 1 to 1.5 h. |
| [93]/2019 | Chest strap ECG sensor | ECG | Max, Min, mean, SD features of HRV; RMSSD, NN; NN20; NN50; pNN20, pNN50 | Applied algorithms such as LR and SVM with 84% and 91% accuracy, respectively. | Stress detection using two classes: rest—stress, using two tasks designed to mimic stressful scenarios, such as transparent box and tangram/tangoes. | Twenty-two participants with ASD |
| [87]/2019 | Empatica E4 | EDA | Arousal | Two different types of noise-attenuating headphones were designed. EDA was recorded continuously with a minimum of 20 min. Decibel levels increased; SCL increased during the stages without intervention. | Evaluate the proof of concept of an intervention to decrease sympathetic activation using EDA | Six participants with ASD between 8–16 years. |
| [18]/2020 | Wristband | PPG; GSR; ST | HRV; BPM; GSR; ST | Not all participants understood the purpose of wearing the device. Each decision to classify a certain situation as stressful was based on symptoms such as behavior, gesture, interaction, voice, and facial features. | Device for monitoring stress levels | Twenty participants between 5–24 years (19 males; 1 female), wearing time: 5 h. |
| [89]/2021 | Shimmer ECG | ECG | HRV; QRS; Pan–Tompkin’s algorithm; mean; median; entropy; kurtosis | HRV and QRS amplitude were classified using KNN, SVM, and ensemble classifier, obtaining accuracy of 81%, 87.4%, and 83.8%. | Protocol to induce positive and negative valence and ECG | Six children between 5 to 11 years |
| [104]/2021 | EEG headband | EEG | EEG; GSR; ACC; ST; HR | GBDT, RF obtained the best prediction accuracies of 86.67% and 99.05%. | Evaluate machine learning models such as GBDT and RF | Thirty-five participants with ASD |
| [107]/2021 | ECG Comftech CozyBaby | ECG | RR; SD1; SD2 | We analyzed a total of 26 therapeutic sessions. | Variability in the therapist’s heart rate and conversational turn-taking during online sessions. | Sixteen participants between 6 to 18 years with ASD level 1. |
| [108]/2021 | Bracelet | EDA; PPG | RR | - | Design a smart bracelet | - |
| [83]/2022 | Polar, Mio Muse and PulseOn | ECG; | HRV | Three wearables (Polar H7, Mio Fuse, and PulseOn) were within a priori sampling fidelity. | Evaluating commercial wearables for elements such as stressor task; comfort | Thirty-two children with ASD between 8–12 years |
| [109]/2022 | _ | ECG; EDA | SCL; SCR; HRV; RMSSD | Affective sliders, and state trait anxiety scale questionnaires were used as self-reports. | Include physiological data to evaluate social interaction behaviors in children with ASD | Seventy-two children between 8–12 years. (female = 12, male = 60) |
| [22]/2022 | Empatica E4 | EDA; PPG; ST | LF; HF | Each interaction session was analyzed in detail in 2–3 min intervals and validated with emotional labels. | Testing physiological-signal-based stress detection to be used in social interaction | Twenty-nine children between 2 to 12 years; 2–11 sessions |
| [110]/2022 | - | PPG | RMSSD | Participant age was significantly negatively correlated with all HRV variables, namely, high-frequency HRV. Some difficulties related with device and problems with home testing of HRV. | Exploring HRV biofeedback | Twenty participants with ASD between 13–24 years |
| [85]/2022 | Hexoskin | ECG | QRS | Ten participants with ASD who have aggressive or disruptive tendencies were monitored for 7 days. In addition, LSTM was applied. | To assess the differences in physiological reaction to stressful stimuli | Twenty participants with ASD between 20–40 years for 7 days |
| [111]/2023 | Empatica E4 | PPG, EDA | Average, SD of HR; SCL; SCR (number of peaks, amplitude, rise time) | A total of 207.6 and 203.5 h of physiological data that reported stressful events, where PPG and EDA were evaluated. | To understand the stress through physiological signals | Eight children between 5–12 years for 2 days |
| [98]/2023 | Polar OH1 or Verity sense | PPG | HRV | If HR increases by more than 2 SD above the average HR, it sends a signal to alert the caregiver. HR response to events is not a specific measure of pain per se. | Heart rate monitoring to detect acute pain in non-verbal patients | Thirty-eight participants with ASD for 2 weeks |
| [84]/2023 | Samsung Galaxy 3 | PPG | HRV, DWT | The average HR of all participants is 96.8 BPM, and max HR is 124 BPM. | Analyze affective states | Nine children between 8–11 years (6 male and 3 female) |
