Rapid In Situ Near-Infrared Assessment of Tetrahydrocannabinolic Acid in Cannabis Inflorescences before Harvest Using Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth & Conditions
2.2. Instrumentation & Parameters
2.3. Sample Preparation for LCMS Analysis
2.4. Statistical Analysis
3. Results
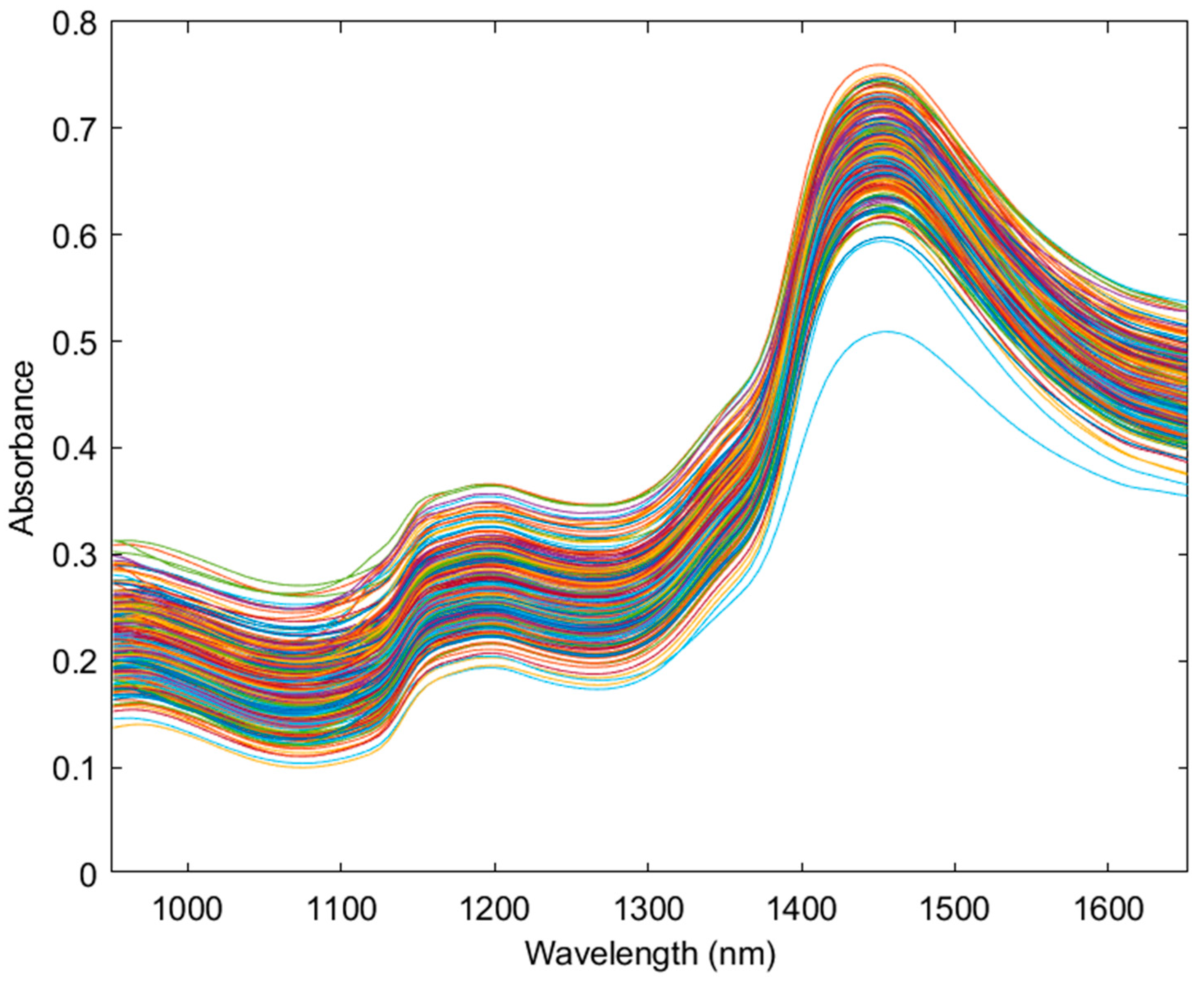
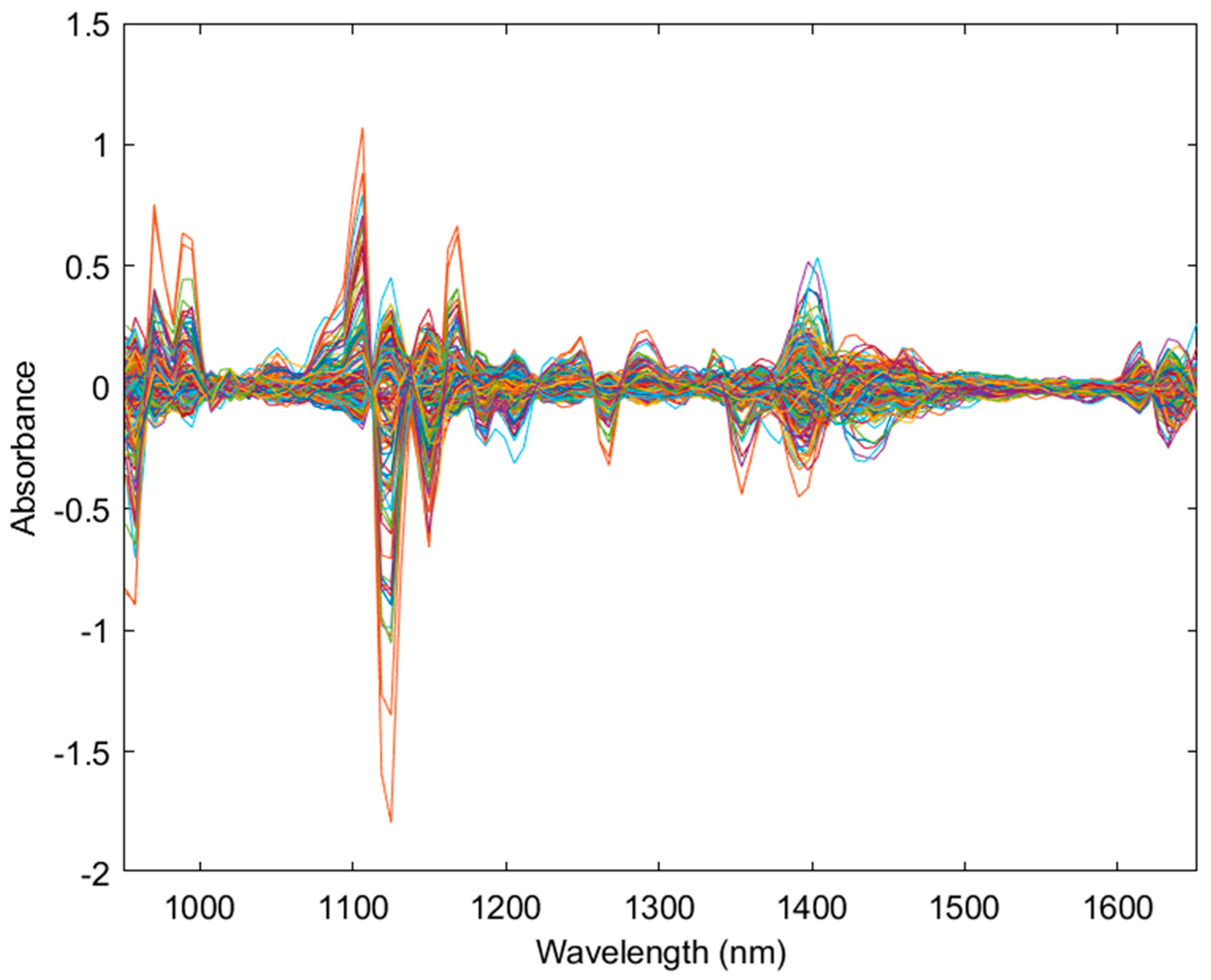
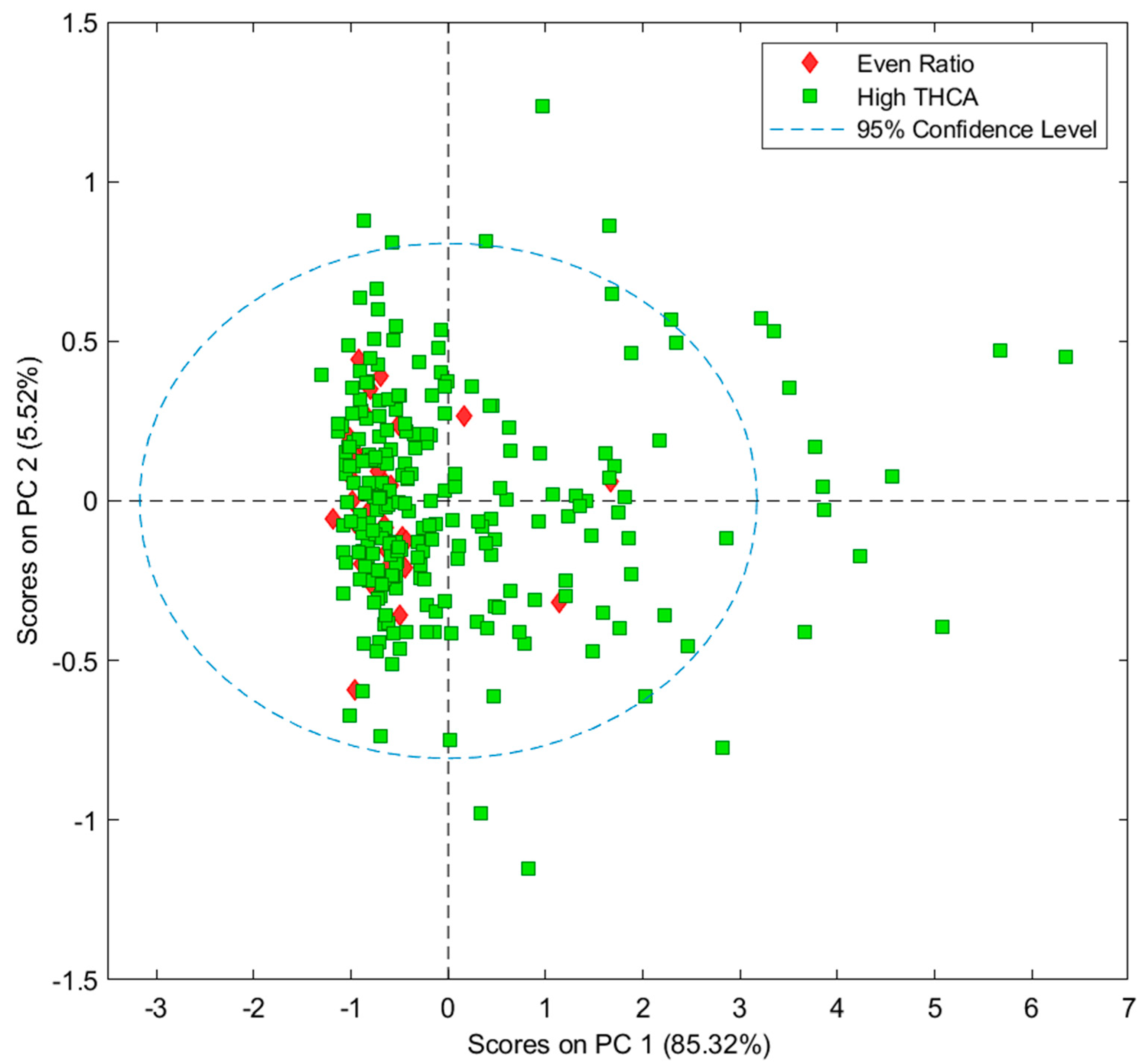
3.1. Principal Component Analysis of NIR Data
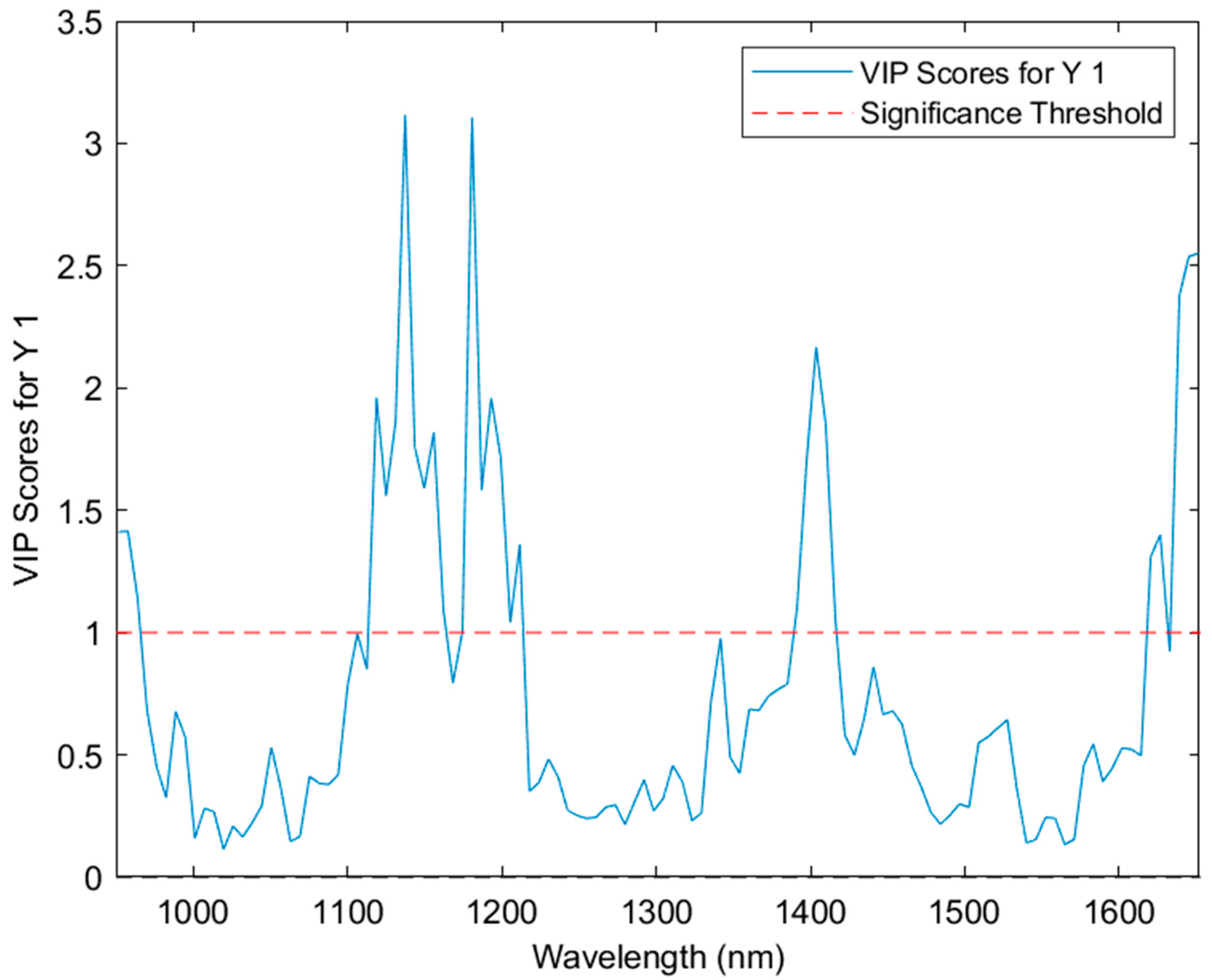
3.2. Partial Least Squares Discriminant Analysis (PLS-DA) Modelling
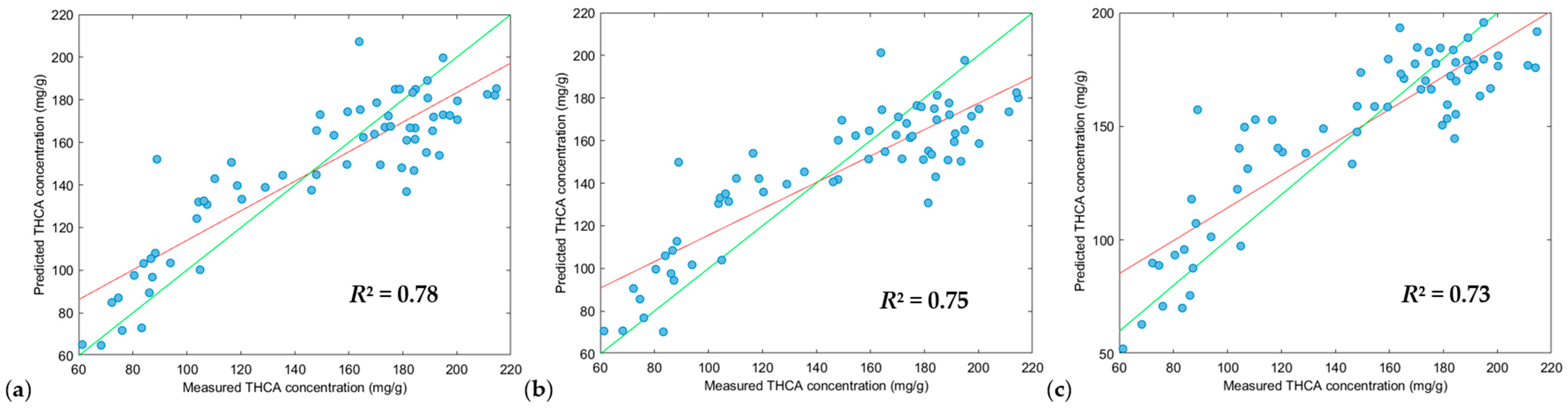
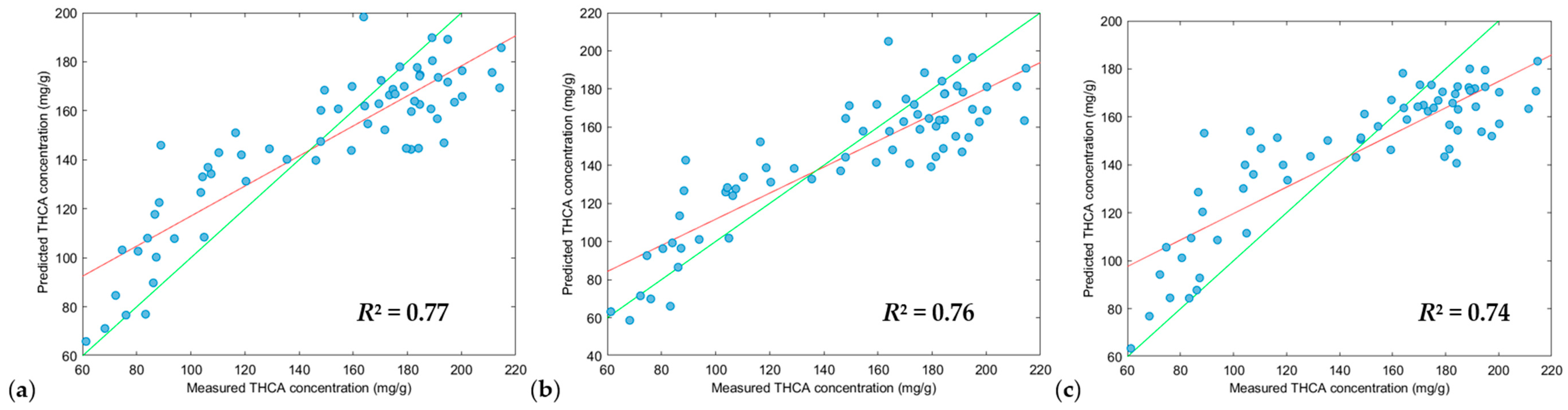
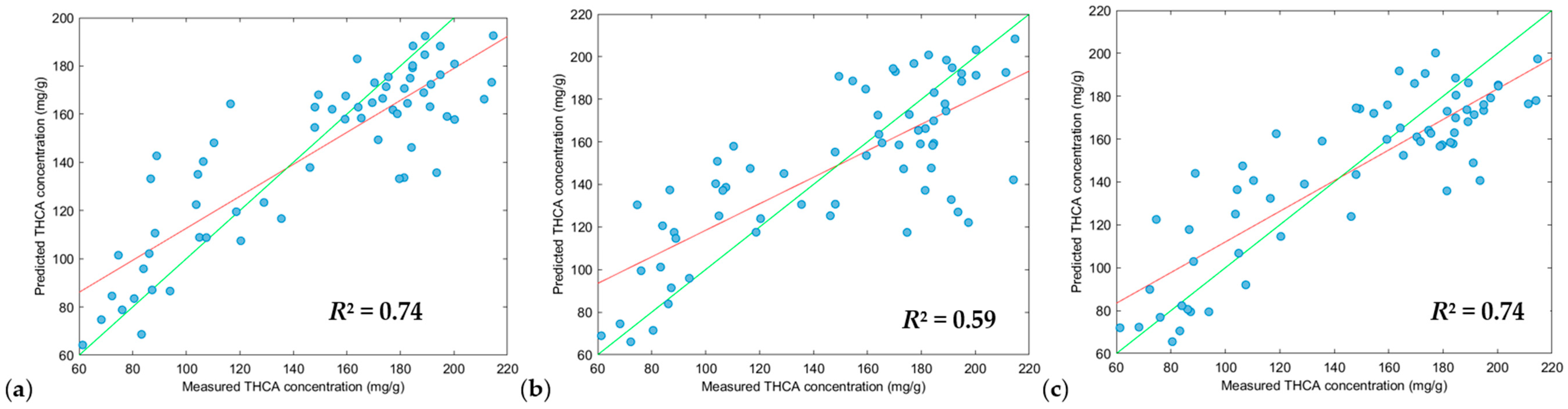
3.3. Partial Least Squares Regression (PLS-R), Support Vector Machine Regression (SVM-R) and XGBoost Regression (XGB-R) Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, Z.D.; Abrams, D.I.; Gust, S.; Salicrup, A.; Throckmorton, D.C. Challenges for Clinical Cannabis and Cannabinoid Research in the United States. J. Natl. Cancer Inst. Monogr. 2021, 2021, 114–122. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. Challenges and Barriers in Conducting Cannabis Research. In The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Farrelly, K.N.; Wardell, J.D.; Marsden, E.; Scarfe, M.L.; Najdzionek, P.; Turna, J.; MacKillop, J. The Impact of Recreational Cannabis Legalization on Cannabis Use and Associated Outcomes: A Systematic Review. Subst. Abuse. 2023, 17, 11782218231172054. [Google Scholar] [CrossRef]
