Electrochemical Sensors for Antibiotic Detection: A Focused Review with a Brief Overview of Commercial Technologies
Abstract
1. Introduction
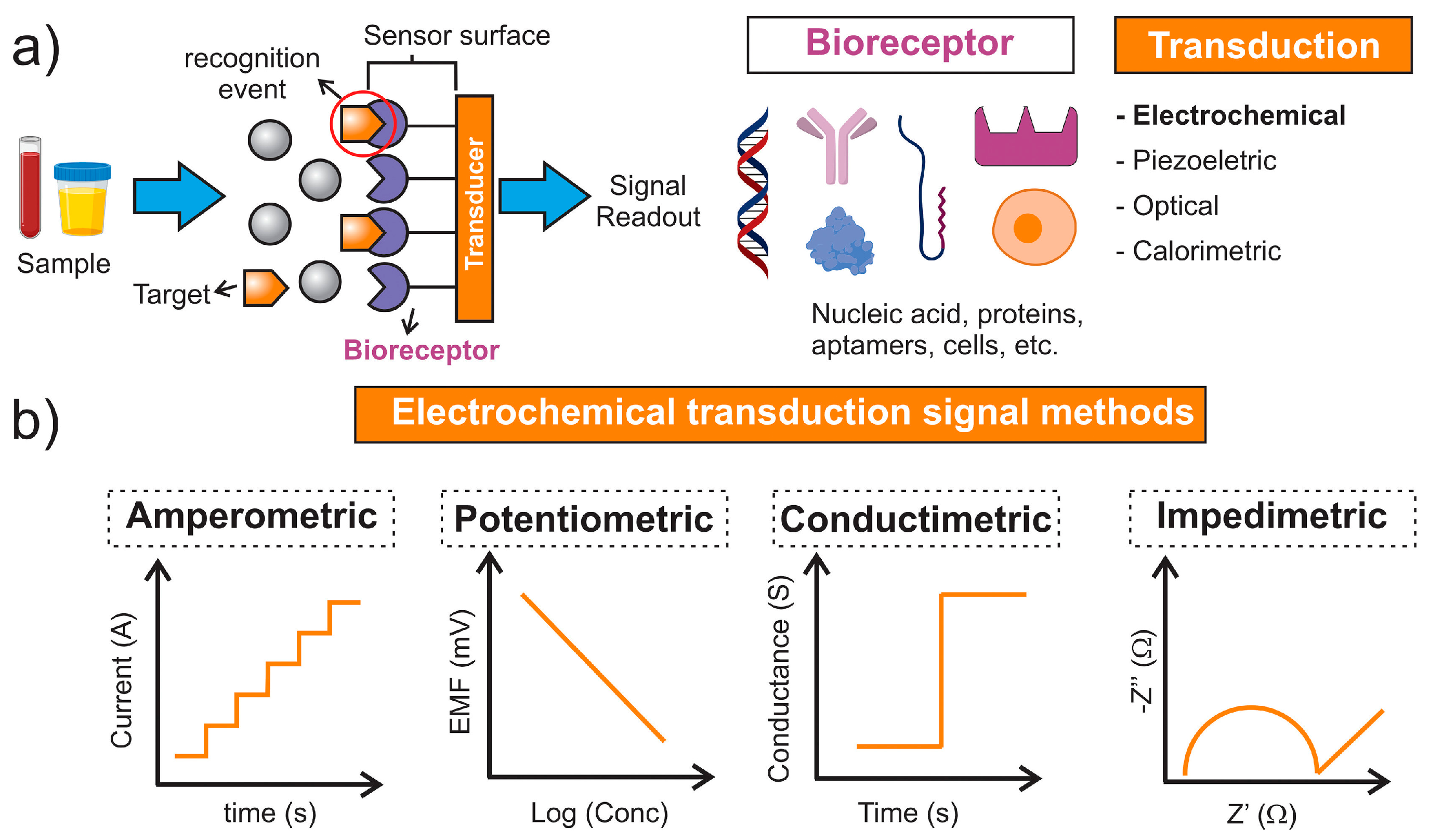
2. General Principles of Electrochemical Biosensors
2.1. Amperometric Biosensors
2.2. Potentiometric Biosensors
2.3. Coulometric Biosensors
2.4. Impedimetric Biosensors
3. Types of Electrochemical Biosensors Used for Antibiotic Detection
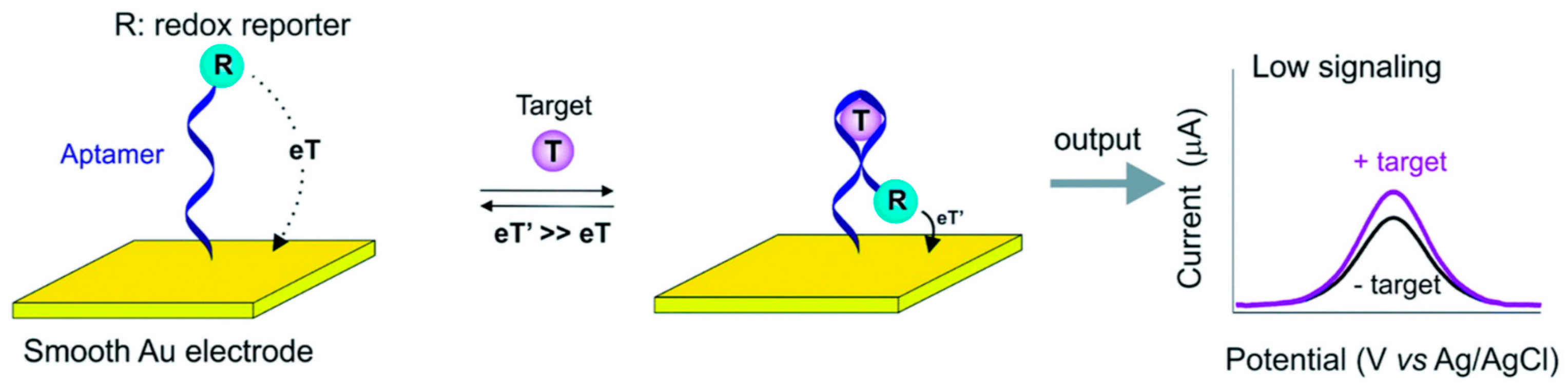
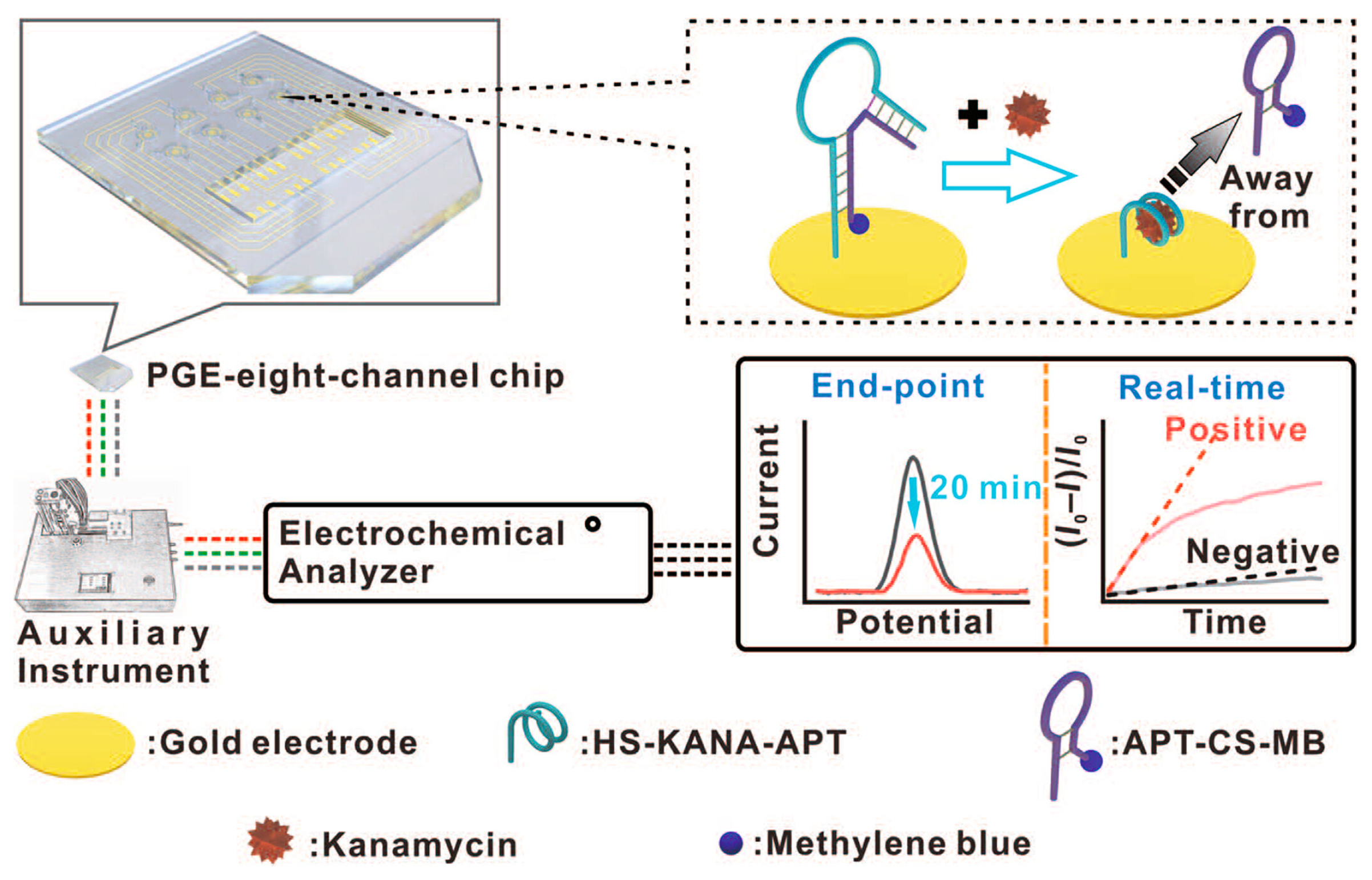
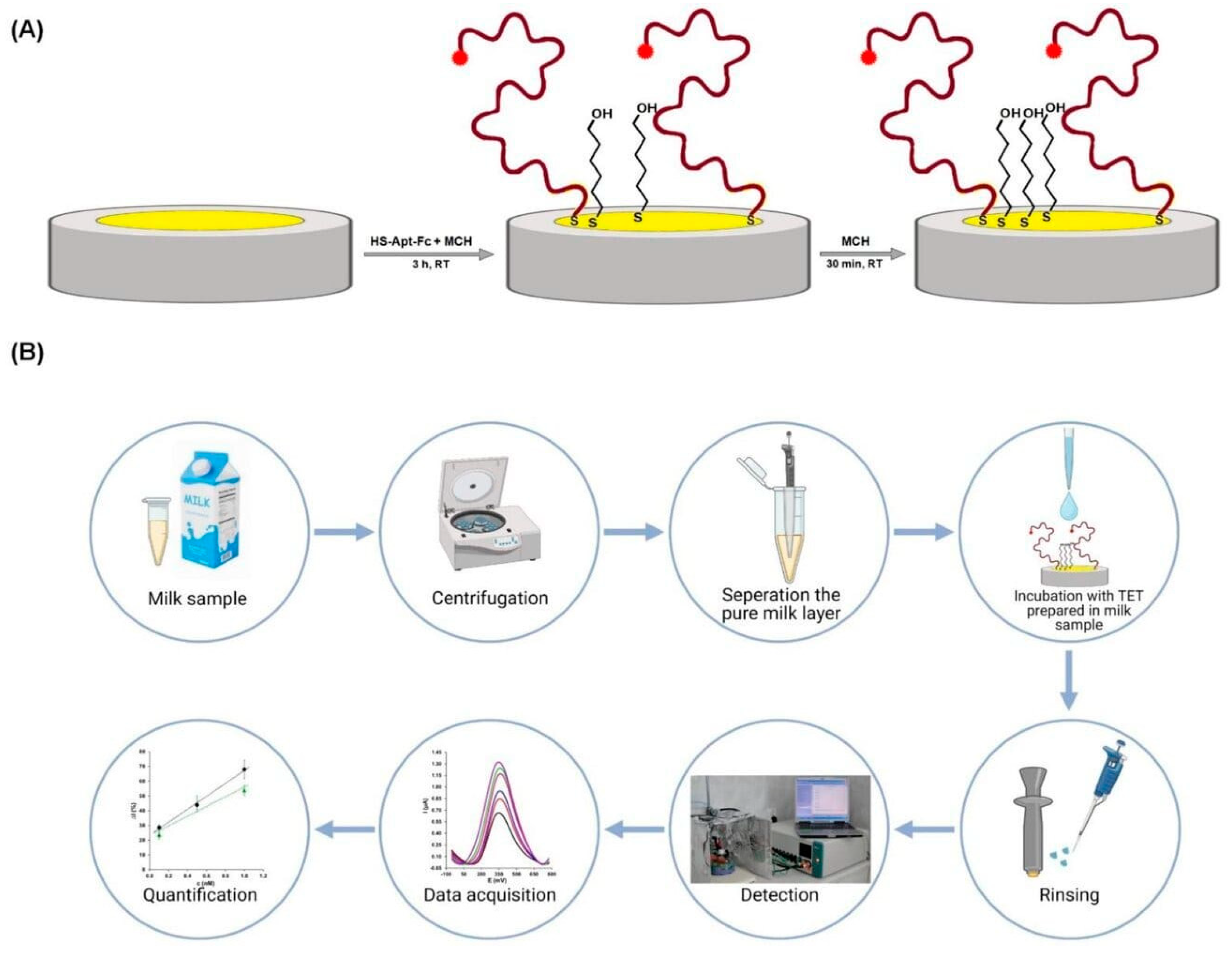
3.1. Aptamer-Based Electrochemical Sensors
3.2. Molecularly Imprinted Polymer-Based Electrochemical Sensors
| Sensor Type | Target Molecule | Method of Detection | Limit of Detection | Sample | Monomer | Ref. |
|---|---|---|---|---|---|---|
| SPE combined with an MIP prepared from dual functional monomers. | Erythromycin Clarithromycin Azithromycin | DPV | 1.1–1.6 nM | Buffer, Tap water samples | m-phenylenediamine | [112] |
| Gold screen-printed electrode (Au-SPE) functionalized via electropolymerization of custom-made conjugated monomer (Th2-NDI-PIA) | Streptomycin sulfate | DPV | 0.190 pM | Buffer, tap water | Th2-NDI-PIA | [113] |
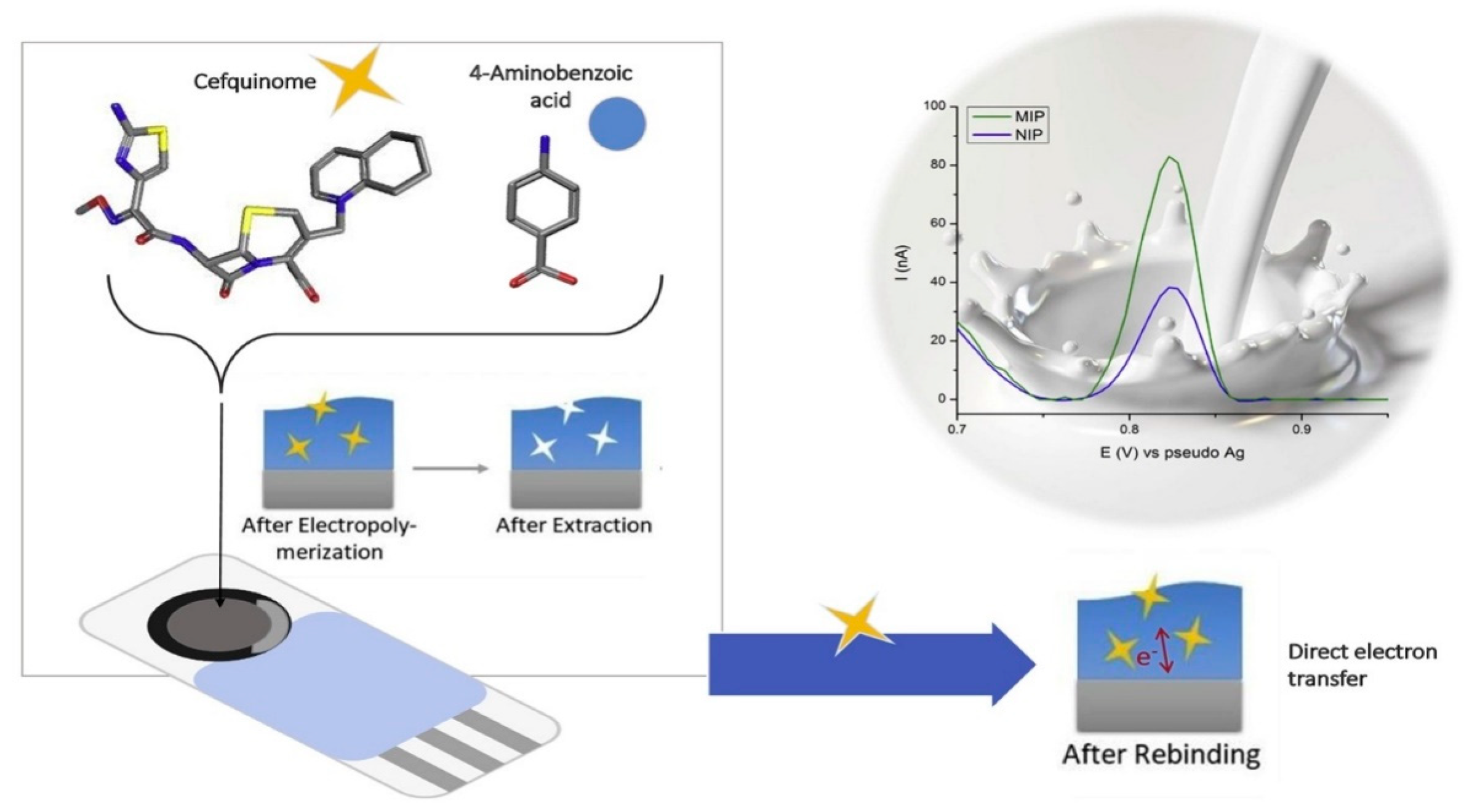
| Electropolymerized MIPs onto a screen-printed carbon electrode (SPCE) | Azithromycin | DPV | 0.08 µM | Water samples | 4-aminobenzoic acid | [114] |
| MIP electrodeposited onto the surface of AuNPs/rGo/single-walled carbon nanotube-modified GCE | Pefloxacin | DPV | 16 nM | Milk | o-phenylenediamine (oPD) | [115] |
| CO2 laser-induced graphene (LIG) with AuNPs and MIPs | Tetracycline | DPV | 0.32 nM 0.85 nM 0.80 nM | Buffer, Milk, Meat | oPD | [116] |
