Surface Acoustic Waves (SAW) Sensors: Tone-Burst Sensing for Lab-on-a-Chip Devices
Abstract
1. Introduction
1.1. Sensors and Actuators
1.2. Biosensors and Types
1.3. Piezoelectrical Biosensors and SAW Devices
1.4. Conventional SAW Device Challenges and Proposed Tone Burst-Based Sensing Platform
2. Model-Assisted Design of SAW Sensor
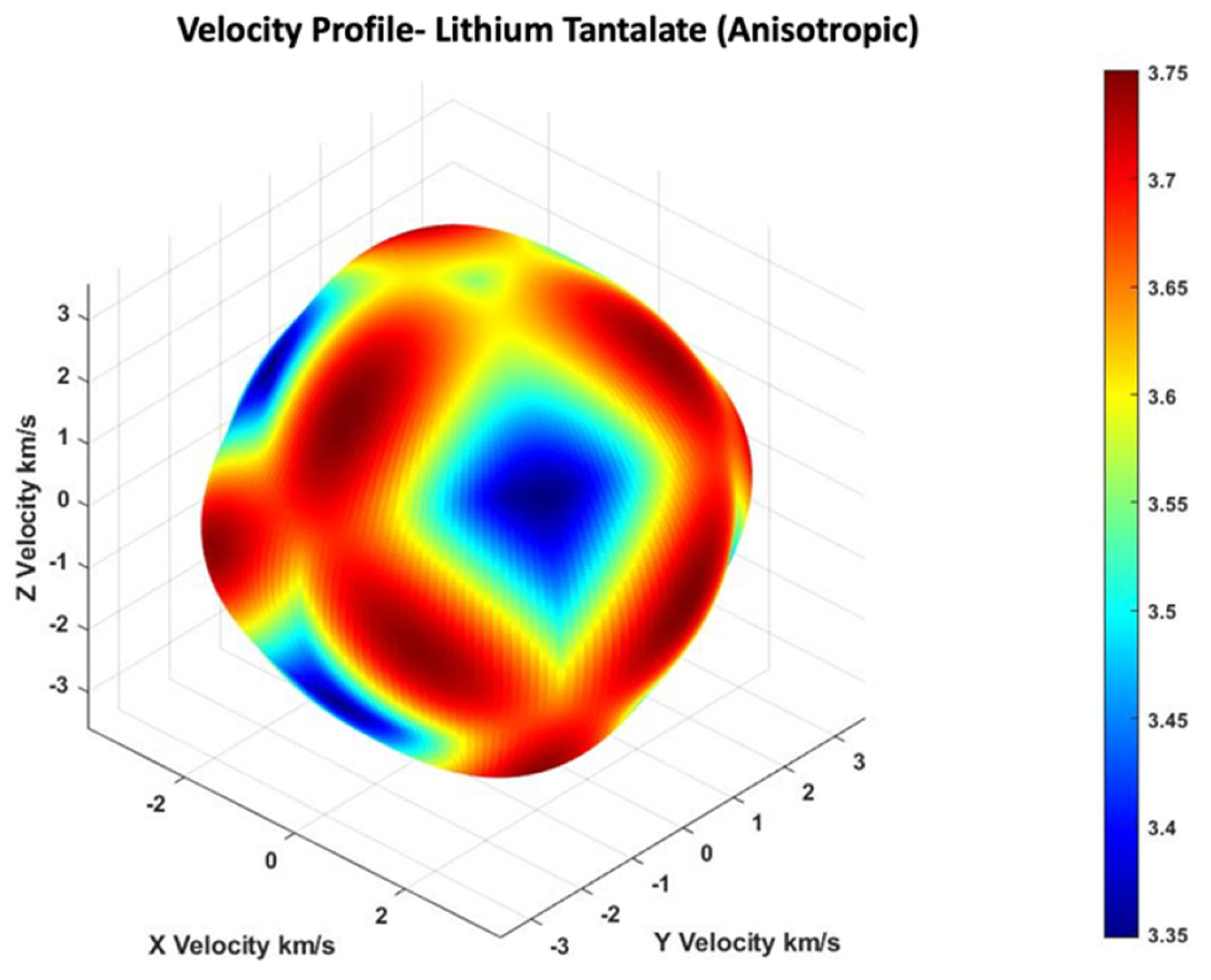
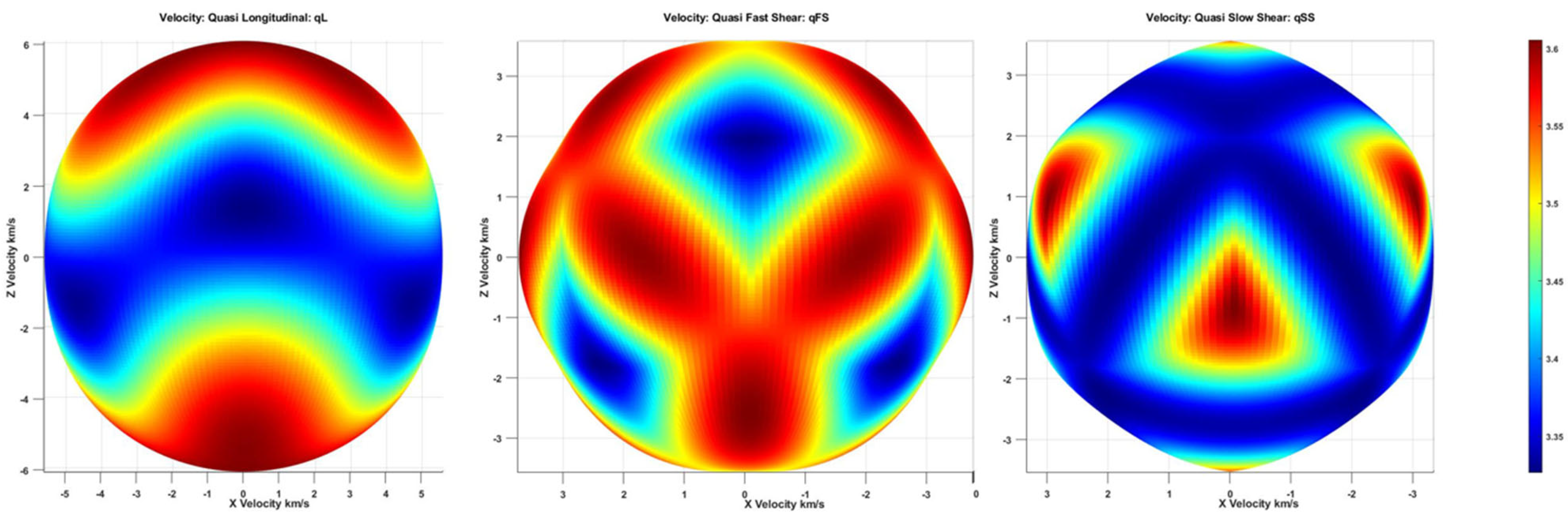
2.1. Bulk Wave in 36° YX Cut-Lithium Tantalate
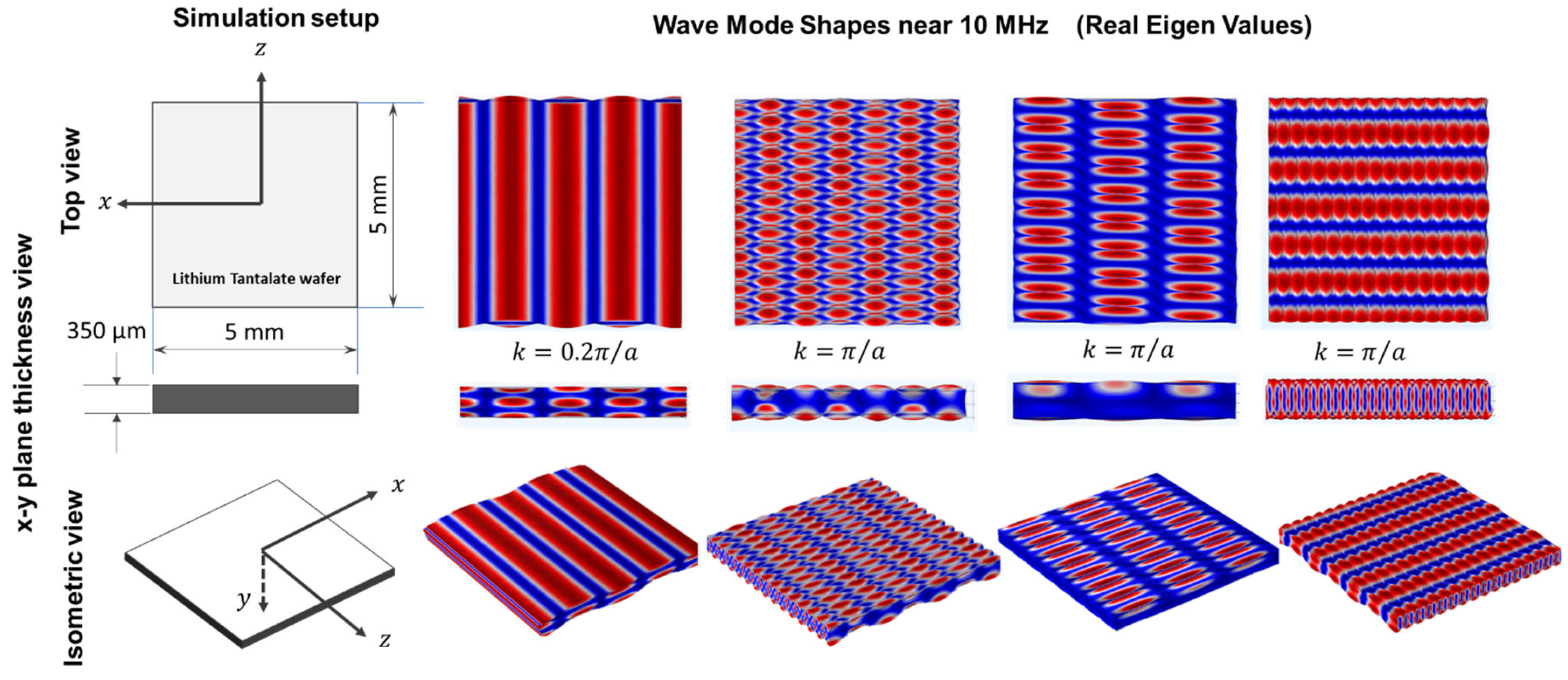
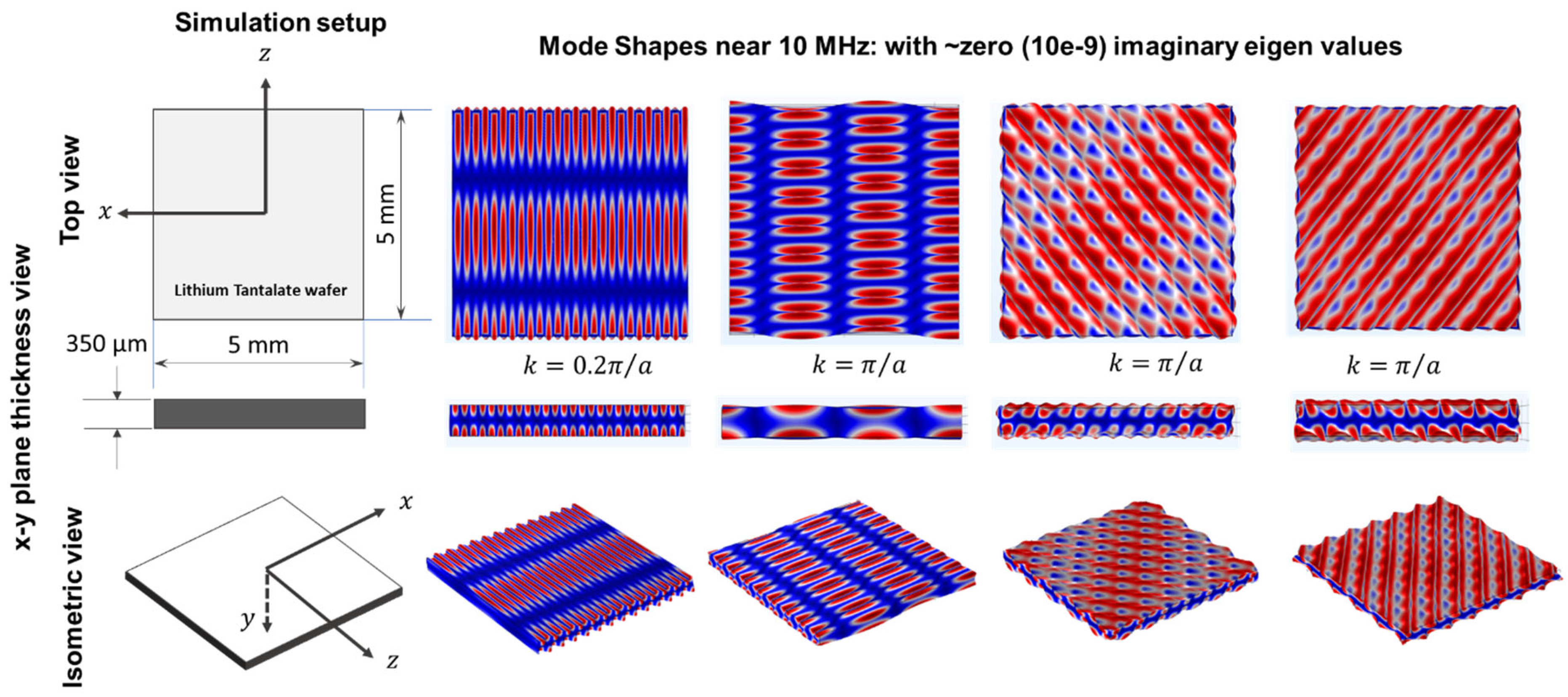
2.2. Guided Wave in 36° YX Cut-Lithium Tantalate
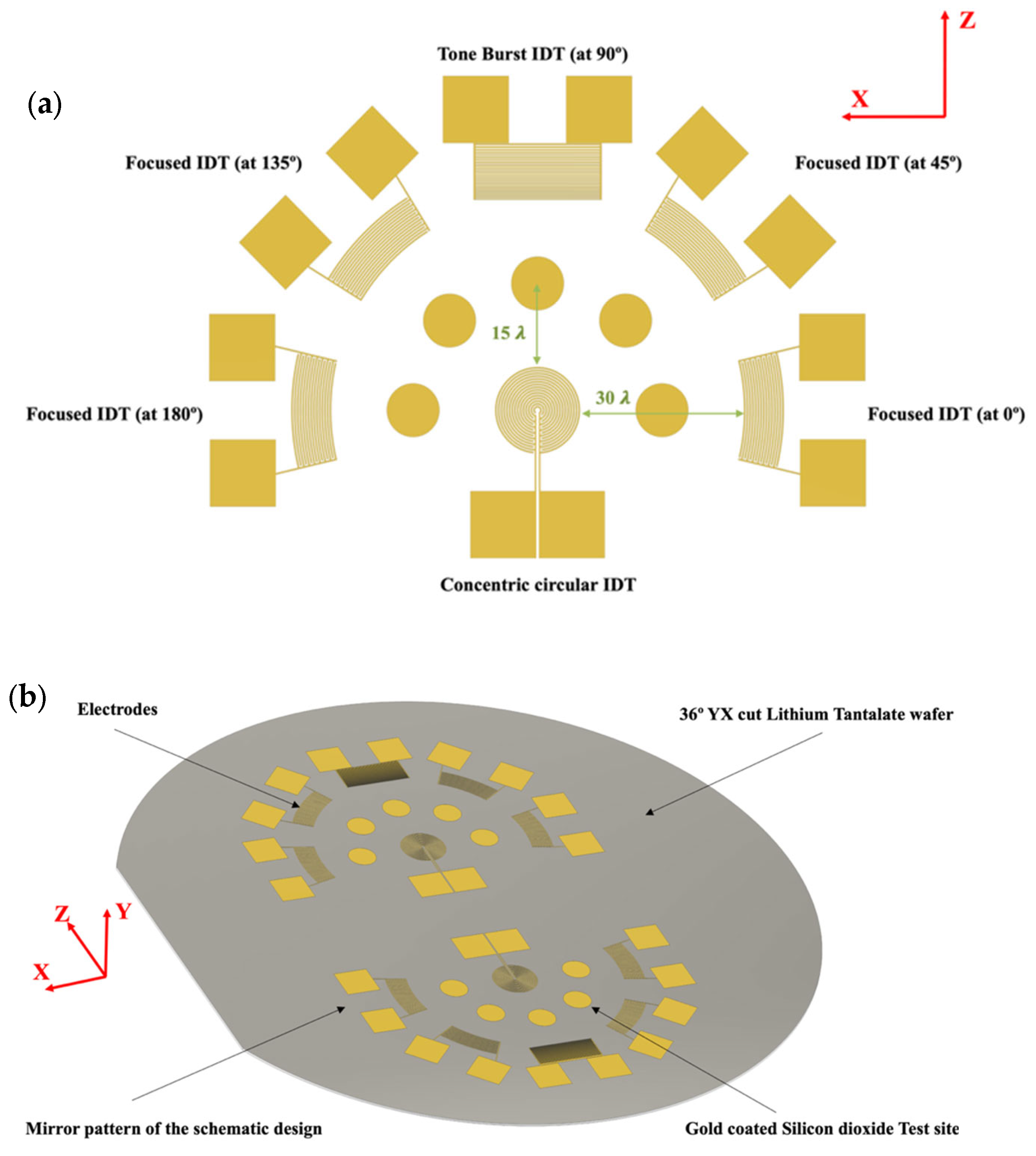
2.3. Design of the Sensing Platform and Computational Simulation of the Sensing Platform
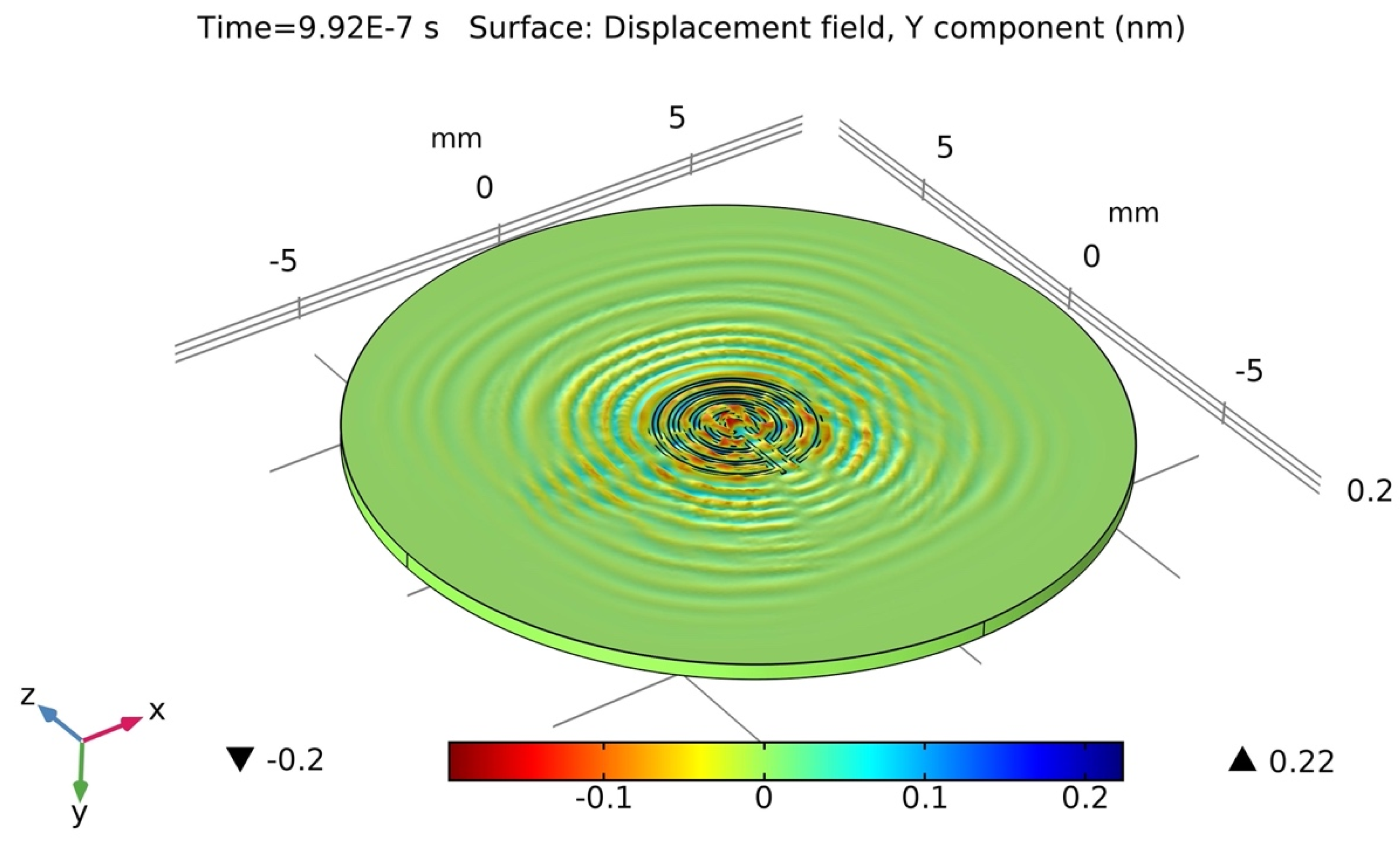
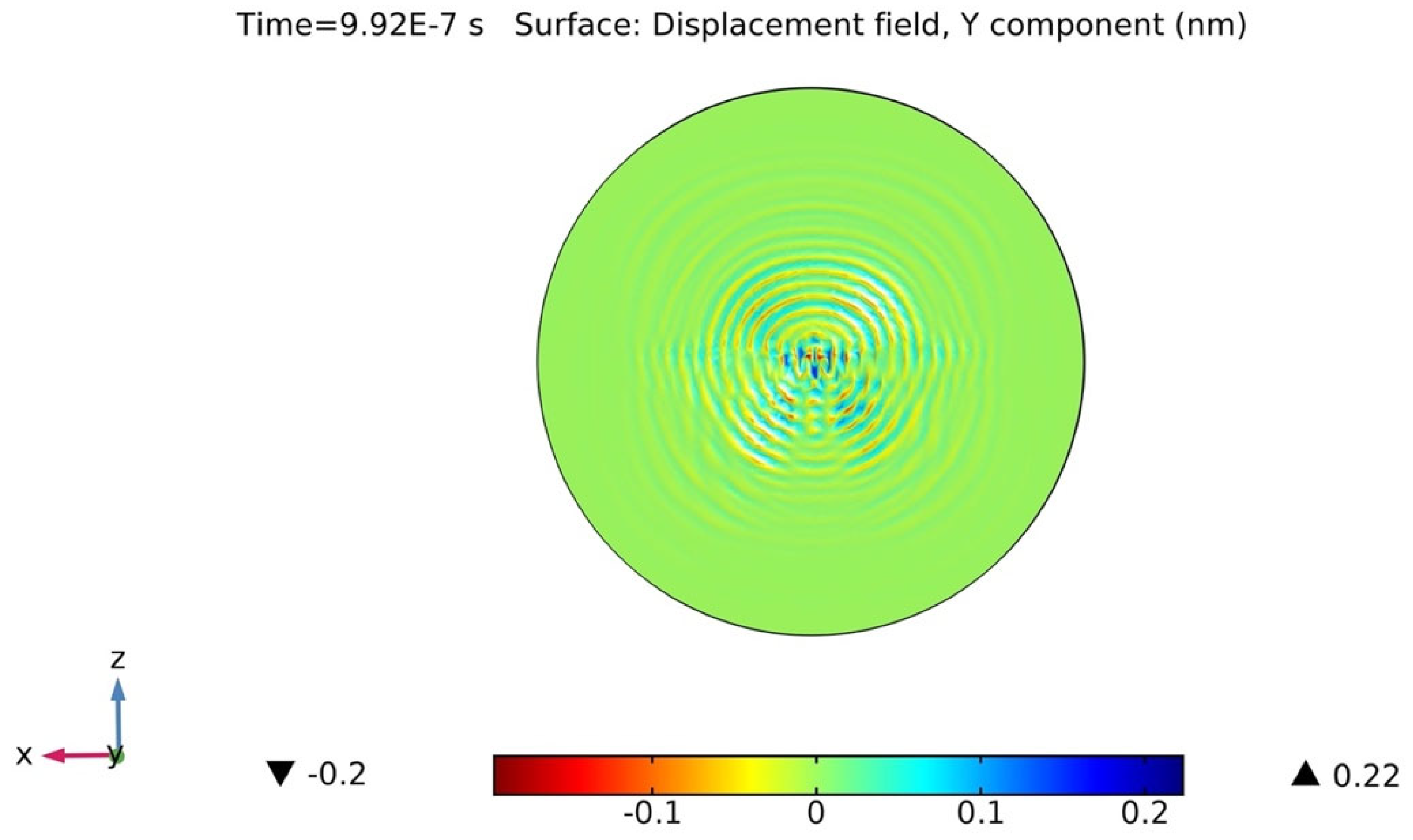
2.4. Wave-Field Modeling in 36° YX Cut-Lithium Tantalate
3. Materials and Methods
3.1. List of Instruments and Materials
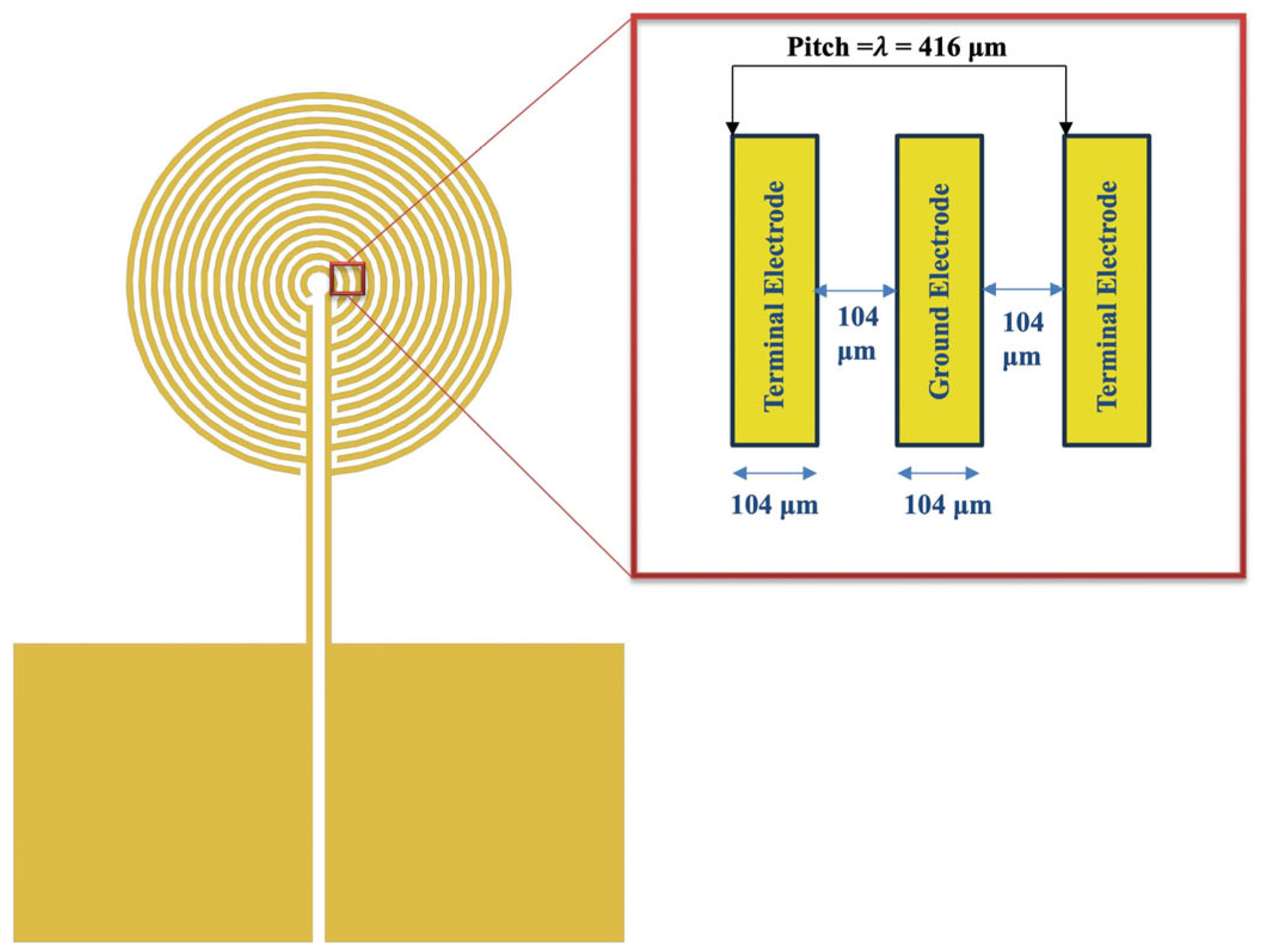
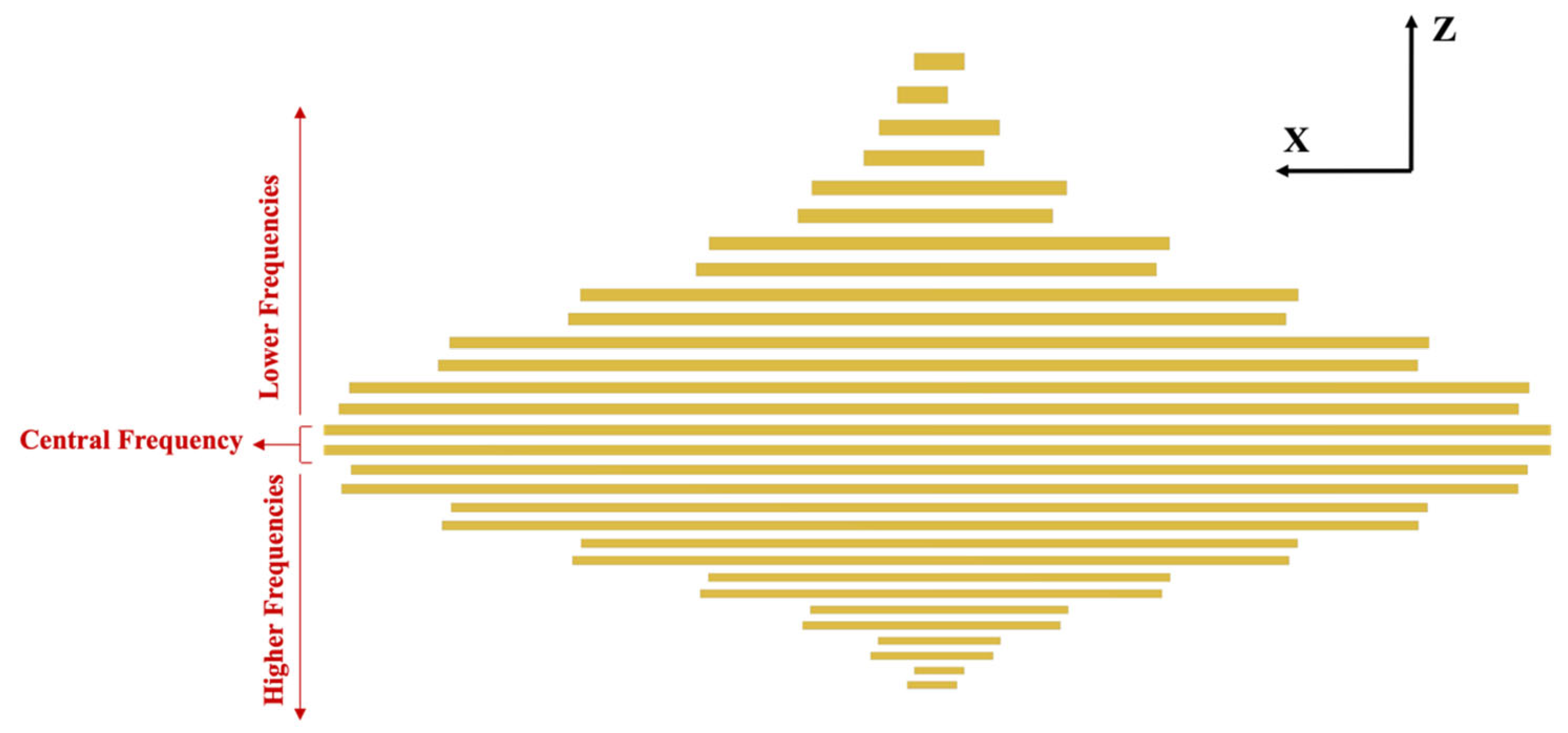
3.2. Tone Burst Interdigitated Electrodes Design
3.3. Sensor Fabrication
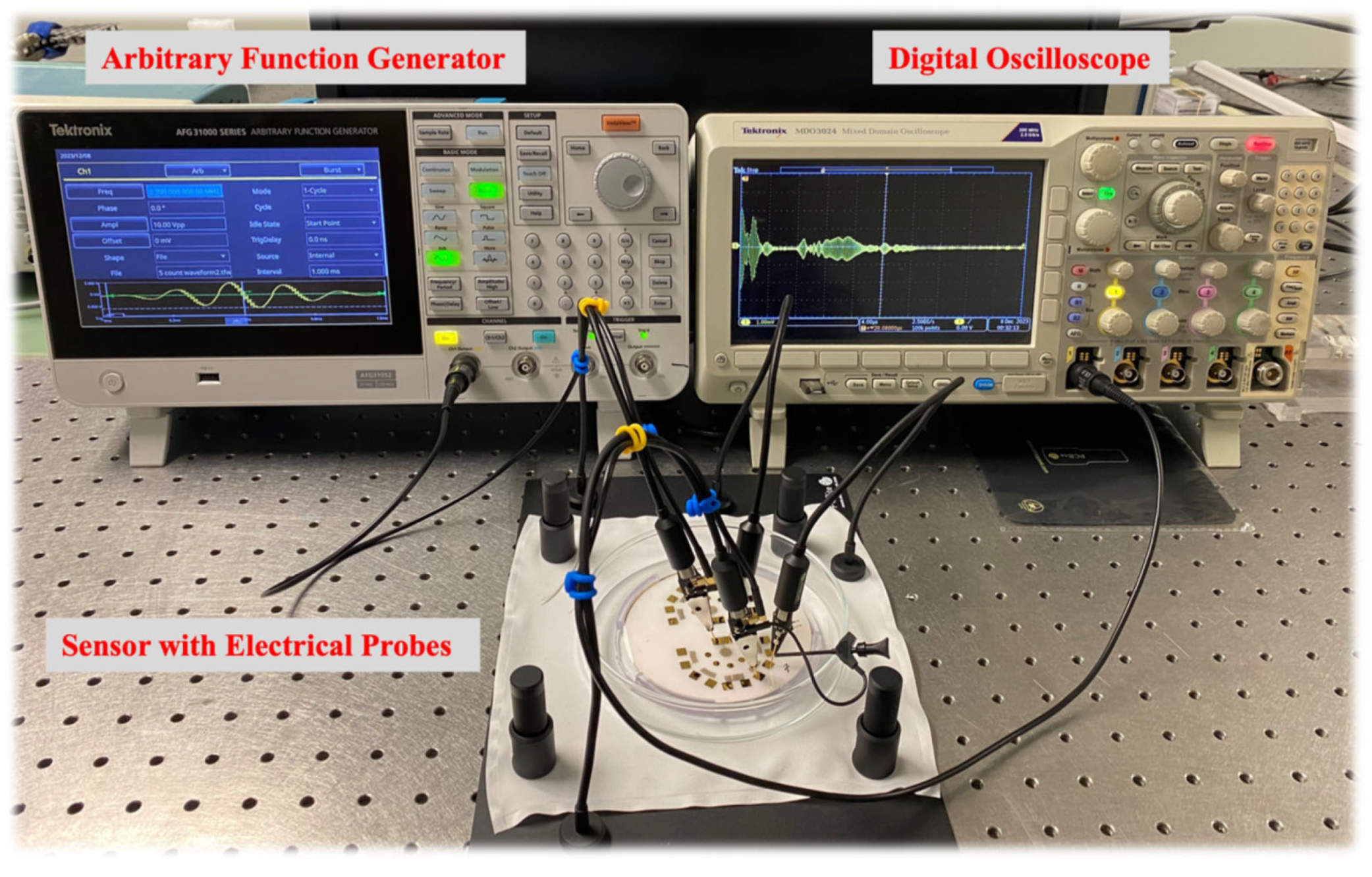
3.4. Experimental Process
4. Results and Discussion
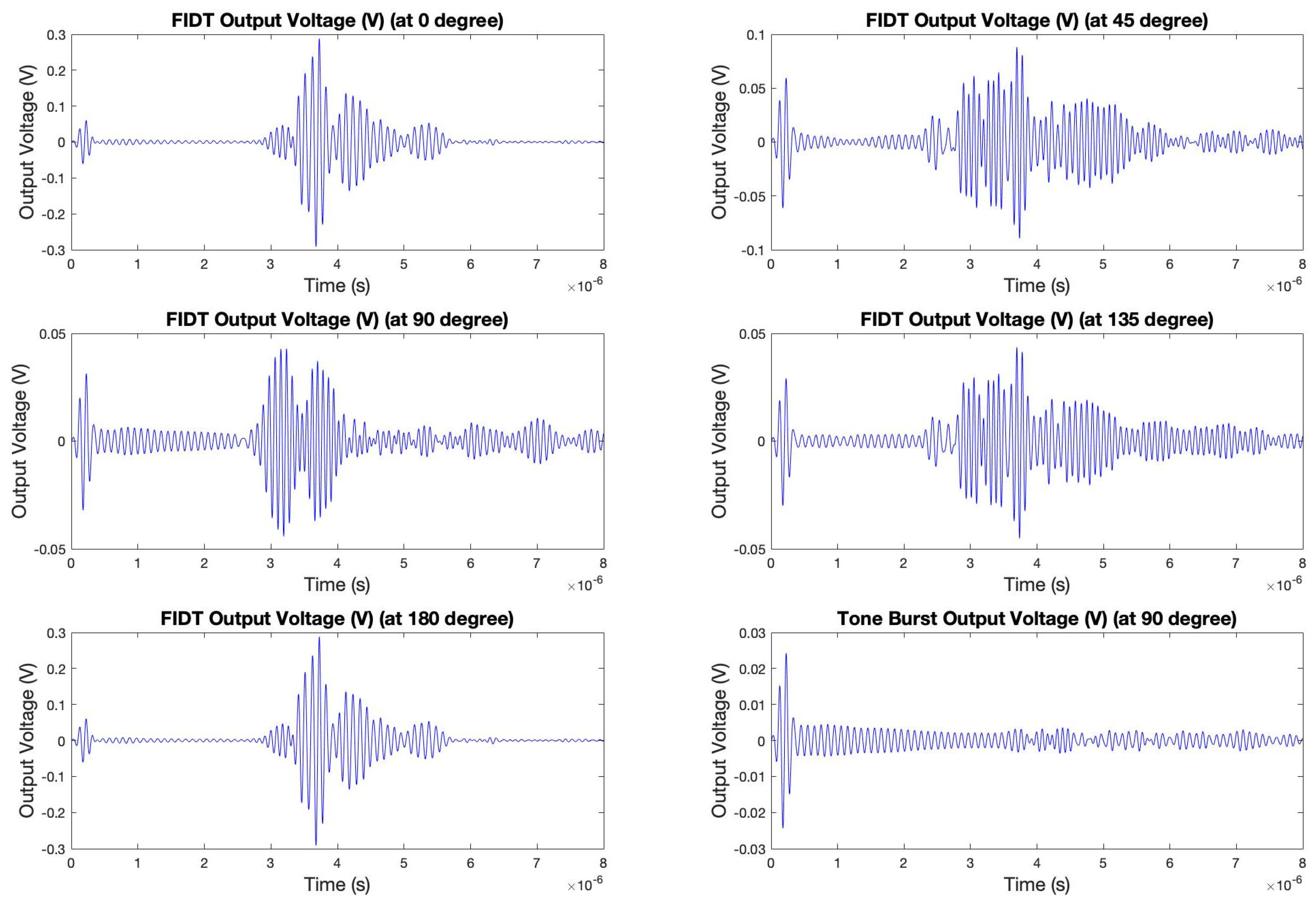
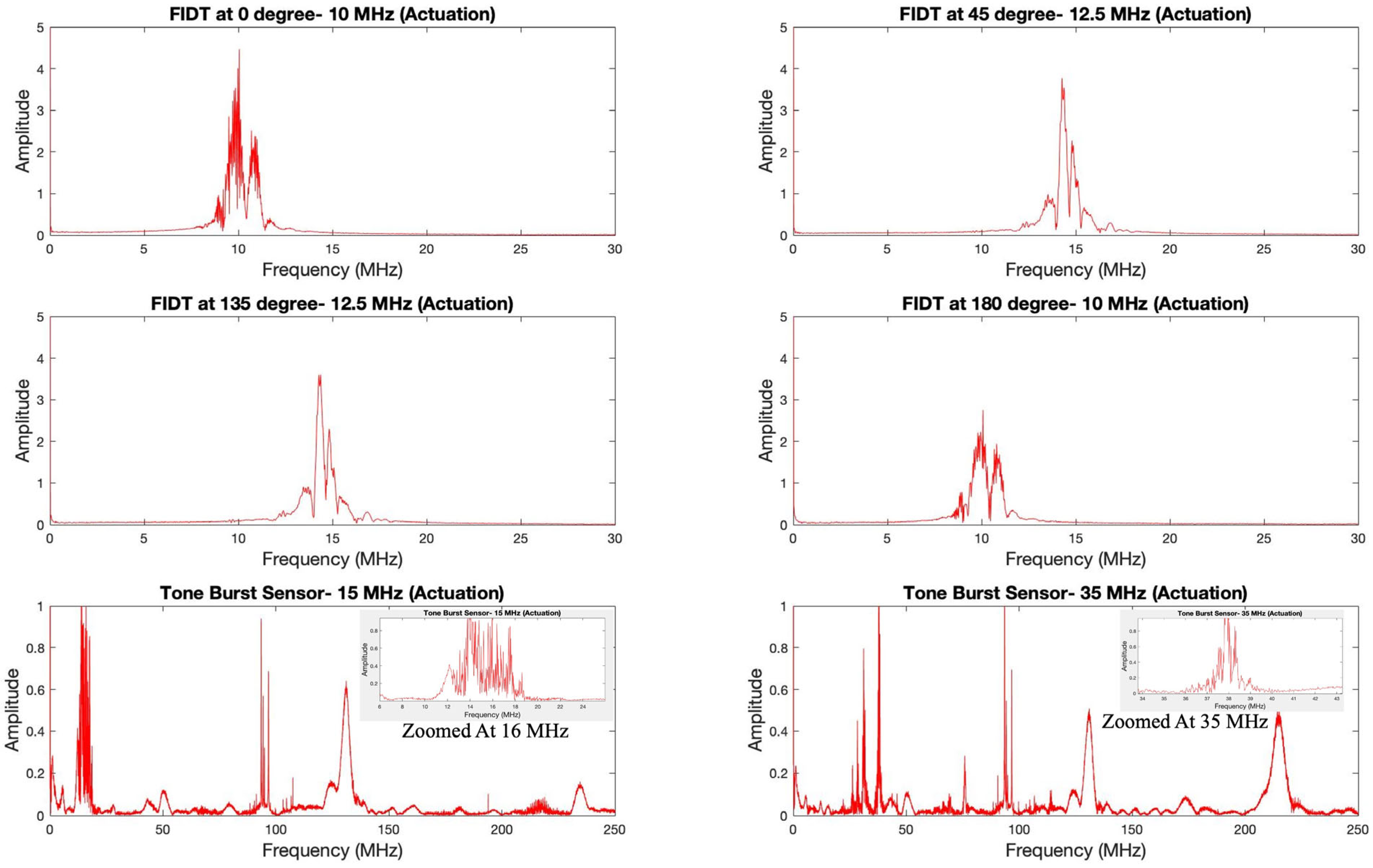
4.1. Actual Signal Analysis of the Sensing Platform