| [112]/2024 | Ballistocardiography | RR; HR | Filters, peak detection. | Results show reasonable frequency estimation performance both for the respiration rate and heart rate. | Emotion detection for individuals with ASD who face difficulties in communicating their emotional stress and discomfort during medical or dental visits. | - |
| [94]/2024 | Sewn-in pockets (BioNomadix ECG) and ACC module; Polar H7; Mio Fuse | PPG; ACC; ECG | QRS; SD, mean; BPM | For all children, mean heart rate and peak heart rate were extracted from the SW for the low-level stress task and resting state periods. | Testing low-level stress through physiological signals | Forty-one children with ASD between 2–4 and 8–12 years |
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ACC | Accelerometer |
| ANS | Autonomic nervous system |
| BP | Blood pressure |
| BVP | Blood volume pulse |
| ECG | Electrocardiography |
| EDA | Electrodermal activity |
| EEG | Electroencephalogram |
| EMG | Electromyography |
| GSR | Galvanic skin response |
| HR | Heart rate |
| HF | High frequency |
| HRV | Heart rate variability |
| IBI | Interbeat interval |
| IMU | Inertial measurement unit |
| KNN | K-nearest neighbor |
| LF | Low frequency |
| MFCC | Mel-frequency cepstral coefficient |
| NN50 | Number of pairs of successive normal-to-normal (NN) |
| pNN50 | Percentage of successive RR intervals that differ by more than 50 ms |
| PPG | Photoplethysmography |
| PSS | Perceived stress scale |
| RR | Respiration sate |
| RMSSD | Root mean square of successive differences between normal heartbeats |
| SCL | Skin Conductance Level |
| SCR | Skin conductance response |
| SDNN | Standard deviation of NN intervals |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Richmond, VA, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Salazar, F.; Baird, G.; Chandler, S.; Tseng, E.; O’sullivan, T.; Howlin, P.; Pickles, A.; Simonoff, E. Co-occurring Psychiatric Disorders in Preschool and Elementary School-Aged Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- White, S.W.; Oswald, D.; Ollendick, T.; Scahill, L. Anxiety in children and adolescents with autism spectrum disorders. Clin. Psychol. Rev. 2009, 29, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Fitzpatrick, L.; Mazefsky, C.A.; Minshew, N.J.; Eack, S.M. The Relationship Between Stress and Social Functioning in Adults With Autism Spectrum Disorder and Without Intellectual Disability. Autism Res. 2015, 8, 164–173. [Google Scholar] [CrossRef]
- Kuhaneck, H.M.; Burroughs, T.; Wright, J.; Lemanczyk, T.; Darragh, A.R. A Qualitative Study of Coping in Mothers of Children with an Autism Spectrum Disorder. Phys. Occup. Ther. Pediatr. 2010, 30, 340–350. [Google Scholar] [CrossRef]
- Hirvikoski, T.; Blomqvist, M. High self-perceived stress and poor coping in intellectually able adults with autism spectrum disorder. Autism 2015, 19, 752–757. [Google Scholar] [CrossRef]
- Fuld, S. Autism Spectrum Disorder: The Impact of Stressful and Traumatic Life Events and Implications for Clinical Practice. Clin. Soc. Work. J. 2018, 46, 210–219. [Google Scholar] [CrossRef]
- Richdale, A.L.; Lawson, L.P.; Chalmers, A.; Uljarević, M.; Morris, E.M.J.; Arnold, S.R.C.; Trollor, J.N. Pathways to Anxiety and Depression in Autistic Adolescents and Adults. Depress. Anxiety 2023, 2023, 5575932. [Google Scholar] [CrossRef]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychol. Med. 2019, 49, 559–572. [Google Scholar] [CrossRef]
- McGowan, J.J.; Mcgregor, I.P. Investigation into Stress Triggers in Autistic Adults for the Development of Technological Self-Interventions. In Proceedings of the 25th International ACM SIGACCESS Conference on Computers and Accessibility, New York, NY, USA, 22–25 October 2023; ACM: New York, NY, USA, 2023; pp. 1–17. [Google Scholar]
- Northrup, C.M.; Lantz, J.; Hamlin, T. Wearable Stress Sensors for Children With Autism Spectrum Disorder With In Situ Alerts to Caregivers via a Mobile Phone. Iproceedings 2016, 2, e9. [Google Scholar] [CrossRef]
- Vincent, A.; Semmer, N.K.; Becker, C.; Beck, K.; Tschan, F.; Bobst, C.; Schuetz, P.; Marsch, S.; Hunziker, S. Does stress influence the performance of cardiopulmonary resuscitation? A narrative review of the literature. J. Crit. Care 2021, 63, 223–230. [Google Scholar] [CrossRef]
- Lumban Gaol, N.T. Teori Stres: Stimulus, Respons, dan Transaksional. bpsi 2016, 24, 1. [Google Scholar] [CrossRef]
- Ross, R.A.; Foster, S.L.; Ionescu, D.F. The Role of Chronic Stress in Anxious Depression. Chronic Stress 2017, 1, 2470547016689472. [Google Scholar] [CrossRef]
