Abstract
Our purpose was to characterize the oxygen uptake kinetics (VO2), energy systems contributions and total energy expenditure during a CrossFit® benchmark workout performed in the extreme intensity domain. Fourteen highly trained male CrossFitters, aged 28.3 ± 5.4 years, with height 177.8 ± 9.4 cm, body mass 87.9 ± 10.5 kg and 5.6 ± 1.8 years of training experience, performed the Isabel workout at maximal exertion. Cardiorespiratory variables were measured at baseline, during exercise and the recovery period, with blood lactate and glucose concentrations, including the ratings of perceived exertion, measured pre- and post-workout. The Isabel workout was 117 ± 10 s in duration and the VO2 peak was 47.2 ± 4.7 mL·kg−1·min−1, the primary component amplitude was 42.0 ± 6.0 mL·kg−1·min−1, the time delay was 4.3 ± 2.2 s and the time constant was 14.2 ± 6.0 s. The accumulated VO2 (0.6 ± 0.1 vs. 4.8 ± 1.0 L·min−1) value post-workout increased substantially when compared to baseline. Oxidative phosphorylation (40%), glycolytic (45%) and phosphagen (15%) pathways contributed to the 245 ± 25 kJ total energy expenditure. Despite the short ~2 min duration of the Isabel workout, the oxygen-dependent and oxygen-independent metabolism energy contributions to the total metabolic energy release were similar. The CrossFit® Isabel requires maximal effort and the pattern of physiological demands identifies this as a highly intensive and effective workout for developing fitness and conditioning for sports.
1. Introduction
Quantifying the dynamic features of oxygen uptake (VO2) kinetics has gained popularity in human physiology as a means of identifying the mechanisms underlying the control of muscle VO2 during exercise [1,2]. Traditionally, the dynamic VO2 response to exercise has been studied in three intensity ranges, i.e., low-moderate (until the anaerobic threshold) [3], heavy (above the anaerobic threshold) [4] and severe (in the area in which maximal VO2 is achieved) domains [5]. More recently, the extreme exercise domain has been proposed for performances leading to exhaustion before maximal VO2 is reached, with VO2 kinetics characterized by the development of a fast component with insufficient time for the appearance of a discernible VO2 slow component [6]. VO2 kinetics at low-severe exercise intensities have been well established in cyclic exercise modes including running, cycling, swimming and rowing [2,7,8,9].
CrossFit® is a multi-modal physical training program that covers functional movement patterns in a single high-intensity training session emphasizing strength and metabolic conditioning [10]. Improvements in metabolic capacity and lung function provided by CrossFit® are functions of the duration, type and intensity of exercise [11,12,13]. To assess and monitor fitness while tracking changes in work capacity over time, CrossFit® has integrated standardized exercises known as benchmark workouts [14,15]. These benchmarks exhibit variations in specific exercise routines/composition, intensity, duration, number/type of exercises and rest periods (see Figure 1) [16]. The manipulation of these parameters will ultimately affect the magnitude of the fitness and performance improvements, and the associated risk of overload [11,12]. Scoring methods typically involve achieving a total number of repetitions within a specified time frame [16]. CrossFit® research is needed to uncover predictive variables for performance in benchmark workouts through conventional laboratory tests [17,18,19]. However, due to the specificity of these workouts, the application of traditional laboratory testing protocols is constrained, as the physiological demands diverge from those encountered in a real-world training context. It is worth noting that only a limited number of pre-established workouts have been systematically characterized, with Cindy [20,21,22] and Fran [11,12,13,17] benchmarks workouts standing out as the most extensively assessed.
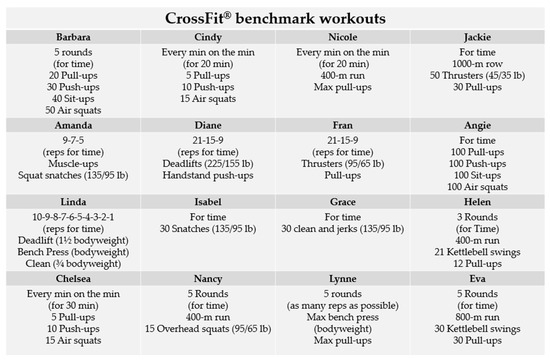
Figure 1.
Overview of exercises and structural elements in CrossFit® benchmark workouts.
Prior studies reveal an acute blood oxidative stress [23] response and heightened concentrations in the indirect blood markers of muscle damage, such as interleukin-6 and creatine kinase, post-CrossFit® sessions [24,25,26]. Diverse CrossFit® workouts correlate positively with elevated blood lactate concentrations ([La−]), highlighting the impact of factors like workout intensity, duration, exercise variety and rest periods on physiological responses [11,13,21,22,27]. In CrossFit® workouts conducted at severe intensity, there is a substantial oxidative phosphorylation energy contribution that should be considered a vital element in the training process. In addition, the optimization of various metabolic pathways is achievable depending on the total duration and performance (intermittent or continuous) strategy of the workout [11,13,21]. Furthermore, an observed reduction in muscle functional capacity underscores the dynamic nature of these workouts [21,22]. Cardiorespiratory and bioenergetic assessments have been primarily assessed in well-controlled environments, particularly in exercise laboratories [28,29]. The number of studies conducted on training and competition conditions is limited [11,13]. Studies not accounting for the oxygen-independent metabolism (glycolytic and phosphagen pathways) contribution at higher exercise intensities result in an underestimation of the total energy expenditure, which negatively impacts the overall understanding of the effects of specific workouts [30].