4.2. Example Actual Sensing Using Microcystin-LR
4.2.1. Functionalization of the Sensing Test Sites
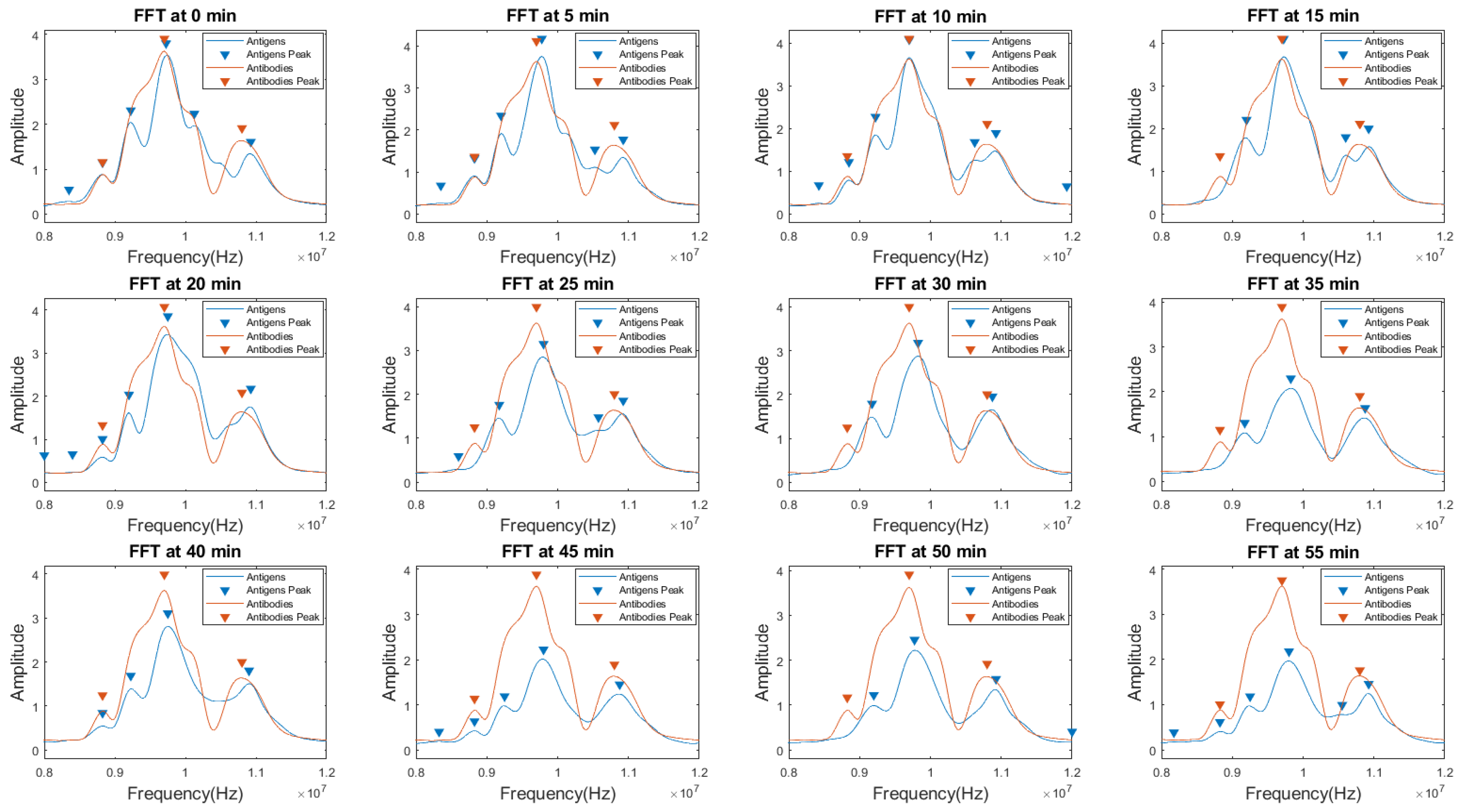
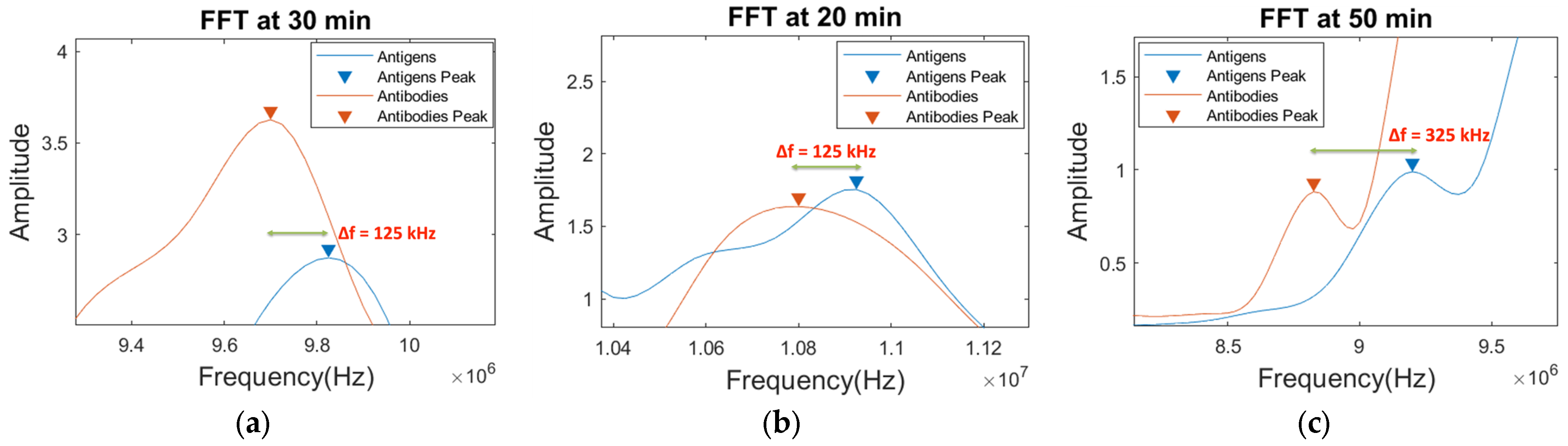
4.2.2. Comparison of Sensor Signals: MC-LR Antibodies and Antigens for the Detection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogue, R. MEMS sensors: Past, present and future. Sens. Rev. 2007, 27, 7–13. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Rab, S.; Singh, R.P.; Suman, R. Sensors for daily life: A review. Sens. Int. 2021, 2, 100121. [Google Scholar] [CrossRef]
- Patranabi, D. Sensors and Tranducers; PHI Learning Pvt. Ltd.: Delhi, India, 2003. [Google Scholar]
- Vetelino, J.; Reghu, A. Introduction to Sensors; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Sekhar, P.; Uwizeye, V. Review of Sensor and Actuator Mechanisms for bioMEMS. In MEMS for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2012; pp. 46–77. [Google Scholar]
- Alegria, F.A.C. Sensors and Actuators; World Scientific: Singapore, 2021. [Google Scholar]
- Carotta, M.; Ferroni, M.; Gnani, D.; Guidi, V.; Merli, M.; Martinelli, G.; Casale, M.; Notaro, M. Nanostructured pure and Nb-doped TiO2 as thick film gas sensors for environmental monitoring. Sens. Actuators B Chem. 1999, 58, 310–317. [Google Scholar] [CrossRef]