- Ingvardsen, C.R.; Brinch-Pedersen, H. Challenges and potentials of new breeding techniques in Cannabis sativa. Front. Plant Sci. 2023, 14, 1154332. [Google Scholar] [CrossRef]
- Stockings, E.; Zagic, D.; Campbell, G.; Weier, M.; Hall, W.D.; Nielsen, S.; Herkes, G.K.; Farrell, M.; Degenhardt, L. Evidence for cannabis and cannabinoids for epilepsy: A systematic review of controlled and observational evidence. J. Neurol. Neurosurg. Psychiatry. 2018, 89, 741–753. [Google Scholar] [CrossRef]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. Guidance for the Use of Medicinal Cannabis in the Treatment of Multiple Scelrosis in Australia; Department of Health, Australia Government: Canberra, Australia, 2017; pp. 9–14.
- Pacitto, R.; Peters, C.; Iadipaolo, A.; Rabinak, C.A. Cannabinoid modulation of brain activation during volitional regulation of negative affect in trauma-exposed adults. Neuropharmacology 2022, 218, 109222. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The anti-inflammatory and analgesic effects of formulated full-spectrum cannabis extract in the treatment of neuropathic pain associated with multiple sclerosis. Inflamm. Res. 2020, 69, 549–558. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagne, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Pourseyed Lazarjani, M.