Positively Charged Organosilanes Covalently Linked to the Silica Network as Modulating Tools for the Salinity Correction of pH Values Obtained with Colorimetric Sensor Arrays (CSAs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
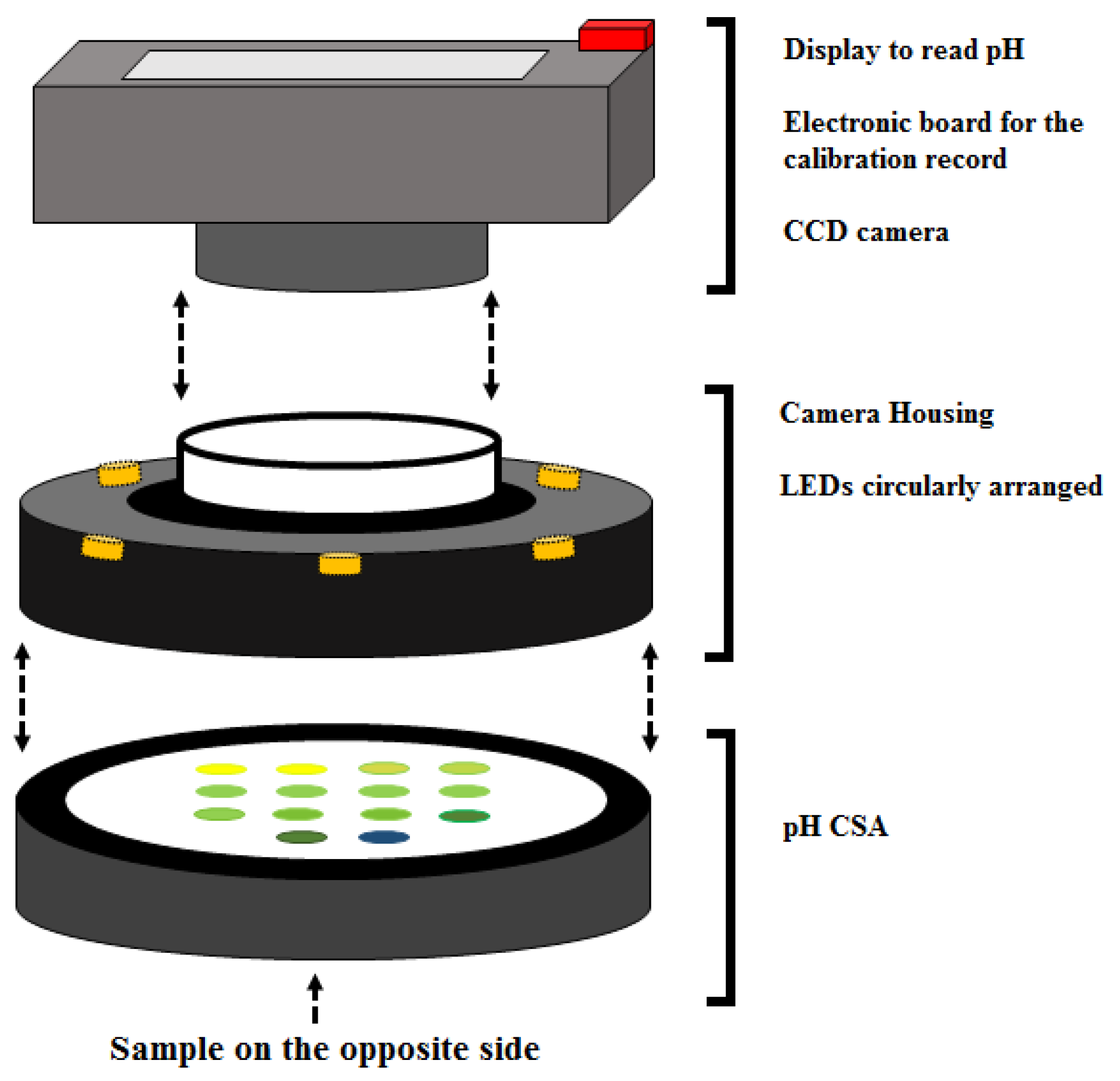
2.2. Measurement Cell
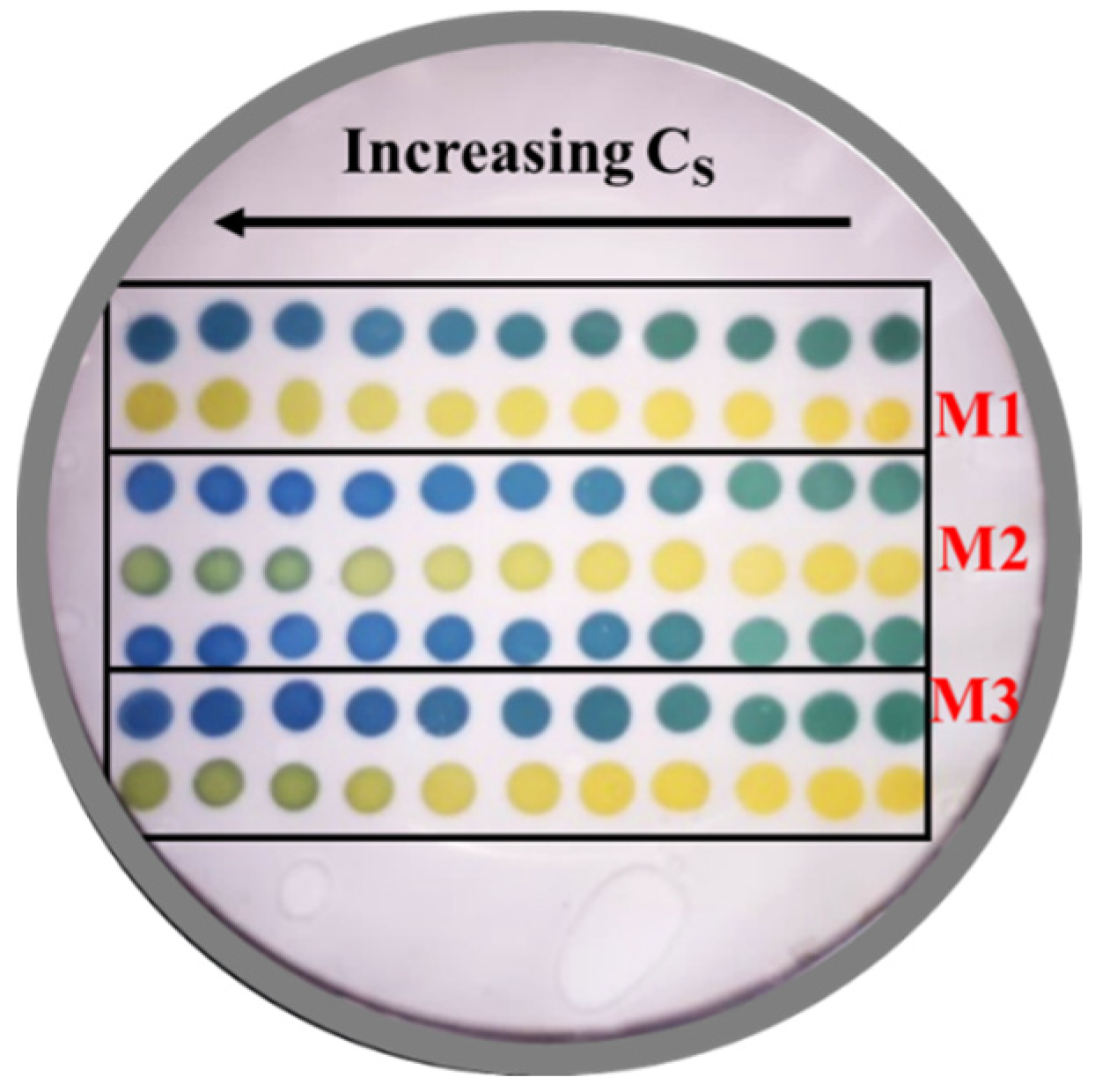
2.3. Preparation of Nitrocellulose-Supported Colorimetric Sensors
