Assessment of Cardiac Autonomic Function by Short-Term Sensor-Based and Long-Term Heart Rate Variability Analyses in Individuals with Spinal Cord Injury After Long-Term Table Tennis Training
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
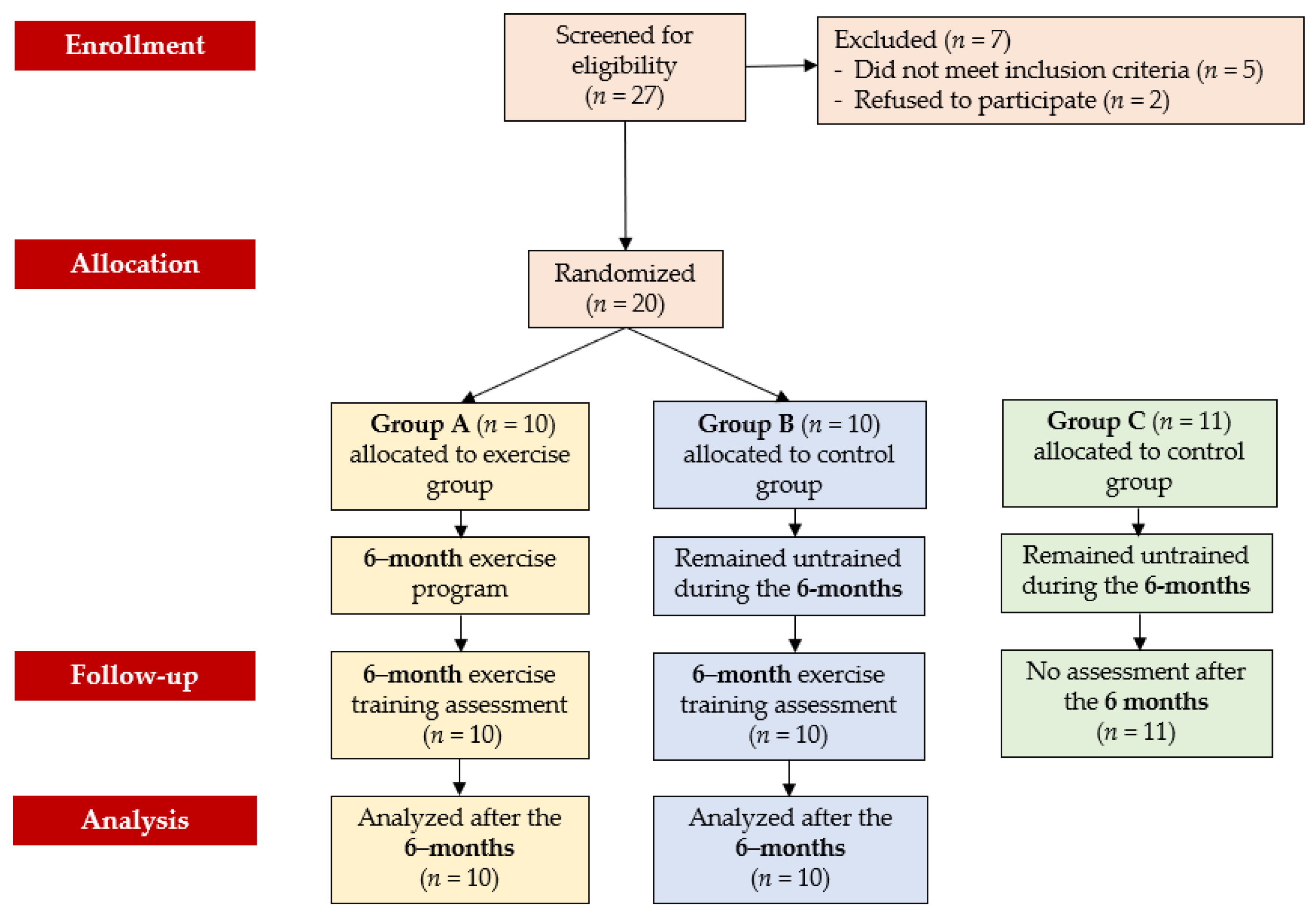
2.2. Study Design
2.3. 24-h Holter Monitoring
- The standard deviation of R-R (the time intervals between two successive heartbeats) intervals (SDNN).
- The standard deviation of R-R intervals calculated every 5 min (SDANN).
- The square root of the mean sum of the squares of the differences between consecutive intervals R-R (rMSSD).