| Nanocomposite molecularly imprinted polymer (nanoMIP) using oxidised MWNCTs and ultrathin overoxidised polypyrrole MIP | Sulfamethoxazole | DPV | 0.41 μM | Buffer | pyrrole | [117] |
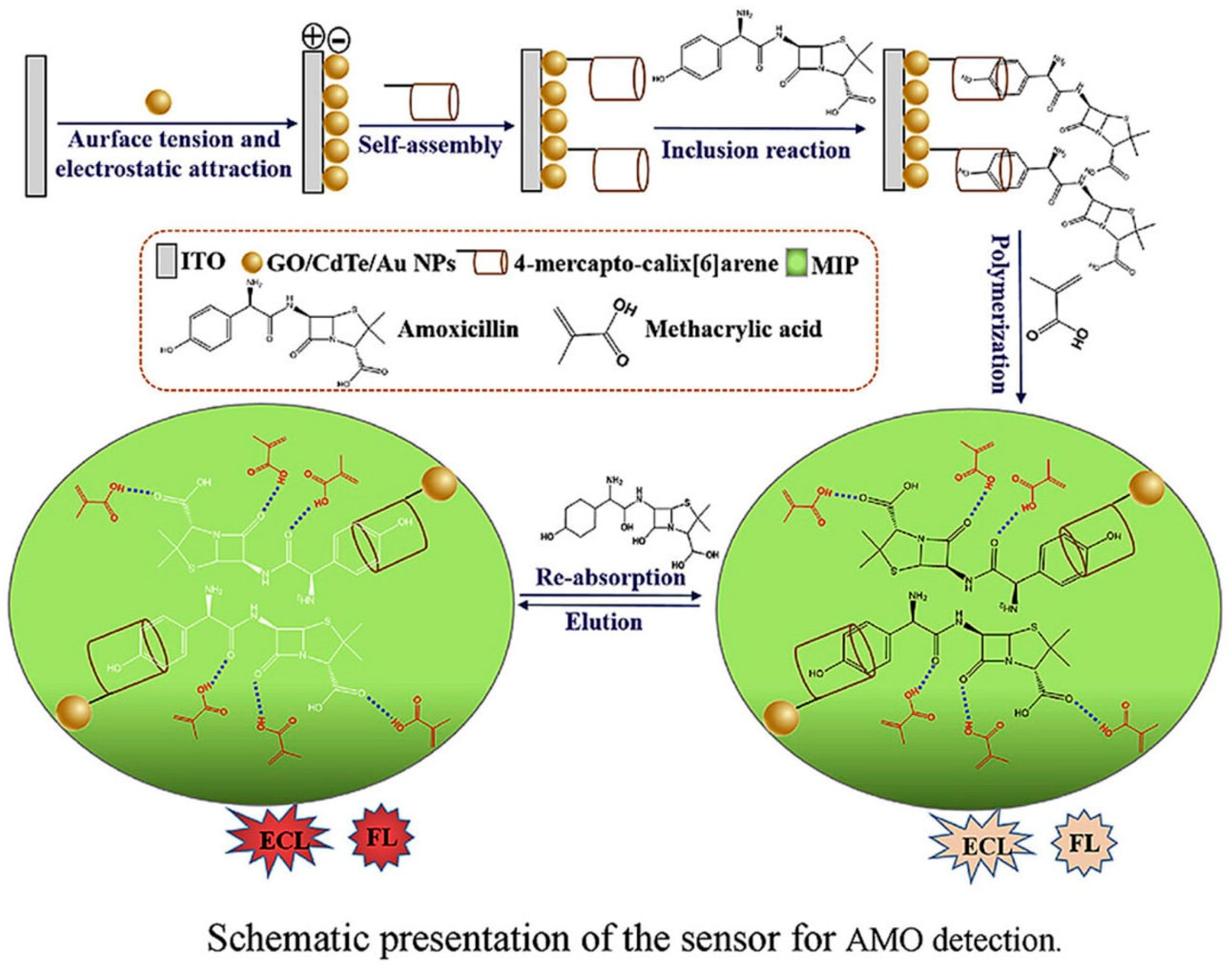
| Dual recognition MIP-coated graphene oxide loaded with CdTe quantum dots/AuNPs (GO/CdTe/AuNPs) on an indium tin oxide (ITO) electrode | Amoxicillin | DPV EIS | 8.3 pM | Buffer | α-methacrylic acid | [110] |
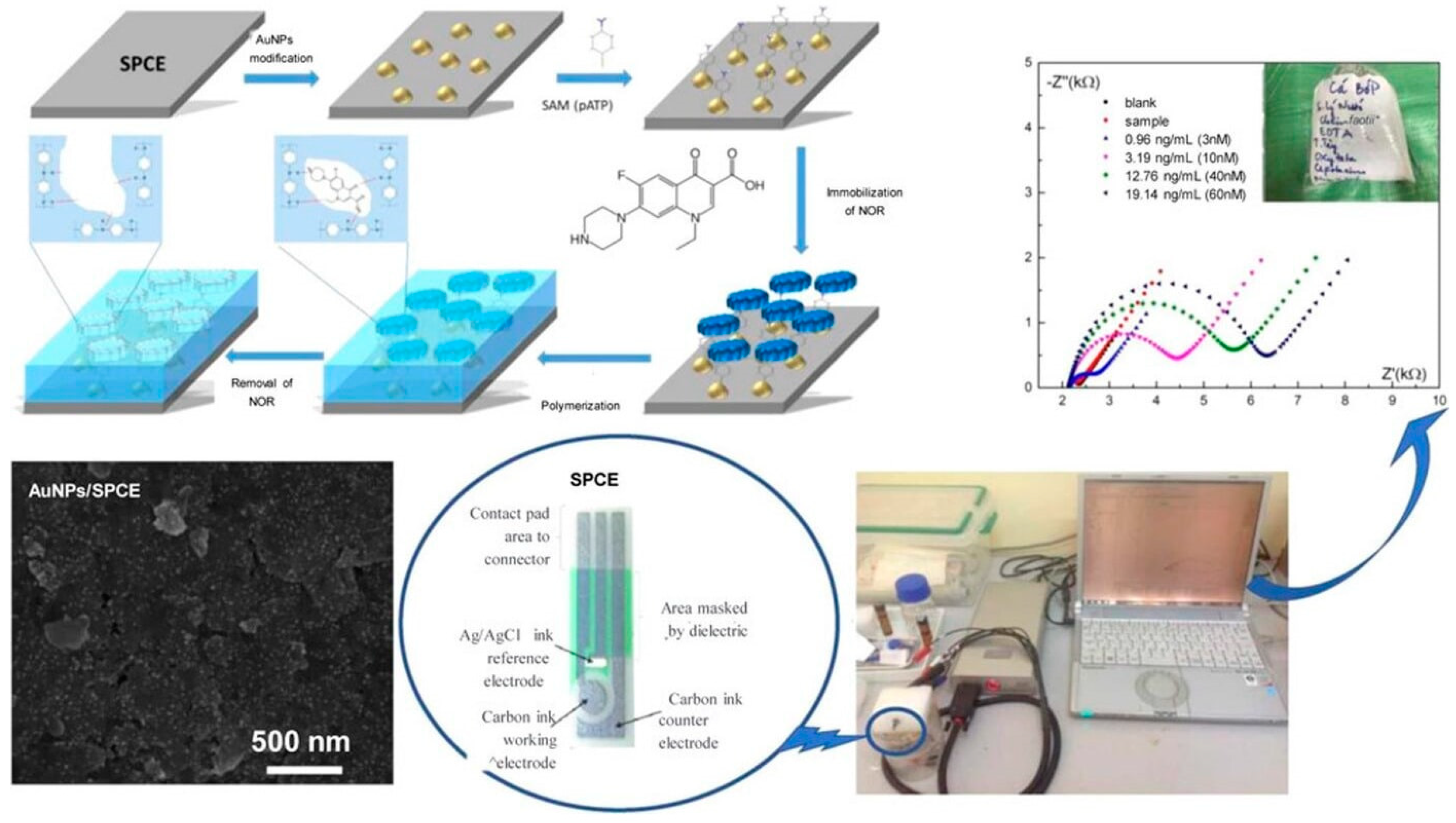
| Gold nanoparticles (AuNPs) and MIP-based electrochemical sensors | Norfloxacin | EIS | 0.15 ng/mL | Aquaculture water | 4-aminothiophenol | [111] |
| Bifunctional dual-template molecularly imprinted polymer-modified electrode | Ceftazidime Avibactam | EIS SWV | 35 μM 0.5 μM | Serum samples | o-PD | [118] |
| Sensor using magnetic nanoparticles (mag) and molecularly imprinted polymer | Tetracycline | SWV | 0.15 μM | Milk | Acrylic acid | [119] |
| Molecularly imprinted electrochemiluminescence (ECL) sensor using amino-functional titanium carbide nanodots (TNDs) and carbon nitride nanosheets (CNNS) | Ciprofloxacin | ECL EIS | 1.2 nM | Food samples including chicken, milk, and pork | o-PD | [120] |
| MIP sensor using electro-polymerization with surface-deposited AuNPs | Sufhaguanidine Sulfamerazine | CV DPV CA | 0.030 µM | Human fluids | oPD | [121] |
| Chitosan gold nanoparticle-decorated MIP (Ch-AuMIP) modified GCE | Ciprofloxacin | CV | 0.210 µM | Water | Methacrylic acid (MAA) | [122] |
| MIP electrochemical sensor using Fe3N-Co2N nanoarray with high electric conductivity and large surface area for MIP growth | Ampicillin | CV EIS | 0.365 nM | Milk samples | N-N-dimethyl bisacrylamide | [123] |
| MIP coated on graphene oxide deposited as a thin film on GCE | Amoxicillin | CV DPV | 0.294 nM | Buffer | APTES + PTES | [124] |