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for quantification of cannabinoids: A narrative review. J. Cannabis Res. 2020, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Elkins, A.C.; Deseo, M.A.; Rochfort, S.; Ezernieks, V.; Spangenberg, G. Development of a validated method for the qualitative and quantitative analysis of cannabinoids in plant biomass and medicinal cannabis resin extracts obtained by super-critical fluid extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1109, 76–83. [Google Scholar] [CrossRef]
- Birenboim, M.; Kengisbuch, D.; Chalupowicz, D.; Maurer, D.; Barel, S.; Chen, Y.; Fallik, E.; Paz-Kagan, T.; Shimshoni, J.A. Use of near-infrared spectroscopy for the classification of medicinal cannabis cultivars and the prediction of their cannabinoid and terpene contents. Phytochemistry 2022, 204, 113445. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Maghirang, E.; Tan, J.W.; Yoon, J.Y.; Armstrong, P.; Kachroo, P.; Hildebrand, D. NIR spectroscopy for rapid measurement of moisture and cannabinoid contents of industrial hemp (Cannabis sativa). Ind. Crops Prod. 2022, 184, 115007. [Google Scholar] [CrossRef]
- Yao, S.; Ball, C.; Miyagusuku-Cruzado, G.; Giusti, M.M.; Aykas, D.P.; Rodriguez-Saona, L.E. A novel handheld FT-NIR spectroscopic approach for real-time screening of major cannabinoids content in hemp. Talanta 2022, 247, 123559. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Vassiliadis, S.; Elkins, A.C.; Cogan, N.O.I.; Rochfort, S.J. Developing Prediction Models Using Near-Infrared Spectroscopy to Quantify Cannabinoid Content in Cannabis Sativa. Sensors 2023, 23, 2607. