The Failure of Molecular Imprinting in Conducting Polymers: A Case Study of Imprinting Picric Acid on Polycarbazole
Abstract
1. Introduction
2. Materials and Methods
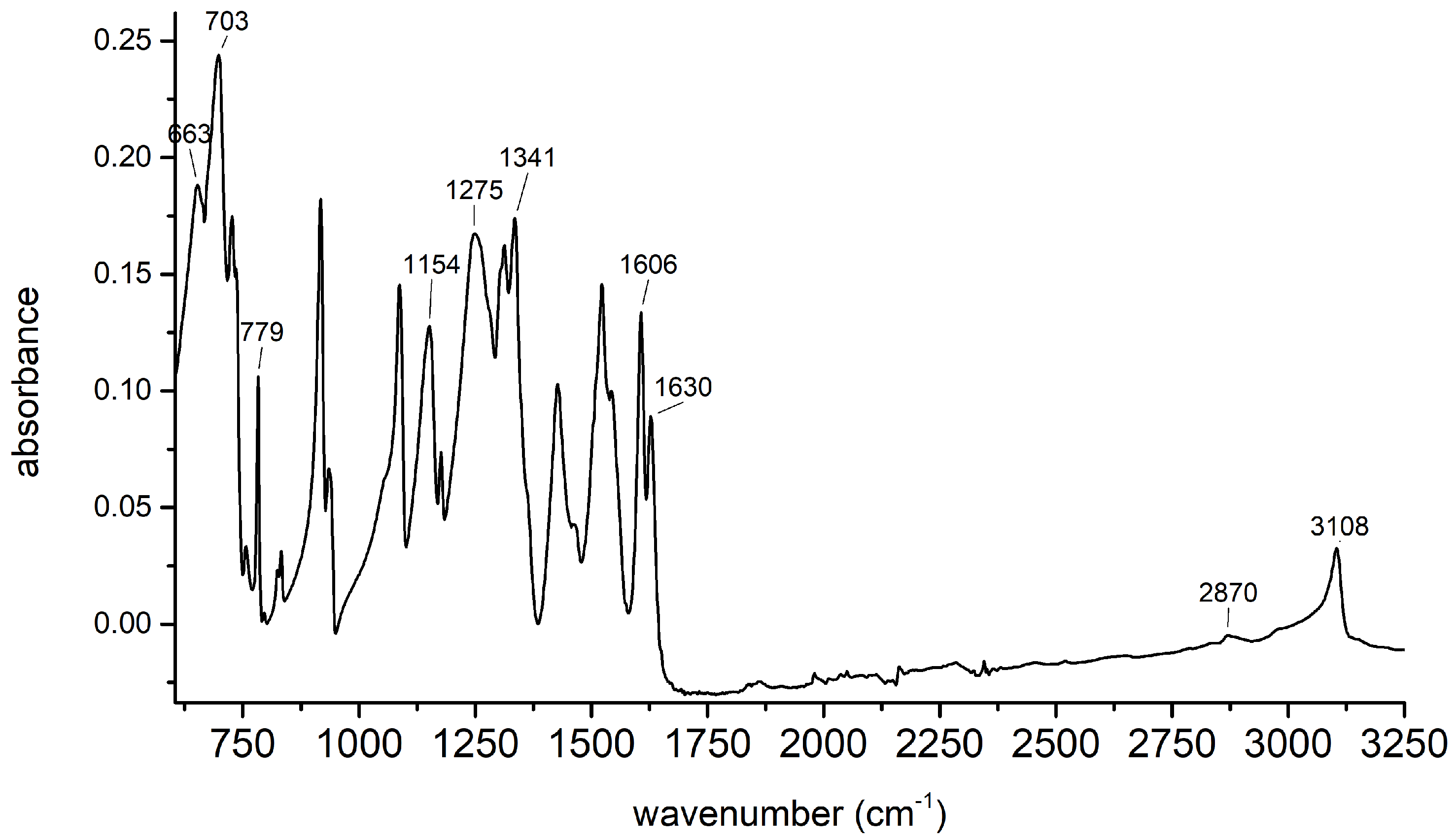
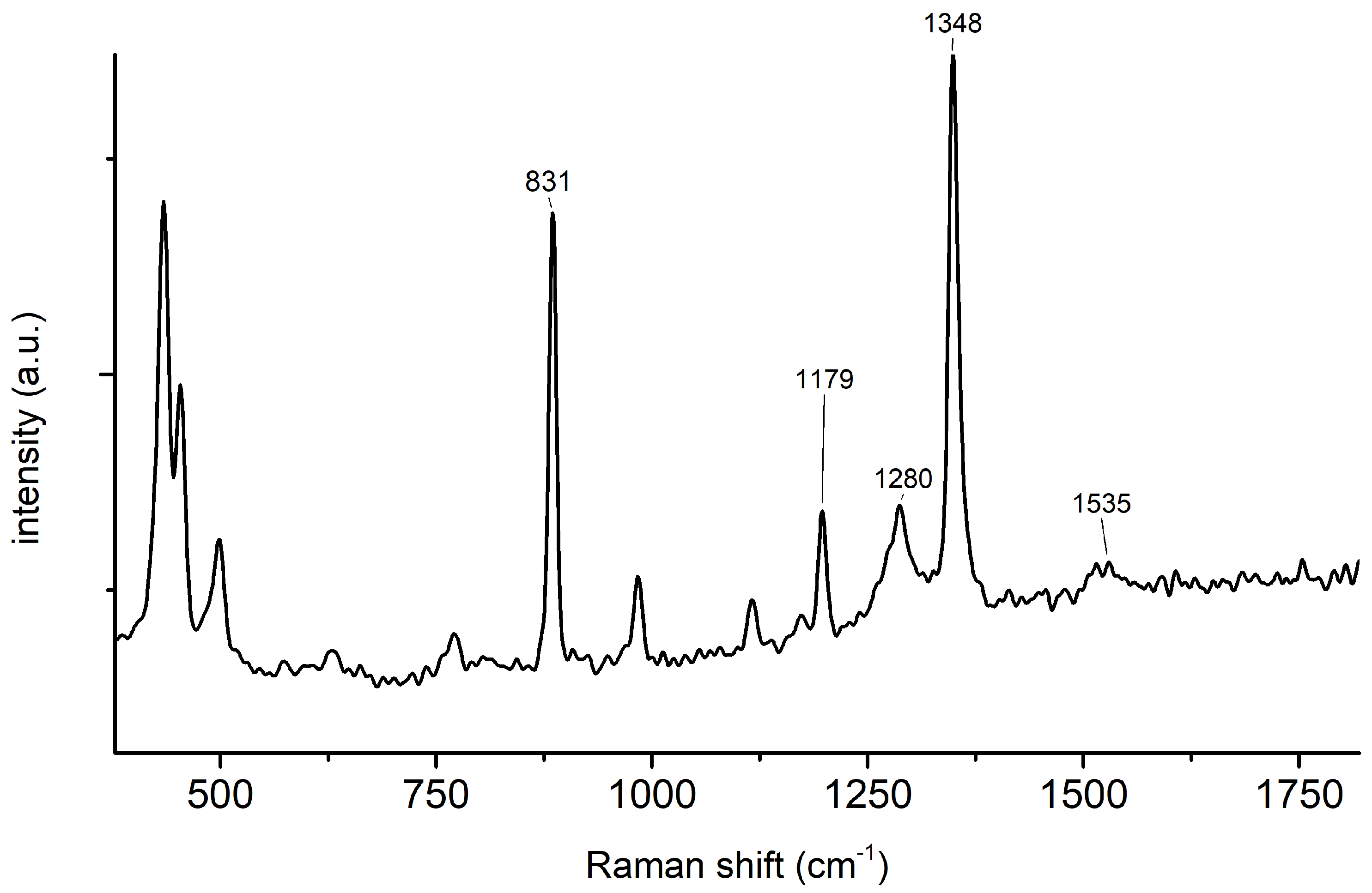
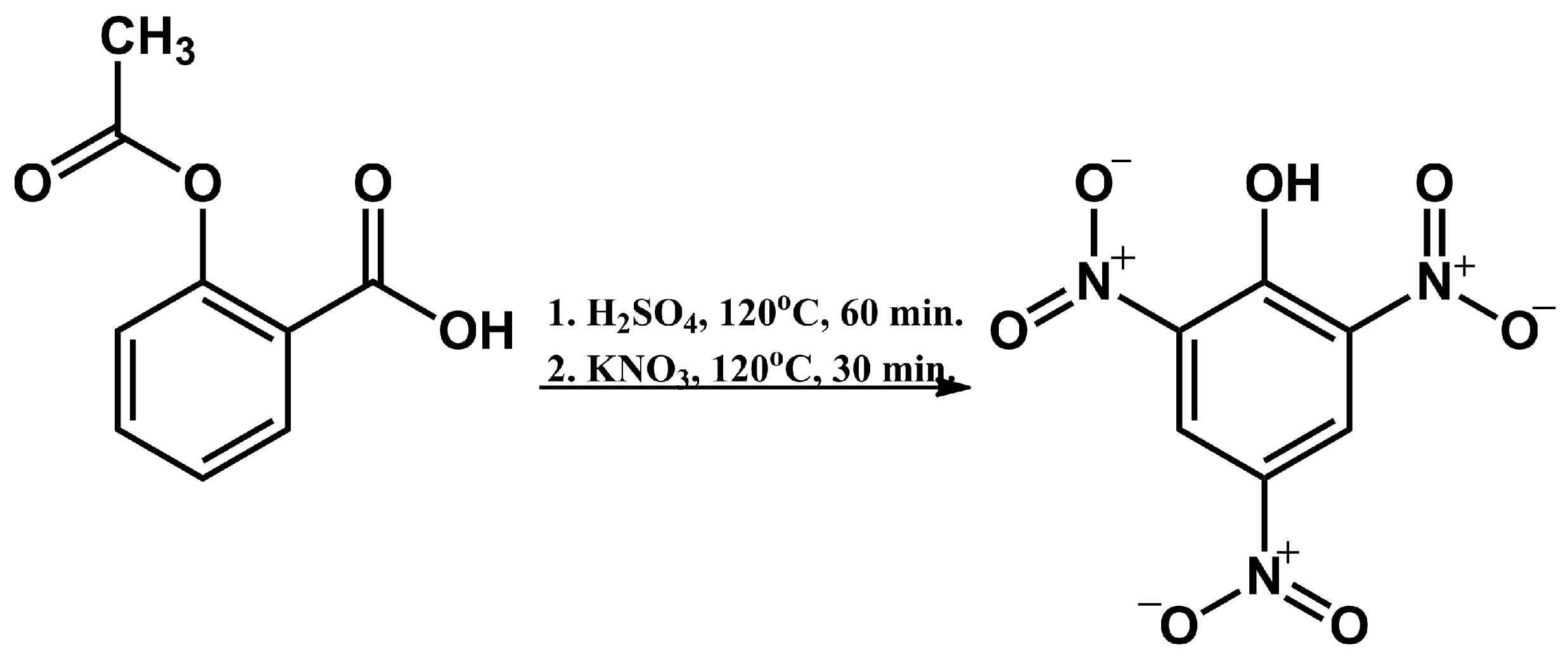
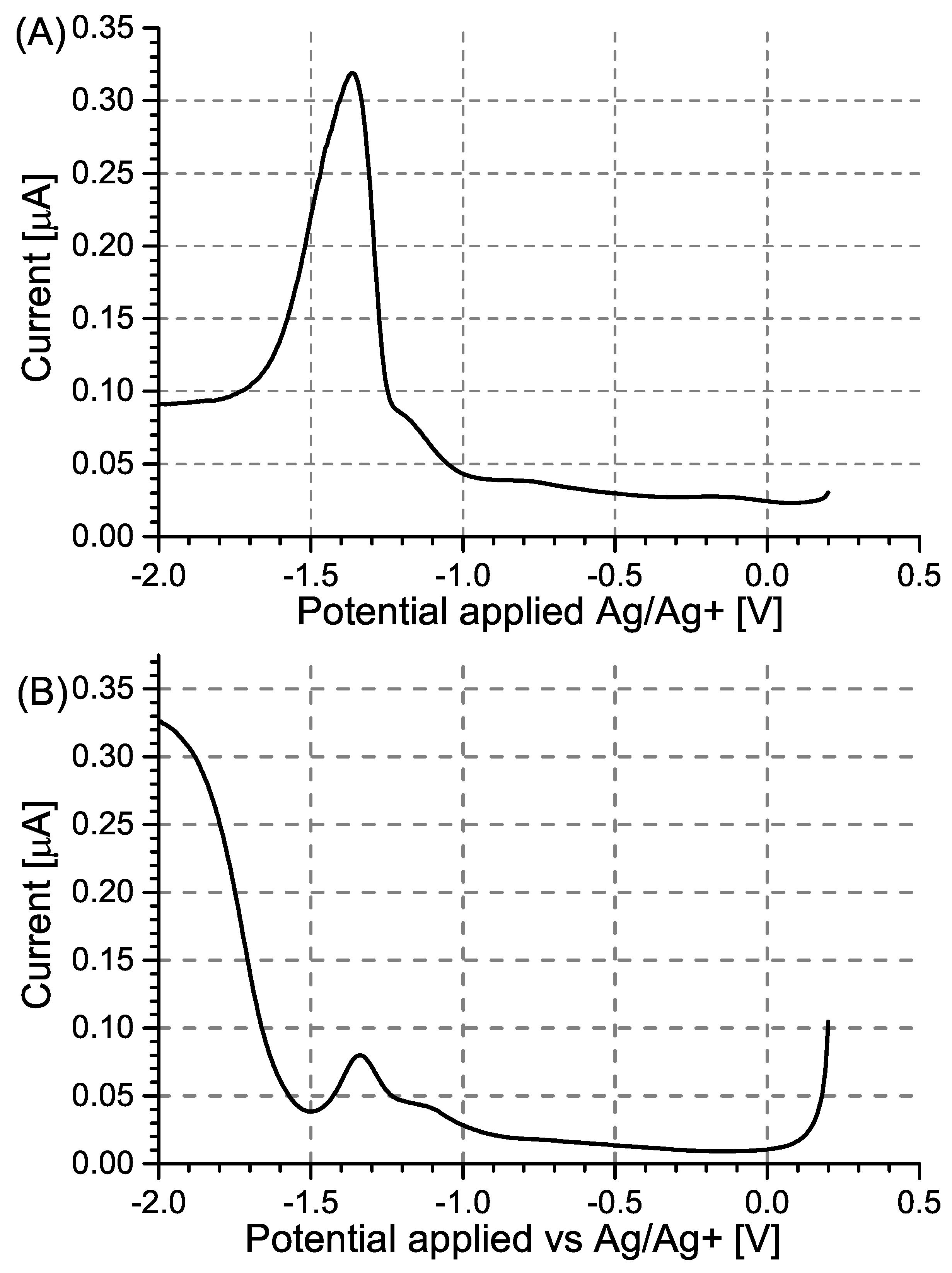
2.1. Synthesis of 2,4,6-Trinitrophenol
2.2. Electrochemical Investigations
2.3. Quantum Chemical Calculations
2.4. SEM Analyses
3. Results and Discussion
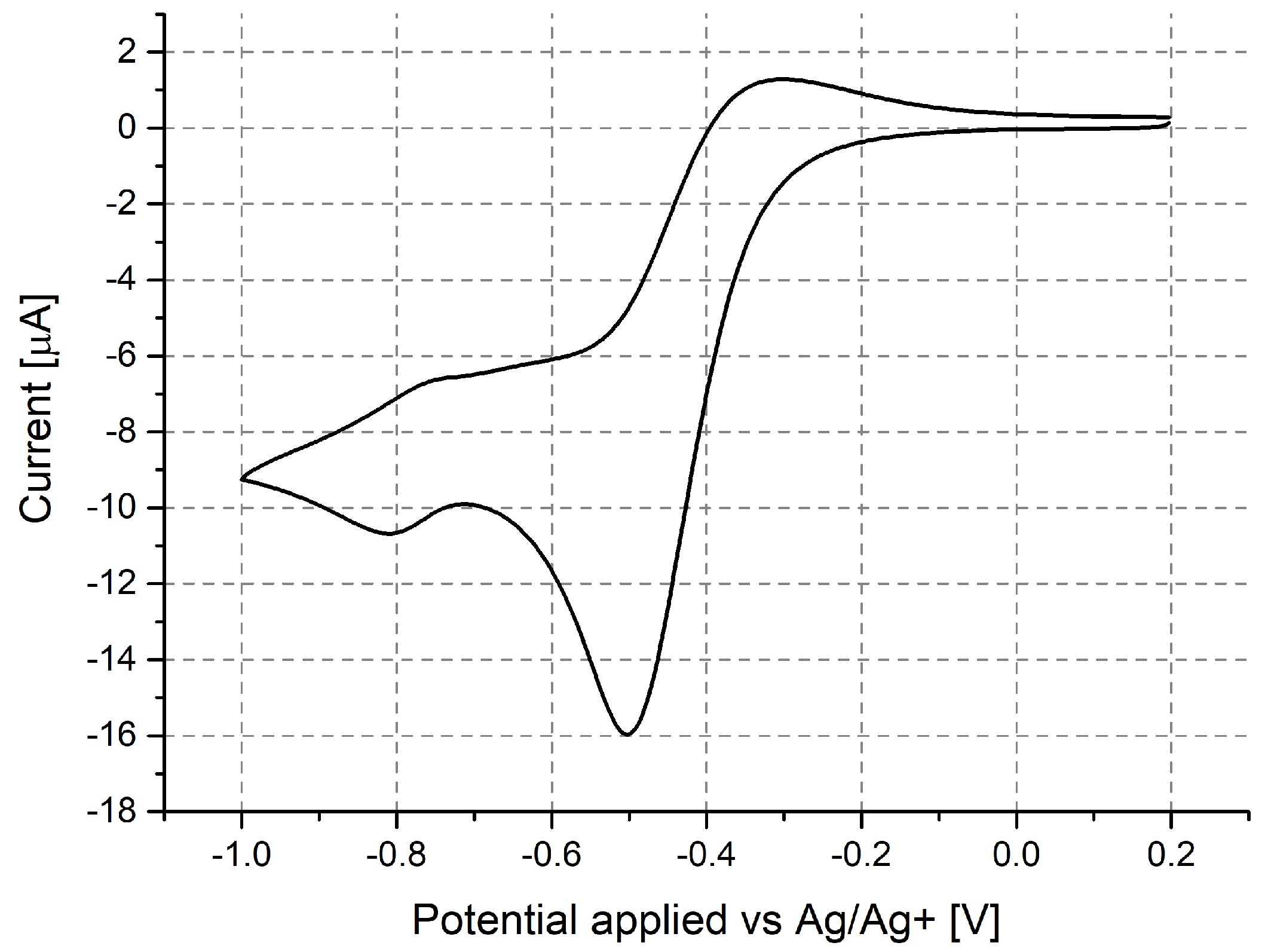
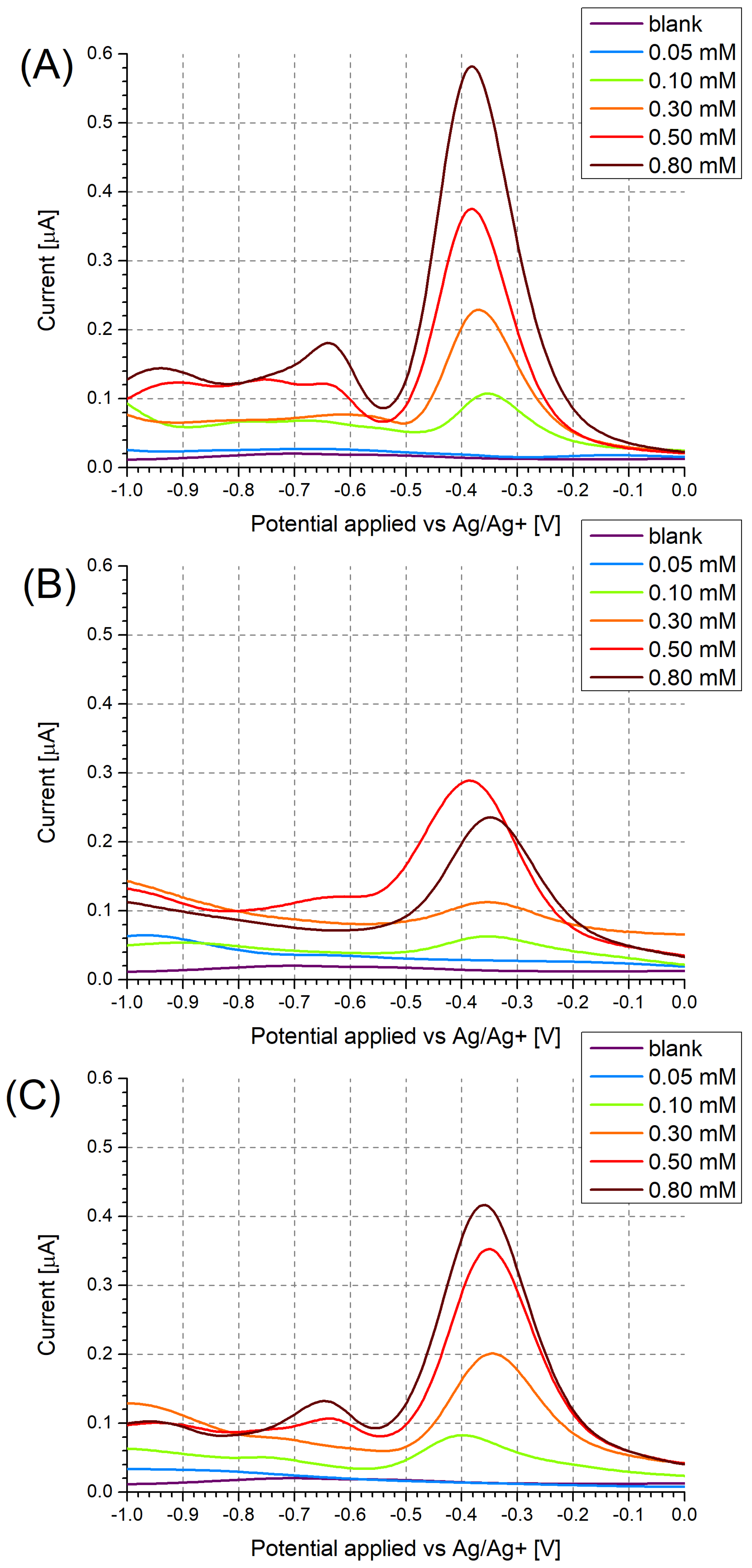
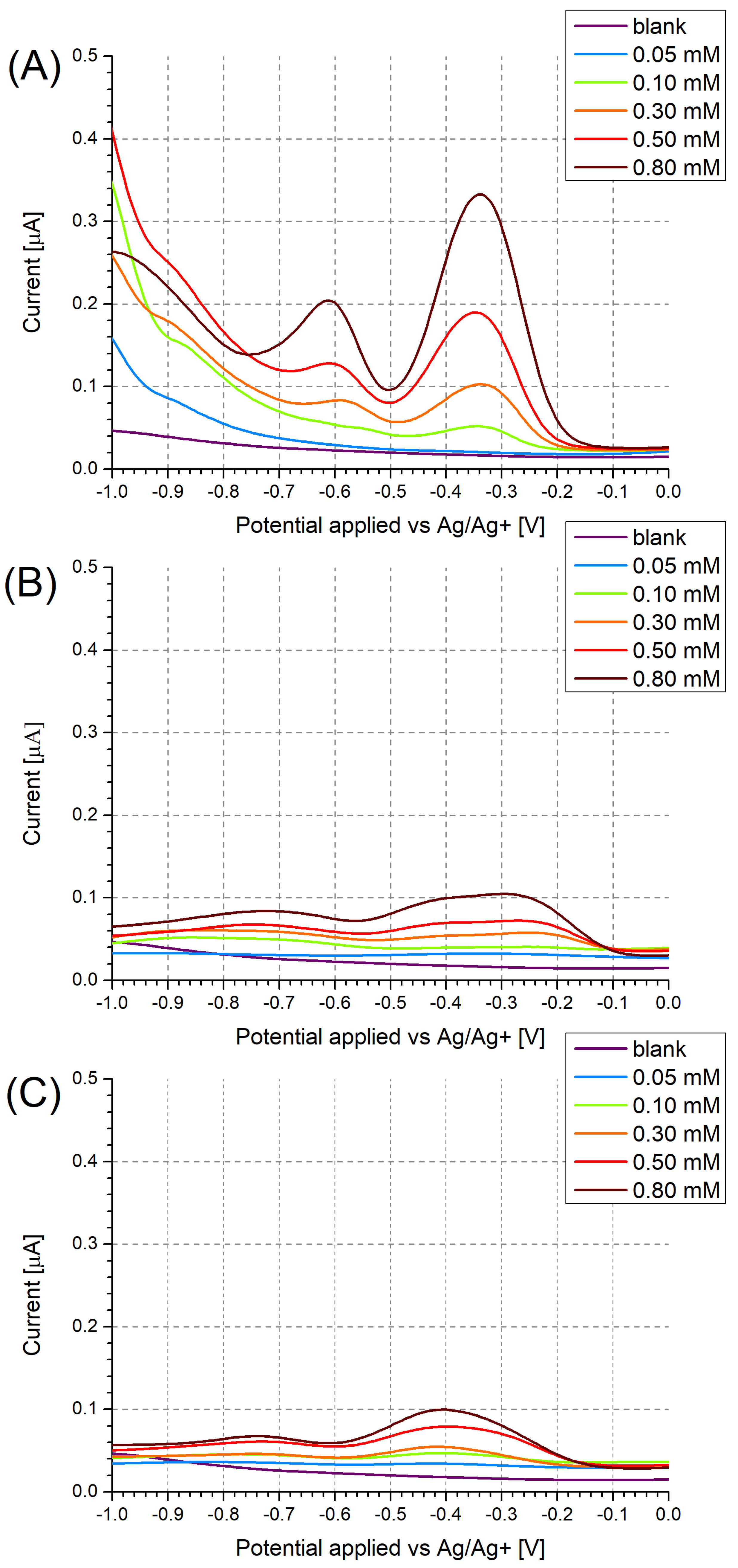
3.1. Investigation of Polymer Layers Deposited on Platinum Electrodes
3.2. Investigation of Polymer Layers Deposited on Glassy Carbon Electrodes
3.3. Investigation of Electrode Cross-Selectivity
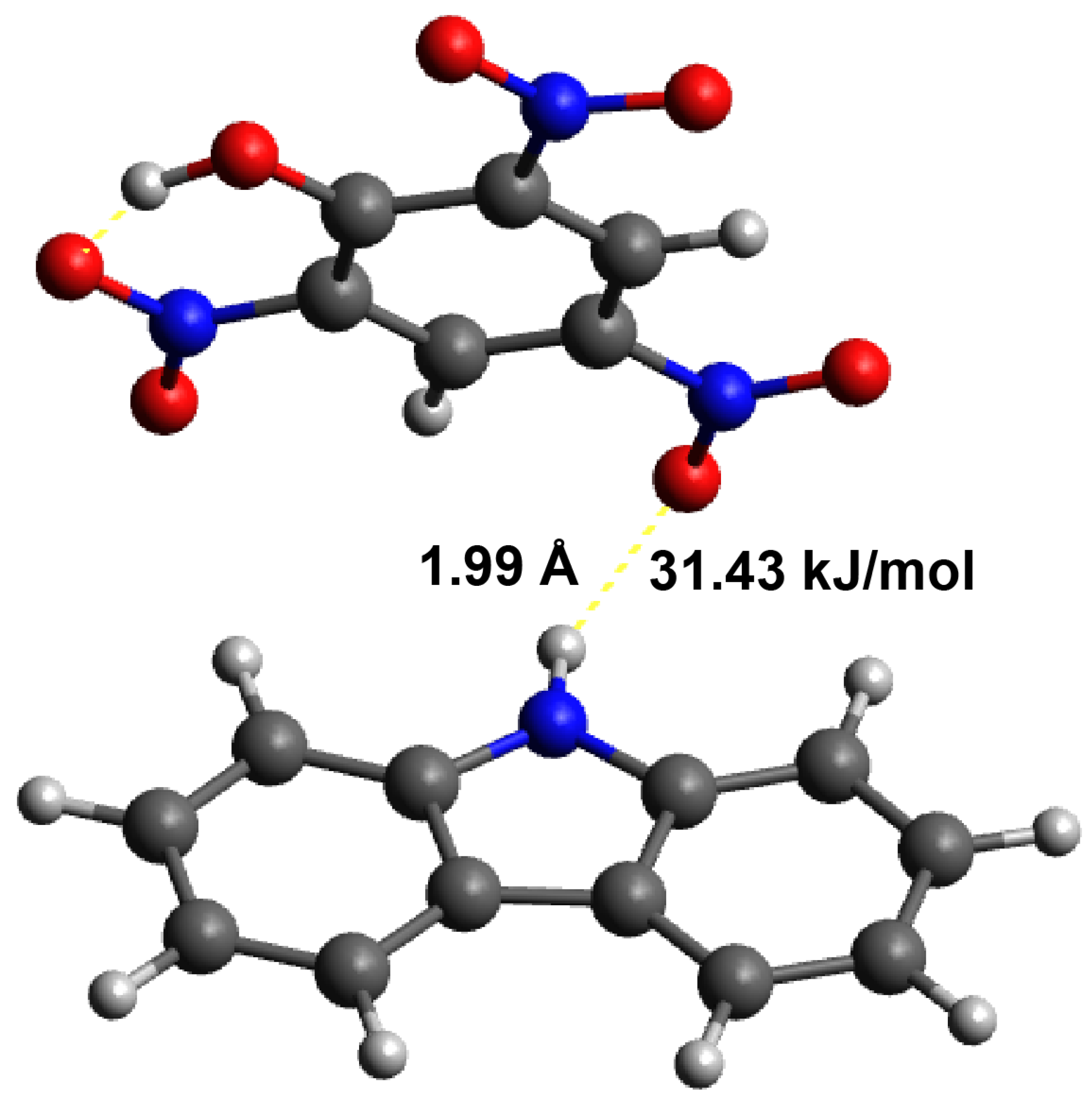