- Dishongh, T.J.; McGrath, M. Wireless Sensor Networks for Healthcare Applications; Artech House: Washington, DC, USA, 2010. [Google Scholar]
- García, C.G.; Meana-Llorián, D.; Lovelle, J.M.C. A review about Smart Objects, Sensors, and Actuators. Int. J. Interact. Multimed. Artif. Intell. 2017, 4, 7–10. [Google Scholar]
- Xie, D.; Chen, L.; Liu, L.; Chen, L.; Wang, H. Actuators and sensors for application in agricultural robots: A review. Machines 2022, 10, 913. [Google Scholar] [CrossRef]
- Edmonds, T. Chemical Sensors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kassal, P.; Steinberg, M.D.; Steinberg, I.M. Wireless chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2018, 266, 228–245. [Google Scholar] [CrossRef]
- Li, X.J.; Zhou, Y. Microfluidic Devices for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Perret, E. Radio Frequency Identification and Sensors: From RFID to Chipless RFID; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Piraino, F.; Selimović, Š. Diagnostic Devices with Microfluidics; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Sberveglieri, G. Gas Sensors: Principles, Operation and Developments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Liu, B.; Chen, X.; Cai, H.; Ali, M.M.; Tian, X.; Tao, L.; Yang, Y.; Ren, T. Surface acoustic wave devices for sensor applications. J. Semicond. 2016, 37, 021001. [Google Scholar] [CrossRef]