- Ovsiannikova, Y.; Pokhilko, D.; Kerdyvar, V.; Krasnokutsky, M.; Kosolapov, O. Peculiarities of the impact of stress on physical and psychological health. Multidiscip. Sci. J. 2024, 6, 2024ss0711. [Google Scholar] [CrossRef]
- Ollander, S.; Godin, C.; Campagne, A.; Charbonnier, S. A comparison of wearable and stationary sensors for stress detection. In Proceedings of the 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, 9–12 October 2016; pp. 004362–004366. [Google Scholar]
- Alhejaili, R.; Alomainy, A. The Use of Wearable Technology in Providing Assistive Solutions for Mental Well-Being. Sensors 2023, 23, 7378. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.T.; Wojcikowski, M.; Pankiewicz, B.; Lubinski, J.; Majchrowicz, J.; Majchrowicz, D.; Walasiewicz, A.; Kilinski, T.; Szczerska, M. Stress Monitoring System for Individuals With Autism Spectrum Disorders. IEEE Access 2020, 8, 228236–228244. [Google Scholar] [CrossRef]
- Yaacob, W.N.W.; Yaacob, L.H.; Muhamad, R.; Zulkifli, M.M. Behind the Scenes of Parents Nurturing a Child with Autism: A Qualitative Study in Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 8532. [Google Scholar] [CrossRef] [PubMed]
- Enea, V.; Rusu, D.M. Raising a Child with Autism Spectrum Disorder: A Systematic Review of the Literature Investigating Parenting Stress. J. Ment. Health Res. Intellect. Disabil. 2020, 13, 283–321. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Penning, R.; Hellhammer, J.; Verster, J.C.; Klaessens, J.H.G.M.; Olivier, B.; Kalkman, C.J. The effect of stress on core and peripheral body temperature in humans. Stress 2013, 16, 520–530. [Google Scholar] [CrossRef]
- Aktas, S.N.B.; Uluer, P.; Coskun, B.; Toprak, E.; Barkana, D.E.; Kose, H.; Zorcec, T.; Robins, B.; Landowska, A. Stress Detection of Children With ASD Using Physiological Signals. In Proceedings of the 2022 30th Signal Processing and Communications Applications Conference (SIU), Safranbolu, Turkey, 15–18 May 2022; pp. 1–4. [Google Scholar]
- Taj-Eldin, M.; Ryan, C.; O’Flynn, B.; Galvin, P. A Review of Wearable Solutions for Physiological and Emotional Monitoring for Use by People with Autism Spectrum Disorder and Their Caregivers. Sensors 2018, 18, 4271. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.C.; Bychkov, D.; Searson, P.C. Wearable Devices for Precision Medicine and Health State Monitoring. IEEE Trans. Biomed. Eng. 2019, 66, 1242–1258. [Google Scholar] [CrossRef]
- Koo, S.H.; Gaul, K.; Rivera, S.; Pan, T.; Fong, D. Wearable Technology Design for Autism Spectrum Disorders. Arch. Des. Res. 2018, 31, 37–55. [Google Scholar] [CrossRef]
- Simm, W.; Ferrario, M.A.; Gradinar, A.; Tavares Smith, M.; Forshaw, S.; Smith, I.; Whittle, J. Anxiety and Autism: Towards Personalized Digital Health. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; ACM: New York, NY, USA, 2016; pp. 1270–1281. [Google Scholar]
- Black, M.H.; Milbourn, B.; Chen, N.T.M.; McGarry, S.; Wali, F.; Ho, A.S.V.; Lee, M.; Bölte, S.; Falkmer, T.; Girdler, S. The use of wearable technology to measure and support abilities, disabilities and functional skills in autistic youth: A scoping review. Scand. J. Child Adolesc. Psychiatry Psychol. 2020, 8, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Kientz, J.A.; Hayes, G.R.; Goodwin, M.S.; Gelsomini, M.; Abowd, G.D. Sensor-Based and Wearable. In Interactive Technologies and Autism, 2nd ed.; Synthesis Lectures on Assistive, Rehabilitative, and Health-Preserving Technologies; Springer International Publishing: Cham, Switzerland, 2020; pp. 107–117. ISBN 978-3-031-00476-6. [Google Scholar]
- McCarthy, C.; Pradhan, N.; Redpath, C.; Adler, A. Validation of the Empatica E4 wristband. In Proceedings of the 2016 IEEE EMBS International Student Conference (ISC), Ottawa, ON, Canada, 29–31 May 2016; pp. 1–4. [Google Scholar]
- Cantin-Garside, K.D.; Kong, Z.; White, S.W.; Antezana, L.; Kim, S.; Nussbaum, M.A. Detecting and Classifying Self-injurious Behavior in Autism Spectrum Disorder Using Machine Learning Techniques. J. Autism Dev. Disord. 2020, 50, 4039–4052. [Google Scholar] [CrossRef] [PubMed]
- Thiel, K.J.; Dretsch, M.N. The Basics of the Stress Response: A Historical Context and Introduction. In The Handbook of Stress; Conrad, C.D., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 1–28. ISBN 978-1-4443-3023-6. [Google Scholar]
- Soylu, A.; Campbell, S.S. Physical and emotional stresses of technology on employees in the workplace. J. Employ. Couns. 2012, 49, 130–139. [Google Scholar] [CrossRef]