3.4. Calculation of the Interactions between the Template and Monomer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Carbazole | |||
|---|---|---|---|
| N | 0.02981 | 1.82223 | −0.00815 |
| C | −1.11644 | 1.02826 | −0.00910 |
| C | −0.71386 | −0.34060 | −0.01061 |
| C | 0.74168 | −0.35128 | −0.01054 |
| C | 1.16430 | 1.01151 | −0.00900 |
| C | 2.51851 | 1.35112 | −0.00852 |
| C | 3.45290 | 0.31688 | −0.00961 |
| C | 3.05074 | −1.03126 | −0.01113 |
| C | 1.69857 | −1.37045 | −0.01161 |
| C | −1.68551 | −1.34569 | −0.01177 |
| C | −3.03260 | −0.98678 | −0.01142 |
| C | −3.41495 | 0.36711 | −0.00993 |
| C | −2.46558 | 1.38760 | −0.00875 |
| H | −2.76961 | 2.42780 | −0.00760 |
| H | −4.46861 | 0.62305 | −0.00968 |
| H | −3.79597 | −1.75596 | −0.01231 |
| H | −1.39387 | −2.39031 | −0.01294 |
| H | 4.51023 | 0.55737 | −0.00926 |
| H | 2.83764 | 2.38678 | −0.00735 |
| H | 1.39172 | −2.41065 | −0.01280 |
| H | 3.80272 | −1.81159 | −0.01195 |