| Fe-doped porous carbon (Fe-PC)-modified Au electrode covered with MIP film electropolymerized onto an electrode | Lomefloxacin | CV DPV EIS | 0.2 nM | Water samples | o-PD | [125] |
| MIP-based biomimetic layer electrodeposited onto a glassy carbon electrode (GCE) | Azithromycin | CV EIS | 0.85 nM | Spiked plasma, tears, and urine samples | 3-thienyl boronic acid | [126] |
| Aggregation-based ECL sensor using ferriferous oxide@Pt NPs for signal amplification | Ciprofloxacin | CV EIS | 0.60 pM | Meat samples | 4-aminothiophenol | [127] |
| Screen-printed electrode | Erythromycin | CV EIS | 0.1 nM | Buffer | MAA | [128] |
3.3. Antibody-Based Electrochemical Sensors
4. Commercially Available Sensors for Antibiotic Detection
4.1. Gold-Standard Technologies
4.2. Optical-Based Sensors
4.3. Immunoassay-Based Sensors
4.4. Electrochemical-Based Biosensors
5. General Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Balloux, F.; van Dorp, L. Q&A: What Are Pathogens, and What Have They Done to and for Us? BMC Biol. 2017, 15, 91. [Google Scholar] [CrossRef]
- Antimicrobial Resistance|Antimicrobial Resistance|CDC. Available online: https://www.cdc.gov/antimicrobial-resistance/index.html (accessed on 2 August 2024).
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Patel, P. Bactericidal and Bacteriostatic Antibiotics. Infect. Sepsis Dev. 2021, 3, 773–774. [Google Scholar] [CrossRef]
- WHO EMRO|What Is the Difference between Antibiotic and Antimicrobial Resistance?|Antimicrobial Resistance|Health Topics. Available online: https://www.emro.who.int/health-topics/drug-resistance/what-is-the-difference-between-antibiotic-and-antimicrobial-resistance.html (accessed on 2 August 2024).