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carnerero Callado, C.; Nunez-Sanchez, N.; Casano, S.; Ferreiro-Vera, C. The potential of near infrared spectroscopy to estimate the content of cannabinoids in Cannabis sativa L.: A comparative study. Talanta 2018, 190, 147–157. [Google Scholar] [CrossRef]
- Birenboim, M.; Chalupowicz, D.; Maurer, D.; Barel, S.; Chen, Y.; Fallik, E.; Paz-Kagan, T.; Rapaport, T.; Sadeh, A.; Kengisbuch, D.; et al. Multivariate classification of cannabis chemovars based on their terpene and cannabinoid profiles. Phytochemistry 2022, 200, 113215. [Google Scholar] [CrossRef] [PubMed]
- Deidda, R.; Coppey, F.; Damergi, D.; Schelling, C.; Coic, L.; Veuthey, J.L.; Sacre, P.Y.; De Bleye, C.; Hubert, P.; Esseiva, P.; et al. New perspective for the in-field analysis of cannabis samples using handheld near-infrared spectroscopy: A case study focusing on the determination of Delta(9)-tetrahydrocannabinol. J. Pharm. Biomed. Anal. 2021, 202, 114150. [Google Scholar] [CrossRef] [PubMed]
- Jarén, C.; Zambrana, P.C.; Pérez-Roncal, C.; López-Maestresalas, A.; Ábrego, A.; Arazuri, S. Potential of NIRS Technology for the Determination of Cannabinoid Content in Industrial Hemp (Cannabis sativa L.). Agronomy 2022, 12, 938. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, B. The diversity in the applications of partial least squares: An overview. J. Chemom. 2015, 30, 4–17. [Google Scholar] [CrossRef]
