Improvement of an Edge-IoT Architecture Driven by Artificial Intelligence for Smart-Health Chronic Disease Management
Abstract
1. Introduction
- Propose a decentralized approach that allows integrated care and long-term management for chronic non-communicable diseases to improve in real-time capabilities;
- Establish an architecture based on the principles of IoT, edge computing, and AI to develop an ecosystem that allows pre-processing at the edge of the network and data analysis in the cloud;
- Build a wearable edge-IoT device that captures and non-invasively pre-processes the parameters of oxygen concentration, pulse rate, skin temperature, and bio-impedance;
- Propose and establish a baseline of AI regression models to compare the non-invasive glucose predictive findings with a capillary blood glucose measurement.
2. Related Works
3. Materials and Methods
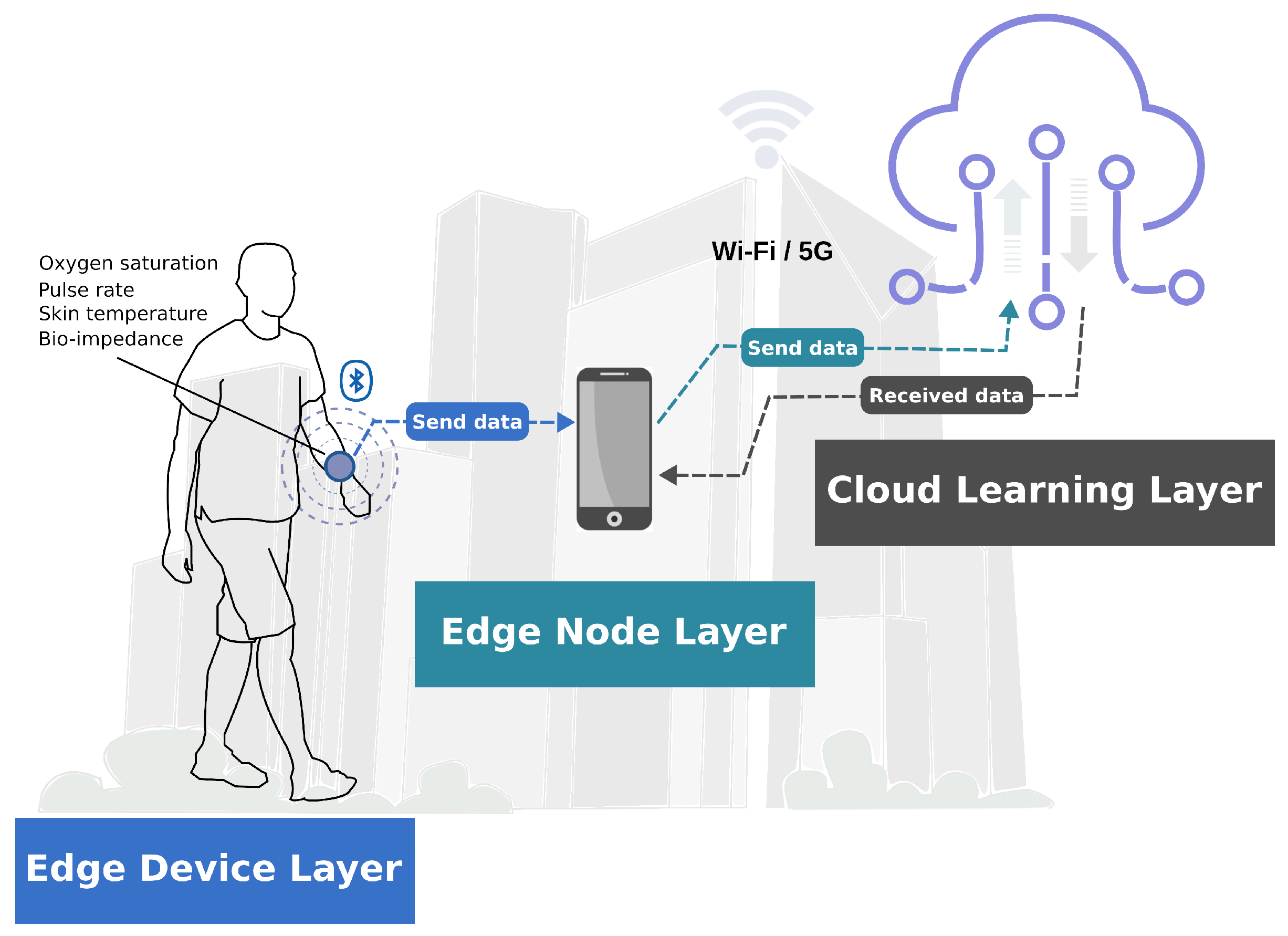
3.1. Edge Device Layer
3.2. Edge Node Layer
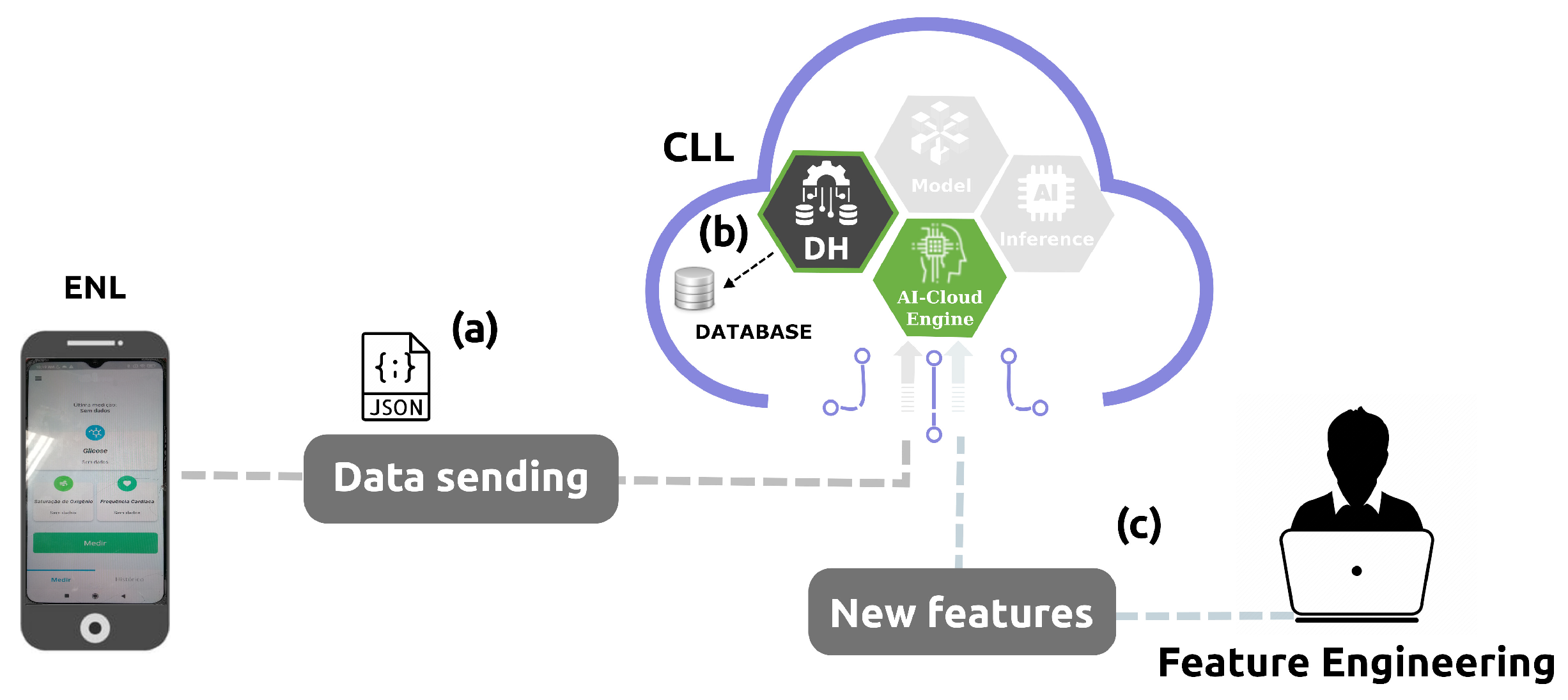
3.3. Cloud Learning Layer
3.3.1. AI Algorithms Baseline
3.3.2. AI Algorithm Evaluation
4. Results