- The percentage of successive RR intervals higher than 50 ms (pNN50).
- The total frequency power (TP).
- The very low-frequency power (VLF) (<0.003–0.04 Hz).
- The low-frequency power (LF) (0.04–0.15 Hz).
- The high-frequency power (HF) (0.15–0.4 Hz).
- The frequency ratio (LF/HF)
- VLF and LF reflect the heart’s sympathetic activity,
- pNN50, rMSSD, and HF represent the heart’s parasympathetic activity,
- SDNN, SDANN, and the LF/HF ratio have been used to index sympathovagal activity [17].
2.4. POLAR S810i HRV Resting and Exercise Monitoring
2.5. Dynamometric Testing
2.6. Arm Cycle Ergometric Exercise Testing
2.7. Table Tennis Training Program
2.8. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. 24 h Holter Monitoring
3.3. POLAR S810i HRV Resting and Exercise Monitoring
3.4. Dynamometric Testing Results
3.5. Arm Cycle Ergometric Fatigue Testing Results
3.6. Linear Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myers, J.; Lee, M.; Kiratli, J. Cardiovascular Disease in Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2007, 86, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wecht, J.M.; Harel, N.Y.; Guest, J.; Kirshblum, S.C.; Forrest, G.F.; Bloom, O.; Ovechkin, A.V.; Harkema, S. Cardiovascular Autonomic Dysfunction in Spinal Cord Injury: Epidemiology, Diagnosis, and Management. Semin. Neurol. 2020, 40, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Wecht, J.M.; Weir, J.P.; DeMeersman, R.E.; Schilero, G.J.; Handrakis, J.P.; LaFountaine, M.F.; Cirnigliaro, C.M.; Kirshblum, S.C.; Bauman, W.A. Cold face test in persons with spinal cord injury: Age versus inactivity. Clin. Auton. Res. 2009, 19, 221–229. [Google Scholar] [CrossRef]
- West, C.R.; Mills, P.; Krassioukov, A.V. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: A meta-analysis. Spinal Cord. 2012, 50, 484–492. [Google Scholar] [CrossRef]
- Krassioukov, A.; Claydon, V.E. The clinical problems in cardiovascular control following spinal cord injury: An overview. Prog. Brain Res. 2006, 152, 223–229. [Google Scholar]
- Hicks, A.L.; Martin, K.A.; Ditor, D.S.; Latimer, A.E.; Craven, C.; Bugaresti, J.; McCartney, N. Long-term exercise training in persons with spinal cord injury: Effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003, 41, 34–43. [Google Scholar] [CrossRef]
- Figoni, S.F. Spinal Cord Disabilities: Paraplegia and Tetraplegia. In ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities; Durstine, J.L., Moore, G.E., Eds.; Human Kinetics: Champaign, IL, USA, 2003; pp. 247–253. [Google Scholar]
- Loan, M.D.V.; McCluer, S.; Loftin, J.M.; Boileau, R.A. Comparison of physiological responses to maximal arm exercise among able-bodied, paraplegics and quadriplegics. Spinal Cord. 1987, 5, 397–405. [Google Scholar] [CrossRef]
- Hicks, A.L.; Martin Ginis, K.A.; Pelletier, C.A.; Ditor, D.S.; Foulon, B.; Wolfe, D.L. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: A systematic review. Spinal Cord. 2011, 49, 1103–1127. [Google Scholar] [CrossRef]
- Buchholz, A.C.; McGillivray, C.F.; Pencharz, P.B. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am. J. Clin. Nutr. 2003, 77, 371–378. [Google Scholar] [CrossRef]