- 2019 Antibiotic Resistance Threats Report|Antimicrobial Resistance|CDC. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 2 August 2024).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE Pathogens in the Environment: Antibiotic Resistance Status, Community-Acquired Infection and Risk to Human Health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Burmeister, A.R. Horizontal Gene Transfer. Evol. Med. Public Heal. 2015, 2015, 193. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2021, 20, 257–269. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic Resistance Genes in Bacteria: Occurrence, Spread, and Control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Medina, E.; Pieper, D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Ellington, M.J. The Emergence of Antibiotic Resistance by Mutation. Clin. Microbiol. Infect. 2007, 13, 5–18. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Hu, M.; Ben, Y.; Wong, M.H.; Zheng, C. Trace Analysis of Multiclass Antibiotics in Food Products by Liquid Chromatography-Tandem Mass Spectrometry: Method Development. J. Agric. Food Chem. 2021, 69, 1656–1666. [Google Scholar] [CrossRef]
- Ben, Y.; Hu, M.; Zhang, X.; Wu, S.; Wong, M.H.; Wang, M.; Andrews, C.B.; Zheng, C. Efficient Detection and Assessment of Human Exposure to Trace Antibiotic Residues in Drinking Water. Water Res. 2020, 175, 115699. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Gilbert, D.N.; Spellberg, B. Seven Ways to Preserve the Miracle of Antibiotics. Clin. Infect. Dis. 2013, 56, 1445–1450. [Google Scholar] [CrossRef]
- Blaser, M.J.; Melby, M.K.; Lock, M.; Nichter, M. Accounting for Variation in and Overuse of Antibiotics among Humans. BioEssays 2021, 43, 2000163. [Google Scholar] [CrossRef]
- Pitt, S.J.; Gunn, A. The One Health Concept. Br. J. Biomed. Sci. 2024, 81, 12366. [Google Scholar] [CrossRef]
- Gorham, J.; Taccone, F.S.; Hites, M. Ensuring Target Concentrations of Antibiotics in Critically Ill Patients through Dose Adjustment. Expert Opin. Drug Metab. Toxicol. 2022, 18, 177–187. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Bissantz, C.; Zampaloni, C.; David-Pierson, P.; Dieppois, G.; Guenther, A.; Trauner, A.; Winther, L.; Stubbings, W. Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective. Antibiotics 2024, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garzón, V.; Bustos, R.H.; Pinacho, D.G. Personalized Medicine for Antibiotics: The Role of Nanobiosensors in Therapeutic Drug Monitoring. J. Pers. Med. 2020, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Request for Advice from the Expert Committee on Selection and Use of Essential Medicines on Prioritization of Medicines Requiring Therapeutic Drug Monitoring; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Huang, J.-W.; Zhong, X.-F.; Gao, Y.-Z. New Antibiotic against Multi-Drug Resistant Bacteria. Innov. Life 2024, 2, 100057. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells Are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Sachi, S.; Ferdous, J.; Sikder, M.H.; Azizul Karim Hussani, S.M. Antibiotic Residues in Milk: Past, Present, and Future. J. Adv. Vet. Anim. Res. 2019, 6, 315–332. [Google Scholar] [CrossRef]
- Pauter, K.; Szultka-Młýnska, M.; Buszewski, B. Determination and Identification of Antibiotic Drugs and Bacterial Strains in Biological Samples. Molecules 2020, 25, 2556. [Google Scholar] [CrossRef]
- Hagel, R.B.; Waysek, E.H.; Cort, W.M. High-Pressure Liquid Chromatography Analysis of Antibiotic Susceptibility Disks. Antimicrob. Agents Chemother. 1979, 16, 372. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, J.Y.; Huang, P.Y.; Sung, H.L.; Tung, Y.C.; Lai, C.C.; Wei, Y.F.; Fu, P.K. Comparing Prolonged Infusion to Intermittent Infusion Strategies for Beta-Lactam Antibiotics in Patients with Gram-Negative Bacterial Infections: A Systematic Review and Meta-Analysis. Expert Rev. Anti. Infect. Ther. 2024, 1–11. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Riezk, A.; Wilson, R.C.; Rawson, T.M.; Vasikasin, V.; Arkel, P.; Ferris, T.J.; Haigh, L.D.; Cass, A.E.G.; Holmes, A.H. A Rapid, Simple, High-Performance Liquid Chromatography Method for the Clinical Measurement of Beta-Lactam Antibiotics in Serum and Interstitial Fluid. Anal. Methods 2023, 15, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, Y.; Liu, L.; Kuang, H.; Li, A.; Xu, C. Multiplex Lateral Flow Immunoassay for Five Antibiotics Detection Based on Gold Nanoparticle Aggregations. RSC Adv. 2016, 6, 7798–7805. [Google Scholar] [CrossRef]
- Frigoli, M.; Lowdon, J.W.; Caldara, M.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Emerging Biomimetic Sensor Technologies for the Detection of Pathogenic Bacteria: A Commercial Viability Study. ACS Omega 2024, 9, 23155–23171. [Google Scholar] [CrossRef]
- Haiping, L.; Jiangyue, W.; Fanping, M.; Aifeng, L. Immunochromatographic Assay for the Detection of Antibiotics in Animal-Derived Foods: A Review. Food Control. 2021, 130, 108356. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, B.; Wang, M.; Si, Y.; Taha, A.Y.; Liu, G.; Pan, T.; Sun, G. Improving the Sensitivity of Nanofibrous Membrane-Based ELISA for On-Site Antibiotics Detection. ACS Sens. 2022, 7, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, M.; Montaseri, M.; Hosseinzadeh, S.; Majlesi, M.; Berizi, E.; Zare, M.; Derakhshan, Z.; Ferrante, M.; Conti, G.O. Antibiotic Residues in Poultry Tissues in Iran: A Systematic Review and Meta-Analysis. Environ. Res. 2022, 204, 112038. [Google Scholar] [CrossRef]
- Aslam, S.; Zhang, Z.; Muhammad, A. A Review on Validation of Enzyme Linked Immunosorbent Assay (Elisa) Techniques for Detection and Quantification of Different Contaminant in Aquatic Environment. J. Bioresour. Manag. 2023, 10, 6. [Google Scholar]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Abu bakr Shabbir, M.; Cheng, G.; Yuan, Z. Current Advances in Immunoassays for the Detection of Antibiotics Residues: A Review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Shanin, I.A.; Shaimardanov, A.R.; Thai, N.T.D.; Eremin, S.A. Determination of Fluoroquinolone Antibiotic Levofloxacin in Urine by Fluorescence Polarization Immunoassay. J. Anal. Chem. 2015, 70, 712–717. [Google Scholar] [CrossRef]
- Pastor-Navarro, N.; Gallego-Iglesias, E.; Maquieira, Á.; Puchades, R. Immunochemical Method for Sulfasalazine Determination in Human Plasma. Anal. Chim. Acta 2007, 583, 377–383. [Google Scholar] [CrossRef]
- Gaspar, V.P.; Ibrahim, S.; Zahedi, R.P.; Borchers, C.H. Utility, Promise, and Limitations of Liquid Chromatography-mass Spectrometry-based Therapeutic Drug Monitoring in Precision Medicine. J. Mass Spectrom. 2021, 56, e4788. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Wai, H.K.F.; Wu, P.; Lai, S.W.; Chan, O.S.K.; Tun, H.M. A Universal LC-MS/MS Method for Simultaneous Detection of Antibiotic Residues in Animal and Environmental Samples. Antibiotics 2022, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Budd, J.; Miller, B.S.; Weckman, N.E.; Cherkaoui, D.; Huang, D.; Decruz, A.T.; Fongwen, N.; Han, G.-R.; Broto, M.; Estcourt, C.S.; et al. Lateral Flow Test Engineering and Lessons Learned from COVID-19. Nat. Rev. Bioeng. 2023, 1, 13–31. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors Applications in Medical Field: A Brief Review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. Applications of Commercial Biosensors in Clinical, Food, Environmental, and Biothreat/Biowarfare Analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and Their Applications—A Review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Frigoli, M.; Lowdon, J.W.; Caldara, M.; Arreguin-Campos, R.; Sewall, J.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Thermal Pyocyanin Sensor Based on Molecularly Imprinted Polymers for the Indirect Detection of Pseudomonas Aeruginosa. ACS Sens. 2023, 8, 353–362. [Google Scholar] [CrossRef]
- Morales, M.A.; Halpern, J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug. Chem. 2018, 29, 3231. [Google Scholar] [CrossRef]
- Fatima, T.; Bansal, S.; Husain, S.; Khanuja, M. Biosensors. In Electrochemical Sensors: From Working Electrodes to Functionalization Miniaturized Devices; Woodhead Publishing: Cambridge, UK, 2022; pp. 1–30. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Lu, L.; Hu, X.; Zhu, Z. Biomimetic Sensors and Biosensors for Qualitative and Quantitative Analyses of Five Basic Tastes. TrAC Trends Anal. Chem. 2017, 87, 58–70. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, M. Optical biosensors: A decade in review. Alexandria. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Qian, W.; Zhou, J.; Chen, Y.; Liu, H.; Ding, P. Label-free electrochemical immunosensor based on staphylococcal protein a and AgNPs-rGO-Nf for sensitive detection of virginiamycin M1. Bioelectrochemistry 2023, 153, 108489. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Glahn-Martinez, B.; Benito-Peña, E.; Moreno-Bondi, M. COptical Biosensors for Label-Free Detection of Small Molecules. Sensors. 2018, 18, 4126. [Google Scholar] [CrossRef] [PubMed]
- Price, C.P. Point-of-Care Testing in Diabetes Mellitus. Clin. Chem. Lab. Med. 2003, 41, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, E.J.; Higgins, I.J.; Turner, A.P.F. Quinoprotein Glucose Dehydrogenase and Its Application in an Amperometric Glucose Sensor. Biosensors 1986, 2, 71–87. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Kravets, V.G.; Grigorenko, A.N. Label-Free Optical Biosensing: Going beyond the Limits. Chem. Soc. Rev. 2023, 52, 6554–6585. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; M, Y.; S, R.; CA, M.; Thirunavookarasu, S.N.; CK, S. Recent Advances in Surface Plasmon Resonance (SPR) Biosensors for Food Analysis: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1055–1077. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, M. Optical Biosensors for Environmental Monitoring: Recent Advances and Future Perspectives in Bacterial Detection. Environ. Res. 2023, 236, 116826. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
- Rojin, S.; Pour, S.; Calabria, D.; Emamiamin, A.; Lazzarini, E.; Pace, A.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Electrochemical vs. Optical Biosensors for Point-of-Care Applications: A Critical Review. Chemosens 2023, 11, 546. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Q.; Chen, T.; Li, J.; Liu, Y.; Shan, X.; Liu, F.; Jia, J. Recent Advances in Electrochemical Sensors for Antibiotics and Their Applications. Chin. Chem. Lett. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhat, A.; Dutta, K.M. Electrochemical Biosensors for the Detection of Antibiotics in Milk: Recent Trends and Future Perspectives. Biosens. 2023, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sun, Y.; Wei, X.; He, Y.; Wang, H.; Cui, Z.; Ma, J.; Liu, X.; Shu, R.; Lin, H.; et al. Detection Methods for Antibiotics in Wastewater: A Review. Bioprocess Biosyst. Eng. 2024, 47, 1433–1451. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400. [Google Scholar] [CrossRef]
- Lopes, L.C.; Santos, A.; Bueno, P.R. An Outlook on Electrochemical Approaches for Molecular Diagnostics Assays and Discussions on the Limitations of Miniaturized Technologies for Point-of-Care Devices. Sens. Actuators Rep. 2022, 4, 100087. [Google Scholar] [CrossRef]
- Adeloju, S.B. Amperometry. Encycl. Anal. Sci. 2005, 70–79. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical Biosensors and Nanobiosensors. Essays Biochem. 2016, 60, 69. [Google Scholar] [CrossRef]
- Pedersen, T.; Fojan, P.; Pedersen, A.K.N.; Magnusson, N.E.; Gurevich, L. Amperometric Biosensor for Quantitative Measurement Using Sandwich Immunoassays. Biosensors 2023, 13, 519. [Google Scholar] [CrossRef]
- Walker, N.L.; Roshkolaeva, A.B.; Chapoval, A.I.; Dick, J.E. Recent Advances in Potentiometric Biosensing. Curr. Opin. Electrochem. 2021, 28, 100735. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Budnikov, H.; Analysis Chemosensors, A.; Rodriguez-Mendez, L. Analytical Capabilities of Coulometric Sensor Systems in the Antioxidants Analysis. Chemosensors 2021, 9, 91. [Google Scholar] [CrossRef]
- Razem, M.; Ding, Y.; Morozova, K.; Mazzetto, F.; Scampicchio, M. Analysis of Phenolic Compounds in Food by Coulometric Array Detector: A Review. Sensors 2022, 22, 7498. [Google Scholar] [CrossRef]
- Basics of EIS: Electrochemical Research-Impedance Gamry Instruments. Available online: https://www.gamry.com/application-notes/EIS/basics-of-electrochemical-impedance-spectroscopy/ (accessed on 2 August 2024).