Isabel stands as a timed CrossFit® benchmark workout, challenging participants to complete 30 snatch repetitions with a 61 kg barbell in the shortest time possible. Widely employed for evaluating performance improvements in CrossFitters, this workout is renowned for its substantial muscular power demands [31]. However, to gain a more comprehensive understanding of the effects of workout intensity on the specific training performance of CrossFitters, more detailed physiological assessments, such as [La−] and cardiorespiratory parameters, are essential [11,12,13,21]. The purpose of the current study was to characterize the VO2 kinetics, estimate the contribution of the different energy systems and calculate the total energy expenditure of the Isabel workout. We expected that the specific cardiorespiratory demands would be consistent with the extreme intensity domain classification.
2. Materials and Methods
2.1. Participants
Fourteen highly trained male CrossFitters of 28.3 ± 5.4 years old with height 177.8 ± 9.4 cm, body mass 87.9 ± 10.5 kg, lean body mass 52.1 ± 4.5%, fat body mass 12.3 ± 4.8%, body mass index 27.8 ± 2.2 and 5.6 ± 1.8 years of training experience volunteered to participate. Subjects were recruited if they had a CrossFit® training frequency of more than five times per week for a minimum of three years before the commencement of the study. Participants were contacted personally and selected based on the following eligibility criteria: (i) ability to perform the Isabel workout <2 min; (ii) age between 18 and 40 years; and (iii) eligibility to exercise according to the Physical Activity Readiness Questionnaire. All CrossFitters were provided with clear instructions to adhere to their typical nutritional habits and explicitly instructed to abstain from consuming alcohol and caffeine, as well as engaging in intense physical activity, in the 48 h prior to the test. Detailed information about the experimental procedures, associated risks, and the benefits of participation was provided to all volunteers. All experiments were approved by the local Ethics Committee (CEFADE212019), with participants reading and signing an informed consent form in accordance with the Declaration of Helsinki and guidelines of the World Medical Association for research with humans.
2.2. Experimental Design
All assessments were conducted in a gym facility, maintaining consistent environmental conditions of 23 °C ambient temperature and 60% humidity. The assessments were supervised by an experienced CrossFit® researcher, ensuring meticulous and precise execution, thereby maintaining consistency and reliability across all participants. Initial measurements of body mass were obtained using the InBody 120 (Seul, Republic of Korea), while height was recorded using the Seca 222 stadiometer (Brussel, Belgium) immediately upon participants’ arrival. A standardized 10 min warm-up, including joint mobility exercises and specific movements with low loads tailored for Isabel, was administered. Subsequently, each CrossFitter engaged in the Isabel workout, exerting maximal effort. Pulmonary gas exchange was monitored breath-by-breath throughout the baseline, during the workout and in post-workout phases using a K5 telemetric portable gas analyzer (Cosmed, Rome, Italy). Simultaneously, continuous heart rate data were captured by a telemetric heart rate monitor belt (Cosmed ANT+), transmitting information to the K5 portable unit (Figure 2). During the recovery, subjects maintained a seated position for subsequent data collection. Capillary blood samples (5 μL) were collected from a fingertip at baseline at the 1st, 3rd, 5th and 7th min post-workout. The initial sample was discarded to eliminate contaminants and ensure measurement accuracy. Capillary blood collection involved applying controlled pressure to the finger, minimizing volume variations for consistent results. Lactate concentration ([La−]) and glucose levels were determined using the Lactate Pro analyzer (Arkay, Inc, Kyoto, Japan) and Accu-Chek Aviva analyzer (Mannheim, Germany), respectively. The participants’ self-reported perceived exertion was assessed using the Borg scale ranging from 6 to 20 (from very, very light to very and very heavy) at both baseline and 30 min post-workout. To ensure accurate interpretation and consistent reporting, the rating of the perceived exertion scale was explained individually to the participants according to the recommendations [32].
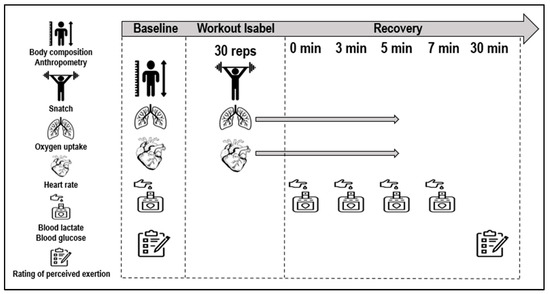
Figure 2.
Workout Isabel data collection set-up.