| H | 0.03719 | 2.83358 | −0.00696 |
| Picric Acid | |||
|---|---|---|---|
| C | 0.00945 | 3.33411 | 0.03651 |
| C | −1.17395 | 2.54845 | 0.02991 |
| C | −1.12209 | 1.17076 | 0.02080 |
| C | 0.10007 | 0.51084 | 0.01801 |
| C | 1.28347 | 1.21765 | 0.02437 |
| C | 1.23781 | 2.60246 | 0.03349 |
| N | 2.49980 | 3.30598 | 0.04006 |
| N | −2.51177 | 3.15454 | 0.03243 |
| N | 0.13368 | −0.94626 | 0.00818 |
| O | −2.59304 | 4.42698 | 0.04244 |
| O | −3.50351 | 2.33420 | 0.02650 |
| O | −0.99687 | −1.54952 | 0.00292 |
| O | 2.47491 | 4.62626 | 0.04753 |
| H | −2.04592 | 0.61352 | 0.01596 |
| H | 2.23067 | 0.70114 | 0.02231 |
| O | −0.01358 | 4.66298 | 0.04530 |
| H | 0.98294 | 4.97739 | 0.04855 |
| O | 3.57888 | 2.63791 | 0.03726 |
| O | 1.29258 | −1.49580 | 0.00624 |
| Carbazole + Picric Acid | |||
|---|---|---|---|
| C | 0.78977 | 3.54467 | 2.65822 |
| C | −0.62494 | 3.42763 | 2.71890 |