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy─A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy and Its Use in Analyzing the Steady-State AC Response of Solid and Liquid Electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 1987, 223, 25–50. [Google Scholar] [CrossRef]
- Caldara, M.; Lowdon, J.W.; van Wissen, G.; Ferrari, A.G.M.; Crapnell, R.D.; Cleij, T.J.; Diliën, H.; Banks, C.E.; Eersels, K.; van Grinsven, B. Dipstick Sensor Based on Molecularly Imprinted Polymer-Coated Screen-Printed Electrodes for the Single-Shot Detection of Glucose in Urine Samples—From Fundamental Study toward Point-of-Care Application. Adv. Mater. Interfaces 2023, 10, 2300182. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for on-Site Analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.H. Aptamers as a Replacement for Antibodies in Enzyme-Linked Immunosorbent Assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Chen, S.; Zhuang, Z.; Zhang, Y.; Jiang, L.; Lin, J.S. SELEX Tool: A Novel and Convenient Gel-Based Diffusion Method for Monitoring of Aptamer-Target Binding. J. Biol. Eng. 2020, 14, 1. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, E.J.; Park, T.K.; Bae, D.W.; Cha, S.S.; Kim, T.W.; Kim, Y.P. Gold Nanoparticle-Assisted SELEX as a Visual Monitoring Platform for the Development of Small Molecule-Binding DNA Aptasensors. Biosens. Bioelectron. 2021, 191, 113468. [Google Scholar] [CrossRef]
- Chrouda, A.; Ayed, D.; Zinoubi, K.; Majdoub, H.; Jaffrezic-Renault, N. Highly Stable and Ultra-Sensitive Amperometric Aptasensor Based on Pectin Stabilized Gold Nanoparticles on Graphene Oxide Modified GCE for the Detection of Aflatoxin M1. Food Chem. Adv. 2022, 1, 100068. [Google Scholar] [CrossRef]
- Radi, A.E.; Abd-Ellatief, M.R. Electrochemical Aptasensors: Current Status and Future Perspectives. Diagnostics 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Pellitero, M.A.; Arroyo-Currás, N. Study of Surface Modification Strategies to Create Glassy Carbon-Supported, Aptamer-Based Sensors for Continuous Molecular Monitoring. Anal. Bioanal. Chem. 2022, 414, 5627. [Google Scholar] [CrossRef]
- Sequeira-Antunes, B.; Ferreira, H.A. Nucleic Acid Aptamer-Based Biosensors: A Review. Biomedicines 2023, 11, 3201. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Song, L.; Gao, Y.; Wu, K.; Guo, R.; Chen, R.; Zhen, J.; Pan, L. Aptamer Sensors for the Detection of Antibiotic Residues— A Mini-Review. Toxics 2023, 11, 513. [Google Scholar] [CrossRef]
- Han, C.; Li, R.; Li, H.; Liu, S.; Xu, C.; Wang, J.; Wang, Y.; Huang, J. Ultrasensitive Voltammetric Determination of Kanamycin Using a Target-Triggered Cascade Enzymatic Recycling Couple along with DNAzyme Amplification. Microchim. Acta 2017, 184, 2941–2948. [Google Scholar] [CrossRef]
- Bao, Y.; Cai, K.; Liu, Y.; Li, B. Aptamer-Based Antibiotic Electrochemical Detection Platform Using Portable Plastic Gold Electrode and Multichannel Chip. Chin. J. Chem. 2024, 42, 171–176. [Google Scholar] [CrossRef]
- Malecka-Baturo, K.; Zaganiaris, A.; Grabowska, I.; Kurzątkowska-Adaszyńska, K. Electrochemical Biosensor Designed to Distinguish Tetracyclines Derivatives by SsDNA Aptamer Labelled with Ferrocene. Int. J. Mol. Sci. 2022, 23, 13785. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Khajehsharifi, H.; Hajihosseini, S. Detection of Oxytetracycline Using an Electrochemical Label-Free Aptamer-Based Biosensor. Biosensors 2022, 12, 468. [Google Scholar] [CrossRef]
- Li, F.; Wu, Y.; Chen, D.; Guo, Y.; Wang, X.; Sun, X. Sensitive Dual-Labeled Electrochemical Aptasensor for Simultaneous Detection of Multi-Antibiotics in Milk. Int. J. Hydrog. Energy 2021, 46, 23301–23309. [Google Scholar] [CrossRef]
- Chen, F.L.; Song, K.H.; Xu, J.T.; Wang, K.Z.; Feng, X.Z.; Han, G.C.; Kraatz, H.B. An Electrochemical Aptamer Sensor for Rapid Quantification Sulfadoxine Based on Synergistic Signal Amplification of Indole and MWCNTs and Its Electrooxidation Mechanism. Sens. Actuators B Chem. 2024, 401, 135008. [Google Scholar] [CrossRef]
- Hosu, O.; Melinte, G.; Ștefan, G.; Casian, M.; Cristea, C. Towards Selective Tetracycline Recognition in Wastewater Based on Gold Nanovoids@aptamer Sensing. Electrochim. Acta 2023, 460, 142556. [Google Scholar] [CrossRef]
- Wei, X.; Sun, Y.; Luo, Y.; Shu, R.; Lin, H.; Xu, D. Electrochemical Biosensor Bi2O3@Au@Apta Based on Bi2O3 Nanofibers for Sensitive Detection of Ampicillin. Biochem. Eng. J. 2023, 200, 109107. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Qu, X.; Li, S.; Zhao, Y.; Liu, S.; Wang, Y.; Huang, J.; Yu, J. Efficient Strand Displacement Amplification via Stepwise Movement of a Bipedal DNA Walker on an Electrode Surface for Ultrasensitive Detection of Antibiotics. Analyst 2020, 145, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Wu, M.; Shi, M.; Tian, Q.; Fu, L.; Tsai, H.S.; Xie, W.F.; Lai, G.; Wang, G.; et al. A Novel Electrochemical Aptasensor Based on Eco-Friendly Synthesized Titanium Dioxide Nanosheets and Polyethyleneimine Grafted Reduced Graphene Oxide for Ultrasensitive and Selective Detection of Ciprofloxacin. Anal. Chim. Acta 2023, 1275, 341607. [Google Scholar] [CrossRef]
- Hu, M.; Yue, F.; Dong, J.; Tao, C.; Bai, M.; Liu, M.; Zhai, S.; Chen, S.; Liu, W.; Qi, G.; et al. Screening of Broad-Spectrum Aptamer and Development of Electrochemical Aptasensor for Simultaneous Detection of Penicillin Antibiotics in Milk. Talanta 2024, 269, 125508. [Google Scholar] [CrossRef]
- Yao, X.; Shen, J.; Liu, Q.; Fa, H.; Yang, M.; Hou, C. A Novel Electrochemical Aptasensor for the Sensitive Detection of Kanamycin Based on UiO-66-NH2/MCA/MWCNT@rGONR Nanocomposites. Anal. Methods 2020, 12, 4967–4976. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Wei, X.; Gu, Q.; Chen, M.; Zhang, J.; Mo, S.; Wang, J.; Xue, L.; Ding, Y.; et al. Amplified Electrochemical Antibiotic Aptasensing Based on Electrochemically Deposited AuNPs Coordinated with PEI-Functionalized Fe-Based Metal-Organic Framework. Microchim. Acta 2021, 188, 286. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Dai, X.; Xu, W.; Zhan, F.; Liu, Y.; Wang, Q. Aptamer Tuned Nanozyme Activity of Nickel-Metal–Organic Framework for Sensitive Electrochemical Aptasensing of Tetracycline Residue. Food Chem. 2024, 430, 137041. [Google Scholar] [CrossRef]
- Reville, E.K.; Sylvester, E.H.; Benware, S.J.; Negi, S.S.; Berda, E.B. Customizable Molecular Recognition: Advancements in Design, Synthesis, and Application of Molecularly Imprinted Polymers. Polym. Chem. 2022, 13, 3387–3411. [Google Scholar] [CrossRef]
- Veloz Martínez, I.; Ek, J.I.; Ahn, E.C.; Sustaita, A.O. Molecularly Imprinted Polymers via Reversible Addition–Fragmentation Chain-Transfer Synthesis in Sensing and Environmental Applications. RSC Adv. 2022, 12, 9186. [Google Scholar] [CrossRef]
- Moro, G.; Bottari, F.; Sleegers, N.; Florea, A.; Cowen, T.; Moretto, L.M.; Piletsky, S.; De Wael, K. Conductive Imprinted Polymers for the Direct Electrochemical Detection of β-Lactam Antibiotics: The Case of Cefquinome. Sens. Actuators B Chem. 2019, 297, 126786. [Google Scholar] [CrossRef]
- Seguro, I.; Rebelo, P.; Pacheco, J.G.; Delerue-Matos, C. Electropolymerized, Molecularly Imprinted Polymer on a Screen-Printed Electrode—A Simple, Fast, and Disposable Voltammetric Sensor for Trazodone. Sensors 2022, 22, 2819. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Pang, C.; Li, H.; Liu, C.; Xu, Z.; Luo, J.; Yang, Y. Novel Molecularly Imprinted Amoxicillin Sensor Based on a Dual Recognition and Dual Detection Strategy. Anal. Chim. Acta 2020, 1127, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Vu, O.T.; Nguyen, Q.H.; Nguy Phan, T.; Luong, T.T.; Eersels, K.; Wagner, P.; Truong, L.T.N. Highly Sensitive Molecularly Imprinted Polymer-Based Electrochemical Sensors Enhanced by Gold Nanoparticles for Norfloxacin Detection in Aquaculture Water. ACS Omega 2022, 8, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.C.; Ayankojo, A.G.; Reut, J.; Rappich, J.; Furchner, A.; Hinrichs, K.; Syritski, V. Molecularly Imprinted Co-Polymer for Class-Selective Electrochemical Detection of Macrolide Antibiotics in Aqueous Media. Sens. Actuators B Chem. 2023, 374, 132768. [Google Scholar] [CrossRef]
- Frigoli, M.; Caldara, M.; Royakkers, J.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Gold Screen-Printed Electrodes Coupled with Molecularly Imprinted Conjugated Polymers for Ultrasensitive Detection of Streptomycin in Milk. Microchem. J. 2024, 200, 110433. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Cordeiro, M.N.D.S.; Melo, A.; Delerue-Matos, C. Azithromycin Electrochemical Detection Using a Molecularly Imprinted Polymer Prepared on a Disposable Screen-Printed Electrode. Anal. Methods 2020, 12, 1486–1494. [Google Scholar] [CrossRef]