- Mandal, D.; Banerjee, S. Surface acoustic wave (SAW) sensors: Physics, materials, and applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef]
- Shiokawa, S.; Kondoh, J. Surface acoustic wave sensors. Jpn. J. Appl. Phys. 2004, 43, 2799. [Google Scholar] [CrossRef]
- Slobodnik, A.J. Surface acoustic waves and SAW materials. Proc. IEEE 1976, 64, 581–595. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Kougianos, E. Biosensors: A tutorial review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Rasooly, A.; Herold, K.E.; Herold, K.E. Biosensors and Biodetection; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Malhotra, B.D. Biosensors: Fundamentals and Applications; Smithers Rapra: Akron, OH, USA, 2017. [Google Scholar]
- Bilitewski, U.; Turner, A. Biosensors in Environmental Monitoring; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Yasmin, J.; Ahmed, M.R.; Cho, B.-K. Biosensors and their applications in food safety: A review. J. Biosyst. Eng. 2016, 41, 240–254. [Google Scholar] [CrossRef]
- Eggins, B.R. Biosensors: An Introduction; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Narang, J.; Pundir, C.S. Biosensors: An Introductory Textbook; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Turner, A.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Pohanka, M. The piezoelectric biosensors: Principles and applications. Int. J. Electrochem. Sci. 2017, 12, 496–506. [Google Scholar] [CrossRef]