- Stone, N.J. Focus on lifestyle change and the metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2004, 33, 493–508. [Google Scholar] [CrossRef]
- Dallmeijer, A.J.; Hopman, M.T.E.; van As, H.H.J.; van der Woude, L.H.V. Physical capacity and physical strain in persons with tetraplegia; The role of sport activity. Spinal Cord. 1996, 34, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Midha, M.; Schmitt, J.K.; Sclater, M. Exercise effect with the wheelchair aerobic fitness trainer on conditioning and metabolic function in disabled persons: A pilot study. Arch. Phys. Med. Rehabil. 1999, 80, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Tordi, N.; Dugue, B.; Klupzinski, D.; Rasseneur, L.; Rouillon, J.; Lonsdorfer, J. Interval training program on a wheelchair ergometer for paraplegic subjects. Spinal Cord. 2001, 19, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Levins, S.M.; Redenbach, D.M.; Dyck, I. Individual and societal influences on participation in physical activity following spinal cord injury: A qualitative study. Phys. Ther. 2004, 84, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Devillard, X.; Rimaud, D.; Roche, F.; Calmels, P. Effects of training programs for spinal cord injury. Ann. Readapt. Med. Phys. 2007, 50, 490–498. [Google Scholar] [CrossRef]
- Sztajzel, J. Heart rate variability: A noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med. Wkly. 2004, 134, 514–522. [Google Scholar]
- Amaral, J.F.; Mancini, M.; Novo Júnior, J.M. Comparison of three hand dynamometers in relation to the accuracy and precision of the measurements. Braz. J. Phys. Ther. 2012, 16, 216–224. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Rogers, S. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar]
- Lasko-McCarthey, P.; Davis, J.A. Protocol dependency of VO2max during arm cycle ergometry in males with quadriplegia. Med. Sci. Sports Exerc. 1991, 23, 1097–1101. [Google Scholar] [CrossRef]
- DiCarlo, S.E. Effect of Arm Ergometry Training on Wheelchair Propulsion Endurance of Individuals with Quadriplegia. Phys. Ther. 1988, 68, 40–44. [Google Scholar] [CrossRef]
- Krassioukov, A.; West, C. The Role of Autonomic Function on Sport Performance in Athletes with Spinal Cord Injury. PM&R 2014, 6, S58–S65. [Google Scholar]
- Shields, R.K. Muscular, Skeletal, and Neural Adaptations Following Spinal Cord Injury. J. Orthop. Sports Phys. Ther. 2002, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Bellantoni, A.; Krassioukov, A. Cardiovascular Function in Individuals with Incomplete Spinal Cord Injury: A Systematic Review. Top. Spinal Cord Inj. Rehabil. 2013, 19, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Calaresu, F.R.; Yardley, C.P. Medullary Basal Sympathetic Tone. Annu. Rev. Physiol. 1988, 50, 511–524. [Google Scholar] [CrossRef]
- Vivodtzev, I.; Taylor, J.A. Cardiac, Autonomic, and Cardiometabolic Impact of Exercise Training in Spinal Cord Injury. J. Cardiopulm Rehabil. Prev. 2021, 41, 6–12. [Google Scholar] [CrossRef]
- Solinsky, R.; Kirshblum, S.C.; Burns, S.P. Exploring detailed characteristics of autonomic dysreflexia. J. Spinal Cord Med. 2018, 41, 549–555. [Google Scholar] [CrossRef]
- Haensel, A.; Mills, P.J.; Nelesen, R.A.; Ziegler, M.G.; Dimsdale, J.E. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 2008, 33, 1305–1312. [Google Scholar] [CrossRef]