- Rodriguez-Perez, R.; Bajorath, J. Evolution of Support Vector Machine and Regression Modeling in Chemoinformatics and Drug Discovery. J. Comput. Aided. Mol. Des. 2022, 36, 355–362. [Google Scholar] [CrossRef]
- Cervantes, J.; Garcia-Lamont, F.; Rodríguez-Mazahua, L.; Lopez, A. A comprehensive survey on support vector machine classification: Applications, challenges and trends. Neurocomputing 2020, 408, 189–215. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Kim, N.; Lee, T.; Quagliato, L. Extreme gradient boosting-inspired process optimization algorithm for manufacturing engineering applications. Mater. Des. 2023, 226, 111625. [Google Scholar] [CrossRef]
- Ma, B.; Meng, F.; Yan, G.; Yan, H.; Chai, B.; Song, F. Diagnostic classification of cancers using extreme gradient boosting algorithm and multi-omics data. Comput. Biol. Med. 2020, 121, 103761. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chang, K.-H.; Wu, G.-J. Application of eXtreme gradient boosting trees in the construction of credit risk assessment models for financial institutions. Appl. Soft Comput. 2018, 73, 914–920. [Google Scholar] [CrossRef]
- Gallagher, N.B.; O’Sullivan, D. Selection of Representative Learning and Test Sets Using the Onion Method. Available online: https://eigenvector.com/wp-content/uploads/2020/01/Onion_SampleSelection.pdf (accessed on 9 September 2022).