- Mandal, D.; Indaleeb, M.M.; Younan, A.; Banerjee, S. Piezoelectric point-of-care biosensor for the detection of SARS-CoV-2 (COVID-19) antibodies. Sens. Bio-Sens. Res. 2022, 37, 100510. [Google Scholar] [CrossRef]
- Go, D.B.; Atashbar, M.Z.; Ramshani, Z.; Chang, H.-C. Surface acoustic wave devices for chemical sensing and microfluidics: A review and perspective. Anal. Methods 2017, 9, 4112–4134. [Google Scholar] [CrossRef]
- Oh, H.; Lee, K.; Eun, K.; Choa, S.-H.; Yang, S.S. Development of a high-sensitivity strain measurement system based on a SH SAW sensor. J. Micromech. Microeng. 2012, 22, 025002. [Google Scholar] [CrossRef]
- Rocha-Gaso, M.I.; March-Iborra, C.; Montoya-Baides, Á.; Arnau-Vives, A. Surface generated acoustic wave biosensors for the detection of pathogens: A review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, C. Low-loss floating electrode unidirectional transducer for SAW sensor. Acoust. Phys. 2019, 65, 178–184. [Google Scholar] [CrossRef]
- Zida, S.I.; Lin, Y.D.; Khung, Y.L. Current trends on surface acoustic wave biosensors. Adv. Mater. Technol. 2021, 6, 2001018. [Google Scholar] [CrossRef]