2.4. Preparation of the Buffer Solutions
2.5. Electronic Board
3. Results and Discussion
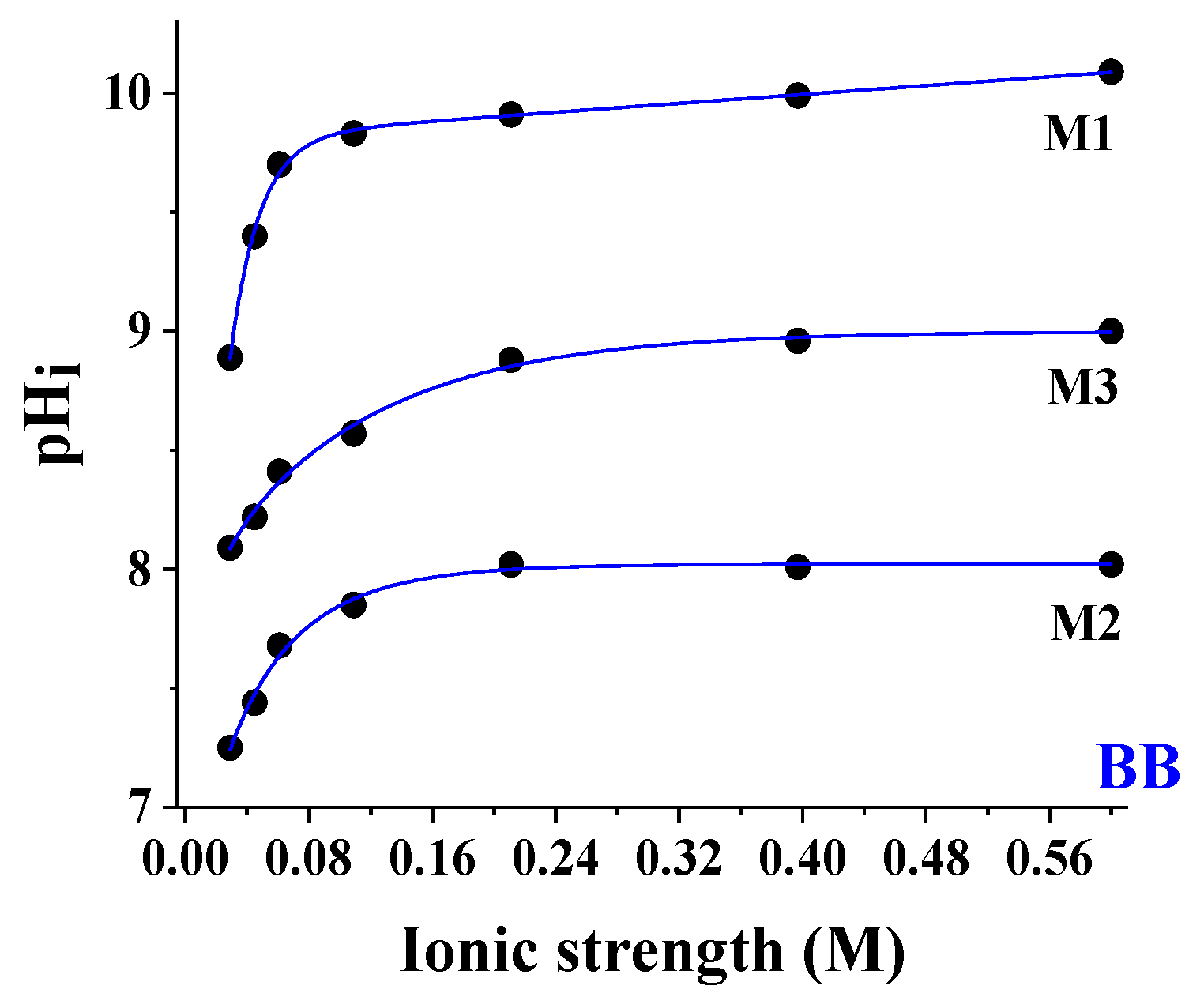
- The pH shift due to the surfactant was lower at large salinity values. Referring to Figure 4a, the BCG indicator, for instance, moved from pH 8.37 to 2.51 (Δ = 5.86) at salinity of 0.029 M (■) and from pH 7.43 to 5.65 (Δ = 1.78) from at salinity of 0.397 M (●). Theoretically, to produce a full-range sensor, the best situation was characterized by the largest pH variation due to the surfactant. This would be advisable at all the ion strengths. A constant trend of the values vs. (see for instance BB at large ion strength) led to having only one or two active spots, rendering the array useless as it read a limited pH interval.