2.3. Methodology
The VO2 peak and ventilatory variables’ mean values were determined by analyzing the data from the final 30 s of exercise [13]. Data were carefully reviewed, and any breaths resulting from coughing or signal interruptions were excluded from the analysis [33,34]. Only values within the range of mean ± 3 standard deviations were considered for further analysis. Subsequently, a smoothing process was applied using a moving average for three breaths and a temporal average for 10 s, respectively [35]. For the estimation of VO2 kinetics parameters, a bootstrapping approach with 1000 samples was employed, with the exclusion of the cardiodynamic phase from the analysis [36]. The on-transient VO2 of Isabel’s workout and the excess post-exercise VO2 were determined using both mono-exponential and bi-exponential models through the VO2FITTING software [37]:
where VO2(t) represents the oxygen uptake normalized to body mass at time t, which is the baseline value for VO2, H denotes the Heaviside step function, Ap and Asc are the amplitudes of the primary and slow component phases, whereas TDp and Tsc, τp and τsc are the corresponding time delays and time constants of the fast and slow components of VO2, respectively [37]. Accumulated VO2 was computed as the ratio of the time integral of net VO2 to the exercise duration [11]. An individual example of the VO2 kinetics during and post-exercise responses is presented in Figure 3.
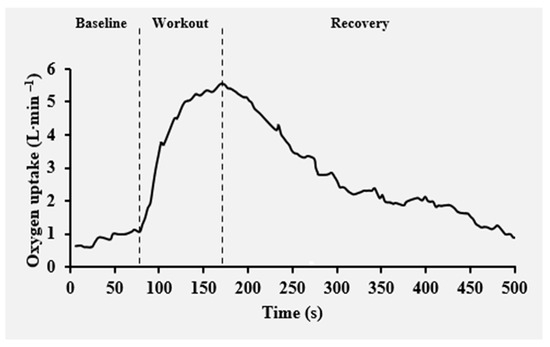
Figure 3.
Example of individual oxygen uptake kinetics as a function of time along the baseline, Isabel’s workout and recovery.
To assess the contribution of the oxidative phosphorylation energy system, the time integral of the net VO2 versus time relationship was examined [11,13]. The oxygen-independent metabolism contribution was approximated as the sum of the energy derived from lactic acid production and phosphocreatine splitting in the contracting muscles [11,38]:
where [La−]net is the peak accumulation of lactate after exercise, β is the constant for O2 equivalent for lactate accumulation in the blood (3 mL·kg−1·mM−1) and M (kg) is the body mass of de CrossFitter [11,38]. The phosphagen pathway contribution was estimated based on the maximal phosphocreatine splitting in the contracting muscle. This estimate assumed an energy equivalent of 0.468 kJ·mM−1 and a phosphate/oxygen ratio of 6.25 [11,34]:
where t represents the time duration, τ is the time constant of phosphocreatine splitting at the onset of workout (23.4 s), M (kg) denotes the mass of the participant and PCr is the assumed phosphocreatine concentration at rest, set at 18.5 mmol·kg−1 [34,38]. Energy system contributions were quantified in kilojoules (kJ), assuming an energy equivalent of 20.9 kJ·L−1 [11,39]. The total energy expenditure during Isabel’s workout was estimated by summing the contributions of the three energy systems [38,39]. To estimate metabolic power, energy expenditure was divided by the total duration (s) of the Isabel workout [11,38]. Caloric expenditure is estimated by multiplying absolute VO2 by 5.05 kcal·L–1 (expressed in kJ by assuming an energy equivalent of 4.184 kJ·L−1) [11,33].
2.4. Statistical Analysis
All calculations were completed using GraphPad Prism 6, with descriptive statistics presented as mean and standard deviation (SD). Data normality was checked through the Shapiro–Wilk test and repeated-measures ANOVA (with a Bonferroni post-hoc test) was applied to compare cardiorespiratory and energetic variables at different time points. A paired sample t-test was applied to compare perceived exertion and metabolic variables before and after the workout. Based on a post-hoc analysis, a sample of 14 subjects, an effect size of 0.8 and a 0.05 overall level of significance, the statistical power (β) obtained was 0.80. The effect size was calculated using Cohen’s d and interpreted as follows: trivial if d < 0.2, medium if 0.2 > d < 0.5 and large if d ≥ 0.5. The statistical significance level was set at 5%.
3. Results
The overall Isabel workout duration was 117 ± 10 s, with an exercise frequency of 0.3 ± 0.0 repetition/s. During the exercise, the VO2 peak, primary component amplitude, time delay and time constant values were 47.2 ± 4.7 and 42.0 ± 6.0 mL·kg−1·min−1, 4.3 ± 2.2 and 14.2 ± 6.0 s (respectively). The cardiorespiratory values at baseline, during the Isabel workout and at recovery are presented in Figure 4. The accumulated VO2 (p = 0.001, d = 5.8), minute ventilation (p = 0.001, d = 7.6), respiratory frequency (p = 0.001, d = 6.2), tidal volume (p = 0.001, d = 5.1), respiratory exchange ratio (p = 0.001, d = 3.3) and heart rate (p = 0.001, d = 12.3) values were substantially elevated from baseline. The excess post-exercise VO2 (p = 0.001, d = 4.7), minute ventilation (p = 0.001, d = 3.5), respiratory frequency (p = 0.001, d = 3.6), tidal volume (p = 0.001, d = 2.5) and heart rate (p = 0.001, d = 3.0) values remained elevated at the post-workout 5 min of recovery compared to baseline, with excess post-exercise VO2 (p = 0.026, d = 0.8) and respiratory exchange ratio (p = 0.001, d = 1.8) values greater than the exercise condition. In contrast, minute ventilation (p = 0.001, d = 2.5), respiratory frequency (p = 0.001, d = 3.1) and heart rate (p = 0.001, d = 3.7) were higher along the exercise compared with the recovery period. Regarding metabolic variables, there was a 14-fold increase in [La−] and a 46% increase in glucose levels in post-exercise values compared to baseline. The perceived exertion was much higher at the 30 min of recovery than at baseline (Table 1).