- Lewis, J.E.; Nash, M.S.; Hamm, L.F.; Martins, S.C.; Groah, S.L. The Relationship Between Perceived Exertion and Physiologic Indicators of Stress During Graded Arm Exercise in Persons with Spinal Cord Injuries. Arch. Phys. Med. Rehabil. 2007, 88, 1205–1211. [Google Scholar] [CrossRef]
- Lasko-McCarthey, P.; Davis, J.A. Effect of work rate increment on peak oxygen uptake during wheelchair ergometry in men with quadriplegia. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 349–353. [Google Scholar] [CrossRef]
- Takahashi, M.; Matsukawa, K.; Nakamoto, T.; Tsuchimochi, H.; Sakaguchi, A.; Kawaguchi, K.; Onari, K. Control of heart rate variability by cardiac parasympathetic nerve activity during voluntary static exercise in humans with tetraplegia. J. Appl. Physiol. 2007, 103, 1669–1677. [Google Scholar] [CrossRef]
- Mathias, C.J.; Christensen, N.J.; Corbett, J.L.; Frankel, H.L.; Goodwins, T.J.; Peart, W.S. Plasma Catecholamines, Plasma Renin Activity and Plasma Aldosterone in Tetraplegic Man, Horizontal and Tilted. Clin. Sci. 1975, 49, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Huonker, M.; Barturen, J.M.; Stahl, F.; Schmidt-Trucksass, A.; Konig, D.; Grathwohl, D.; Lehmann, M.; Keul, J. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J. Appl. Physiol. 1998, 85, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Dela, F.; Mohr, T.; Jensen, C.M.; Haahr, H.L.; Secher, N.H.; Biering-Sørensen, F.; Kjaer, M. Cardiovascular Control During Exercise. Circulation 2003, 107, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Vanderlei, L.C.M.; Silva, R.A.; Pastre, C.M.; Azevedo, F.M.; Godoy, M.F. Comparison of the Polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz. J. Med. Biol. Res. 2008, 41, 854–859. [Google Scholar] [CrossRef]
- Nunan, D.; Donovan, G.; Jakovljevic, D.; Hodges, L.; Sandercock, G.; Brodie, D. Validity and Reliability of Short-Term Heart-Rate Variability from the Polar S810. Med. Sci. Sports Exerc. 2009, 41, 243–250. [Google Scholar] [CrossRef]
- Gamelin, F.X.; Baquet, G.; Berthoin, S.; Bosquet, L. Validity of the Polar S810 to Measure R-R Intervals in Children. Int. J. Sports Med. 2008, 29, 134–138. [Google Scholar] [CrossRef]
- Vanderbilt, D.; Bushley, T.; Young, R.; Frank, D.A. Acute Posttraumatic Stress Symptoms Among Urban Mothers with Newborns in the Neonatal Intensive Care Unit: A Preliminary Study. J. Dev. Behav. Pediatr. 2009, 30, 50–56. [Google Scholar] [CrossRef]
- Romero Ávila, J.L.; Brizuela Costa, G.A. Efecto Inmediato del Ejercicio Sobre la Variabilidad de la Frecuencia Cardíaca en Personas con Tetraplejia; Departamento de Educación Física y Deportiva, Universitat de València: Valencia, Spain, 2011; Available online: http://hdl.handle.net/10550/25857 (accessed on 8 September 2024).
- Wecht, J.; Marsico, R.; Weir, J.; Spungen, A.; Bauman, W.; De Meersman, R. Autonomic Recovery from Peak Arm Exercise in Fit and Unfit Individuals with Paraplegia. Med. Sci. Sports Exerc. 2006, 38, 1223–1228. [Google Scholar] [CrossRef]
- Agiovlasitis, S.; Heffernan, K.S.; Jae, S.Y.; Ranadive, S.M.; Lee, M.; Mojtahedi, M.C.; Fernhall, B. Effects of Paraplegia on Cardiac Autonomic Regulation During Static Exercise. Am. J. Phys. Med. Rehabil. 2010, 89, 817–823. [Google Scholar] [CrossRef]