- Sivaratnam, C.S.; Newman, L.K.; Tonge, B.J.; Rinehart, N.J. Attachment and Emotion Processing in Children with Autism Spectrum Disorders: Neurobiological, Neuroendocrine, and Neurocognitive Considerations. Rev. J. Autism Dev. Disord. 2015, 2, 222–242. [Google Scholar] [CrossRef]
- Goodwin, M.S.; Mazefsky, C.A.; Ioannidis, S.; Erdogmus, D.; Siegel, M. Predicting aggression to others in youth with autism using a wearable biosensor. Autism Res. 2019, 12, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Suresh, L.; Yang, J.; Zhang, X.; Tan, S.C. Augmenting Sensor Performance with Machine Learning Towards Smart Wearable Sensing Electronic Systems. Adv. Intell. Syst. 2022, 4, 2100194. [Google Scholar] [CrossRef]
- Rochette, L.; Vergely, C. Hans Selye and the stress response: 80 years after his “letter” to the Editor of Nature. Annales De Cardiologie Et d’Angéiologie 2017, 66, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Corbett, B.A.; Schupp, C.W.; Simon, D.; Ryan, N.; Mendoza, S. Elevated cortisol during play is associated with age and social engagement in children with autism. Mol. Autism 2010, 1, 13. [Google Scholar] [CrossRef]
- Corbett, B.A.; Muscatello, R.A.; Kim, A.; Patel, K.; Vandekar, S. Developmental effects in physiological stress in early adolescents with and without autism spectrum disorder. Psychoneuroendocrinology 2021, 125, 105115. [Google Scholar] [CrossRef] [PubMed]
- Lindau, M.; Almkvist, O.; Mohammed, A.H. Effects of Stress on Learning and Memory. In Stress: Concepts, Cognition, Emotion, and Behavior; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–160. ISBN 978-0-12-800951-2. [Google Scholar]
- Tomchek, S.D.; Dunn, W. Sensory Processing in Children With and Without Autism: A Comparative Study Using the Short Sensory Profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef]
- Miller, L.J.; Schoen, S.A.; Mulligan, S.; Sullivan, J. Identification of Sensory Processing and Integration Symptom Clusters: A Preliminary Study. Occup. Ther. Int. 2017, 2017, 2876080. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.A.; Beidel, D.C. The Impact of Children with High-Functioning Autism on Parental Stress, Sibling Adjustment, and Family Functioning. Behav. Modif. 2009, 33, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Engel-Yeger, B.; Dunn, W. The Relationship between Sensory Processing Difficulties and Anxiety Level of Healthy Adults. Br. J. Occup. Ther. 2011, 74, 210–216. [Google Scholar] [CrossRef]
- Engel-Yeger, B.; Dunn, W. Exploring the Relationship between Affect and Sensory Processing Patterns in Adults. Br. J. Occup. Ther. 2011, 74, 456–464. [Google Scholar] [CrossRef]
- Ben-Avi, N.; Almagor, M.; Engel-Yeger, B. Sensory Processing Difficulties and Interpersonal Relationships in Adults: An Exploratory Study. Psychology 2012, 03, 70–77. [Google Scholar] [CrossRef]
- Cardon, G.; Bradley, M. Uncertainty, sensory processing, and stress in autistic children during the COVID-19 pandemic. Res. Autism Spectr. Disord. 2023, 106, 102202. [Google Scholar] [CrossRef] [PubMed]
- Pop-Jordanova, N.; Pop-Jordanov, J. Electrodermal Activity and Stress Assessment. PRILOZI 2020, 41, 5–15. [Google Scholar] [CrossRef]
- Francese, R.; Yang, X. Supporting autism spectrum disorder screening and intervention with machine learning and wearables: A systematic literature review. Complex. Intell. Syst. 2022, 8, 3659–3674. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Murugappan, M.; Yaacob, S. Detection Of Human Stress Using Short-Term ECG and HRV Signals. J. Mech. Med. Biol. 2013, 13, 1350038. [Google Scholar] [CrossRef]
- Lee, M.; Moon, J.; Cheon, D.; Lee, J.; Lee, K. Respiration signal based two layer stress recognition across non-verbal and verbal situations. In Proceedings of the 35th Annual ACM Symposium on Applied Computing, Brno, Czech Republic, 30 March–3 April 2020; ACM: New York, NY, USA, 2020; pp. 638–645. [Google Scholar]
- Riaz, R.; Naz, N.; Javed, M.; Naz, F.; Toor, H. Effect of Mental Workload Related Stress on Physiological Signals. In Proceedings of the 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Langkawi Island, Malaysia, 1–3 March 2021; pp. 339–344. [Google Scholar]
- Lecavalier, L.; Leone, S.; Wiltz, J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J. Intellect. Disabil. Res. 2006, 50, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Corbett, B.A.; Muscatello, R.A.; Blain, S.D. Impact of Sensory Sensitivity on Physiological Stress Response and Novel Peer Interaction in Children with and without Autism Spectrum Disorder. Front. Neurosci. 2016, 10, 278. [Google Scholar] [CrossRef]