4.1. Data Source
4.2. Feature Scaling and Selection
4.3. Performance of the Best AI Algorithms
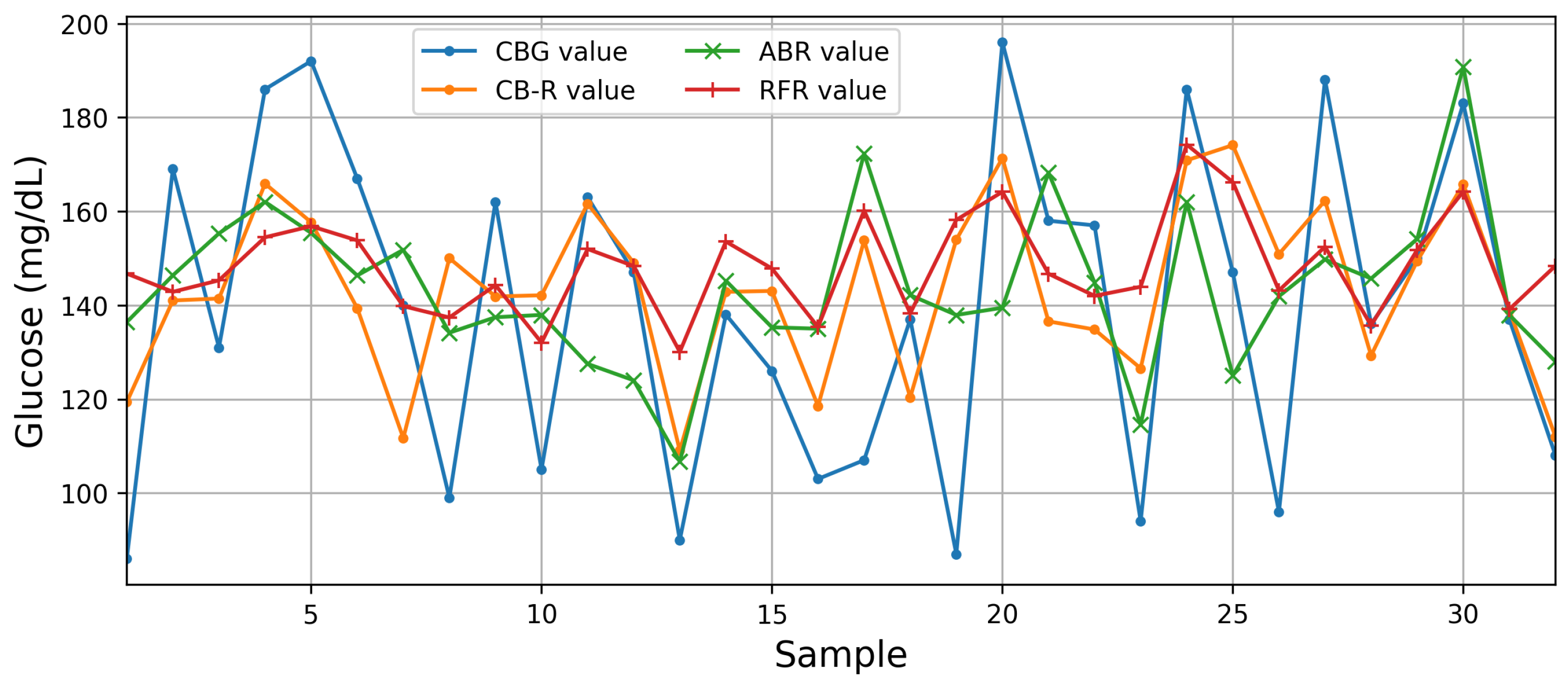
4.4. Performance of the Proposed Architecture
5. Discussions and Conclusions
- Ref. [29] proposes a system consisting of five components: patient data collection, the generation of patient primary reports, hospital patient care, pharmacist patient care, and diagnostics.
- Ref. [30] only proposes a software architecture for an IoT system for a healthcare application in a smart city context.
- Ref. [31], in the context of applications of edge-AI and healthcare in smart cities, is the only study to implement a quantitative study case proposing a cloud-based s-health monitoring model.
- Ref. [33] does not mention works for diabetes prediction and/or management.
- Ref. [32] is a literature review that discusses in detail how AI-powered IoT and wireless sensor networks are applied in the healthcare sector. The research is a baseline study to understand the role of the IoT in smart cities.
- Ref. [34] presents an overview of IoT-based healthcare. Three works concern diabetes. The first concerns type 2 diabetes detection and monitoring. The smart sensors used are heart rate, blood pressure, activity, and blood glucose sensors. The fog/edge device used is a laptop computer. The AI method adopted is a hybrid deep-learning model for type 2 diabetes prediction, and results are not presented. The second one performs the real-time remote monitoring of diabetes patients. The smart sensors used are ECG, blood glucose, and movement sensors. The fog/edge device used is a smartphone. The AI method adopted is a decision tree for diabetes risk prediction classification. Only the cloud was used for centralized storage and fog devices to perform data collection, pre-processing, feature extraction, data compression, security, and analytics. The third one performs stress prediction and classification for monitoring heart rate and diabetes. The smart sensors use various wearable body sensors and embedded devices. The fog/edge device is a computer. The AI method adopted is a DL classifier for predicting early symptoms of type 2 diabetes.