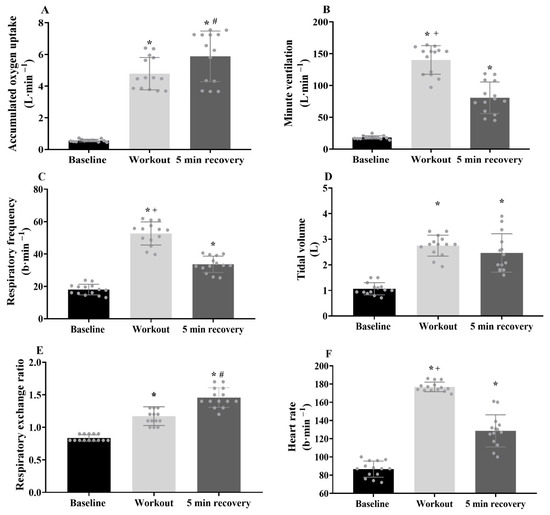
Figure 4.
(A–F) Cardiorespiratory variables assessed during baseline, Isabel workout and recovery with the respective differences identified by *, #, +, respectively (p ≤ 0.05). Individual and mean ± SD values.

Table 1.
Baseline and Isabel workout metabolic demands and perceived exertion.
The absolute and relative energy contribution values for the total workout are presented in Figure 5, with the oxidative phosphorylation (p = 0.001, d = 4.0) and glycolytic pathway (p = 0.001, d = 4.7) systems values contributing substantially more than the phosphagen pathway system (with higher values for glycolytic pathway contribution than the oxidative phosphorylation system). Total energy expenditure and metabolic power values during the Isabel workout were 245 ± 25 kJ and 2.0 ± 0.2 kW, and the caloric expenditure was lower during the workout than during the recovery period (101 ± 22 vs. 124 ± 34 kJ; p = 0.026, d = 0.8).
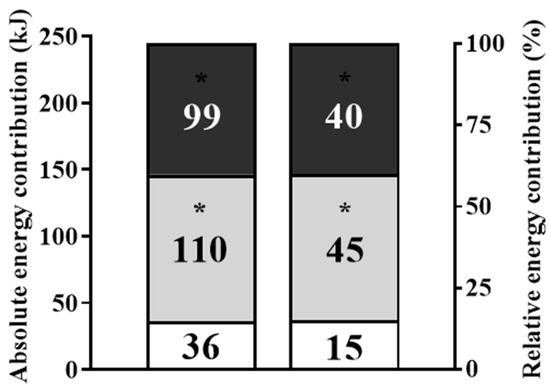
Figure 5.
Isabel workout (absolute and relative) oxidative phosphorylation, glycolytic pathway and phosphagen pathway energy contributions (identified by dark grey, light grey and white). * Differences from phosphagen pathway (p < 0.001).
4. Discussion
We characterized the VO2 kinetics, estimated the energy system contributions and evaluated the total energy expenditure of the Isabel CrossFit® workout. This exercise is typically performed in ~120 s and generates energetic demands in the extreme intensity domain [6]. Our main findings are summarized as follows: (i) a fast VO2 increase occurred at the beginning of the workout and continued to rise during the exercise yielding a high accumulated VO2; (ii) a greater contribution of the oxygen-independent metabolism (~60% of the total energy release) was observed (as a sum of both the glycolytic and phosphagen pathways); and (iii) the total energy expenditure values were high. The velocity of muscle contraction during the workout resulted in higher cardiorespiratory and metabolic stress (evidenced by the excess post-exercise VO2, [La−] and glucose values) compared to baseline, which ultimately affected the return to homeostasis. These high metabolic demands confirm the utility of the CrossFit® Isabel workout as a very effective high-intensity training modality for enhancing fitness and conditioning.
It is well established that performing an a priori test in a standardized protocol, such as an intermittent incremental treadmill test, allows the collection of [La−] analysis and, in conjunction with gas exchange assessments, provides a comprehensive physiological characterization of exercise in the low, moderate, heavy and severe intensity domains [8]. However, advocating an a priori standardized testing protocol to determine maximal VO2 (e.g., cycling, treadmill and rowing ergometer) and comparing it to the Isabel performance that includes specific strength training elements compromises the principle of modality specificity in sports training. For this reason, we chose not to perform a priori tests to determine the maximal VO2 of CrossFitters. Instead, we compared their values with cardiorespiratory data from athletes participating in sports performed at the same intensity.