- Szymanska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
- Grasel, F.S.; Ferrão, M.F. A rapid and non-invasive method for the classification of natural tannin extracts by near-infrared spectroscopy and PLS-DA. Anal. Methods 2016, 8, 644–649. [Google Scholar] [CrossRef]
- Williams, P.; Manley, M.; Antoniszyn, J. Near-Infrared Technology: Getting the Best out of Light, 1st ed.; African Sun Media: Stellenbosch, South Africa, 2019; pp. 69–102. [Google Scholar]
- Minansy, B.; McBratney, A.B. Why you don’t need to use RPD. Pedometron 2013, 33, 14–15. [Google Scholar]
- Garcia Martin, J.F. Potential of Near-Infrared Spectroscopy for the Determination of Olive Oil Quality. Sensors 2022, 22, 2831. [Google Scholar] [CrossRef] [PubMed]
- Leardi, R.; Nørgaard, L. Sequential application of backward interval partial least squares and genetic algorithms for the selection of relevant spectral regions. J. Chemom. 2004, 18, 486–497. [Google Scholar] [CrossRef]
- Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Andriamananjara, A.; Rabenarivo, M.; Asai, H.; Rakotoson, T.; Razafimbelo, T. Laboratory Visible and Near-Infrared Spectroscopy with Genetic Algorithm-Based Partial Least Squares Regression for Assessing the Soil Phosphorus Content of Upland and Lowland Rice Fields in Madagascar. Remote Sens. 2019, 11, 506. [Google Scholar] [CrossRef]


| High THCA 1 | Even Ratio 2 | |
|---|---|---|
| Sensitivity (Cal) 3 | 1.00 | 1.00 |
| Specificity (Cal) | 1.00 | 1.00 |