- Ref. [35] proposes a framework model with three main layers, including the sensor layer, edge layer, and cloud layer. In the summary of AI-based edge-assisted smart healthcare solutions, none were found for diabetes application cases. This work does not include a study case.
- Ref. [37] discusses the current state-of-the-art smart healthcare systems. In the summary of works on smart health monitoring systems based on wearable devices, none were found for diabetes. The communication media/protocol only describes works using Wi-Fi, HTTP, MQTT, and Bluetooth hc-06. None were found to use 5G. In the summary of works on smart health monitoring systems based on smartphones, there exists only one for glucose levels through video analysis. In the summary of works on diabetes detection frameworks in IoT health environments, eight were found. In all works, the AI task was classification. In the works, no user prototype was shown, detailed data collection procedures were not mentioned, a glucose sensor and Arduino could not be operated at the same time, the latency was comparatively high, and real-time cases were not found.
- Ref. [50] introduces an edge-of-things computing framework for secure and smart healthcare surveillance services and a case study with electrocardiogram ECG data. The architecture comprises four main entities: community members (includes patients and wired/wireless sensors); a medical services gateway and an IoT gateway to collect data, perform local processing, encrypt health data, and perform transmission to the cloud; a cloud-enabled database to store encrypted healthcare data; and an abnormality detection model to analyze the encrypted data. Thus, the proposed system architecture shows an edge computing layer that is internally composed of two edge devices: the medical services gateway and the IoT gateway. The biosignal data are captured via sensors and transmitted over a 5G network to the medical services gateway. After that, this edge device again transmits the data over a 5G network to the IoT gateway, which finally sends the data to the cloud.
- Increased speed and lower latency eliminate the need for back-and-forth communication with the cloud. An edge-AI-IoT saves data-processing and bandwidth costs by running on-device, reducing power consumption and prolonging battery life.
- Federated learning enables edge-AI-IoT to share end-user information without the need to take their data to central cloud storage and to access the appropriate set of data locally and experience learning activities without disruptions.
- AI models are on the device, and there is no need to send sensitive data to the cloud for processing. Personal data remain on personal devices.
- AI models are trained on user inputs and are thereby optimized for individual users. Furthermore, AI functions are available offline and can be accessed at any time.
- Federated learning allows edge-AI-IoT devices to learn a shared prediction model collaboratively. Since prediction takes place on the edge device, real-time prediction is feasible. Thus, the prediction method works even though there is no internet connection because the models are stored on the device.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Bank Group. Demographic Trends and Urbanization; World Bank Group: Washington, DC, USA, 2021. [Google Scholar]
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; WHO Press: Geneva, Switzerland, 2013. [Google Scholar]
- Bennett, J.E.; Stevens, G.A.; Mathers, C.D.; Bonita, R.; Rehm, J.; Kruk, M.E.; Riley, L.M.; Dain, K.; Kengne, A.P.; Chalkidou, K.; et al. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef] [PubMed]
- WHO. Noncommunicable Diseases: Progress Monitor 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Vansimaeys, C.; Benamar, L.; Balagué, C. Digital health and management of chronic disease: A multimodal technologies typology. Int. J. Health Plan. Manag. 2021, 36, 1107–1125. [Google Scholar] [CrossRef] [PubMed]
- Stübinger, J.; Schneider, L. Understanding Smart City—A Data-Driven Literature Review. Sustainability 2020, 12, 8460. [Google Scholar] [CrossRef]