Although VO2 kinetics is well described in the literature, especially in cyclic sports [2,9], few attempts have been made in the CrossFit® literature to evaluate VO2 kinetics using direct oximetry protocols under real exercise conditions. When the Isabel workout is performed at maximal effort, CrossFitters begin exercising at a very high intensity. From the onset of the exercise, the requirement for oxygen in muscles triggers an instantaneous and sudden increase in the accumulated VO2, resulting in a high peak VO2, which is consistent with recently reported data obtained in trained CrossFitters performing the CrossFit® Fran workout [11,13]. The current primary component amplitude and time constant values were similar (but with smaller values for the time delay) than those previously reported for rowing, running and cycling at maximal intensity [39]. Faster VO2 kinetics is related to a shorter time lag in the imbalance of VO2 demand and supply, implying an increased oxidative contribution to energy transfer [40]. In addition, given that the CrossFitters were trained, it likely influenced a shorter time constant (associated with higher fatigue tolerance) contributing to better performance [40,41].
Cardiorespiratory outcomes during the Isabel workout yielded similar values for minute ventilation, respiratory frequency, tidal volume, respiratory exchange ratio and heart rate compared with running and cycling at maximal intensity [8,39]. However, the accumulated VO2 and heart rate values were lower than those obtained in the previous evaluation of the Fran workout [11,13]. The differences in accumulated VO2 values can be explained by the greater exercise volume involved in this latter effort (90 repetitions of thrusters plus pull-ups) that ends up demanding a greater pulmonary function [11,13]. In support of this assertion, the short duration of the Isabel workout (combined with the extreme intensity) limited the increase in heart rate and prevented it from reaching a higher value [12]. The [La−] and glucose concentrations in response to the extreme intensity of the Isabel workout were higher than the Grace and Fran CrossFit® workouts [13,20], reflecting a greater glycolytic pathway contribution and involvement of carbohydrate metabolism. These effects would likely reflect a greater amount of glucose at the muscle level [30]. The perceived exertion was lower in the Cindy [21] and Fran [11] workout sessions compared to the Isabel workout, which was classified as extremely hard. A higher rating of perceived exertion value during an intense workout session indicates that sufficient stimuli are present to promote resistance adaptation, as the rating of perceived exertion value has been used as a marker of psychophysiological response to a training session [42].
The oxidative phosphorylation contribution determined in the current study was lower than the values previously reported for Fran workout [13], running, cycling [39] and rowing [43], but higher than the values reported for strength training [35]. In contrast, the oxygen-independent metabolism (phosphagen and glycolytic pathways) contribution during Isabel workout in the present study was higher than that reported for the other types of training [11,43], although these differences could be attributed to the shorter total duration (117 s) and high net [La−] accumulation (1.5–20.7 mmol∙L−1) of the Isabel workout [30]. In addition, specific mechanical factors (e.g., the muscle contraction scheme and the resulting muscle fiber recruitment profile itself) might have influenced the energy contribution of the workout, which in turn largely depends on the type of training performed [33]. Nevertheless, some caution should be exercised when interpreting data, as different methodological procedures can easily influence the energy contribution [30]. The gold standard for assessing oxygen-independent metabolism release involves a highly invasive muscle biopsy, quantifying energy sources and metabolite accumulation inside muscle cells. However, the technique’s limitation lies in sampling only a small portion of human muscle tissue, requiring multiple samples from different depths to reflect muscle heterogeneity [34].
Studies that have examined total energy expenditure, metabolic power and caloric expenditure assessment under CrossFit® training conditions, particularly at extreme intensity, are few in number. Total energy expenditure during the Isabel workout was lower than the Cindy and Fran workouts [11,20], but higher than those reported for strength training [44] and rowing [43]. Metabolic power reported in this study was higher compared to the Fran workout [11] and rowing [43], while caloric expenditure was lower compared to strength training [45], Cindy [20] and Fran workouts [11,13]. These differences could be due to intensity, volume, the number of repetitions and different types of exercises [33], and provide a framework for the prescription of training in these settings.
Cardiorespiratory function remained elevated during the recovery period (as expected) compared with baseline values, consistent with other exercises [13,46]. This elevation is justifiable because strength training can induce Valsalva maneuvers and an increase in cardiovascular demands, which may yield a compensatory rise in minute ventilation and VO2 during the recovery period [47,48]. In addition, the higher minute ventilation and VO2 could also be interpreted as part of the compensation to normalize the lowered pH caused by increased [La−] levels post-workout [46,48]. The higher respiratory exchange ratio during the recovery compared to baseline and exercise conditions is likely explained by the explosive nature of the Isabel workout. The Isabel requires a rapid production of adenosine triphosphate via the oxygen-independent metabolism (60%), resulting in a greater involvement of carbohydrate metabolism [30].