- Banerjee, S.; Leckey, C.A. Computational Nondestructive Evaluation Handbook: Ultrasound Modeling Techniques; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Bisoffi, M.; Hjelle, B.; Brown, D.; Branch, D.; Edwards, T.; Brozik, S.; Bondu-Hawkins, V.; Larson, R. Detection of viral bioagents using a shear horizontal surface acoustic wave biosensor. Biosens. Bioelectron. 2008, 23, 1397–1403. [Google Scholar] [CrossRef]
- Fertier, L.; Cretin, M.; Rolland, M.; Durand, J.-O.; Raehm, L.; Desmet, R.; Melnyk, O.; Zimmermann, C.; Déjous, C.; Rebière, D. Love wave immunosensor for antibody recognition using an innovative semicarbazide surface functionalization. Sens. Actuators B Chem. 2009, 140, 616–622. [Google Scholar] [CrossRef]
- Puiu, M.; Gurban, A.-M.; Rotariu, L.; Brajnicov, S.; Viespe, C.; Bala, C. Enhanced sensitive love wave surface acoustic wave sensor designed for immunoassay formats. Sensors 2015, 15, 10511–10525. [Google Scholar] [CrossRef]
- Rupp, S.; Vonschickfus, M.; Hunklinger, S.; Eipel, H.; Priebe, A.; Enders, D.; Pucci, A. A shear horizontal surface acoustic wave sensor for the detection of antigen—Antibody reactions for medical diagnosis. Sens. Actuators B Chem. 2008, 134, 225–229. [Google Scholar] [CrossRef]