- Buttazzoni, A.; Veenhof, M.; Minaker, L. Smart City and High-Tech Urban Interventions Targeting Human Health: An Equity-Focused Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2325. [Google Scholar] [CrossRef] [PubMed]
- Ristvej, J.; Lacinák, M.; Ondrejka, R. On Smart City and Safe City Concepts. Mob. Netw. Appl. 2020, 25, 836–845. [Google Scholar] [CrossRef]
- Mohammadzadeh, Z.; Saeidnia, H.R.; Lotfata, A.; Hassanzadeh, M.; Ghiasi, N. Smart city healthcare delivery innovations: A systematic review of essential technologies and indicators for developing nations. BMC Health Serv. Res. 2023, 23, 1180. [Google Scholar] [CrossRef]
- Pérez-Roman, E.; Alvarado, M.; Barrett, M. Personalizing Healthcare in Smart Cities. In Smart Cities in Application: Healthcare, Policy, and Innovation; McClellan, S., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–18. [Google Scholar] [CrossRef]
- Nastjuk, I.; Trang, S.; Papageorgiou, E.I. Smart cities and smart governance models for future cities. Electron. Mark. 2022, 32, 1917–1924. [Google Scholar] [CrossRef]
- Solanas, A.; Patsakis, C.; Conti, M.; Vlachos, I.S.; Ramos, V.; Falcone, F.; Postolache, O.; Perez-martinez, P.A.; Pietro, R.D.; Perrea, D.N.; et al. Smart health: A context-aware health paradigm within smart cities. IEEE Commun. Mag. 2014, 52, 74–81. [Google Scholar] [CrossRef]
- Ben Dhaou, I.; Ebrahimi, M.; Ben Ammar, M.; Bouattour, G.; Kanoun, O. Edge Devices for Internet of Medical Things: Technologies, Techniques, and Implementation. Electronics 2021, 10, 2104. [Google Scholar] [CrossRef]
- Vishnu, S.; Ramson, S.J.; Jegan, R. Internet of Medical Things (IoMT)—An overview. In Proceedings of the 2020 5th International Conference on Devices, Circuits and Systems (ICDCS), Coimbatore, India, 5–6 March 2020; pp. 101–104. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef]
- Smuck, M.; Odonkor, C.A.; Wilt, J.K.; Schmidt, N.; Swiernik, M.A. The emerging clinical role of wearables: Factors for successful implementation in healthcare. NPJ Digit. Med. 2021, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- El-Gayar, O.F.; Ambati, L.S.; Nawar, N. Wearables, Artificial intelligence, and the Future of Healthcare. In AI and Big Data’s Potential for Disruptive Innovation; Strydom, M., Buckley, S., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 104–129. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910. [Google Scholar] [CrossRef] [PubMed]
- Casselman, J.; Onopa, N.; Khansa, L. Wearable healthcare: Lessons from the past and a peek into the future. Telemat. Inform. 2017, 34, 1011–1023. [Google Scholar] [CrossRef]
- Mostafa, H.; Kerstin, T.; Regina, S. Wearable Devices in Medical Internet of Things: Scientific Research and Commercially Available Devices. Healthc. Inform. Res. 2017, 23, 4–15. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in healthcare wearable devices. NPJ Flex. Electron. 2021, 5, 14. [Google Scholar] [CrossRef]
- Geetanjali; Malviya, R.; Awasthi, R.; Sharma, P.K.; Kala, N.; Kumar, V.; Yadav, S.K. Applications of Artificial Intelligence, Blockchain, and Internet-of-Things inManagement of Chronic Disease. In Cognitive Intelligence and Big Data in Healthcare; John Wiley Sons, Ltd.: Hoboken, NJ, USA, 2022; Chapter 13; pp. 349–365. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, L.; Gao, F.; He, J.S.; Zhao, J.H.; Fang, Y.; Yang, M.J.; An, Y.; Ye, W.Z.; Dong, Z. Integration of Artificial Intelligence, Blockchain, and Wearable Technology for Chronic Disease Management: A New Paradigm in Smart Healthcare. Curr. Med. Sci. 2021, 41, 1123–1133. [Google Scholar] [CrossRef]
- Kristoffersson, A.; Lindén, M. Wearable Sensors for Monitoring and Preventing Noncommunicable Diseases: A Systematic Review. Information 2020, 11, 521. [Google Scholar] [CrossRef]
- Shabong Lyngdoh, L.E. Applications of Internet of things in Non-Communicable Disease Prevention and Management: A Review. Indian J. Public Health Res. Dev. 2018, 9, 55–60. [Google Scholar] [CrossRef]
- Cisco. Cisco Global Cloud Index: Forecast and Methodology, 2016–2021. 2018. Available online: https://virtualization.network/Resources/Whitepapers/0b75cf2e-0c53-4891-918e-b542a5d364c5_white-paper-c11-738085.pdf (accessed on 3 September 2024).