- BB was less affected than BCG.
- The pH shift due to the salinity was positive for spots with larger values and negative for the less concentrated ones, leading to intersection points representing the independence of the measured pH from salinity. These points can be taken as references to correct the dependence on salinity using suitable theoretical models. Considering that the working interval was around 2.50 pH units for each spot, the pH interval in which it is possible to perform an autocorrection with suitable software integrated with the pH-CSA device was between pH = 5.00 and 12.00. To achieve an accurate measurement below pH = 5.00, it was necessary to use another pH indicator or a second device like a conductivity probe.
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelis, J.L.D.; Bura, L.; Zhao, Y.; Burkin, K.M.; Rafferty, K.; Elliott, C.T.; Campbell, K. The Efficiency of Color Space Channels to Quantify Color and Color Intensity Change in Liquids, PH Strips, and Lateral Flow Assays with Smartphones. Sensors 2019, 19, 5104. [Google Scholar] [CrossRef]
- Kuswandi, B.; Asih, N.P.N.; Pratoko, D.K.; Kristiningrum, N.; Moradi, M. Edible pH Sensor Based on Immobilized Red Cabbage Anthocyanins into Bacterial Cellulose Membrane for Intelligent Food Packaging. Packag. Technol. Sci. 2020, 33, 321–332. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Alberti, G.; Zanoni, C.; Guembe-Garcia, M.; Quadrelli, P.; Biesuz, R. Chemometric-Assisted Litmus Test: One Single Sensing Platform Adapted from 1–13 to Narrow pH Ranges. Sensors 2023, 23, 1696. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Capone, F.; Zanoni, C.; Alberti, G.; Quadrelli, P.; Biesuz, R. Colorimetric Sensor Array for Monitoring, Modelling and Comparing Spoilage Processes of Different Meat and Fish Foods. Foods 2020, 9, 684. [Google Scholar] [CrossRef]
- Kamer, D.D.A.; Kaynarca, G.B.; Yücel, E.; Gümüş, T. Development of Gelatin/PVA Based Colorimetric Films with a Wide pH Sensing Range Winery Solid by-Product (Vinasse) for Monitor Shrimp Freshness. Int. J. Biol. Macromol. 2022, 220, 627–637. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Hu, W.; Liu, Y.; Dong, C.; Chen, Q. Monitoring Black Tea Fermentation Using a Colorimetric Sensor Array-Based Artificial Olfaction System. J. Food Process. Preserv. 2018, 42, e13348. [Google Scholar] [CrossRef]
- Comuzzo, P.; Battistutta, F. Acidification and PH Control in Red Wines; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128144008. [Google Scholar]
- Zhang, D.; Wang, S.; Yang, F.; Li, Z.; Huang, W. Visual Inspection of Acidic pH and Bisulfite in White Wine Using a Colorimetric and Fluorescent Probe. Food Chem. 2023, 408, 135200. [Google Scholar] [CrossRef]
- Mehrdel, P.; Karimi, S.; Farré-LLadós, J.; Casals-Terré, J. Portable 3D-Printed Sensor to Measure Ionic Strength and pH in Buffered and Non-Buffered Solutions. Food Chem. 2021, 344, 128583. [Google Scholar] [CrossRef]
- Clarke, J.S.; Achterberg, E.P.; Rérolle, V.M.C.; Abi Kaed Bey, S.; Floquet, C.F.A.; Mowlem, M.C. Characterisation and Deployment of an Immobilised PH Sensor Spot towards Surface Ocean pH Measurements. Anal. Chim. Acta 2015, 897, 69–80. [Google Scholar] [CrossRef]