Our results indicate that Isabel’s workout, completed in less than two min, triggers a substantial and effective cardiorespiratory response. This regimen could serve as an excellent training option for optimizing both oxygen-independent metabolism and oxidative phosphorylation pathways. Certain limitations in our study warrant acknowledgment. First, the relatively small number of participants and the absence of dietary control before the test must be acknowledged. Caution should be used when extrapolating the results of the current study to other cohorts or individuals with different training experiences, as only healthy, experienced, male participants were recruited for this study. Several variables, including active muscle mass percentage, a time constant of VO2 on the response at the muscle level and the concentration of phosphocreatine splitting per kilogram of wet muscle, can influence the values obtained using the phosphagen pathway method [34]. Finally, this study lacks biochemical markers (e.g., creatine kinase, total antioxidant status and malondialdehyde) to elucidate the elevated physiological stress induced by a single training session of Isabel.
In future cross-sectional studies, comparing cardiorespiratory responses and energy utilization between novice and experienced CrossFitters is valuable. Furthermore, future research should assess the metabolic profile of novice participants to understand the impact of strategies employed during the Isabel workout in this cohort. It is crucial to explore variations in these responses between males and females, emphasizing adaptations in both central (stroke volume) and peripheral (oxidative capacity) aspects. Longitudinal studies analyzing the effects of prescribed training interventions over weeks in response to the consecutive days of CrossFit® training, using similar methods and including biochemical markers and biomechanics analysis, are crucial for a comprehensive understanding of how to accurately quantify and monitor CrossFit® training load.
5. Conclusions
During the extremely intense Isabel workout, there was an immediate and sudden increase in VO2 at the beginning of the exercise that persisted until the end, highlighting the contribution of oxidative phosphorylation energy metabolism during short and very intense CrossFit® workouts. The majority (~60%) of the total energy was obtained from oxygen-independent metabolism, and both the glycolytic and phosphagen pathway energy systems should be strengthened to improve the performance of trained male CrossFitters. The Isabel workout is an excellent high-intensity training option for CrossFitters and other athletes seeking to improve their fitness and conditioning.
Author Contributions
Conceptualization: M.R., D.B.P., D.M.-G. and R.J.F.; methodology: M.R., V.M.R., D.M.-G. and R.J.F.; formal analysis: M.R., K.M.B. and F.C.; investigation: M.R., K.M.B. and F.C.; resources: M.R. and R.J.F.; writing—original draft preparation, M.R.; writing—review and editing: M.R., K.M.B., F.C., D.B.P., V.M.R., D.M.-G. and R.J.F.; visualization: M.R.; supervision: D.M.-G. and R.J.F.; project administration: M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Portuguese Foundation for Science and Technology to Manoel Rios (FCT202104701BD) and Victor Machado Reis (UIDB/04045/2020).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Sport of the University of Porto (CEFADE212019) and the guidelines of the World Medical Association for research on humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors would like to acknowledge all study participants and collaborators.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Whipp, B.; Ward, S. Physiological determinants of pulmonary gas exchange kinetics during exercise. Med. Sci. Sports Exerc. 1990, 22, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Jones, A.M. Oxygen uptake kinetics. Compr. Physiol. 2012, 2, 933–996. [Google Scholar] [PubMed]
- Robergs, R.A. A critical review of the history of low-to moderate-intensity steady-state VO2 kinetics. Sports Med. 2014, 44, 641–653. [Google Scholar] [CrossRef]
- Pringle, J.S.; Doust, J.H.; Carter, H.; Tolfrey, K.; Campbell, I.T.; Jones, A.M. Oxygen uptake kinetics during moderate, heavy and severe intensity’submaximal’exercise in humans: The influence of muscle fibre type and capillarisation. Eur. J. Appl. Physiol. 2003, 89, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Poole, D.C. The slow component of oxygen uptake kinetics in humans. Exerc. Sport Sci. Rev. 1996, 24, 35–70. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.W.; Poole, D.C.; Smith, J.C. The relationship between power and the time to achieve V̇O2max. Med. Sci. Sports Exerc. 2002, 34, 709–714. [Google Scholar]
- Pringle, J.; Carter, H.; Doust, J.; Jones, A. Oxygen uptake kinetics during horizontal and uphill treadmill running in humans. Eur. J. Appl. Physiol. 2002, 88, 163–169. [Google Scholar] [CrossRef]