- Shi, X.; Zuo, Y.; Jia, X.; Wu, X.; Jing, N.; Wen, B.; Mi, X. A Novel Molecularly Imprinted Sensor Based on Gold Nanoparticles/Reduced Graphene Oxide/Single-Walled Carbon Nanotubes Nanocomposite for the Detection of Pefloxacin. Int. J. Electrochem. Sci. 2020, 15, 9683–9697. [Google Scholar] [CrossRef]
- Abera, B.D.; Ortiz-gómez, I.; Shkodra, B.; Romero, F.J.; Cantarella, G.; Petti, L.; Salinas-castillo, A.; Lugli, P.; Rivadeneyra, A. Laser-induced Graphene Electrodes Modified with a Molecularly Imprinted Polymer for Detection of Tetracycline in Milk and Meat. Sensors 2022, 22, 269. [Google Scholar] [CrossRef]
- Turco, A.; Corvaglia, S.; Pompa, P.P.; Malitesta, C. An Innovative and Simple All Electrochemical Approach to Functionalize Electrodes with a Carbon Nanotubes/Polypyrrole Molecularly Imprinted Nanocomposite and Its Application for Sulfamethoxazole Analysis. J. Colloid Interface Sci. 2021, 599, 676–685. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, J.; Qu, J.; Huang, J.; Tan, R.; Yu, Y.; Wu, J.; Yang, J.; Li, Y.; et al. A Bifunctional Electrochemical Sensor for Simultaneous Determination of Electroactive and Non-Electroactive Analytes: A Universal yet Very Effective Platform Serving Therapeutic Drug Monitoring. Biosens. Bioelectron. 2022, 208, 114233. [Google Scholar] [CrossRef]
- Zeb, S.; Wong, A.; Khan, S.; Hussain, S.; Sotomayor, M.D.P.T. Using Magnetic Nanoparticles/MIP-Based Electrochemical Sensor for Quantification of Tetracycline in Milk Samples. J. Electroanal. Chem. 2021, 900, 115713. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Cai, L.; Wang, H.; Zhang, B.; Fang, G.; Wang, S. A “Signal on/off” Biomimetic Electrochemiluminescence Sensor Using Titanium Carbide Nanodots as Co-Reaction Accelerator for Ultra-Sensitive Detection of Ciprofloxacin. Anal. Chim. Acta 2022, 1206, 339690. [Google Scholar] [CrossRef] [PubMed]
- Koçak, I. ZnO and Au Nanoparticles Supported Highly Sensitive and Selective Electrochemical Sensor Based on Molecularly Imprinted Polymer for Sulfaguanidine and Sulfamerazine Detection. J. Pharm. Biomed. Anal. 2023, 234, 115518. [Google Scholar] [CrossRef] [PubMed]
- Surya, S.G.; Khatoon, S.; Lahcen, A.A.; Nguyen, A.T.H.; Dzantiev, B.B.; Tarannum, N.; Salama, K.N. A Chitosan Gold Nanoparticles Molecularly Imprinted Polymer Based Ciprofloxacin Sensor. RSC Adv. 2020, 10, 12823–12832. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, T.; Zhang, Y.; Ren, X.; Wang, Y.; Ma, H.; Wei, Q. Electrochemical Assay of Ampicillin Using Fe3N-Co2N Nanoarray Coated with Molecularly Imprinted Polymer. Microchim. Acta 2020, 187, 442. [Google Scholar] [CrossRef]
- Güney, S.; Arslan, T.; Yanık, S.; Güney, O. An Electrochemical Sensing Platform Based on Graphene Oxide and Molecularly Imprinted Polymer Modified Electrode for Selective Detection of Amoxicillin. Electroanalysis 2021, 33, 46–56. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Ma, J.; Wei, S.; Zhang, H. A Novel Electrochemical Sensor Based on Molecularly Imprinted Polymer with Binary Functional Monomers at Fe-Doped Porous Carbon Decorated Au Electrode for the Sensitive Detection of Lomefloxacin. Ionics 2020, 26, 4183–4192. [Google Scholar] [CrossRef]
- Stoian, I.A.; Iacob, B.C.; Dudaș, C.L.; Barbu-Tudoran, L.; Bogdan, D.; Marian, I.O.; Bodoki, E.; Oprean, R. Biomimetic Electrochemical Sensor for the Highly Selective Detection of Azithromycin in Biological Samples. Biosens. Bioelectron. 2020, 155, 112098. [Google Scholar] [CrossRef]
- Li, S.; Pang, C.; Ma, X.; Wu, Y.; Wang, M.; Xu, Z.; Luo, J. Aggregation-Induced Electrochemiluminescence and Molecularly Imprinted Polymer Based Sensor with Fe3O4@Pt Nanoparticle Amplification for Ultrasensitive Ciprofloxacin Detection. Microchem. J. 2022, 178, 107345. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly Imprinted Polymer-Based Sensor for Electrochemical Detection of Erythromycin. Talanta 2020, 209, 120502. [Google Scholar] [CrossRef] [PubMed]
- Wilkirson, E.C.; Singampalli, K.L.; Li, J.; Dixit, D.D.; Jiang, X.; Gonzalez, D.H.; Lillehoj, P.B. Affinity-Based Electrochemical Sensors for Biomolecular Detection in Whole Blood. Anal. Bioanal. Chem. 2023, 415, 3983. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, X.; Shen, Y.; Zhang, C. Immobilization of Antibodies on Aldehyde-Functionalized Polymer/Graphene Films for the Fabrication of a Label-Free Electrochemical Immunosensor. J. Electroanal. Chem. 2015, 759, 67–71. [Google Scholar] [CrossRef]
- Aymard, C.; Kanso, H.; Serrano, M.J.; Pagán, R.; Noguer, T.; Istamboulie, G. Development of a New Dual Electrochemical Immunosensor for a Rapid and Sensitive Detection of Enrofloxacin in Meat Samples. Food Chem. 2022, 370, 131016. [Google Scholar] [CrossRef]
- Police Patil, A.V.; Chuang, Y.S.; Li, C.; Wu, C.C. Recent Advances in Electrochemical Immunosensors with Nanomaterial Assistance for Signal Amplification. Biosensors 2023, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; de la Escosura-Muñiz, A. Nanoparticles as Emerging Labels in Electrochemical Immunosensors. Sensors 2019, 19, 5137. [Google Scholar] [CrossRef]
- Pollap, A.; Kochana, J. Electrochemical Immunosensors for Antibiotic Detection. Biosensors 2019, 9, 61. [Google Scholar] [CrossRef]
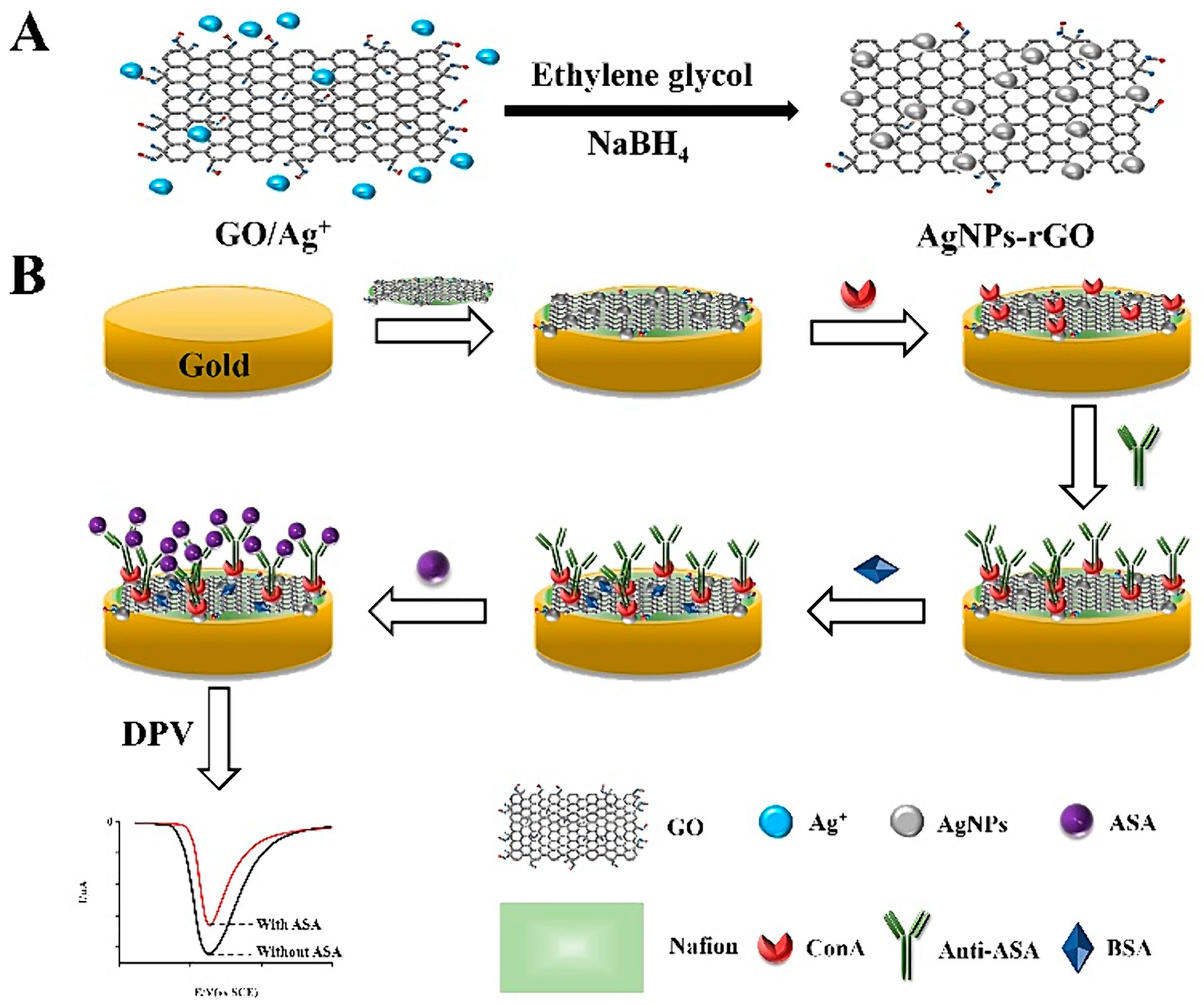
- You, X.; Chen, Y.; Zhou, J.; Liu, H.; Liu, Y.; Qi, Y.; Liang, C.; Ding, P.; Zhu, X.; Wang, A.; et al. Concanavalin A as Carrier for Sensitive Electrochemical Immunosensor Based on AgNPs-RGO Signal Amplification. J. Electroanal. Chem. 2024, 961, 118214. [Google Scholar] [CrossRef]
- Kumar, R.; Lakshmi, G.B.V.S.; Dhiman, T.K.; Singh, K.; Solanki, P.R. Highly Sensitive Amoxicillin Immunosensor Based on Aqueous Vanadium Disulphide Quantum Dots. J. Electroanal. Chem. 2021, 892, 115266. [Google Scholar] [CrossRef]
- Kolhe, P.; Roberts, A.; Gandhi, S. Fabrication of an Ultrasensitive Electrochemical Immunosensor Coupled with Biofunctionalized Zero-Dimensional Graphene Quantum Dots for Rapid Detection of Cephalexin. Food Chem. 2023, 398, 133846. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Verma, D.; Lakshmi, G.B.V.S.; Eremin, S.; Solanki, P.R. Fabrication of Label-Free and Ultrasensitive Electrochemical Immunosensor Based on Molybdenum Disulfide Nanoparticles Modified Disposable ITO: An Analytical Platform for Antibiotic Detection in Food Samples. Food Chem. 2021, 363, 130245. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhu, L.; Ding, Y.; Wang, J.; Li, J.; Du, X. Determination of Sulfamethoxazole in Foods Based on CeO2/Chitosan Nanocomposite-Modified Electrodes. Mater. Sci. Eng. C 2012, 32, 2623–2627. [Google Scholar] [CrossRef]
- Sanli, S. Single-Drop Electrochemical Immunosensor with 3D-Printed Magnetic Attachment for Onsite Smartphone Detection of Amoxicillin in Raw Milk. Food Chem. 2024, 437, 137823. [Google Scholar] [CrossRef]
- Chomthong, K.; Kunpatee, K.; Pimpitak, U.; Puthong, S.; Komolpis, K.; Wonsawat, W.; Nuanualsuwan, S.; Yakoh, A.; Khongchareonporn, N.; Ruecha, N.; et al. Label-Free Simultaneous Detection of Quinolone Antibiotic Residues Using an Origami Paper–Based Electrochemical Immunosensor. Sens. Actuators B Chem. 2024, 410, 135667. [Google Scholar] [CrossRef]
- Valera, E.; Muriano, A.; Pividori, I.; Sánchez-Baeza, F.; Marco, M.P. Development of a Coulombimetric Immunosensor Based on Specific Antibodies Labeled with CdS Nanoparticles for Sulfonamide Antibiotic Residues Analysis and Its Application to Honey Samples. Biosens. Bioelectron. 2013, 43, 211–217. [Google Scholar] [CrossRef]
- Himanshu, J.K.; Lakshmi, G.B.V.S.; Singh, A.K.; Solanki, P.R. Reduced Graphene Oxide-Gadolinium Oxide-Functionalized Paper Based Immunosensor for Electrochemical Detection of Gentamicin. Biosens. Bioelectron. X 2024, 17, 100442. [Google Scholar] [CrossRef]
- Chaudhary, N.; Yadav, A.K.; Verma, D.; Sharma, J.G.; Solanki, P.R. An Electrochemical Immunosensor Based on a Nanostructured Lanthanum Oxide-Substituted Reduced Graphene Oxide Interface for Ultralow Ciprofloxacin Detection in Milk Samples. Mater. Adv. 2024, 5, 1597–1613. [Google Scholar] [CrossRef]
- Prusty, A.K.; Bhand, S. A Capacitive Immunosensor for Tetracycline Estimation Using Antibody Modified Polytyramine-Alkanethiol Ultra-Thin Film on Gold. J. Electroanal. Chem. 2020, 863, 114055. [Google Scholar] [CrossRef]
- High-End UHPLC System, 1290 Infinity II LC System|Agilent. Available online: https://www.agilent.com/en/product/liquid-chromatography/hplc-systems/analytical-hplc-systems/1290-lc-system (accessed on 2 August 2024).