- Yap, C.Y.; Ng, K.H.; Cheah, Y.; Lim, S.Y.; Price, J.; De Vries, M. App4Autism: An Integrated Assistive Technology with Heart Rate Monitoring for Children with Autism. In Advances in Visual Informatics; Badioze Zaman, H., Smeaton, A.F., Shih, T.K., Velastin, S., Terutoshi, T., Mohamad Ali, N., Ahmad, M.N., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2019; Volume 11870, pp. 498–512. ISBN 978-3-030-34031-5. [Google Scholar]
- Gunasekara, N.; Gaeta, G.; Levy, A.; Boot, E.; Tachtsidis, I. fNIRS neuroimaging in olfactory research: A systematic literature review. Front. Behav. Neurosci. 2022, 16, 1040719. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Matsuda, S.; Suzuki, K. A Smart Clothe for ECG Monitoring of Children with Autism Spectrum Disorders. In Computers Helping People with Special Needs; Miesenberger, K., Bühler, C., Penaz, P., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2016; Volume 9758, pp. 555–562. ISBN 978-3-319-41263-4. [Google Scholar]
- Pourmohammadi, S.; Maleki, A. Stress detection using ECG and EMG signals: A comprehensive study. Comput. Methods Programs Biomed. 2020, 193, 105482. [Google Scholar] [CrossRef] [PubMed]
- Chaspari, T.; Tsiartas, A.; Duker, L.I.S.; Cermak, S.A.; Narayanan, S.S. EDA-gram: Designing electrodermal activity fingerprints for visualization and feature extraction. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 403–406. [Google Scholar]
- Ahmad, N.; Ghazilla, R.A.R.; Khairi, N.M.; Kasi, V. Reviews on Various Inertial Measurement Unit (IMU) Sensor Applications. Int. J. Signal Process. Syst. 2013, 1, 256–262. [Google Scholar] [CrossRef]
- Ghamari, M. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Wei, X. Monitoring heart and respiratory rates at radial artery based on PPG. Optik 2013, 124, 3954–3956. [Google Scholar] [CrossRef]
- Bolanos, M.; Nazeran, H.; Haltiwanger, E. Comparison of Heart Rate Variability Signal Features Derived from Electrocardiography and Photoplethysmography in Healthy Individuals. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 4289–4294. [Google Scholar]
- Mohan, P.M.; Nagarajan, V.; Das, S.R. Stress measurement from wearable photoplethysmographic sensor using heart rate variability data. In Proceedings of the 2016 International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, Tamilnadu, India, 6–8 April 2016; pp. 1141–1144. [Google Scholar]
- Nguyen, J.; Cardy, R.E.; Anagnostou, E.; Brian, J.; Kushki, A. Examining the effect of a wearable, anxiety detection technology on improving the awareness of anxiety signs in autism spectrum disorder: A pilot randomized controlled trial. Mol. Autism 2021, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, M.V.; Zapirain, B.G.; Zorrilla, A.M. A Stress Sensor Based on Galvanic Skin Response (GSR) Controlled by ZigBee. Sensors 2012, 12, 6075–6101. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Lagopoulos, J.; Sachdev, P.S.; Ivanovski, B.; Shnier, R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005, 7, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Kipli, K.; Latip, A.A.A.; Lias, K.; Bateni, N.; Yusoff, S.M.; Tajudin, N.M.A.; Jalil, M.A.; Ray, K.; Shamim Kaiser, M.; Mahmud, M. GSR Signals Features Extraction for Emotion Recognition. In Proceedings of Trends in Electronics and Health Informatics; Kaiser, M.S., Bandyopadhyay, A., Ray, K., Singh, R., Nagar, V., Eds.; Lecture Notes in Networks and Systems; Springer Nature: Singapore, 2022; Volume 376, pp. 329–338. ISBN 9789811688256. [Google Scholar]
- Shukla, J.; Barreda-Angeles, M.; Oliver, J.; Nandi, G.C.; Puig, D. Feature Extraction and Selection for Emotion Recognition from Electrodermal Activity. IEEE Trans. Affect. Comput. 2021, 12, 857–869. [Google Scholar] [CrossRef]
- Noelke, C.; McGovern, M.; Corsi, D.J.; Jimenez, M.P.; Stern, A.; Wing, I.S.; Berkman, L. Increasing ambient temperature reduces emotional well-being. Environ. Res. 2016, 151, 124–129. [Google Scholar] [CrossRef]
- Ishaque, S.; Khan, N.; Krishnan, S. Physiological Signal Analysis and Stress Classification from VR Simulations Using Decision Tree Methods. Bioengineering 2023, 10, 766. [Google Scholar] [CrossRef]
- Bianchi, W.; Dugas, A.F.; Hsieh, Y.-H.; Saheed, M.; Hill, P.; Lindauer, C.; Terzis, A.; Rothman, R.E. Revitalizing a Vital Sign: Improving Detection of Tachypnea at Primary Triage. Ann. Emerg. Med. 2013, 61, 37–43. [Google Scholar] [CrossRef]