- O’Brien, P.A.; Morrow, K.M.; Willis, B.L.; Bourne, D.G. Implications of Ocean Acidification for Marine Microorganisms from the Free-Living to the Host-Associated. Front. Mar. Sci. 2016, 3, 47. [Google Scholar] [CrossRef]
- Bressan, M.; Chinellato, A.; Munari, M.; Matozzo, V.; Manci, A.; Marčeta, T.; Finos, L.; Moro, I.; Pastore, P.; Badocco, D.; et al. Does Seawater Acidification Affect Survival, Growth and Shell Integrity in Bivalve Juveniles? Mar. Environ. Res. 2014, 99, 136–148. [Google Scholar] [CrossRef]
- Carter, B.R.; Radich, J.A.; Doyle, H.L.; Dickson, A.G. An Automated System for Spectrophotometric Seawater PH Measurements. Limnol. Oceanogr. Methods 2013, 11, 16–27. [Google Scholar] [CrossRef]
- Li, Z.; Suslick, K.S. Colorimetric Sensor Array for Monitoring CO and Ethylene. Anal. Chem. 2019, 91, 797–802. [Google Scholar] [CrossRef]
- Lagasse, M.K.; Rankin, J.M.; Askim, J.R.; Suslick, K.S. Colorimetric Sensor Arrays: Interplay of Geometry, Substrate and Immobilization. Sens. Actuators B Chem. 2014, 197, 116–122. [Google Scholar] [CrossRef]
- Paghi, A.; Corsi, M.; La Mattina, A.A.; Egri, G.; Dähne, L.; Barillaro, G. Wireless and Flexible Optoelectronic System for In Situ Monitoring of Vaginal pH Using a Bioresorbable Fluorescence Sensor. Adv. Mater. Technol. 2023, 8, 2201600. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Zanoni, C.; Bancalari, E.; Hadj Saadoun, J.; Alberti, G.; Quadrelli, P.; Biesuz, R. pH-Sensitive Colorimetric Sensors at Work on Chicken Freshness: Do Sensors Development on Real Samples Really Makes the Difference? SSRN Electron. J. 2022, 1–13. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Alberti, G.; Pazzi, B.M.; Zanoni, C.; Biesuz, R. A Green-PAD Array Combined with Chemometrics for pH Measurements. N. J. Chem. 2022, 46, 19460–19467. [Google Scholar] [CrossRef]
- Dickson, A.G. The Measurement of Sea Water PH. Mar. Chem. 1993, 44, 131–142. [Google Scholar] [CrossRef]
- Illingworth, J.A. A Common Source of Error in pH Measurements. Biochem. J. 1981, 195, 259–262. [Google Scholar] [CrossRef]
- Oláh, K. On the Theory of the Alkaline Error. Period. Polytech. Chem. Eng. 1960, 4, 141–156. [Google Scholar]
- Morris, D.; Coyle, S.; Wu, Y.; Lau, K.T.; Wallace, G.; Diamond, D. Bio-Sensing Textile Based Patch with Integrated Optical Detection System for Sweat Monitoring. Sens. Actuators B Chem. 2009, 139, 231–236. [Google Scholar] [CrossRef]
- Dickson, A.G. pH Buffers for Sea Water Media Based on the Total Hydrogen Ion Concentration Scale. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 107–118. [Google Scholar] [CrossRef]
- Anes, B.; Bettencourt da Silva, R.J.N.; Oliveira, C.; Camões, M.F. Seawater pH Measurements with a Combination Glass Electrode and High Ionic Strength TRIS-TRIS HCl Reference Buffers—An Uncertainty Evaluation Approach. Talanta 2019, 193, 118–122. [Google Scholar] [CrossRef]
- Liu, S.; Butman, D.E.; Raymond, P.A. Evaluating CO2 Calculation Error from Organic Alkalinity and pH Measurement Error in Low Ionic Strength Freshwaters. Limnol. Oceanogr. Methods 2020, 18, 606–622. [Google Scholar] [CrossRef]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical PH Sensor Covering the Range from pH 0-14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef]