- Whiting, R.B.; Dreisinger, T.E.; Dalton, R.B.; Londeree, B.R. Improved physical fitness and work capacity in quadriplegics by wheelchair exercise. J. Cardiol. Rehabil. 1983, 3, 251–255. [Google Scholar]
- DiCarlo, S.E. Improved Cardiopulmonary Status after a Two-Month Program of Graded Arm Exercise in a Patient with C6 Quadriplegia. Phys. Ther. 1982, 62, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Kondrič, M.; Zagatto, A.M.; Sekulić, D. The physiological demands of table tennis: A review. J. Sports Sci. Med. 2013, 12, 362–370. [Google Scholar] [PubMed]
- Picabea, J.; Cámara, J.; Nakamura, F.; Yanci, J. Comparison of Heart Rate Variability Before and After a Table Tennis Match. J. Hum. Kinet. 2021, 77, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Richards, H.; Kondric, M. A Multidisciplinary Investigation of the Effects of Competitive State Anxiety on Serve Kinematics in Table Tennis. J. Hum. Kinet. 2017, 55, 83–95. [Google Scholar] [CrossRef]
- Katsikadelis, M.; Pilianidis, T.; Mantzouranis, N.; Fatouros, I.; Agelousis, N. Heart rate variability of young table tennis players with the use of the multiball training. J. Biol. Exerc. 2014, 10, 25–35. [Google Scholar] [CrossRef]

| Participants with SCI | Healthy Inividuals | A vs. B | A vs. C | ||
|---|---|---|---|---|---|
| Group A (nA = 10) | Group B (nB = 10) | Group C (nC = 11) | p-Value | p-Value | |
| Age (years) | 37.71 ± 4.38 | 38.30 ± 4.02 | 39.71 ± 5.87 | p = 0.184 | p = 0.150 |
| Height (cm) | 1.75 ± 0.05 | 1.76 ± 0.05 | 1.77 ± 0.05 | p = 0.213 | p = 0.402 |
| Weight (Kg) | 80.20 ± 7.88 | 80.70 ± 5.81 | 84.14 ± 6.54 | p = 0.573 | p = 0.134 |
| BMI (Kg/m2) | 26.12 ± 3.32 | 25.97 ± 2.50 | 26.45 ± 3.28 | p = 0.347 | p = 0.568 |
| SCI duration (years) | 12.80 ± 2.09 | 12.90 ± 3.28 | - | p = 0.423 | - |
| Group A | Group B | Group C | Group A vs. B | Group A vs. C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6-Months | p-Value | Baseline | After 6-Months | p-Value | Baseline | Pre | Post | Pre | Post | |
| HR (bpm) | 57.06 ± 8.27 | 59.6 ± 6.20 | p = 0.118 | 56.11 ± 9.33 | 56.03 ± 9.15 | p = 0.352 | 75.11 ± 13.70 | p = 0.817 | p = 0.261 | p = 0.075 | p = 0.001 *** |
| TP (ms2) | 1939.33 ± 367.51 | 2048.89 ± 451.70 | p = 0.394 | 2000.95 ± 111.17 | 2000.61 ± 162.08 | p = 0.990 | 4100.56 ± 714.09 | p = 0.768 | p = 0.754 | p < 0.001 *** | p < 0.001 *** |
| SDNN (ms) | 110.79 ± 8.89 | 126.26 ± 11.76 | p = 0.007 * | 109.55 ± 9.57 | 109.68 ± 9.38 | p = 0.858 | 149.99 ± 7.16 | p = 0.800 | p = 0.001 ** | p < 0.001 *** | p < 0.001 *** |
| SDANN (ms) | 100.00 ± 7.04 | 108.35 ± 8.68 | p = 0.007 * | 100.90 ± 8.56 | 100.61 ± 9.05 | p = 0.619 | 130.33 ± 5.29 | p = 0.871 | p = 0.033 ** | p < 0.001 *** | p < 0.001 *** |
| rMSSD (ms) | 42.01 ± 5.28 | 41.23 ± 3.14 | p = 0.530 | 41.64 ± 4.75 | 41.31 ± 4.97 | p = 0.457 | 40.80 ± 1.54 | p = 0.979 | p = 0.966 | p = 0.496 | p = 0.703 |
| pNN50 (%) | 8.24 ± 0.81 | 8.68 ± 1.21 | p = 0.064 | 8.23 ± 0.89 | 8.23 ± 0.95 | p = 1.000 | 11.88 ± 0.99 | p = 0.740 | p = 0.370 | p < 0.001 *** | p < 0.001 *** |