| C | −1.39194 | 3.36173 | 1.57606 |
| C | −0.79390 | 3.39592 | 0.32093 |
| C | 0.57713 | 3.48852 | 0.20030 |
| C | 1.35118 | 3.57474 | 1.34362 |
| N | 2.77608 | 3.71222 | 1.16349 |
| N | −1.34928 | 3.37843 | 3.99743 |
| N | −1.61719 | 3.36607 | −0.86974 |
| O | −0.66607 | 3.42812 | 5.07247 |
| O | −2.62944 | 3.28279 | 3.91711 |
| O | −2.87847 | 3.25082 | −0.71841 |
| O | 3.53374 | 3.77284 | 2.23971 |
| H | −2.46434 | 3.29022 | 1.67508 |
| H | 1.05438 | 3.49186 | −0.76596 |
| O | 1.54920 | 3.62212 | 3.74411 |
| H | 2.53593 | 3.70336 | 3.41606 |
| O | 3.25032 | 3.76931 | −0.01722 |
| O | −1.01095 | 3.48576 | −2.01032 |
| C | 0.77076 | 7.08961 | −1.22926 |
| N | −0.20516 | 6.21119 | −1.69641 |
| C | −1.41935 | 6.88531 | −1.82469 |
| C | −1.22820 | 8.24325 | −1.43832 |
| C | 0.17149 | 8.37596 | −1.05892 |
| C | 0.94539 | 9.44130 | −0.58998 |
| C | 2.29053 | 9.22493 | −0.29392 |