- Jarchi, D.; Rodgers, S.J.; Tarassenko, L.; Clifton, D.A. Accelerometry-Based Estimation of Respiratory Rate for Post-Intensive Care Patient Monitoring. IEEE Sens. J. 2018, 18, 4981–4989. [Google Scholar] [CrossRef]
- Mogera, U.; Sagade, A.A.; George, S.J.; Kulkarni, G.U. Ultrafast response humidity sensor using supramolecular nanofibre and its application in monitoring breath humidity and flow. Sci. Rep. 2014, 4, 4103. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.G.; Ahmed, M.M.; Walsleben, J.A.; Rapoport, D.M. Detection of Respiratory Events During NPSG: Nasal Cannula/Pressure Sensor Versus Thermistor. Sleep 1997, 20, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- De Santos Sierra, A.; Avila, C.S.; Guerra Casanova, J.; Bailador Del Pozo, G.; Jara Vera, V. Two Stress Detection Schemes Based on Physiological Signals for Real-Time Applications. In Proceedings of the 2010 Sixth International Conference on Intelligent Information Hiding and Multimedia Signal Processing, Darmstadt, Germany, 15–17 October 2010; pp. 364–367. [Google Scholar]
- Zhang, T.; Huang, Z.; Li, R.; Zhao, J.; Jin, Q. Multimodal Fusion Strategies for Physiological-emotion Analysis. In Proceedings of the 2nd on Multimodal Sentiment Analysis Challenge, Virtual Event, China, 20–24 October 2021; ACM: New York, NY, USA, 2021; pp. 43–50. [Google Scholar]
- Kim, K.H.; Bang, S.W.; Kim, S.R. Emotion recognition system using short-term monitoring of physiological signals. Med. Biol. Eng. Comput. 2004, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Gay, V.; Leijdekkers, P. Design of emotion-aware mobile apps for autistic children. Health Technol. 2014, 4, 21–26. [Google Scholar] [CrossRef]
- Planalp, E.M.; Van Hulle, C.; Gagne, J.R.; Goldsmith, H.H. The Infant Version of the Laboratory Temperament Assessment Battery (Lab-TAB): Measurement Properties and Implications for Concepts of Temperament. Front. Psychol. 2017, 8, 846. [Google Scholar] [CrossRef]
- Pittella, E.; Piuzzi, E.; Rizzuto, E.; Del Prete, Z.; Fioriello, F.; Maugeri, A.; Sogos, C. Wearable heart rate monitoring as stress response indicator in children with neurodevelopmental disorder. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–5. [Google Scholar]
- Nuske, H.J.; Goodwin, M.S.; Kushleyeva, Y.; Forsyth, D.; Pennington, J.W.; Masino, A.J.; Finkel, E.; Bhattacharya, A.; Tan, J.; Tai, H.; et al. Evaluating commercially available wireless cardiovascular monitors for measuring and transmitting real-time physiological responses in children with autism. Autism Res. 2022, 15, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Shah, S.; Hughes, C.E. In-the-Wild Affect Analysis of Children with ASD Using Heart Rate. Sensors 2023, 23, 6572. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, M.; Romano, A.; Hoffman, H.; Lotan, M.; Tesler, R. Development and validation of a system for the prediction of challenging behaviors of people with autism spectrum disorder based on a smart wearable shirt: A mixed-methods design. Front. Behav. Neurosci. 2022, 16, 948184. [Google Scholar] [CrossRef] [PubMed]
- Pollreisz, D.; TaheriNejad, N. A simple algorithm for emotion recognition, using physiological signals of a smart watch. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Republic of Korea, 11–15 July 2017; pp. 2353–2356. [Google Scholar]
- Pfeiffer, B.; Stein Duker, L.; Murphy, A.; Shui, C. Effectiveness of Noise-Attenuating Headphones on Physiological Responses for Children With Autism Spectrum Disorders. Front. Integr. Neurosci. 2019, 13, 65. [Google Scholar] [CrossRef]
- Torrado, J.C.; Gomez, J.; Montoro, G. Emotional Self-Regulation of Individuals with Autism Spectrum Disorders: Smartwatches for Monitoring and Interaction. Sensors 2017, 17, 1359. [Google Scholar] [CrossRef]
- Bagirathan, A.; Selvaraj, J.; Gurusamy, A.; Das, H. Recognition of positive and negative valence states in children with autism spectrum disorder (ASD) using discrete wavelet transform (DWT) analysis of electrocardiogram signals (ECG). J. Ambient. Intell. Human. Comput. 2021, 12, 405–416. [Google Scholar] [CrossRef]
- Umair, M.; Chalabianloo, N.; Sas, C.; Ersoy, C. HRV and Stress: A Mixed-Methods Approach for Comparison of Wearable Heart Rate Sensors for Biofeedback. IEEE Access 2021, 9, 14005–14024. [Google Scholar] [CrossRef]