| Sensitivity (CV) 3 | 1.00 | 1.00 |
| Specificity (CV) | 1.00 | 1.00 |
| Sensitivity (Pred) 3 | 1.00 | 1.00 |
| Specificity (Pred) | 1.00 | 1.00 |
| Class. Err 4 (Cal) | 0.00 | 0.00 |
| Class. Err (CV) | 0.00 | 0.00 |
| Class. Err (Pred) | 0.00 | 0.00 |
| RMSEC 5 | 0.15 | 0.15 |
| RMSECV 6 | 0.15 | 0.15 |
| RMSEP 7 | 0.12 | 0.12 |
| Bias | 0.00 | 0.00 |
| CV Bias | 0.00 | 0.00 |
| Pred Bias | 0.01 | −0.01 |
| R2Cal 8 | 0.83 | 0.83 |
| R2CV | 0.82 | 0.82 |
| R2Pred 9 | 0.79 | 0.79 |
| Model | Figure Key | Region (nm) 1 | Scatter Correction 2 | Derivative 2a | N 3 | RMSEC 4 | R2Cal 5 | RMSECV 6 | R2CV 7 | RMSEP 8 | Pred Bias 9 | R2Pred 10 | RPD 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLS-R | (a) | 950–1650 | DT, SNV and MC | 2, 2, 5 | 264 | 26.34 | 0.62 | 28.87 | 0.54 | 21.49 | −0.68 | 0.78 | 2.08 |
| (b) | 950–1650 | DT, SNV and MC | 2, 2, 7 | 264 | 28.00 | 0.57 | 30.49 | 0.50 | 23.49 | −2.61 | 0.75 | 1.91 | |
| (c) | 950–1650 | DT, SNV and MC | 2, 2, 3 | 264 | 26.41 | 0.62 | 30.93 | 0.48 | 23.34 | 0.77 | 0.73 | 1.93 | |
| SVM-R | (a) | 950–1650 | DT, SNV and MC | 2, 2, 5 | 264 | 24.87 | 0.68 | 28.48 | 0.56 | 22.95 | −1.47 | 0.77 | 1.96 |
| (b) | 950–1650 | DT, SNV and MC | 2, 2, 7 | 264 | 23.87 | 0.70 | 29.40 | 0.53 | 22.49 | −3.38 | 0.76 | 2.00 | |
| (c) | 950–1650 | DT, SNV and MC | 2, 2, 3 | 264 | 25.11 | 0.68 | 30.34 | 0.51 | 24.87 | −1.80 | 0.74 | 1.81 | |