| C | 2.86441 | 7.95078 | −0.45292 |
| C | 2.11506 | 6.86993 | −0.91808 |
| C | −2.66552 | 6.40365 | −2.23297 |
| C | −3.72888 | 7.30395 | −2.26060 |
| C | −3.55676 | 8.65113 | −1.88917 |
| C | −2.31301 | 9.12582 | −1.47445 |
| H | −2.18901 | 10.16273 | −1.18224 |
| H | −4.40587 | 9.32389 | −1.92215 |
| H | −4.70834 | 6.95814 | −2.57062 |
| H | −2.79492 | 5.36025 | −2.49568 |
| H | 2.90159 | 10.04348 | 0.06773 |
| H | 0.50375 | 10.42276 | −0.45749 |
| H | 2.56567 | 5.88758 | −1.00781 |
| H | 3.90976 | 7.80228 | −0.20651 |
| H | −0.08465 | 5.24726 | −1.98864 |
References
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef]
- Jamieson, O.; Soares, T.C.; de Faria, B.A.; Hudson, A.; Mecozzi, F.; Rowley-Neale, S.J.; Banks, C.E.; Gruber, J.; Novakovic, K.; Peeters, M.; et al. Screen printed electrode based detection systems for the antibiotic amoxicillin in aqueous samples utilising molecularly imprinted polymers as synthetic receptors. Chemosensors 2019, 8, 5. [Google Scholar] [CrossRef]
- Stringer, R.C.; Gangopadhyay, S.; Grant, S.A. Detection of nitroaromatic explosives using a fluorescent-labeled imprinted polymer. Anal. Chem. 2010, 82, 4015–4019. [Google Scholar] [CrossRef]
- Bal, M.; Köse, A.; Özpaça, Ö.; Köse, M. Pyrene, Anthracene, and Naphthalene-Based Azomethines for Fluorimetric Sensing of Nitroaromatic Compounds. J. Fluoresc. 2023, 33, 1–13. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of fluorescent sensors based on azaheterocyclic push-pull systems towards nitroaromatic explosives and related compounds: A review. Dyes Pigments 2020, 180, 108414. [Google Scholar] [CrossRef]
- Goerlitz, D. Analysis of Picric Acid in Water by High-Performance Liquid Chromatography; Technical Report; US Geological Survey: Reston, VA, USA, 1979.