- Dutheil, F.; Chambres, P.; Hufnagel, C.; Auxiette, C.; Chausse, P.; Ghozi, R.; Paugam, G.; Boudet, G.; Khalfa, N.; Naughton, G.; et al. ‘Do Well B.’: Design Of WELL Being monitoring systems. A study protocol for the application in autism. BMJ Open 2015, 5, e007716. [Google Scholar] [CrossRef] [PubMed]
- Krupa, N.; Anantharam, K.; Sanker, M.; Datta, S.; Sagar, J.V. Recognition of emotions in autistic children using physiological signals. Health Technol. 2016, 6, 137–147. [Google Scholar] [CrossRef]
- Masino, A.J.; Forsyth, D.; Nuske, H.; Herrington, J.; Pennington, J.; Kushleyeva, Y.; Bonafide, C.P. m-Health and Autism: Recognizing Stress and Anxiety with Machine Learning and Wearables Data. In Proceedings of the 2019 IEEE 32nd International Symposium on Computer-Based Medical Systems (CBMS), Cordoba, Spain, 5–7 June 2019; pp. 714–719. [Google Scholar]
- Finkel, E.; Sah, E.; Spaulding, M.; Herrington, J.D.; Tomczuk, L.; Masino, A.; Pang, X.; Bhattacharya, A.; Hedley, D.; Kushleyeva, Y.; et al. Physiological and communicative emotional disconcordance in children on the autism spectrum. J. Neurodev. Disord. 2024, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Licht, C.M.M.; De Geus, E.J.C.; Van Dyck, R.; Penninx, B.W.J.H. Association between Anxiety Disorders and Heart Rate Variability in The Netherlands Study of Depression and Anxiety (NESDA). Psychosom. Med. 2009, 71, 508–518. [Google Scholar] [CrossRef]
- Jiménez-Mijangos, L.P.; Rodríguez-Arce, J.; Martínez-Méndez, R.; Reyes-Lagos, J.J. Advances and challenges in the detection of academic stress and anxiety in the classroom: A literature review and recommendations. Educ. Inf. Technol. 2023, 28, 3637–3666. [Google Scholar] [CrossRef]
- Nuske, H.J.; Finkel, E.; Hedley, D.; Parma, V.; Tomczuk, L.; Pellecchia, M.; Herrington, J.; Marcus, S.C.; Mandell, D.S.; Dissanayake, C. Heart rate increase predicts challenging behavior episodes in preschoolers with autism. Stress 2019, 22, 303–311. [Google Scholar] [CrossRef]
- Kildal, E.S.M.; Quintana, D.S.; Szabo, A.; Tronstad, C.; Andreassen, O.; Nærland, T.; Hassel, B. Heart rate monitoring to detect acute pain in non-verbal patients: A study protocol for a randomized controlled clinical trial. BMC Psychiatry 2023, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.T.; Wójcikowski, M.; Listewnik, P.; Pankiewicz, B.; Majchrowicz, D.; Jędrzejewska-Szczerska, M. Support for Employees with ASD in the Workplace Using a Bluetooth Skin Resistance Sensor—A Preliminary Study. Sensors 2018, 18, 3530. [Google Scholar] [CrossRef]
- Elyazidi, S.; Escamilla-Ambrosio, P.J.; Gallegos-Garcia, G.; Rodríguez-Mota, A. Accelerometer Based Body Area Network Sensor Authentication. In Smart Technology; Torres Guerrero, F., Lozoya-Santos, J., Gonzalez Mendivil, E., Neira-Tovar, L., Ramírez Flores, P.G., Martin-Gutierrez, J., Eds.; Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer International Publishing: Cham, Switzerland, 2018; Volume 213, pp. 151–164. ISBN 978-3-319-73322-7. [Google Scholar]
- Di Palma, S.; Tonacci, A.; Narzisi, A.; Domenici, C.; Pioggia, G.; Muratori, F.; Billeci, L. Monitoring of autonomic response to sociocognitive tasks during treatment in children with Autism Spectrum Disorders by wearable technologies: A feasibility study. Comput. Biol. Med. 2017, 85, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Samson, C.; Koh, A. Stress Monitoring and Recent Advancements in Wearable Biosensors. Front. Bioeng. Biotechnol. 2020, 8, 1037. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Jones, R.M.; Wilder-Smith, O.; Wormwood, J.B.; Akcakaya, M.; Quigley, K.S.; Lord, C.; Goodwin, M.S. Simple, Transparent, and Flexible Automated Quality Assessment Procedures for Ambulatory Electrodermal Activity Data. IEEE Trans. Biomed. Eng. 2018, 65, 1460–1467. [Google Scholar] [CrossRef]
- Deng, L.; Rattadilok, P.; Xiong, R. A Machine Learning-Based Monitoring System for Attention and Stress Detection for Children with Autism Spectrum Disorders. In Proceedings of the 2021 International Conference on Intelligent Medicine and Health, Macau, China, 13–15 August 2021; ACM: New York, NY, USA, 2021; pp. 23–29. [Google Scholar]
- Villarroel, M.J.; Villarroel, C.H. Wireless smart environment in Ambient Assisted Living for people that suffer from cognitive disabilities. Ingeniare Rev. Chil. Ing. 2014, 22, 158–168. [Google Scholar] [CrossRef]
- Baker, J.K.; Fenning, R.M.; Erath, S.A.; Baucom, B.R.; Moffitt, J.; Howland, M.A. Sympathetic Under-Arousal and Externalizing Behavior Problems in Children with Autism Spectrum Disorder. J. Abnorm. Child. Psychol. 2018, 46, 895–906. [Google Scholar] [CrossRef] [PubMed]