- Tian, S.; Yang, W.; Grange, J.M.L.; Wang, P.; Huang, W.; Ye, Z. Smart healthcare: Making medical care more intelligent. Glob. Health J. 2019, 3, 62–65. [Google Scholar] [CrossRef]
- Sanghavi, J. Review of Smart Healthcare Systems and Applications for Smart Cities. In Proceedings of the ICCCE 2019; Kumar, A., Mozar, S., Eds.; Springer: Singapore, 2020; pp. 325–331. [Google Scholar]
- Gera, S.; Mridul, M.; Sharma, S. IoT based Automated Health Care Monitoring System for Smart City. In Proceedings of the 2021 5th International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 8–10 April 2021; pp. 364–368. [Google Scholar] [CrossRef]
- Gavrilović, N.; Mishra, A. Software architecture of the internet of things (IoT) for smart city, healthcare and agriculture: Analysis and improvement directions. J. Ambient. Intell. Humaniz. Comput. 2021, 12, 1315–1336. [Google Scholar] [CrossRef]
- Kamruzzaman, M.M. New Opportunities, Challenges, and Applications of Edge-AI for Connected Healthcare in Smart Cities. In Proceedings of the 2021 IEEE Globecom Workshops (GC Wkshps), Madrid, Spain, 7–11 December 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Ghazal, T.M.; Hasan, M.K.; Alshurideh, M.T.; Alzoubi, H.M.; Ahmad, M.; Akbar, S.S.; Al Kurdi, B.; Akour, I.A. IoT for Smart Cities: Machine Learning Approaches in Smart Healthcare—A Review. Future Internet 2021, 13, 218. [Google Scholar] [CrossRef]
- Alshehri, F.; Muhammad, G. A Comprehensive Survey of the Internet of Things (IoT) and AI-Based Smart Healthcare. IEEE Access 2021, 9, 3660–3678. [Google Scholar] [CrossRef]
- Amin, S.U.; Hossain, M.S. Edge Intelligence and Internet of Things in Healthcare: A Survey. IEEE Access 2021, 9, 45–59. [Google Scholar] [CrossRef]
- Hayyolalam, V.; Aloqaily, M.; Ozkasap, O.; Guizani, M. Edge Intelligence for Empowering IoT-Based Healthcare Systems. IEEE Wirel. Commun. 2021, 28, 6–14. [Google Scholar] [CrossRef]
- Makina, H.; Ben Letaifa, A. Bringing intelligence to Edge/Fog in Internet of Things-based healthcare applications: Machine learning/deep learning-based use cases. Int. J. Commun. Syst. 2023, 36, e5484. [Google Scholar] [CrossRef]
- Nasr, M.; Islam, M.M.; Shehata, S.; Karray, F.; Quintana, Y. Smart Healthcare in the Age of AI: Recent Advances, Challenges, and Future Prospects. IEEE Access 2021, 9, 145248–145270. [Google Scholar] [CrossRef]