- Cardoso, F.; Monteiro, A.S.; Vilas-Boas, J.P.; Pinho, J.C.; Pyne, D.B.; Fernandes, R.J. Effects of wearing a 50% lower jaw advancement splint on biophysical and perceptual responses at low to severe running intensities. Life 2022, 12, 253. [Google Scholar] [CrossRef]
- Sousa, A.; Rodríguez, F.A.; Machado, L.; Vilas-Boas, J.P.; Fernandes, R.J. Exercise modality effect on oxygen uptake off-transient kinetics at maximal oxygen uptake intensity. Exp. Physiol. 2015, 100, 719–729. [Google Scholar] [CrossRef]
- Dominski, F.H.; Tibana, R.A.; Andrade, A. “Functional Fitness Training”, CrossFit, HIMT, or HIFT: What is the preferable terminology? Front. Sports Act. Living 2022, 4, 882195. [Google Scholar] [CrossRef]
- Rios, M.; Zacca, R.; Azevedo, R.; Fonseca, P.; Pyne, D.B.; Reis, V.M.; Moreira-Gonçalves, D.; Fernandes, R.J. Bioenergetic Analysis and Fatigue Assessment During the Workout Fran in Experienced Crossfitters. Int. J. Sports Physiol. Perform. 2023, 18, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; De Sousa, N.M.F.; Prestes, J.; Voltarelli, F.A. Lactate, heart rate and rating of perceived exertion responses to shorter and longer duration CrossFit® training sessions. J. Funct. Morphol. Kinesiol. 2018, 3, 60. [Google Scholar] [CrossRef]
- Rios, M.; Becker, K.M.; Monteiro, A.S.; Fonseca, P.; Pyne, D.B.; Reis, V.M.; Moreira-Gonçalves, D.; Fernandes, R.J. Effect of Fran CrossFit Workout on Oxygen Uptake Kinetics, Energetics, and Postexercise Muscle Function in Trained CrossFitters. Int. J. Sports Physiol. Perform. 2024, 1–8. [Google Scholar] [CrossRef]
- Claudino, J.G.; Gabbett, T.J.; Bourgeois, F.; Souza, H.d.S.; Miranda, R.C.; Mezêncio, B.; Soncin, R.; Cardoso Filho, C.A.; Bottaro, M.; Hernandez, A.J. CrossFit overview: Systematic review and meta-analysis. Sports Med. Open 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Meier, N.; Rabel, S.; Schmidt, A. Determination of a CrossFit® benchmark performance profile. Sports 2021, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Meier, N.; Schlie, J.; Schmidt, A. CrossFit®:‘Unknowable’or Predictable?—A Systematic Review on Predictors of CrossFit® Performance. Sports 2023, 11, 112. [Google Scholar] [CrossRef]
- Leitão, L.; Dias, M.; Campos, Y.; Vieira, J.G.; Sant’Ana, L.; Telles, L.G.; Tavares, C.; Mazini, M.; Novaes, J.; Vianna, J. Physical and physiological predictors of FRAN CrossFit® WOD athlete’s performance. Int. J. Environ. Res. Public Health 2021, 18, 4070. [Google Scholar] [CrossRef]
- Zeitz, E.K.; Cook, L.F.; Dexheimer, J.D.; Lemez, S.; Leyva, W.D.; Terbio, I.Y.; Tran, J.R.; Jo, E. The relationship between crossfit® performance and laboratory-based measurements of fitness. Sports 2020, 8, 112. [Google Scholar] [CrossRef]
- Dexheimer, J.D.; Schroeder, E.T.; Sawyer, B.J.; Pettitt, R.W.; Aguinaldo, A.L.; Torrence, W.A. Physiological performance measures as indicators of crossfit® performance. Sports 2019, 7, 93. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; Buresh, R.; Bechke, E.; Williamson, C. Metabolic biomarkers following a short and long bout of high-intensity functional training in recreationally trained men. J. Hum. Sport Exerc. 2017, 12, 710–718. [Google Scholar] [CrossRef]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; Cañuelo-Márquez, A.M.; Guodemar-Pérez, J.; García-Fernández, P.; Lozano-Estevan, M.d.C.; Alonso-Melero, R.; Sánchez-Calabuig, M.A.; Ruíz-López, M. Cardiometabolic and muscular fatigue responses to different crossfit® workouts. J. Sports Sci. Med. 2018, 17, 668. [Google Scholar] [PubMed]
- Forte, L.D.; Freire, Y.G.; Júnior, J.S.; Melo, D.A.; Meireles, C.L. Physiological responses after two different CrossFit workouts. Biol. Sport 2022, 39, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.; Macan, T.; Stevanović-Silva, J.; Nhusawi, K.; Fernandes, R.J.; Beleza, J.; Ascensão, A.; Magalhães, J. Acute CrossFit® workout session impacts blood redox marker modulation. Physiologia 2021, 1, 13–21. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; John, Q.C.; Daniel, B.L.; Gretchen, O.D.; Michael, E.R.; Kyle, T.J. Acute exercise and oxidative stress: CrossFit™ vs. treadmill bout. J. Hum. Kinet. 2015, 47, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.; Novaes, J.S.; Behm, D.G.; Vieira, J.G.; Dias, M.R.; Vianna, J.M. Characterization of hormonal, metabolic, and inflammatory responses in CrossFit® training: A systematic review. Front. Physiol. 2020, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; de Almeida, L.M.; Frade de Sousa, N.M.; Nascimento, D.d.C.; Neto, I.V.d.S.; de Almeida, J.A.; de Souza, V.C.; Lopes, M.d.F.T.; Nobrega, O.d.T.; Vieira, D.C. Two consecutive days of extreme conditioning program training affects pro and anti-inflammatory cytokines and osteoprotegerin without impairments in muscle power. Front. Physiol. 2016, 7, 260. [Google Scholar] [CrossRef]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; García-Fernández, P.; Garnacho-Castaño, M.V.; Domínguez, R. Muscular fatigue in response to different modalities of CrossFit sessions. PLoS ONE 2017, 12, e0181855. [Google Scholar] [CrossRef]