- López-Florit, L.; García-Cuesta, E.; Gracia-Expósito, L.; García-García, G.; Iandolo, G. Physiological Reactions in the Therapist and Turn-Taking during Online Psychotherapy with Children and Adolescents with Autism Spectrum Disorder. Brain Sci. 2021, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Ortolan-Soto, A.; Castro-García, J.A.; Molina-Cantero, A.J.; Merino-Monge, M.; Gómez-González, I.M. Smart Bracelet for Emotional Enhancement in Children with Autism Spectrum Disorder. Eng. Proc. 2021, 7, 7. [Google Scholar] [CrossRef]
- Sayis, B.; Ramirez, R.; Pares, N. Mixed reality or LEGO game play? Fostering social interaction in children with Autism. Virtual Real. 2022, 26, 771–787. [Google Scholar] [CrossRef]
- Coulter, H.; Donnelly, M.; Mallett, J.; Kernohan, W.G. Heart Rate Variability Biofeedback to Treat Anxiety in Young People With Autism Spectrum Disorder: Findings From a Home-Based Pilot Study. JMIR Form. Res. 2022, 6, e37994. [Google Scholar] [CrossRef]
- Yu, Z.; Sherpa, M.T.; Iadarola, S.; Shamlian, K.; Daley, S.; Levine, G.; Bajorski, P.; Zheng, Z. Understanding Stress in Children with ASD and Their Caregivers in Daily Life: A Feasibility Study Using Mobile Devices. In Proceedings of the 25th International Conference on Mobile Human-Computer Interaction, Athens, Greece, 26–29 September 2023; ACM: New York, NY, USA, 2023; pp. 1–7. [Google Scholar]
- Salice, F.; Maggi, M.; Varesi, A.; Masciadri, A.; Comai, S. SMED: SMart Chair for Emotion Detection. In Computers Helping People with Special Needs; Miesenberger, K., Peňáz, P., Kobayashi, M., Eds.; Lecture Notes in Computer Science; Springer Nature: Cham, Switzerland, 2024; Volume 14751, pp. 201–207. ISBN 978-3-031-62848-1. [Google Scholar]
- Kumari, P.; Mathew, L.; Syal, P. Increasing trend of wearables and multimodal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef]
- Motti, V.G.; Caine, K. Human Factors Considerations in the Design of Wearable Devices. Proc. Human. Factors Ergon. Soc. Annu. Meet. 2014, 58, 1820–1824. [Google Scholar] [CrossRef]
- Francés-Morcillo, L.; Morer-Camo, P.; Rodríguez-Ferradas, M.I.; Cazón-Martín, A. Wearable Design Requirements Identification and Evaluation. Sensors 2020, 20, 2599. [Google Scholar] [CrossRef]
- Koumpouros, Y.; Kafazis, T. Wearables and mobile technologies in Autism Spectrum Disorder interventions: A systematic literature review. Res. Autism Spectr. Disord. 2019, 66, 101405. [Google Scholar] [CrossRef]
- Benssassi, E.M.; Gomez, J.-C.; Boyd, L.E.; Hayes, G.R.; Ye, J. Wearable Assistive Technologies for Autism: Opportunities and Challenges. IEEE Pervasive Comput. 2018, 17, 11–21. [Google Scholar] [CrossRef]
- Ramasamy, S.; Balan, A. Wearable sensors for ECG measurement: A review. Sens. Rev. 2018, 38, 412–419. [Google Scholar] [CrossRef]
- Indikawati, F.I.; Winiarti, S. Stress Detection from Multimodal Wearable Sensor Data. IOP Conf. Ser. Mater. Sci. Eng. 2020, 771, 012028. [Google Scholar] [CrossRef]
- Coşkun, B.; Ay, S.; Erol Barkana, D.; Bostanci, H.; Uzun, İ.; Oktay, A.B.; Tuncel, B.; Tarakci, D. A physiological signal database of children with different special needs for stress recognition. Sci. Data 2023, 10, 382. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, S.; Cubillos, C.; Alfaro, R.; Romo, A.; García, M.; Moreira, F. Wearable Solutions Using Physiological Signals for Stress Monitoring on Individuals with Autism Spectrum Disorder (ASD): A Systematic Literature Review. Sensors 2024, 24, 8137. https://doi.org/10.3390/s24248137
Cano S, Cubillos C, Alfaro R, Romo A, García M, Moreira F. Wearable Solutions Using Physiological Signals for Stress Monitoring on Individuals with Autism Spectrum Disorder (ASD): A Systematic Literature Review. Sensors. 2024; 24(24):8137. https://doi.org/10.3390/s24248137
Chicago/Turabian StyleCano, Sandra, Claudio Cubillos, Rodrigo Alfaro, Andrés Romo, Matías García, and Fernando Moreira. 2024. "Wearable Solutions Using Physiological Signals for Stress Monitoring on Individuals with Autism Spectrum Disorder (ASD): A Systematic Literature Review" Sensors 24, no. 24: 8137. https://doi.org/10.3390/s24248137
APA StyleCano, S., Cubillos, C., Alfaro, R., Romo, A., García, M., & Moreira, F. (2024). Wearable Solutions Using Physiological Signals for Stress Monitoring on Individuals with Autism Spectrum Disorder (ASD): A Systematic Literature Review. Sensors, 24(24), 8137. https://doi.org/10.3390/s24248137