| VLF (ms2) | 816.02 ± 130.63 | 873.93 ± 118.72 | p = 0.042 * | 811.51 ± 118.51 | 816.01 ± 113.01 | p = 0.481 | 1784.29 ± 402.65 | p = 0.939 | p = 0.001 ** | p < 0.001 *** | p < 0.001 *** |
| LF (ms2) | 717.31 ± 169.81 | 792.73 ± 136.15 | p = 0.009 * | 719.18 ± 82.23 | 720.43 ± 83.47 | p = 0.742 | 1362.31 ± 255.73 | p = 0.975 | p = 0.046 ** | p < 0.001 *** | p < 0.001 *** |
| LF (n.u.) | 72.23 ± 10.29 | 77.16 ± 10.38 | p = 0.002 * | 71.54 ± 5.66 | 71.92 ± 5.57 | p = 0.443 | 73.71 ± 8.36 | p = 0.855 | p = 0.023 ** | p = 0.728 | p = 0.424 |
| HF (ms2) | 358.02 ± 132.59 | 318.91 ± 133.01 | p = 0.390 | 355.85 ± 42.66 | 354.14 ± 49.69 | p = 0.698 | 1102.07 ± 137.43 | p = 0.961 | p = 0.443 | p < 0.001 *** | p < 0.001 *** |
| HF (n.u.) | 38.42 ± 11.87 | 37.71 ± 10.90 | p = 0.698 | 37.52 ± 3.48 | 36.98 ± 5.11 | p = 0.425 | 43.73 ± 3.72 | p = 0.821 | p = 0.850 | p = 0.194 | p = 0.116 |
| LF/HF | 2.12 ± 0.50 | 2.27 ± 0.89 | p = 0.335 | 1.91 ± 0.21 | 2.05 ± 0.40 | p = 0.306 | 1.72 ± 0.24 | p = 0.316 | p = 0.355 | p = 0.164 | p = 0.060 |
| Group A | Group B | Group C | Group A vs. B | Group A vs. C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6-Months | p-Value | Baseline | After 6-Months | p-Value | Baseline | Pre | Post | Pre | Post | |
| Resting HRV | |||||||||||
| rMSSD (ms) | 49.94 ± 3.91 | 48.93 ± 3.00 | p = 0.651 | 49.28 ± 5.31 | 49.04 ± 5.02 | p = 0.373 | 40.25 ± 5.62 | p = 0.588 | p = 0.480 | p = 0.817 | p = 0.671 |
| pNN50 (%) | 31.63 ± 3.19 | 30.67 ± 4.54 | p = 0.298 | 30.81 ± 2.29 | 30.55 ± 2.47 | p = 0.653 | 37.14 ± 6.80 | p = 0.893 | p = 0.479 | p = 0.041 *** | p = 0.003 *** |
| LF (n.u.) | 52.77 ± 5.62 | 57.09 ± 5.41 | p < 0.001 * | 52.27 ± 3.82 | 51.91 ± 3.92 | p = 0.174 | 126.42 ± 13.87 | p = 0.818 | p = 0.118 | p < 0.001 *** | p < 0.001 *** |
| HF (n.u.) | 37.64 ± 2.62 | 34.69 ± 2.93 | p = 0.008 * | 36.87 ± 2.51 | 35.61 ± 2.12 | p = 0.061 | 89.01 ± 11.75 | p = 0.794 | p = 0.886 | p < 0.001 *** | p < 0.001 *** |
| LF/HF | 1.47 ± 0.21 | 1.65 ± 0.19 | p = 0.002 * | 1.42 ± 0.15 | 1.46 ± 0.11 | p = 0.284 | 1.42 ± 0.23 | p = 0.977 | p = 0.002 ** | p = 0.062 | p = 0.002 *** |
| Exercise HRV | |||||||||||
| rMSSD (ms) | 14.57 ± 1.29 | 12.85 ± 1.52 | p = 0.002 * | 14.77 ± 1.03 | 14.68 ± 1.27 | p = 0.494 | 11.56 ± 1.67 | p = 0.964 | p = 0.735 | p = 0.035 *** | p = 0.024 *** |
| pNN50 (%) | 0.79 ± 0.18 | 0.58 ± 0.17 | p = 0.037 * | 0.78 ± 0.17 | 0.77 ± 0.09 | p = 0.511 | 0.56 ± 0.11 | p = 0.237 | p = 0.035 ** | p = 0.001 *** | p < 0.001 *** |
| LF (n.u.) | 60.49 ± 2.43 | 65.57 ± 4.05 | p = 0.001 * | 61.07 ± 4.03 | 61.75 ± 2.80 | p = 0.137 | 187.44 ± 23.07 | p = 0.139 | p = 0.029 ** | p < 0.001 *** | p < 0.001 *** |
| HF (n.u.) | 26.22 ± 2.74 | 18.03 ± 2.52 | p < 0.001 * | 26.00 ± 2.62 | 25.92 ± 2.71 | p = 0.343 | 36.99 ± 5.48 | p = 0.853 | p = 0.001 ** | p < 0.001 *** | p < 0.001 *** |
| LF/HF | 2.41 ± 0.32 | 3.61 ± 0.80 | p < 0.001 * | 2.36 ± 0.24 | 2.39 ± 0.22 | p = 0.100 | 5.06 ± 0.71 | p = 0.710 | p = 0.026 ** | p < 0.001 *** | p < 0.001 *** |
| Group A | Group B | Group C | Group A vs. B | Group A vs. C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6-Months | p-Value | Baseline | After 6-Months | p-Value | Baseline | Pre | Post | Pre | Post | |