- Nipper, M.; Carr, R.S.; Biedenbach, J.M.; Hooten, R.L.; Miller, K. Fate and effects of picric acid and 2,6-DNT in marine environments: Toxicity of degradation products. Mar. Pollut. Bull. 2005, 50, 1205–1217. [Google Scholar] [CrossRef]
- Hebert, R.M.; Jackovitz, A.M. Wildlife toxicity assessment for picric acid (2, 4, 6-trinitrophenol). In Wildlife Toxicity Assessments for Chemicals of Military Concern; Elsevier: Amsterdam, The Netherlands, 2015; pp. 271–277. [Google Scholar]
- Nagarkar, S.S.; Desai, A.V.; Samanta, P.; Ghosh, S.K. Aqueous phase selective detection of 2, 4, 6-trinitrophenol using a fluorescent metal–organic framework with a pendant recognition site. Dalton Trans. 2015, 44, 15175–15180. [Google Scholar] [CrossRef]
- Wyman, J.F.; Serve, M.P.; Hobson, D.W.; Lee, L.H.; Uddin, D.E. Acute toxicity, distribution, and metabolism of 2,4,6-trinitrophenol (picric acid) in Fischer 344 rats. J. Toxicol. Environ. Health 1992, 37, 313–327. [Google Scholar] [CrossRef]
- Ludwig, H.R.; Cairelli, S.G.; Whalen, J.J. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHS); National Institute for Occupational Safety and Health, Division of Standards Development and Technology Transfer: Cincinnati, OH, USA, 1994.
- Agarwal, P.; Goyal, A.; Vaishnav, R. Chemical hazards in pharmaceutical industry: An overview. Asian J. Pharm. Clin. Res. 2018, 11, 27–35. [Google Scholar] [CrossRef][Green Version]
- Barron, L.; Gilchrist, E. Ion chromatography-mass spectrometry: A review of recent technologies and applications in forensic and environmental explosives analysis. Anal. Chim. Acta 2014, 806, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; He, Y.; Ding, L.; Su, B. Highly ordered binary assembly of silica mesochannels and surfactant micelles for extraction and electrochemical analysis of trace nitroaromatic explosives and pesticides. Anal. Chem. 2015, 87, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, H.; Peng, S.; Wang, L. Highly selective and sensitive fluorescent paper sensor for nitroaromatic explosive detection. Anal. Chem. 2012, 84, 8415–8421. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Liu, Z.X.; Huang, C.Z. Highly selective and sensitive detection of 2, 4, 6-trinitrophenol by using newly developed blue–green photoluminescent carbon nanodots. Talanta 2016, 161, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Feng, X.; Wang, B.; Yang, L. Explosives in the cage: Metal–organic frameworks for high-energy materials sensing and desensitization. Adv. Mater. 2017, 29, 1701898. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, K.; Mukherjee, P.S. A fluorescent organic cage for picric acid detection. Chem. Commun. 2014, 50, 15788–15791. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, S.; Li, R.; Pan, L.; Li, L.; Qi, Z.; Li, C. Highly selective detection of nitroaromatic explosive 2,4,6-trinitrophenol (TNP) using N-doped carbon dots. Res. Chem. Intermed. 2021, 47, 2421–2431. [Google Scholar] [CrossRef]
- Surya, S.G.; Raval, H.N.; Ahmad, R.; Sonar, P.; Salama, K.N.; Rao, V.R. Organic field effect transistors (OFETs) in environmental sensing and health monitoring: A review. TrAC Trends Anal. Chem. 2019, 111, 27–36. [Google Scholar] [CrossRef]
- Saglam, S.; Uzer, A.; Apak, R. Direct determination of peroxide explosives on polycarbazole/gold nanoparticle-modified glassy carbon sensor electrodes imprinted for molecular recognition of TATP and HMTD. Anal. Chem. 2022, 94, 17662–17669. [Google Scholar] [CrossRef]
- Bekkar, F.; Bettahar, F.; Moreno, I.; Meghabar, R.; Hamadouche, M.; Hernáez, E.; Vilas-Vilela, J.L.; Ruiz-Rubio, L. Polycarbazole and its derivatives: Synthesis and applications. A review of the last 10 years. Polymers 2020, 12, 2227. [Google Scholar] [CrossRef]
- Pernites, R.; Ponnapati, R.; Felipe, M.J.; Advincula, R. Electropolymerisation molecularly imprinted polymer (E-MIP) SPR sensing of drug molecules: Pre-polymerisation complexed terthiophene and carbazole electroactive monomers. Biosens. Bioelectron. 2011, 26, 2766–2771. [Google Scholar] [CrossRef]
- Karthika, P.; Shanmuganathan, S.; Viswanathan, S. Electrochemical sensor for picric acid by using molecularly imprinted polymer and reduced graphene oxide modified pencil graphite electrode. Proc. Indian Natl. Sci. Acad. 2022, 88, 263–276. [Google Scholar] [CrossRef]
- Huynh, T.P.; Wojnarowicz, A.; Kelm, A.; Woznicki, P.; Borowicz, P.; Majka, A.; D’Souza, F.; Kutner, W. Chemosensor for selective determination of 2, 4, 6-trinitrophenol using a custom designed imprinted polymer recognition unit cross-linked to a fluorophore transducer. Acs Sens. 2016, 1, 636–639. [Google Scholar] [CrossRef]