- TSQ QuantisTM Triple Quadrupole Mass Spectrometer. Available online: https://www.thermofisher.com/order/catalog/product/TSQ02-10001 (accessed on 2 August 2024).
- ROSA Lateral Flow Mycotoxin Strips|Charm Sciences. Available online: https://www.charm.com/products/test-and-kits/mycotoxin-tests/rosa-lateral-flow/ (accessed on 2 August 2024).
- The Next Generation of Rapid Microbial Testing System|Neogen. Available online: https://www.neogen.com/soleris-next-generation/ (accessed on 2 August 2024).
- PA 800 Plus Pharmaceutical Analysis System. Available online: https://sciex.com/products/capillary-electrophoresis/pa-800-plus-pharmaceutical-analysis-system (accessed on 2 August 2024).
- UV-1900i: SHIMADZU (Shimadzu Corporation). Available online: https://www.shimadzu.com/an/products/molecular-spectroscopy/uv-vis/uv-vis-nir-spectroscopy/uv-1900i/index.html (accessed on 2 August 2024).
- Vanquish HPLC and UHPLC Systems—NL. Available online: https://www.thermofisher.com/uk/en/home/products-and-services/promotions/industrial/vanquish-hplc-uhplc-systems.html (accessed on 2 August 2024).
- Sciences, C. Antibiotic Testing|Milk Antibiotic Test Kits. Available online: https://www.charm.com/products/test-and-kits/antibiotic-tests/ (accessed on 2 August 2024).
- Sciex Antibiotic Testing. Available online: https://sciex.com/applications/food-and-beverage-testing/residue-analysis/antibiotic-testing (accessed on 2 August 2024).
- Bruker Beta-Lactamase Activity Detection|Bruker. Available online: https://www.bruker.com/en/products-and-solutions/microbiology-and-diagnostics/microbial-identification/beta-lactamase-activity-detection.html (accessed on 2 August 2024).
- Jeol The NMR Application for Medicinal Screening|Applications Notes|JEOL Ltd. Available online: https://www.jeol.com/solutions/applications/details/NM170019.php (accessed on 2 August 2024).
- Waters Method Development of Ten Antibiotic Compounds Using a Systematic Screening Protocol on an ACQUITY Arc with UV and QDa Detection|Waters. Available online: https://www.waters.com/nextgen/ee/en/library/application-notes/2021/method-development-of-ten-antibiotic-compounds-using-a-systematic-screening-protocol-on-an-acquity-arc-with-uv-and-qda-detection.html (accessed on 2 August 2024).
- Charm II System|Charm Sciences Food Safety Solutions. Available online: https://www.charm.com/products/equipment/monitoring-systems/charm-ii-system/ (accessed on 2 August 2024).
- RIDASCREEN® Sulfonamide—Food & Feed Analysis. Available online: https://food.r-biopharm.com/products/ridascreen-sulfamethazin/ (accessed on 2 August 2024).
- Neogen Releases Easiest Dairy Antibiotic Tests|Neogen. Available online: https://www.neogen.com/en-gb/neocenter/press-releases/neogen-releases-easiest-dairy-antibiotic-tests/ (accessed on 2 August 2024).
- Q Exactive Orbitrap Mass Spectrometers|Thermo Fisher Scientific—NL. Available online: https://www.thermofisher.com/nl/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-systems/orbitrap-lc-ms/q-exactive-orbitrap-mass-spectrometers.html (accessed on 2 August 2024).
- Chen, Q.; Pan, X.D.; Huang, B.F.; Han, J.L.; Zhou, B. Screening of Multi-Class Antibiotics in Pork Meat by LC-Orbitrap-MS with Modified QuEChERS Extraction. RSC Adv. 2019, 9, 28119–28125. [Google Scholar] [CrossRef]
- SNAP Beta-Lactam ST Test: Detects Beta-Lactam Residues—IDEXX US. Available online: https://www.idexx.com/en/milk/dairy-tests/snap-beta-lactam-st/ (accessed on 2 August 2024).
- RIDASCREEN® Chloramphenicol—Food & Feed Analysis. Available online: https://food.r-biopharm.com/products/ridascreen-chloramphenicol/ (accessed on 2 August 2024).
- MaxSignal® Beta-Lactam ELISA Kit|PerkinElmer. Available online: https://www.perkinelmer.com/product/maxsignal-beta-lactam-elisa-kit-food-1065-01d (accessed on 2 August 2024).
- AMP’D® ELISA Signal Amplification Kit—Enzo. Available online: https://www.enzo.com/product/ampd-elisa-signal-amplification-kit/ (accessed on 2 August 2024).
- Meizheng Accurate Antibiotics ELISA Test Kits|Meizheng. Available online: https://mzfoodtest.com/product-category/technologies/elisa-test-kits-technologies/antibiotics-elisa-test-kits/ (accessed on 2 August 2024).
- Meizheng Nitroimidazole ELISA Test Kit for Animal Tissues and Eggs. Available online: https://mzfoodtest.com/product/nitroimidazole-elisa-test-kit-for-animal-tissues-and-eggs/ (accessed on 2 August 2024).
- Diagnostics, C. ELISA Kits for Drug Residues Detection—Creative Diagnostics. Available online: https://www.creative-diagnostics.com/news-elisa-kits-for-drug-residues-detection-73.htm (accessed on 2 August 2024).
- Neogen Dairy Antibiotic Testing|Food Safety|Neogen. Available online: https://www.neogen.com/categories/dairy-residues/ (accessed on 2 August 2024).
- Gold Standard Diagnostic SENSISpec Tetracycline ELISA, 96 Tests. Available online: https://www.goldstandarddiagnostics.com/products/veterinary-drug-residues/sensispec-tetracycline-elisa.html (accessed on 2 August 2024).
- Abcam Kanamycin ELISA Kit (Ab287806/K4210-100)|Abcam, Netherlands. Available online: https://www.abcam.com/en-nl/products/elisa-kits/kanamycin-elisa-kit-ab287806 (accessed on 2 August 2024).
- MP Multi Panel Drug Tests—Test Kits for Screening Drugs in Urine & Testing Strips. Available online: https://www.mpbio.com/us/diagnostics/drugs-of-abuse (accessed on 2 August 2024).
- Biovision Gentamicin ELISA Kit, BioVision, Inc.|VWR. Available online: https://us.vwr.com/store/product/23608718/gentamicin-elisa-kit-biovision-inc (accessed on 2 August 2024).
- Randox General Biochip—Antimicrobial Array I Ultra—Randox Food. Available online: https://www.randoxfood.com/biochip/antimicrobial-array-i-ultra/ (accessed on 2 August 2024).
- Sciences, C. SLBL—Charm SL Beta-Lactam Test—Charm Sciences. Available online: https://www.charm.com/products/test-and-kits/antibiotic-tests/rosa-lateral-flow/slbl-charm-sl-beta-lactam-test/ (accessed on 2 August 2024).
- Metrohm Gentamicin Sulfate Using Pulsed Amperometric Detection in Accordance with Ph. Eur.|Metrohm. Available online: https://www.metrohm.com/en_nl/applications/application-notes/aa-p-001-100/an-p-037.html (accessed on 2 August 2024).
- PalmSens PalmSens4—PalmSens. Available online: https://www.palmsens.com/product/palmsens4/ (accessed on 2 August 2024).
- Metrohm Screen-Printed Electrodes Archivos—Metrohm. Available online: https://metrohm-dropsens.com/category/electrodes/screen-printed-electrodes/ (accessed on 2 August 2024).
- PalmSens Sensit Smart—PalmSens. Available online: https://www.palmsens.com/product/sensit-smart/ (accessed on 2 August 2024).
- ZP ZP—Commercializing a Sensor for Antibiotic Detection|Zimmer & Peacock AS. Available online: https://www.zimmerpeacock.com/2020/07/12/zp-commercializing-a-sensor-for-antibiotic-detection/ (accessed on 2 August 2024).