| XGB-R | (a) | 950–1650 | DT, SNV and MC | 2, 2, 5 | 264 | 12.27 | 0.93 | 31.10 | 0.48 | 23.31 | −3.46 | 0.74 | 1.88 |
| (b) | 950–1650 | DT, SNV and MC | 2, 2, 3 | 264 | 0.02 | 1.00 | 34.61 | 0.37 | 28.77 | 0.44 | 0.59 | 1.56 | |
| (c) | 950–1650 | DT and MC | 2, 2, 5 | 264 | 0.25 | 1.00 | 34.09 | 0.38 | 23.02 | −1.64 | 0.74 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, J.; Vassiliadis, S.; Elkins, A.C.; Cogan, N.O.O.; Rochfort, S.J. Rapid In Situ Near-Infrared Assessment of Tetrahydrocannabinolic Acid in Cannabis Inflorescences before Harvest Using Machine Learning. Sensors 2024, 24, 5081. https://doi.org/10.3390/s24165081
Tran J, Vassiliadis S, Elkins AC, Cogan NOO, Rochfort SJ. Rapid In Situ Near-Infrared Assessment of Tetrahydrocannabinolic Acid in Cannabis Inflorescences before Harvest Using Machine Learning. Sensors. 2024; 24(16):5081. https://doi.org/10.3390/s24165081
Chicago/Turabian StyleTran, Jonathan, Simone Vassiliadis, Aaron C. Elkins, Noel O. O. Cogan, and Simone J. Rochfort. 2024. "Rapid In Situ Near-Infrared Assessment of Tetrahydrocannabinolic Acid in Cannabis Inflorescences before Harvest Using Machine Learning" Sensors 24, no. 16: 5081. https://doi.org/10.3390/s24165081
APA StyleTran, J., Vassiliadis, S., Elkins, A. C., Cogan, N. O. O., & Rochfort, S. J. (2024). Rapid In Situ Near-Infrared Assessment of Tetrahydrocannabinolic Acid in Cannabis Inflorescences before Harvest Using Machine Learning. Sensors, 24(16), 5081. https://doi.org/10.3390/s24165081