- Greco, L.; Percannella, G.; Ritrovato, P.; Tortorella, F.; Vento, M. Trends in IoT based solutions for health care: Moving AI to the edge. Pattern Recognit. Lett. 2020, 135, 346–353. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Mishra, S.; González-Briones, A. Integration of Machine Learning and IoT in Healthcare Domain. In Hybrid Artificial Intelligence and IoT in Healthcare; Kumar Bhoi, A., Mallick, P.K., Narayana Mohanty, M., Albuquerque, V.H.C.d., Eds.; Springer: Singapore, 2021; pp. 223–244. [Google Scholar] [CrossRef]
- Makroum, M.A.; Adda, M.; Bouzouane, A.; Ibrahim, H. Machine Learning and Smart Devices for Diabetes Management: Systematic Review. Sensors 2022, 22, 1843. [Google Scholar] [CrossRef]
- Hartmann, M.; Hashmi, U.S.; Imran, A. Edge computing in smart health care systems: Review, challenges, and research directions. Trans. Emerg. Telecommun. Technol. 2022, 33, e3710. [Google Scholar] [CrossRef]
- Oniani, S.; Marques, G.; Barnovi, S.; Pires, I.M.; Bhoi, A.K. Artificial Intelligence for Internet of Things and Enhanced Medical Systems. In Bio-Inspired Neurocomputing; Bhoi, A.K., Mallick, P.K., Liu, C.M., Balas, V.E., Eds.; Springer: Singapore, 2021; pp. 43–59. [Google Scholar]
- WHO. World Health Statistics 2014; WHO Press: Geneva, Switzerland, 2014. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF Press: Brussels, Belgium, 2021. [Google Scholar]
- Naresh, M.; Nagaraju, V.S.; Kollem, S.; Kumar, J.; Peddakrishna, S. Non-invasive glucose prediction and classification using NIR technology with machine learning. Heliyon 2024, 10, e28720. [Google Scholar] [CrossRef]
- Rajeswari, S.V.K.R.; Vijayakumar, P. Development of sensor system and data analytic framework for non-invasive blood glucose prediction. Sci. Rep. 2024, 14, 9206. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Joshi, A.M.; Mohanty, S.P. iGLU 4.0: Intelligent Non-invasive Glucose Measurement and Its Control with Physiological Parameters. SN Comput. Sci. 2024, 5, 368. [Google Scholar] [CrossRef]
- Geng, Z.; Tang, F.; Ding, Y.; Li, S.; Wang, X. Noninvasive Continuous Glucose Monitoring Using a Multisensor-Based Glucometer and Time Series Analysis. Sci. Rep. 2017, 7, 12650. [Google Scholar] [CrossRef] [PubMed]
- Sanai, F.; Sahid, A.S.; Huvanandana, J.; Spoa, S.; Boyle, L.H.; Hribar, J.; Wang, D.T.Y.; Kwan, B.; Colagiuri, S.; Cox, S.J.; et al. Evaluation of a Continuous Blood Glucose Monitor: A Novel and Non-Invasive Wearable Using Bioimpedance Technology. J. Diabetes Sci. Technol. 2023, 17, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Alabdulatif, A.; Khalil, I.; Yi, X.; Guizani, M. Secure Edge of Things for Smart Healthcare Surveillance Framework. IEEE Access 2019, 7, 31010–31021. [Google Scholar] [CrossRef]