- Bobrov, P.V.; Tarantov, Y.A.; Krause, S.; Moritz, W. Chemical Sensitivity of an ISFET with Ta2O5 Membrane in Strong Acid and Alkaline Solutions. Sens. Actuators B Chem. 1991, 3, 75–81. [Google Scholar] [CrossRef]
- Zhang, C.; Suslick, K.S. A Colorimetric Sensor Array for Organics in Water. J. Am. Chem. Soc. 2005, 127, 11548–11549. [Google Scholar] [CrossRef]
- Janzen, M.C.; Ponder, J.B.; Bailey, D.P.; Ingison, C.K.; Suslick, K.S. Colorimetric Sensor Arrays for Volatile Organic Compounds. Anal. Chem. 2006, 78, 3591–3600. [Google Scholar] [CrossRef]
- Ko, Y.; Jeong, H.Y.; Kwon, G.; Kim, D.; Lee, C.; You, J. pH-Responsive Polyaniline/Polyethylene Glycol Composite Arrays for Colorimetric Sensor Application. Sens. Actuators B Chem. 2020, 305, 127447. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Wang, H.; Xiao, S.; Wang, J.; Feng, L. Sensitivity Enhancement of pH Indicator and Its Application in the Evaluation of Fish Freshness. Talanta 2015, 143, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The Key Role of 3-Glycidoxypropyltrimethoxysilane Sol–Gel Precursor in the Development of Wearable Sensors for Health Monitoring. J. Sol-Gel Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Optical Monitoring of Sweat pH by a Textile Fabric Wearable Sensor Based on Covalently Bonded Litmus-3-Glycidoxypropyltrimethoxysilane Coating. Sens. Actuators B Chem. 2016, 222, 213–220. [Google Scholar] [CrossRef]
- Frankær, C.G.; Hussain, K.J.; Rosenberg, M.; Jensen, A.; Laursen, B.W.; Sørensen, T.J. Biocompatible Microporous Organically Modified Silicate Material with Rapid Internal Diffusion of Protons. ACS Sens. 2018, 3, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Capel-Cuevas, S.; Cuéllar, M.P.; de Orbe-Payá, I.; Pegalajar, M.C.; Capitán-Vallvey, L.F. Full-Range Optical pH Sensor Based on Imaging Techniques. Anal. Chim. Acta 2010, 681, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kodeh, F.S.; El-Nahhal, I.M.; Abd el-salam, F.H. Sol–Gel Encapsulation of Thymol Blue in Presence of Some Surfactants. Chem. Afr. 2019, 2, 67–76. [Google Scholar] [CrossRef]
- Kassal, P.; Šurina, R.; Vrsaljko, D.; Steinberg, I.M. Hybrid Sol-Gel Thin Films Doped with a pH Indicator: Effect of Organic Modification on Optical PH Response and Film Surface Hydrophilicity. J. Sol-Gel Sci. Technol. 2014, 69, 586–595. [Google Scholar] [CrossRef]
- Martinez-Olmos, A.; Capel-Cuevas, S.; López-Ruiz, N.; Palma, A.J.; De Orbe, I.; Capitán-Vallvey, L.F. Sensor Array-Based Optical Portable Instrument for Determination of PH. Sens. Actuators B Chem. 2011, 156, 840–848. [Google Scholar] [CrossRef]
- Chung, S.; Park, T.S.; Park, S.H.; Kim, J.Y.; Park, S.; Son, D.; Bae, Y.M.; Cho, S.I. Colorimetric Sensor Array for White Wine Tasting. Sensors 2015, 15, 18197–18208. [Google Scholar] [CrossRef]
- Pastore, A.; Badocco, D.; Bogialli, S.; Cappellin, L.; Pastore, P. pH Colorimetric Sensor Arrays: Role of the Color Space Adopted for the Calculation of the Prediction Error. Sensors 2020, 20, 6036. [Google Scholar] [CrossRef]
- Cappellin, L.; Pastore, P.; Badocco, D.; Pastore, A. Colorimetric Sensor for pH Measurements. IT102019000013878. Available online: https://www.knowledge-share.eu/en/patent/colorimetric-sensor-array-for-ph-measurements/ (accessed on 29 December 2023).