| Right hand (kg) | 29.80 ± 9.01 | 38.10 ± 11.13 | p < 0.001 * | 28.68 ± 4.43 | 28.30 ± 4.47 | p = 0.169 | 77.90 ± 9.64 | p = 0.729 | p = 0.019 ** | p < 0.001 *** | p < 0.001 *** |
| Left hand (kg) | 20.85 ± 7.95 | 23.15 ± 9.16 | p = 0.084 | 20.10 ± 7.51 | 20.20 ± 7.49 | p = 0.343 | 66.10 ± 11.87 | p = 0.831 | p = 0.441 | p < 0.001 *** | p < 0.001 *** |
| Right fingers (kg) | 15.60 ± 3.71 | 16.00 ± 3.16 | p = 0.711 | 15.25 ± 1.96 | 14.80 ± 2.39 | p = 0.330 | 25.70 ± 4.32 | p = 0.795 | p = 0.351 | p < 0.001 *** | p < 0.001 *** |
| Left fingers (kg) | 14.00 ± 1.69 | 14.90 ± 1.37 | p = 0.068 | 13.70 ± 1.76 | 13.10 ± 2.23 | p = 0.081 | 23.20 ± 2.52 | p = 0.703 | p = 0.043 ** | p < 0.001 *** | p < 0.001 *** |
| Group A | Group B | Group C | Group A vs. B | Group A vs. C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 6-Months | p-Value | Baseline | After 6-Months | p-Value | Baseline | Pre | Post | Pre | Post | |
| HRrest (bpm) | 54.00 ± 10.86 | 54.50 ± 11.21 | p = 0.863 | 54.40 ± 5.60 | 54.90 ± 4.72 | p = 0.537 | 70.8 ± 10.30 | p = 0.919 | p = 0.918 | p = 0.002 *** | p = 0.003 *** |
| Exercise time (min) | 1.84 ± 3.32 | 3.32 ± 0.87 | p < 0.001 * | 1.83 ± 0.42 | 1.84 ± 0.39 | p = 0.936 | 5.25 ± 2.27 | p = 0.964 | p < 0.001 ** | p < 0.001 *** | p = 0.022 *** |
| Maximum load (watt) | 6.50 ± 2.41 | 11.00 ± 2.10 | p < 0.001 * | 6.50 ± 2.41 | 6.90 ± 2.02 | p = 0.674 | 14.00 ± 4.59 | p = 1.000 | p < 0.001 ** | p < 0.001 *** | p = 0.077 |
| HRmax (bpm) | 101.80 ± 12.81 | 106.70 ± 7.97 | p = 0.235 | 100.70 ± 11.12 | 101.50 ± 9.44 | p = 0.837 | 146.30 ± 17.58 | p = 0.840 | p = 0.200 | p < 0.001 *** | p < 0.001 *** |
| Borg’s scale score | 15.30 ± 0.67 | 15.90 ± 1.10 | p = 0.193 | 15.00 ± 0.94 | 14.80 ± 1.26 | p = 0.104 | 15.60 ± 1.17 | p = 0.424 | p = 0.125 | p = 0.492 | p = 0.563 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogiatzi, G.; Michou, V.; Malliaropoulos, N.; Tsimaras, V.; Deligiannis, A.; Kouidi, E. Assessment of Cardiac Autonomic Function by Short-Term Sensor-Based and Long-Term Heart Rate Variability Analyses in Individuals with Spinal Cord Injury After Long-Term Table Tennis Training. Sensors 2024, 24, 7167. https://doi.org/10.3390/s24227167
Vogiatzi G, Michou V, Malliaropoulos N, Tsimaras V, Deligiannis A, Kouidi E. Assessment of Cardiac Autonomic Function by Short-Term Sensor-Based and Long-Term Heart Rate Variability Analyses in Individuals with Spinal Cord Injury After Long-Term Table Tennis Training. Sensors. 2024; 24(22):7167. https://doi.org/10.3390/s24227167
Chicago/Turabian StyleVogiatzi, Georgia, Vasiliki Michou, Nikos Malliaropoulos, Vasileios Tsimaras, Asterios Deligiannis, and Evangelia Kouidi. 2024. "Assessment of Cardiac Autonomic Function by Short-Term Sensor-Based and Long-Term Heart Rate Variability Analyses in Individuals with Spinal Cord Injury After Long-Term Table Tennis Training" Sensors 24, no. 22: 7167. https://doi.org/10.3390/s24227167
APA StyleVogiatzi, G., Michou, V., Malliaropoulos, N., Tsimaras, V., Deligiannis, A., & Kouidi, E. (2024). Assessment of Cardiac Autonomic Function by Short-Term Sensor-Based and Long-Term Heart Rate Variability Analyses in Individuals with Spinal Cord Injury After Long-Term Table Tennis Training. Sensors, 24(22), 7167. https://doi.org/10.3390/s24227167