- Clyne, T.W.; Hull, D. An Introduction to Composite Materials; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Coey, J.M. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Fung, Y.-C. Biomechanics: Mechanical Properties of Living Tissues; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kittel, C.; McEuen, P. Introduction to Solid State Physics; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Mallick, P.K. Fiber-Reinforced Composites: Materials, Manufacturing, and Design; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Banerjee, S. Metamaterials in Topological Acoustics; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Kovacs, G.; Anhorn, M.; Engan, H.E.; Visintini, G.; Ruppel, C.C.W. Improved Material Constants for LiNbO/sub 3/and LiTaO/sub 3. In IEEE Symposium on Ultrasonics; IEEE: New York, NY, USA, 1990. [Google Scholar]
- Brzozowski, E.; Stańczyk, B.; Przyborowska, K.; Kozłowski, A. Resonator with transverse surface wave on lithium tantalate crystal for applications in viscosity and temperature sensors. Mater. Elektron. 2016, 44, 3. [Google Scholar]
- Evans, C.; Stanley, S.; Percival, C.; McHale, G.; Newton, M. Lithium tantalate layer guided plate mode sensors. Sens. Actuators A Phys. 2006, 132, 241–244. [Google Scholar] [CrossRef]
- Roditi. Lithium Tantalate Properties. Available online: https://www.roditi.com/SingleCrystal/Lithium-Tantalate/LiTaO3-Properties.html (accessed on 10 November 2023).