- Pastore, A.; Badocco, D.; Cappellin, L.; Pastore, P. Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays. Chemosensors 2022, 10, 87. [Google Scholar] [CrossRef]
- Pastore, A.; Badocco, D.; Pastore, P. Reversible and High Accuracy pH Colorimetric Sensor Array Based on a Single Acid-Base Indicator Working in a Wide pH Interval. Talanta 2020, 219, 121251. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.; Erenas, M.M.; De Orbe-Payá, I.; Capitán-Vallvey, L.F. Use of the Hue Parameter of the Hue, Saturation, Value Color Space as a Quantitative Analytical Parameter for Bitonal Optical Sensors. Anal. Chem. 2010, 82, 531–542. [Google Scholar] [CrossRef]
- Pastore, A.; Badocco, D.; Pastore, P. High Accuracy OrMoSil (Polyvinylidene Fluoride)-Supported Colorimetric Sensor: Novel Approach for the Calculation of the pH Prediction Error. Talanta 2020, 213, 120840. [Google Scholar] [CrossRef] [PubMed]
- Mya, K.Y.; Sirivat, A.; Jamieson, A.M. Effect of Ionic Strength on the Structure of Polymer-Surfactant Complexes. J. Phys. Chem. B 2003, 107, 5460–5466. [Google Scholar] [CrossRef]
- Salvatore, F.; Ferri, D.; Palombari, R. Salt Effect on the Dissociation Constant of Acid-Base Indicators. J. Solut. Chem. 1986, 15, 423–431. [Google Scholar] [CrossRef]
- Pastore, A.; Badocco, D.; Pastore, P. Determination of the Relevant Equilibrium Constants Working in pH Colorimetric Sensor Arrays (CSAs). Microchem. J. 2022, 177, 107288. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]



| BCG | Sol1 + EtOH (g) | Sol2 + EtOH (g) | BB | Sol1 + EtOH (g) | Sol2 + EtOH (g) |
|---|---|---|---|---|---|
| 1 | 0.939 | 0 | 1 | 0.930 | 0 |
| 2 | 0.599 | 0.359 | 2 | 0.610 | 0.352 |
| 3 | 0.405 | 0.557 | 3 | 0.400 | 0.566 |
| 4 | 0.307 | 0.653 | 4 | 0.296 | 0.668 |
| 5 | 0.210 | 0.750 | 5 | 0.202 | 0.763 |
| 6 | 0.103 | 0.877 | 6 | 0.124 | 0.861 |
| 7 | 0 | 0.955 | 7 | 0 | 0.947 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastore, A.; Badocco, D.; Cappellin, L.; Tubiana, M.; Pastore, P. Positively Charged Organosilanes Covalently Linked to the Silica Network as Modulating Tools for the Salinity Correction of pH Values Obtained with Colorimetric Sensor Arrays (CSAs). Sensors 2024, 24, 417. https://doi.org/10.3390/s24020417
Pastore A, Badocco D, Cappellin L, Tubiana M, Pastore P. Positively Charged Organosilanes Covalently Linked to the Silica Network as Modulating Tools for the Salinity Correction of pH Values Obtained with Colorimetric Sensor Arrays (CSAs). Sensors. 2024; 24(2):417. https://doi.org/10.3390/s24020417
Chicago/Turabian StylePastore, Andrea, Denis Badocco, Luca Cappellin, Mauro Tubiana, and Paolo Pastore. 2024. "Positively Charged Organosilanes Covalently Linked to the Silica Network as Modulating Tools for the Salinity Correction of pH Values Obtained with Colorimetric Sensor Arrays (CSAs)" Sensors 24, no. 2: 417. https://doi.org/10.3390/s24020417
APA StylePastore, A., Badocco, D., Cappellin, L., Tubiana, M., & Pastore, P. (2024). Positively Charged Organosilanes Covalently Linked to the Silica Network as Modulating Tools for the Salinity Correction of pH Values Obtained with Colorimetric Sensor Arrays (CSAs). Sensors, 24(2), 417. https://doi.org/10.3390/s24020417