- Analytik Jena Multi EA 5100—CNSX Micro-Elemental Analyzer—Analytik Jena. Available online: https://www.analytik-jena.com/products/chemical-analysis/combustion-elemental-analysis/cnsx/multi-ea-5100-for-micro-elemental-analysis/ (accessed on 2 August 2024).
| Biorecognition Element | Advantages | Disadvantages |
|---|---|---|
| Antibody Enzyme | Selectivity, Reusability, High specificity, Versatility | Reproducibility, Batch variations, Processing stability, Cost; Limited shelf-life |
| Nucleic Acids Aptamers | Sensitivity, Reproducibility | Cost, Non-specific binding interactions, Stability |
| Molecularly imprinted polymers | Stability, Reusability, Low cost | Reproducibility, Template removal from cavities |
| Surface imprinted polymers | Selectivity, Robustness | Scalability, Template availability |
| Sensor Type | Target Molecule | Method of Detection | Limit of Detection | Sample | Ref. |
|---|---|---|---|---|---|
| Modified nanocomposite including multi-walled carbon nanotubes (MWCNTs), gold nanoparticles (AuNPs), reduced graphene oxide (rGO), chitosan (CS), and modified nanosheets to bind with OTC-specific aptamer | Oxytetracycline | DPV | 30 pM | Spiked milk samples | [95] |
| Dual-labeled multiple aptasensor using RNA-based aptamer strands, semiconductor quantum dots (QDs), and gold nanoshells (AuNSs). Conjugation of biotinylated aptamers to SA-coated sadmium sulfide (CdS) and lead sulfide (PbS) QDs. | Kanamycin Tobramycin | DPV | 0.12 nM 0.49 nM | Spiked milk | [96] |
| Aptamer (Apt) BSA/Apt/indole/MWCNTs/GCE with the synergistic amplification of multi-walled carbon nanotubes (MWCNTs) and indole | Sulfadoxine | DPV | 0.033 μM | buffer | [97] |
| Novel 3D honeycombed goldnanovoids@aptamer nanostructured platform with ferrocene labeling on aptamer strands | Tetracycline | DPV | 1.2 nM | Wastewater | [98] |
| Aptamer sequence bonded onto bismuth oxide nanofibers paired with AuNPs | Ampicillin | DPV | 0.88 nM | Water and milk | [99] |
| Exonuclease III (Exo III)-aided target-aptamer binding recycling (ETBR) activated bipedal DNA machine | Kanamycin | CV DPV | 7.1fM | buffer | [100] |
| Polyethyleneimine grafted rGO and titanium dioxide nanocomposite material | Ciprofloxacin | DPV EIS | 0.7 nM | Real water samples | [101] |
| Aptasensor prepared using AuNPs combined with ferroferric oxide-multi walled carbon nanotube (Fe3O4-MWCNTs) complex | Penicillin antibiotics (PENs) | CV DPV EIS | 0.667 nM | Spiked milk samples | [102] |
| DNA aptamer and partially complementary short chain assembled onto integrated portable plastic gold electrode (PGE) through Au-S bonds. | Kanamycin | SWV CV | 0.40 μM | Buffer | [93] |
| Thiolated aptamer labeled with ferrocene covalently co-immobilized onto a gold electrode surface with 6-mercaptohexan-1-ol | Tetracycline | SWV | 0.20 nM | Spiked milk | [94] |
| Nanocomposite comprising a functionalized MOF, a MWCNT@reduced graphene oxide nanoribbon, and a covalent organic framework (COF). cDNA strands with terminal amino groups anchored on the surface, as well as penetration into the pores | Kanamycin | SWV CV | 13 nM | Fish Meat Milk | [103] |
| Amplified aptasensor using Immobilized DNA strands on AuNPs/Fe-based metal organic framework (MOF) | Tobramycin | SWV | 56 pM | Spiked milk samples | [104] |
| MOF of Ni2+-2,3,6,7,10,11-hexahydroxytriphenylene (Ni-HHTP) coated on a SPE, followed by non-covalent adsorption of tetracycline aptamer (TC-Apt) through the π-stacking | Tetracycline | CV SWV | 1.9 pM | buffer | [105] |
| Sensor Type | Target Molecule | Method of Detection | Limit of Detection | Sample | Ref. |
|---|---|---|---|---|---|
| A composite o0066 chitosan (CH) and thioglycolic acid capped vanadium sulfide quantum dots (TGA-VS2QDs) was constructed on glass substrate coated with ITO film to form electrodes on which monoclonal antibodies (mAb) for amoxicillin were immobilized | Amoxicillin | DPV | 1.65 pM | Spiked fish | [136] |
| Immunosensor based on AgNPs-reduced graphene oxide (AgNPs-rGO) and staphylococcal protein A (SPA) that was targeted to immobilize mAb | Virginiamycin M1 | DPV | 0.18 ng mL−1 | Meat | [58] |
| A cephalexin–bovine serum albumin (CFX-BSA) conjugate was developed to create antibodies (Abs). Graphene quantum dots (GQDs) were used for signal enhancement | Cephalexin | DPV | 0.53 fM | Spiked animal source food | [137] |
| Anti-quinolone Ab immobilized onto screen-printed dual carbon electrodes | Enrofloxacin | DPV | 3 ng mL−1 | Meat | [131] |
| molybdenum disulfide nanoparticles (nMoS2NPs) deposited on ITO-coated glass substrate with Abs bonded through amide linkages | Ampicillin | DPV | 0.028 µg mL−1 | Milk, orange juice, and tap water | [138] |
| Sensor based on AgNPs-rGO nanocomposite and concanavalin A (ConA) that was bound to mAbs through lectin–sugar interactions | Arsanilic acid | DPV | 0.008 ng mL−1 | Buffer, chicken, eggs | [135] |
| Nanocomposite-modified glass carbon electrode (GCE) with a biospecific CeO2-chitosan (CHIT)-modified nanocomposite on which polyclonal Abs were immobilized | Sulfamethoxazole | DPV | 1.3 nM | Buffer or food | [139] |
| Immunosensor platform based on Ab-conjugated magnetic particles on an electrode surface that uses a 3D cell to accumulate the analyte | Amoxicillin | SWV | 0.44 µM | Raw milk | [140] |
| Origami paper-based analytical device (oPAD) with multiple Ab zones for simultaneous quinolone residue detection | Norfloxacin, Enrofloxacin | SWV | 2.02 ng mL−1 1.70 ng mL−1 | Food | [141] |
| A graphite composite electrode (GEC), biofunctionalized magnetic μ-particles, and electrochemical nanoprobes prepared by labeling specific antibodies with CdS nanoparticles (CdSNP). | Sulfapyridine | SWV | 0.11 μg kg−1 | Honey | [142] |
| rGO-gadolinium oxide nanocomposite (rGO@Gd2O3 NC) with suspended mAbs on a SPE | Gentamicin | CV | 0.424 pM | Milk | [143] |
| Nanoconstructed lanthanum oxide nanoparticle-decorated reduced graphene oxide nanocomposite (nLa2O3 NP@rGO)-based platform functionalized with 3-aminopropyltriethoxysilane (APTES) and attachment on ITO-coated substrate. mAbs immobilized onto surface. | Ciprofloxacin | CV DPV EIS | 0.055 μg mL−1 | Milk | [144] |
| Tyramine (TA) electropolymerized resulting in an ultrathin polytyramine (PTA) film on a gold-coated silicon electrode (AuE) modified with polyclonal antibodies | Tetracycline | EIS | 0.01 μg L−1 | Spiked buffer | [145] |
| Company | Technology | Product | LoD | Time Required | Sample Types | Ref |
|---|---|---|---|---|---|---|
| ThermoFisher Scientific | HPLC and MS | Vanquish UHPLC and Orbitrap MS | Ppb levels | 30–60 min per sample | Food, water, biological fluids | [152] |
| Charm Sciences | Microbiological Assays | ROSA Lateral Flow System | Low ppb levels | 1–2 h per batch | Food, dairy products | [153] |
| Sciex | CE | Capillary Electrophoresis System | Low ng/mL range | 20–40 min per sample | Water, biological fluids, pharmaceuticals | [154] |
| Bruker Corporation (Billerica, MA, USA) | MS | MBT STAR-Carba IVD | Low ppb levels | 10–20 min per sample | Food, water, pharmaceuticals | [155] |
| JEOL Ltd. (Tokyo, Japan) | NMR | ECZ500R NMR Spectrometer | Ppb levels | 1–2 h per sample | Biological fluids, complex mixtures | [156] |
| Waters Corporation | LC-MS/UV–Vis | Xevo TQ-S micro LC-MS/MS System | Ppt levels | 30–60 min per sample | Food, biological fluids, environmental samples | [157] |
| Company | Product | Sample Types | Time Required | LoD | Ref |
|---|---|---|---|---|---|
| Meizheng (Rizhoa, China) | ELISA Kit | Food, water, biological tissues | 1–2 h | 0.05 ppb | [167] |
| Meizheng | Nitroimidazole ELISA Test Kit | Animal tissues and eggs | 1–2 h | 0.2 ppb | [168] |
| Creative Diagnostics (Shirley, NY, USA) | ELISA Kits for Drug Residues Detection | Water, food, Biological tissues | 2 h | 0.01–1.5 ppb | [169] |
| Neogen Corporation | Veratox for Tetracyclines | Dairy | 30 min | 1 ppb | [170] |
| Gold Standard Diagnostics (Warminster, PA, USA) | SENSISpec Tetracycline ELISA | Meat, milk, shrimp, and honey | 1–2 h | 0.05–2 ppb | [171] |
| Abcam (Cambridge, UK) | Antibiotic Residue Detection ELISA Kit | Tissues, Milk | 1.5 h | 0.1 ppb | [172] |
| MP Biomedicals (Irvine, CA, USA) | Quick Test Kit for Antibiotics | Milk, meat, seafood | 1 h | 0.2 ppb | [173] |
| BioVision (Zurich, Switzerland) | Antibiotic Residue ELISA Kit | Milk, Tissues | 2 h | 0.3 ppb | [174] |
| Company | Product | Sample Types | Time Required | LoD | Ref |
|---|---|---|---|---|---|
| Randox Food Diagnostics | Antibiotic Array | Milk, meat, fish | 30 min | 0.5 ppb | [175] |
| Charm Sciences Inc. | SLBL Beta-Lactam Test | Dairy products | ~5 min | 1 ppb | [176] |
| Metrohm (Bangkok, Thailand) | AN-P-037 | Food, water | 1 h | 0.1 ppb | [177] |
| PalmSens | PalmSens4 Potentiostat | Food, environmental samples | ~30 min | 0.05 ppb | [178] |
| Dropsens (Metrohm) | Screen-Printed Electrodes | Food, water | ~20 min | 0.01–10 ppb | [179] |
| PalmSens | Sensit Smart | Food, clinical samples | ~45 min | 0.1 ppb | [180] |
| ZP (Zimmer and Peacock) (Skoppum, Norway) | ZP Anapot | Environmental samples | 30 min | 0.05 ppb | [181] |
| Analytik Jena (Jena, Germany) | Multi EA 5100 | Water, food, biological samples | 1 h | 0.2 ppb | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigoli, M.; Krupa, M.P.; Hooyberghs, G.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Electrochemical Sensors for Antibiotic Detection: A Focused Review with a Brief Overview of Commercial Technologies. Sensors 2024, 24, 5576. https://doi.org/10.3390/s24175576
Frigoli M, Krupa MP, Hooyberghs G, Lowdon JW, Cleij TJ, Diliën H, Eersels K, van Grinsven B. Electrochemical Sensors for Antibiotic Detection: A Focused Review with a Brief Overview of Commercial Technologies. Sensors. 2024; 24(17):5576. https://doi.org/10.3390/s24175576
Chicago/Turabian StyleFrigoli, Margaux, Mikolaj P. Krupa, Geert Hooyberghs, Joseph W. Lowdon, Thomas J. Cleij, Hanne Diliën, Kasper Eersels, and Bart van Grinsven. 2024. "Electrochemical Sensors for Antibiotic Detection: A Focused Review with a Brief Overview of Commercial Technologies" Sensors 24, no. 17: 5576. https://doi.org/10.3390/s24175576
APA StyleFrigoli, M., Krupa, M. P., Hooyberghs, G., Lowdon, J. W., Cleij, T. J., Diliën, H., Eersels, K., & van Grinsven, B. (2024). Electrochemical Sensors for Antibiotic Detection: A Focused Review with a Brief Overview of Commercial Technologies. Sensors, 24(17), 5576. https://doi.org/10.3390/s24175576