- Giurgiutiu, V. Structural Health Monitoring: With Piezoelectric Wafer Active Sensors; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Han, C.; Doepke, A.; Cho, W.; Likodimos, V.; de la Cruz, A.A.; Back, T.; Heineman, W.R.; Halsall, H.B.; Shanov, V.N.; Schulz, M.J.; et al. A multiwalled-carbon-nanotube-based biosensor for monitoring microcystin-LR in sources of drinking water supplies. Adv. Funct. Mater. 2013, 23, 1807–1816. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, D.; Bovender, T.; Geil, R.D.; Banerjee, S. Surface Acoustic Waves (SAW) Sensors: Tone-Burst Sensing for Lab-on-a-Chip Devices. Sensors 2024, 24, 644. https://doi.org/10.3390/s24020644
Mandal D, Bovender T, Geil RD, Banerjee S. Surface Acoustic Waves (SAW) Sensors: Tone-Burst Sensing for Lab-on-a-Chip Devices. Sensors. 2024; 24(2):644. https://doi.org/10.3390/s24020644
Chicago/Turabian StyleMandal, Debdyuti, Tally Bovender, Robert D. Geil, and Sourav Banerjee. 2024. "Surface Acoustic Waves (SAW) Sensors: Tone-Burst Sensing for Lab-on-a-Chip Devices" Sensors 24, no. 2: 644. https://doi.org/10.3390/s24020644
APA StyleMandal, D., Bovender, T., Geil, R. D., & Banerjee, S. (2024). Surface Acoustic Waves (SAW) Sensors: Tone-Burst Sensing for Lab-on-a-Chip Devices. Sensors, 24(2), 644. https://doi.org/10.3390/s24020644