- Murugan, K.; Jothi, V.K.; Rajaram, A.; Natarajan, A. Novel metal-free fluorescent sensor based on molecularly imprinted polymer N-CDs@ MIP for highly selective detection of TNP. ACS Omega 2021, 7, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Martín-Esteban, A. Molecularly-imprinted polymers as a versatile, highly selective tool in sample preparation. TrAC Trends Anal. Chem. 2013, 45, 169–181. [Google Scholar] [CrossRef]
- Karadurmus, L.; Bilge, S.; Sınağ, A.; Ozkan, S.A. Molecularly imprinted polymer (MIP)-Based sensing for detection of explosives: Current perspectives and future applications. TrAC Trends Anal. Chem. 2022, 155, 116694. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors affecting preparation of molecularly imprinted polymer and methods on finding template-monomer interaction as the key of selective properties of the materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, F.; Chen, W.; Wang, J.; Li, K. Influence of intramolecular hydrogen bond of templates on molecular recognition of molecularly imprinted polymers. Anal. Chim. Acta 2001, 450, 53–61. [Google Scholar] [CrossRef]
- Liu, H.; Jin, P.; Zhu, F.; Nie, L.; Qiu, H. A review on the use of ionic liquids in preparation of molecularly imprinted polymers for applications in solid-phase extraction. TRAC Trends Anal. Chem. 2021, 134, 116132. [Google Scholar] [CrossRef]
- Turner, N.W.; Holdsworth, C.I.; Donne, S.W.; McCluskey, A.; Bowyer, M.C. Microwave induced MIP synthesis: Comparative analysis of thermal and microwave induced polymerisation of caffeine imprinted polymers. New J. Chem. 2010, 34, 686–692. [Google Scholar] [CrossRef]
- Hillberg, A.L.; Brain, K.R.; Allender, C.J. Design and evaluation of thin and flexible theophylline imprinted polymer membrane materials. J. Mol. Recognit. Interdiscip. J. 2009, 22, 223–231. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yan, M.; Zhang, M.; Liu, Z.; Li, Y. A computational and experimental investigation of the interaction between the template molecule and the functional monomer used in the molecularly imprinted polymer. Anal. Chim. Acta 2005, 542, 186–192. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit—A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Olson, E.J.; Isley III, W.C.; Brennan, J.E.; Cramer, C.J.; Bühlmann, P. Electrochemical reduction of 2, 4-dinitrotoluene in aprotic and pH-buffered media. J. Phys. Chem. C 2015, 119, 13088–13097. [Google Scholar] [CrossRef]
- Janderka, P.; Fischer, O.; Fischerová, E. Effect of the solvent basicity and additives on the electroreduction of picric acid and tetramethylammonium picrate in aprotic media. Collect. Czechoslov. Chem. Commun. 1997, 62, 581–596. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Yu, S.; Zhang, J.; Zheng, W.; Niu, L.; Li, G. Poly (3, 6-diamino-9-ethylcarbazole) based molecularly imprinted 396 polymer sensor for ultra-sensitive and selective detection of 17-β-estradiol in biological fluids. Biosens. Bioelectron. 2018, 104, 79–86. [Google Scholar] [CrossRef]






| MIP Receptor Layer | Media | LOD | Ref. |
|---|---|---|---|
| MIP/rGO/PGE a | Water and soil | 1.4 mol/L | [24] |
| BTAM b | Acetonitrle-to-toluene (95:5) | 0.2 ng/L | [25] |
| N-CDs@MIP c | Water | 0.15 nM | [26] |
| Electrode | b Factor a | Limit of Detection (LOD) b |
|---|---|---|
| Unmodified Pt | 9.8 × 10−7 | 0.09 mM |
| NIP PCz/Pt | 3.8 × 10−7 | 0.62 mM |
| MIP PCz/Pt | 7.6 × 10−7 | 0.26 mM |
| Unmodified GC | 5.4 × 10−7 | 0.11 mM |
| NIP PCz/GC | 1.4 × 10−7 | 0.12 mM |
| MIP PCz/GC | 1.4 × 10−7 | 0.57 mM |
| Compound | NIP Cz/Pt | MIP Cz/Pt | IF Pt | MIP Cz/GC | IF GC |
|---|---|---|---|---|---|
| PA (0.8 mM) | 0.235 A | 0.416 A | 1.77 | 0.099 A | 0.95 |
| Nitrobenzene (9 mM) | 0.056 A | 0.319 A | 5.70 | 0.616 A | - |
| Nitromethane (18 mM) | 0.136 A | 0.080 A | 0.59 | 0.060 A | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głosz, K.; Fabin, M.; Janasik, P.; Kołodziej, W.; Stolarczyk, A.; Jarosz, T. The Failure of Molecular Imprinting in Conducting Polymers: A Case Study of Imprinting Picric Acid on Polycarbazole. Sensors 2024, 24, 424. https://doi.org/10.3390/s24020424
Głosz K, Fabin M, Janasik P, Kołodziej W, Stolarczyk A, Jarosz T. The Failure of Molecular Imprinting in Conducting Polymers: A Case Study of Imprinting Picric Acid on Polycarbazole. Sensors. 2024; 24(2):424. https://doi.org/10.3390/s24020424
Chicago/Turabian StyleGłosz, Karolina, Magdalena Fabin, Patryk Janasik, Weronika Kołodziej, Agnieszka Stolarczyk, and Tomasz Jarosz. 2024. "The Failure of Molecular Imprinting in Conducting Polymers: A Case Study of Imprinting Picric Acid on Polycarbazole" Sensors 24, no. 2: 424. https://doi.org/10.3390/s24020424
APA StyleGłosz, K., Fabin, M., Janasik, P., Kołodziej, W., Stolarczyk, A., & Jarosz, T. (2024). The Failure of Molecular Imprinting in Conducting Polymers: A Case Study of Imprinting Picric Acid on Polycarbazole. Sensors, 24(2), 424. https://doi.org/10.3390/s24020424