- Butcher, S.J.; Neyedly, T.J.; Horvey, K.J.; Benko, C.R. Do physiological measures predict selected CrossFit® benchmark performance? Open Access J. Sports Med. 2015, 6, 241. [Google Scholar] [CrossRef]
- Bellar, D.; Hatchett, A.; Judge, L.W.; Breaux, M.; Marcus, L. The relationship of aerobic capacity, anaerobic peak power and experience to performance in in CrossFit exercise. Biol. Sport 2015, 32, 315–320. [Google Scholar] [CrossRef]
- Gastin, P. Energy System Interaction and Relative Contribution During Maximal Exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef]
- Peña, J.; Moreno-Doutres, D.; Peña, I.; Chulvi-Medrano, I.; Ortegón, A.; Aguilera-Castells, J.; Buscà, B. Predicting the Unknown and the Unknowable. Are Anthropometric Measures and Fitness Profile Associated with the Outcome of a Simulated CrossFit® Competition? Int. J. Environ. Res. Public Health 2021, 18, 3692. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Reis, V.M.; Garrido, N.D.; Vianna, J.; Sousa, A.C.; Alves, J.V.; Marques, M.C. Energy cost of isolated resistance exercises across low-to high-intensities. PLoS ONE 2017, 12, e0181311. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.; Reis, V.M.; Soares, S.; Moreira-Gonçalves, D.; Fernandes, R.J. Pros and Cons of Two Methods of Anaerobic Alactic Energy Assessment in a High-Intensity CrossFit® Workout. Oxygen 2022, 2, 621–627. [Google Scholar] [CrossRef]
- Vianna, J.; Lima, J.; Saavedra, F.; Reis, V. Aerobic and anaerobic energy during resistance exercise at 80% 1 RM. J. Hum. Kinet. 2011, 29, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Koppo, K.; Bouckaert, J.; Jones, A.M. Oxygen uptake kinetics during high-intensity arm and leg exercise. Respir. Physiol. Neurobiol. 2002, 133, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Zacca, R.; Azevedo, R.; Figueiredo, P.; Vilas-Boas, J.P.; Castro, F.A.d.S.; Pyne, D.B.; Fernandes, R.J. VO2FITTING: A free and open-source software for modelling oxygen uptake kinetics in swimming and other exercise modalities. Sports 2019, 7, 31. [Google Scholar] [CrossRef]
- Zamparo, P.; Capelli, C.; Pendergast, D. Energetics of swimming: A historical perspective. Eur. J. Appl. Physiol. 2011, 111, 367–378. [Google Scholar] [CrossRef]
- Sousa, A.; Figueiredo, P.; Zamparo, P.; Pyne, D.B.; Vilas-Boas, J.P.; Fernandes, R.J. Exercise modality effect on bioenergetical performance at V̇O2max intensity. Med. Sci. Sports Exerc. 2015, 47, 1705–1713. [Google Scholar] [CrossRef]
- Burnley, M.; Jones, A.M. Oxygen uptake kinetics as a determinant of sports performance. Eur. J. Sport Sci. 2007, 7, 63–79. [Google Scholar] [CrossRef]
- Whipp, B.J.; Rossiter, H.; Ward, S. Exertional oxygen uptake kinetics: A stamen of stamina? Biochem. Soc. Trans. 2002, 30, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.L.; Lixandrão, M.E.; Ugrinowitsch, C.; Moreira, A.; Tricoli, V.; Roschel, H. Session rating of perceived exertion as an efficient tool for individualized resistance training progression. J. Strength Cond. Res. 2022, 36, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Rios, M.; Carvalho, D.D.; Monteiro, A.S.; Soares, S.; Abraldes, J.A.; Gomes, B.B.; Boas, J.P.V.; Fernandes, R.J. Mechanics and energetic analysis of rowing with Big blades with Randall foils. Int. J. Sports Med. 2022, 44, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.B.; Leighton, B.H.; Ahearn, K.J.; McManus, J.J. Aerobic, anaerobic, and excess postexercise oxygen consumption energy expenditure of muscular endurance and strength: 1-set of bench press to muscular fatigue. J. Strength Cond. Res. 2011, 25, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.B.; Booher, B.M.; Lawton, N. Comparison of acute energy expenditure and rating of perceived exertion in equivalent bouts of circuit training and treadmill running. J. Strength Cond. Res. 2021, 35, 680–687. [Google Scholar] [CrossRef]
- Thornton, M.K.; Potteiger, J.A. Effects of resistance exercise bouts of different intensities but equal work on EPOC. Med. Sci. Sports Exerc. 2002, 34, 715–722. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Rosenberg, J.G.; Kang, J.; Sundberg, S.; Izer, K.A.; Levowsky, J.; Rzeszutko, C.; Ross, R.E.; Faigenbaum, A.D. Acute oxygen uptake and resistance exercise performance using different rest interval lengths: The influence of maximal aerobic capacity and exercise sequence. J. Strength Cond. Res. 2014, 28, 1875–1888. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Kang, J.; Kuper, J.D.; O’Grady, E.A.; Ellis, N.L.; Vought, I.T.; Culleton, E.; Bush, J.A.; Faigenbaum, A.D. Acute Cardiorespiratory and Metabolic Effects of a Sandbag Resistance Exercise Protocol. J. Strength Cond. Res. 2018, 32, 1491–1502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).