- Lodderstedt, T.; Bradley, J.; Labunets, A.; Fett, D. OAuth 2.0 Security Best Current Practice. Internet-Draft Draft-Ietf-Oauth-Security-Topics-29. Internet Engineering Task Force. 2024. Available online: https://datatracker.ietf.org/doc/html/draft-ietf-oauth-security-topics (accessed on 15 September 2024).



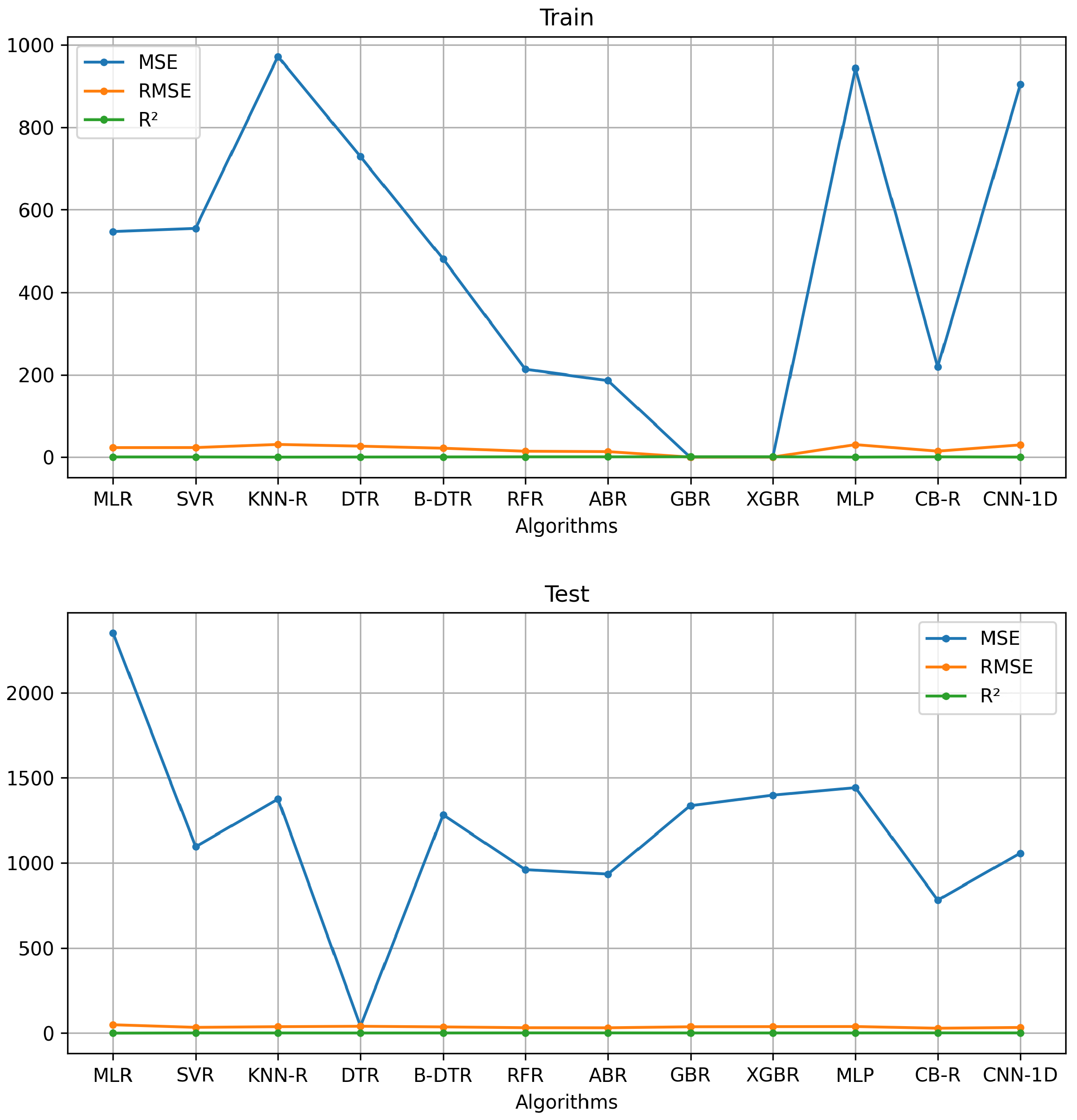


| Model | Features | MSE | RMSE | ||||
|---|---|---|---|---|---|---|---|
| Training | Test | Training | Test | Training | Test | ||
| MLR | 35 | 547.54 | 2353.33 | 23.40 | 48.51 | 0.53 | –1.06 |
| SVR | 20 | 555.28 | 1095.70 | 23.56 | 33.10 | 0.52 | –0.08 |
| KNN-R | 15 | 971.31 | 1375.59 | 31.17 | 37.09 | 0.16 | –0.20 |
| DTR | 15 | 728.72 | 39.60 | 26.99 | 39.59 | 0.37 | –0.37 |
| B-DTR | 15 | 481.01 | 1282.91 | 21.93 | 35.81 | 0.59 | –0.26 |
| RFR*** | 20 | 212.95 | 960.34 | 14.59 | 30.98 | 0.82 | 0.16 |
| ABR** | 15 | 186.19 | 934.03 | 13.65 | 30.56 | 0.84 | 0.18 |
| GBR | 15 | 0.03 | 1336.85 | 0.17 | 36.56 | 0.99 | –0.17 |
| XGBR | 20 | 0.11 | 1399.40 | 0.34 | 37.41 | 0.99 | –0.22 |
| MLP | 15 | 942.75 | 1443.72 | 30.70 | 37.99 | 0.18 | –0.26 |
| CB-R* | 15 | 218.91 | 782.30 | 14.80 | 27.97 | 0.81 | 0.31 |
| CNN-1D | 15 | 903.86 | 1057.93 | 30.06 | 32.53 | 0.22 | 0.09 |
| Reference | Technology | AI Algorithm | Performace Metric |
|---|---|---|---|
| [45] | NIR | FFNN | MAPE (%)—1.94 MAE (mg/dL)—2.49 MSE (mg/dL)—9.16 RMSE—3.02 —0.99 |
| [46] | NIR | RR | Fingertip Wrist MAE—0.15, 0.66 MSE—0.2287, 0.006 —0.9902, 0.9996 |
| [47] | NIR | FFNN | MARD—12.50% —0.97 |
| [48] | Impedance spectroscopy | MATS | RMSE—14.61 MAPE—0.11 |
| [49] | Bioimpedance | GB | MARD—17.9% |
| Our work | Bioimpedance | CB-R | MSE—218.91/782.30 RMSE—14.80/27.87 —0.81/0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Castañeda, W.A.; Bertemes Filho, P. Improvement of an Edge-IoT Architecture Driven by Artificial Intelligence for Smart-Health Chronic Disease Management. Sensors 2024, 24, 7965. https://doi.org/10.3390/s24247965
Cruz Castañeda WA, Bertemes Filho P. Improvement of an Edge-IoT Architecture Driven by Artificial Intelligence for Smart-Health Chronic Disease Management. Sensors. 2024; 24(24):7965. https://doi.org/10.3390/s24247965
Chicago/Turabian StyleCruz Castañeda, William Alberto, and Pedro Bertemes Filho. 2024. "Improvement of an Edge-IoT Architecture Driven by Artificial Intelligence for Smart-Health Chronic Disease Management" Sensors 24, no. 24: 7965. https://doi.org/10.3390/s24247965
APA StyleCruz Castañeda, W. A., & Bertemes Filho, P. (2024). Improvement of an Edge-IoT Architecture Driven by Artificial Intelligence for Smart-Health Chronic Disease Management. Sensors, 24(24), 7965. https://doi.org/10.3390/s24247965








